The current insights in macrophage heterogeneity and the evolving role of pancreas macrophages during organogenesis, tissue injury, and repair are elaborated. Additional identification of macrophage subtypes and of their secreted factors might ultimately translate into novel therapeutic strategies for both type 1 and type 2 diabetes mellitus.
Keywords: Pancreas, β Cell, Development, Injury, Regeneration, Macrophage
Abstract
Macrophages are classically considered detrimental for pancreatic β-cell survival and function, thereby contributing to β-cell failure in both type 1 (T1D) and 2 (T2D) diabetes mellitus. In addition, adipose tissue macrophages negatively influence peripheral insulin signaling and promote obesity-induced insulin resistance in T2D. In contrast, recent data unexpectedly uncovered that macrophages are not only able to protect β cells during pancreatitis but also to orchestrate β-cell proliferation and regeneration after β-cell injury. Moreover, by altering their activation state, macrophages are able to improve insulin resistance in murine models of T2D. This review will elaborate on current insights in macrophage heterogeneity and on the evolving role of pancreas macrophages during organogenesis, tissue injury, and repair. Additional identification of macrophage subtypes and of their secreted factors might ultimately translate into novel therapeutic strategies for both T1D and T2D.
Significance
Diabetes mellitus is a pandemic disease, characterized by severe acute and chronic complications. Macrophages have long been considered prime suspects in the pathogenesis of both type 1 and 2 diabetes mellitus. In this concise review, current insights in macrophage heterogeneity and on the, as yet, underappreciated role of alternatively activated macrophages in insulin sensing and β-cell development/repair are reported. Further identification of macrophage subtypes and of their secreted factors might ultimately translate into novel therapeutic strategies for diabetes mellitus.
Macrophage Origin, Diversity, and Function
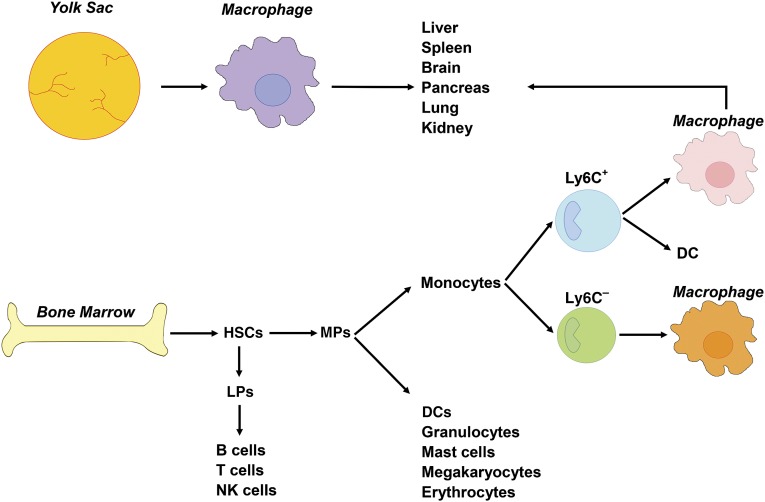
Macrophages, originally recognized for their pivotal role in innate immunity, classically belong to the mononuclear phagocytic system and are derived from circulating monocytes, in turn descending from myeloid-committed bone marrow progenitors. Monocytes are a heterogeneous population consisting of a Ly6C+ inflammatory and a Ly6C− resident or patrolling subset. After tissue infiltration, Ly6C+ monocytes can give rise to either dendritic cells or macrophages. Although less documented, Ly6C− monocytes appear to be equally able to differentiate into macrophages [1, 2] (Fig. 1).
Figure 1.
Macrophage origin in the mouse. Classically, macrophages derive from circulating monocytes, in turn descending from bone marrow progenitors. In the bone marrow, HSCs differentiate into LPs or MPs. LPs further differentiate into B cells, T cells, and NK cells. MPs give rise to DCs, granulocytes, mast cells, megakaryocytes, and erythrocytes, or to Ly6C+ inflammatory and Ly6C− resident or patrolling monocytes. After tissue infiltration, Ly6C+ monocytes give rise to either DCs or macrophages. Although less documented, Ly6C− monocytes can also differentiate into macrophages. Alternatively, adult tissue macrophages can also descend from yolk sac-derived macrophages, independently of HSCs. As such, tissue macrophages in the adult are mainly a mix of bone marrow-derived and yolk sac-derived macrophages. Abbreviations: DCs, dendritic cells; HSCs, hematopoietic stem cells; LPs, lymphoid committed precursors; MPs, myeloid committed precursors; NK, natural killer (cells).
This classic, bone marrow-dependent view of monocyte/macrophage origin has recently been challenged by the demonstration that a subpopulation of adult tissue macrophages descends from yolk sac-derived precursors during embryonic development, independently of hematopoietic stem cells [3]. In addition, fetal liver monocytes can seed embryonic tissues and give rise to several tissue macrophage populations. Interestingly, even under steady state conditions, some tissue macrophages are exclusively derived from embryonic precursors (e.g., microglia and Langerhans cells), and others are, to a large extent, monocyte-derived (e.g., gut macrophages) [4]. As such, tissue macrophages in adults are derived from both adult bone marrow and prebirth progenitors. In addition, tissue macrophages are primarily maintained through replication under steady state conditions [5].
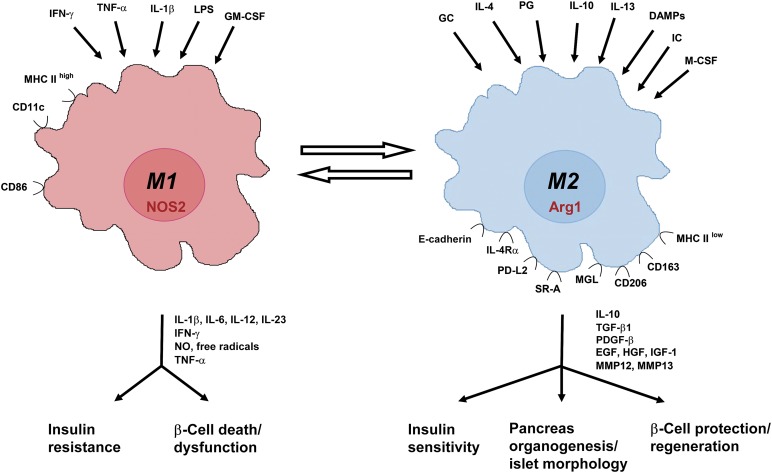
Macrophages are highly plastic cells, capable of changing their activation state in response to microenvironmental cues. Based on their functional phenotype, they can be subdivided into (a) classically activated or proinflammatory M1 macrophages, (b) alternatively activated macrophages, and (c) regulatory or anti-inflammatory macrophages. M1 macrophages are typically activated by interferon (IFN)-γ and are involved in T helper 1 (TH1)-related immune responses during infection. Alternatively, activated macrophages are induced by interleukin-4 (IL-4), IL-10, and IL-13 and are mainly present during the resolution phase of inflammation, in tumors where they sustain tumor growth, in the gut where they mediate antiparasitic actions, and in trophic processes during development and tissue repair. Regulatory macrophages, producing IL-10 and transforming growth factor-β (TGF-β), are activated by Toll-like receptor (TLR) agonists, IgG immune complexes, apoptotic cells, and prostaglandins. Similar to alternatively activated macrophages, regulatory macrophages are mainly anti-inflammatory and are involved in TH2 and regulatory T cell responses [6]. Collectively, alternatively activated and regulatory macrophages are often referred to as M2 macrophages [7]. Recently, an expert panel suggested a novel nomenclature for macrophage activation based on macrophage origin, activators, and a consensus collection of markers [8], taking into consideration the trend toward a spectrum model of macrophage activation [9]. This ongoing discussion on macrophage activation nomenclature reflects that the M1/M2 classification system is not absolute and underscores the inherent capacity of macrophages to continuously adapt their functional phenotype in response to dynamic changes in their microenvironment [6]. In addition, macrophages with a mixed phenotype have been described [10]. Therefore, the M1/M2 classification should be regarded as a dynamic spectrum of activation states, rather than as a fixed set of distinct activation states [6]. Nonetheless, for the sake of simplicity, we will continue to use the M1 and M2 subdivision throughout this report.
M1 and M2 macrophages can be recognized by the expression of typical activation-associated genes and proteins. M1 macrophages typically produce the proinflammatory cytokines IL-1β, IL-6, IL-12, and IL-23, tumor necrosis factor-α (TNF-α), and inducible nitric oxide synthase 2 (NOS2) [6]. In addition, CD11c is primarily expressed by M1, rather than M2, macrophages [11]. M2 macrophages typically produce higher amounts of IL-10 and TGF-β [6] and express genes that are associated with wound healing and angiogenesis, such as arginase-1 (Arg1). Several membrane-bound proteins have also been associated with M2 macrophage activation, including CD206 and the lectins macrophage galactose lectin 1 (MGL1) and MGL2 [11, 12] (Fig. 2).
Figure 2.
M1 versus M2 macrophages: inducers, markers, effector molecules, and function. Summary of the most important inducers and markers of M1 and M2 macrophages and their role in pancreas development, insulin sensitivity, and β-cell death, dysfunction, and regeneration. Abbreviations: Arg, arginase; CD, cluster of differentiation; DAMPs, damage associated molecular patterns; EGF, epidermal growth factor; GCs, glucocorticoids; GM-CSF, granulocyte-macrophage colony-stimulating factor; HGF, hepatocyte growth factor; ICs, immune complexes; IFN, interferon; IGF, insulin-like growth factor; IL, interleukin; IL-4Rα, IL-4 receptor α; LPS, lipopolysaccharide; M-CSF, macrophage colony-stimulating factor; MGL, macrophage galactose-type lectin; MHC, major histocompatibility complex; MMP, matrix metalloproteinase; NO, nitric oxide; NOS, nitric oxide synthase; PDGF, platelet-derived growth factor; PD-L, programmed death-ligand; PG, prostaglandin; SR, scavenger receptor; TGF, transforming growth factor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Macrophages During Pancreas Development
A better understanding of macrophage heterogeneity revealed their importance in nonimmune functions such as organ development [13]. In embryonic mice, maximal accumulation of tissue macrophages generally correlates with the peak period of organogenesis and/or cell turnover [14]. Mouse models deficient in macrophages display disturbances in key developmental processes such as tissue remodeling, apoptosis, trophic support, ductal branching, and angiogenesis [15].
Macrophages play an important role during pancreas development. Similar to the mammary gland, macrophages are recruited to the branching multipotent ductal epithelial tissue, mainly at sites at which new islets of Langerhans seem to originate (bud) from ducts [16, 17]. The importance of macrophage accumulation near sites of ongoing pancreatic cell differentiation was demonstrated in macrophage-deficient Csf1op/op mice, which carry an inactivating mutation in the Csf1 gene and which display a major β-cell mass deficit in the developing and adult pancreas, abnormal postnatal islet morphogenesis, and impaired pancreatic cell proliferation [17]. Furthermore, exogenous colony-stimulating factor 1 (CSF1; also known as macrophage [M]-CSF) stimulates an increase in β-cell number in pancreas explant cultures, concomitant with an increase in macrophage number [16]. These findings strongly indicate that macrophages are required to provide a suitable microenvironment for proper islet cell development. Vascular-derived signals are also known to play a pivotal role during pancreas development and endocrine adaptation [18–20]. Since vascular remodeling is one of the key mechanisms exerted by macrophages during development [21], macrophages likely contribute to β-cell development and adaptation, at least partially, via their effect on blood vessels. Additional research should elaborate on the exact role of macrophages and their secreted factors during branching morphogenesis and islet formation in the developing endocrine pancreas.
Macrophages in Type 1 Diabetes Mellitus
Because of the detrimental effect of macrophages on β cells and on the insulin sensitivity of liver, muscle, and fat, macrophages have become prime suspects in the pathogenesis of type 1 (T1D) and 2 (T2D) diabetes mellitus. During onset of T1D, macrophages, together with CD4+ and CD8+ autoreactive T cells, are among the first cells to infiltrate the islets of Langerhans and contribute to β-cell apoptosis and necrosis. In addition, via major histocompatibility complex class II surface expression, macrophages present β-cell-specific autoantigens [22, 23]. Depletion of macrophages by clodronate-loaded liposomes results in a reduction of inflammation and insulitis and arrests disease progression in nonobese diabetic (NOD) mice, a murine model of T1D [24, 25].
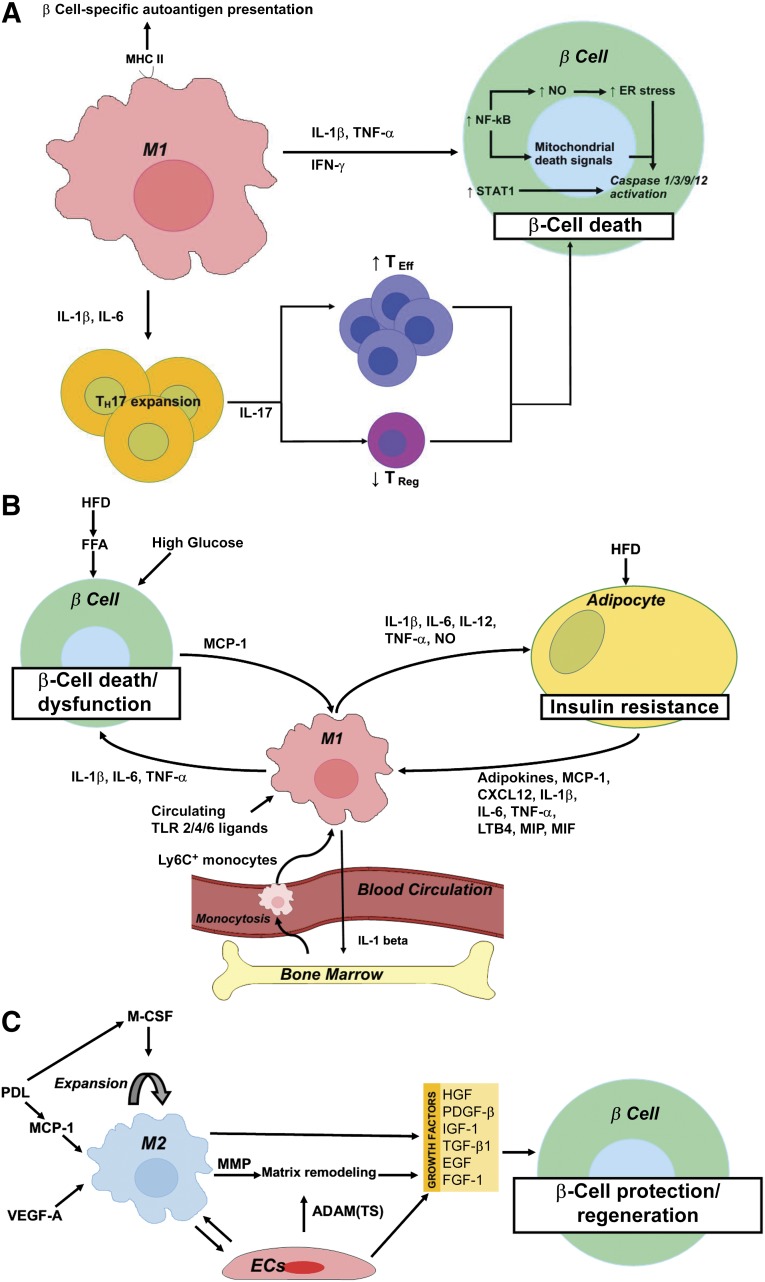
Analysis of the infiltrating immune cells in islets of patients with T1D revealed that, during the initial phase of β-cell death, CD8+ T cells constitute the most abundant immune cell population. Nonetheless, significant numbers of macrophages were detected within these early infiltrates, and their numbers remained fairly constant during all phases of insulitis [26]. In the infiltrated islets, immune cells produce cytokines, including IL-1β, TNF-α, and IFN-γ. IL-1β and/or TNF-α plus IFN-γ induce β-cell apoptosis via the activation of β-cell gene networks under the control of the transcription factors nuclear factor κB (NF-κB) and STAT1. NF-κB activation subsequently leads to production of nitric oxide (NO) and chemokines and to depletion of endoplasmic reticulum (ER) calcium stores. Initiation of β-cell death then occurs through activation of mitogen-activated protein kinases via triggering of ER stress and the release of mitochondrial cytochrome c. This acts as a mitochondrial death signal that sequentially activates cytosolic caspase 9 and 3, thereby promoting β-cell death (reviewed in [27]). Moreover, monocytes/macrophages play a crucial role in the induction of a TH1/TH17 bias, which is a hallmark of autoimmune diseases, including T1D [28, 29]. Monocytes isolated from the blood of patients with T1D secrete IL-1β and IL-6, which induce and expand IL-17-producing CD4+ TH cells. These TH17 cells contribute to the progression of T1D by promoting an imbalance between the effector and regulatory T cells, thereby potentiating inflammatory and proapoptotic responses [28, 30]. IL-17 neutralization, either by anti-IL-17 or by recombinant IL-25, was able to prevent the development of autoimmune diabetes in NOD mice [29]. Taken together, these reports have unequivocally demonstrated the crucial role for macrophages in T1D (summarized in Fig. 3A).
Figure 3.
Schematic representation summarizing the role of macrophages in the pathogenesis of type 1 diabetes mellitus (A), type 2 diabetes mellitus (T2D) (B), and during β-cell protection and regeneration (C). (A): M1 macrophages contribute to β-cell death through (a) MHC II-mediated presentation of β-cell-specific autoantigens, (b) IL-1β and IL-6-mediated TH17 expansion and subsequent IL-17-mediated TEff/TReg imbalance, and (c) a direct cytotoxic effect of IL-1β and/or TNF-α plus IFN-γ with downstream activation of NF-κB and STAT1. NF-κB activation results in NO production and increased ER stress and cytochrome c release from mitochondria, the latter acting as a mitochondrial death signal. Ultimately, NF-κB and STAT1 activation results in caspase 1/3/9/12 activation and subsequent β-cell death. (B): High circulating glucose and FFAs contribute to β-cell death in T2D. In addition, a HFD and circulating FFAs promote MCP-1 secretion from β cells and subsequent intraislet accumulation of M1-like macrophages. Moreover, a HFD and onset of T2D correlate with elevated circulating levels of TLR2 and -4 ligands, which stimulate the secretion of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) from these recruited macrophages, thereby further contributing to β-cell death and dysfunction. A high-fat diet and lipid accumulation also promote adipo- (leptin, resistin), chemo-, and cytokine (IL-1β, IL-6, TNF-α, MCP-1, LTB4, CXCL12, MIP, and MIF) secretion from adipocytes, thereby promoting the recruitment to and activation of Ly6C+ monocytes and M1 macrophages in adipose tissue. These exert detrimental effects on peripheral insulin signaling through secretion of IL-1β, IL-6, IL-12, and TNF-α, and production of NO. Finally, M1 adipose tissue macrophages promote myelopoiesis and monocytosis through IL-1β secretion, thereby further enhancing macrophage accumulation in inflamed adipose tissue and, thus, establishing a positive feedback loop in T2D pathogenesis. (C): M2 macrophages accumulate in the pancreas after transgenic, β cell-specific VEGF-A overexpression or partial PDL, in the latter via MCP-1-mediated recruitment and M-CSF-dependent proliferation of macrophages. M2 macrophages promote β-cell protection and regeneration through secretion of several growth factors (i.e., TGF-β1, EGF, PDGF-β, IGF-1) in concert with endothelial cell-derived growth factors (i.e., HGF, FGF-1, IGF-1, TGF-β1, and PDGF-β). M2 macrophages and endothelial cells moreover produce matrix remodeling factors—MMP (i.e., MMP12, MMP13) and ADAM and ADAM(TS) (i.e., ADAM12, ADAMTS9), respectively—facilitating growth factor bioavailability. Abbreviations: ADAM, a disintegrin and metalloproteinase; ADAM(TS), a disintegrin and metalloproteinase with thrombospondin motifs; CXCL, chemokine (C-X-C motif) ligand; EGF, epidermal growth factor; ER, endoplasmic reticulum; FFA, free fatty acid; FGF, fibroblast growth factor; HFD, high-fat diet; IGF, insulin-like growth factor; IL, interleukin; LTB, leukotriene B; MCP, monocyte chemoattractant protein; M-CSF, macrophage colony-stimulating factor; MIF, macrophage migration inhibitory factor; MIP, macrophage inflammatory protein; MMP, matrix metalloproteinase; NF-κB, nuclear factor κB; NO, nitric oxide; PDGF, platelet-derived growth factor; PDL, pancreatic duct ligation; TEff, T effector; TH, T helper; TReg, T regulator; TLR, Toll-like receptor; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Macrophages in Type 2 Diabetes Mellitus
Just as in T1D, an increase in the number of islet-associated inflammatory cells and macrophages has been observed in T2D models [31]. Emerging evidence suggests that, concomitant with gluco- and lipotoxicity, proinflammatory macrophages contribute to β-cell dysfunction and loss in T2D. During the progression of high fat diet (HFD)-induced T2D in mice, fatty acids stimulate β cells to produce chemokines that promote intraislet accumulation of M1-like monocytes/macrophages [32]. A similar association was found in rats [33]. HFD and the onset of T2D correlates with elevated circulating levels of TLR2 and -4 ligands in both mice and humans. Interestingly, TLR2- and TLR4-deficient mice are protected from the metabolic consequences of a HFD. Mechanistically, TLR2/6 and TLR4 ligands stimulate the secretion of IL-1β and IL-6 from bone marrow-derived macrophages. This subsequently decreases β-cell insulin gene expression and secretion [34]. The pathogenic role of macrophages in β-cell dysfunction and destruction has been further demonstrated by a gain-of-function experiment in which transgenic overexpression of the chemokine monocyte chemo-attractant protein 1 (MCP-1) in β cells resulted in monocyte/macrophage accumulation in transgenic islets and subsequent β-cell injury and the development of diabetes [35].
Macrophages also contribute to the onset and progression of T2D by their detrimental effect on peripheral insulin signaling. Macrophages constitute an important fraction of nonadipocyte cells within the white adipose tissue (WAT), ranging from less than 10% in lean mice and humans to more than 50% in extremely obese, leptin-deficient mice to nearly 40% in obese humans [36]. These adipose tissue macrophages exert tissue surveillance and remodeling functions and are associated with maintenance of WAT insulin sensitivity. Recent work has also demonstrated a role for macrophages in regulating brown adipose tissue thermogenesis [37], thereby promoting blood glucose disposal, insulin sensitivity, and energy expenditure [38]. In nonobese mice, resident adipose tissue macrophages display an alternatively activated M2 phenotype. In mice with macrophage-specific deletion of the peroxisome proliferator activated receptor (PPAR)-γ, maturation of the M2 macrophages is disturbed, resulting in the development of diet-induced obesity, insulin resistance, and glucose intolerance [39]. Interestingly, acute accumulation of monocytes/macrophages within adipose tissue promotes adipogenesis, adipose tissue function, and insulin sensitivity [40]. However, a long-term HFD and obesity results in chronic adipocyte inflammation, subsequent downregulation of glucose transporter-4, and the development of insulin resistance [41]. Mechanistically, a HFD induces lipid accumulation inside adipocytes, which renders them proinflammatory owing to their overexpression of adipokines (leptin, resistin) and chemokines/cytokines (IL-1β, IL-6, TNF-α, MCP-1, leukotriene B4 (LTB4), C-X-C chemokine ligand 12 [42], macrophage inflammatory protein (MIP), and macrophage migration inhibitory factor (MIF) [43]). These cytokines subsequently promote the recruitment of Ly6C+ monocytes and the activation of macrophages [36, 44]. The macrophages accumulating in obese WAT are skewed toward an inflammatory CD11c+ M1 phenotype and secrete IL-1β, IL-6, IL-12, TNF-α, and nitric oxide [41, 45]. Heme oxygenase 1 has been discovered as one of the crucial genes regulating inflammatory skewing and NF-κB amplification in adipose tissue macrophages [46]. A positive feedback loop in obesity, in which adipose tissue macrophages promote myelopoiesis and monocytosis through IL-1 β secretion, further enhances macrophage accumulation in inflamed adipose tissue [47].
In obese patients, the accumulation of proinflammatory CD11c+ adipose tissue macrophages also correlates with systemic insulin resistance [48]. Gastric bypass surgery reduces the number of adipose tissue macrophages, thereby likely contributing to the increased insulin sensitivity observed in these patients [49].
Macrophages have also been recognized as crucial mediators of insulin resistance in skeletal muscle and liver. MCP-1 appears to be one of the key chemokines responsible for macrophage accumulation in both tissue types [50].
Taken together, compelling evidence suggests that macrophages play a pivotal role in the onset and progression of insulin resistance and β-cell dysfunction, thereby contributing to the pathogenesis of T2D (summarized in Fig. 3B). Several other cell types, including eosinophils and different subsets of T cells, have been identified as coeffectors that influence adipose tissue inflammation and macrophage polarization (reviewed in [51]).
Macrophages in β-Cell Protection and Regeneration
Based on these findings, macrophages have long been attributed exclusively proinflammatory and diabetogenic features. In contrast, during the past decade, macrophage-depletion studies have demonstrated the critical involvement of macrophages during tissue repair after skin [52], liver [53], kidney [54], and muscle injury [55]. Several M2-associated factors, among which hepatocyte growth factor (HGF), Wnt3/7, vascular endothelial growth factor (VEGF)-A, and platelet-derived growth factor (PDGF)-β, have been linked to specific processes during tissue repair and regeneration (reviewed in [10, 56]). M1 macrophages metabolize arginine via NOS2 to produce nitric oxide; however, in M2 macrophages, ARG1 converts arginine to polyamines that are necessary for collagen synthesis and cellular proliferation [10]. In addition, these polyamines further amplify the M2/trophic nature of macrophages [57].
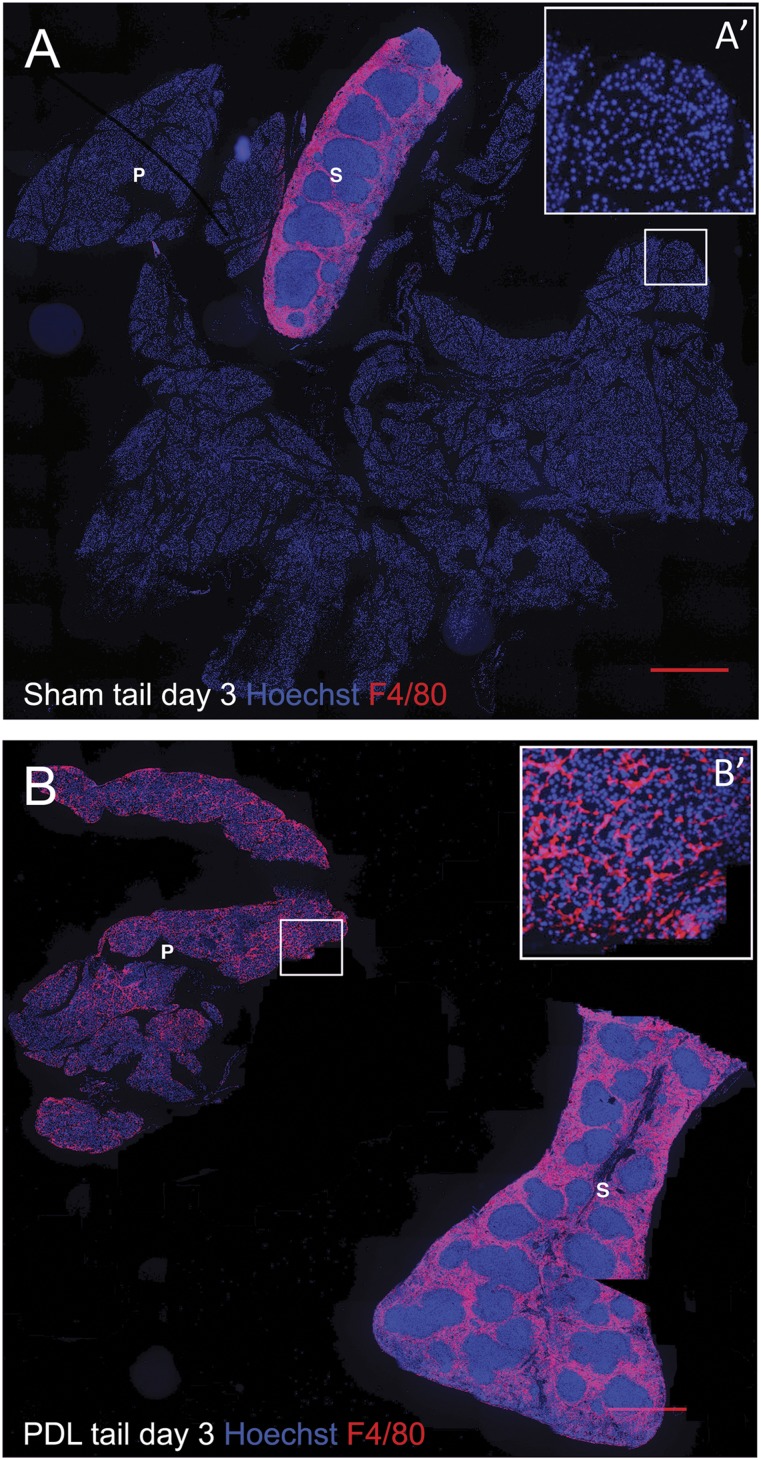
Emerging data have also revealed a role for trophic macrophages during β-cell protection, repair, and regeneration. In human cadaver pancreata, a correlation between β-cell protection/proliferation and pancreatic infiltration of macrophages was found [58]. Moreover, in a murine model of chronic pancreatitis, infiltrated macrophages promoted islet angiogenesis and islet cell proliferation. Macrophage depletion resulted in endocrine cell loss and subsequent diabetes, suggesting a critical role for macrophages in endocrine maintenance during chronic pancreatitis-induced pancreas degeneration [59]. Finally, on helminth infection, an important TH2 response is elicited, with a skew in the macrophage phenotype from the classic proinflammatory toward alternatively activated [60]. Interestingly, helminth infection increases islet infiltration of alternatively activated macrophages. This results in reduced insulitis and endocrine cell loss, thereby preventing the onset of diabetes in NOD [61], multiple low dose streptozotocin (a β-cell toxin)-treated [62], and HFD mice [63]. Two recent studies revealed that M2 macrophages not only protect β cells but also stimulate their expansion/regeneration [64, 65]. In the first report, macrophages were recruited to injured/regenerating islets through VEGF-A signaling. Bone marrow irradiation abrogated macrophage recruitment and subsequent β-cell repair. Transcriptome analysis suggested that both macrophage-derived effector molecules (i.e., matrix metalloproteinase 12 [MMP12], MMP13, HGF, insulin-like growth factor 1 [IGF-1], PDGF-β, TGF-β1) and endothelial-cell derived signals (i.e., a disintegrin and metalloproteinase 12 [ADAM12], ADAM with thrombospondin motifs 9, IGF-1, PDGF-β, fibroblast growth factor 1 [FGF-1]) acted in concert to promote β-cell regeneration [64]. The second report showed that M2 macrophages, recruited to the pancreas after tissue injury by partial duct ligation (PDL), stimulate β-cell proliferation by secretion of TGF-β1 and epidermal growth factor. Both factors subsequently induced SMAD7 and SMAD2 signaling in β cells, which resulted in an increase in the cell cycle activators cyclin D1/2 and nuclear exclusion of the cell cycle inhibitor p27 [65]. The concept that macrophages contribute to β-cell mass increase after PDL originates from seminal observations by our research group in which a massive influx and accumulation of macrophages was observed in the PDL pancreas before progenitor cell activation [66] (Fig. 4). In addition, our own work suggests that macrophage accumulation in PDL is mediated through MCP-1-dependent monocyte recruitment and M-CSF-dependent macrophage proliferation [67]. Interestingly, resident macrophages, rather than recruited monocyte/macrophages, appear crucial for β-cell proliferation in PDL [67].
Figure 4.
Massive macrophage infiltration in the pancreas after partial pancreatic duct ligation. Pancreas tail and spleen sections from day 3 sham-operated (A) and PDL (B) mice, stained for nuclei (Hoechst, blue) and F4/80 (red). (A′, B′): Higher magnification of the area depicted by the squares in (A) and (B). Scale bars = 500 μm. Notably, PDL results in acinar cell loss and thus a decrease in the total pancreas tail area versus sham-operated mice. Abbreviations: P, pancreas tail; PDL, pancreatic duct ligation; S, spleen.
In conclusion, the classic view on the interaction between macrophages and β cells should be reconsidered, because recent reports have unequivocally demonstrated a beneficial role for M2 macrophages in β-cell protection and regeneration (summarized in Fig. 3C).
Clinical Implications
Current knowledge on the role of macrophages in β-cell physiology (summarized in Figs. 2, 3) can be exploited to track and manipulate macrophages to develop novel diagnostic and therapeutic strategies for both T1D and T2D. More specifically, additional identification of trophic macrophage subpopulations and their secreted factors might ultimately translate into strategies to stimulate β-cell regeneration in patients with diabetes via macrophage cell or growth factor therapy. For instance, macrophages could be isolated from diabetic patients and manipulated ex vivo to serve as cell therapy in an autologous transplantation context. Human macrophages can be obtained via in vitro differentiation of blood monocytes using M-CSF, by default leading to M2-like macrophage activation [68, 69]. Their functional activation state can also be manipulated in vitro using IL-4, equally resulting in activation of an M2-like differentiation program [69]. M2 macrophage transfer or skewing could potentially stimulate β-cell proliferation in situ. Coengraftment of M2 macrophages with islets of Langerhans could also be envisioned as a possible strategy to improve the outcome of β-cell transplantation.
However, a better understanding of macrophage heterogeneity and of the mechanism by which some macrophage subsets exert their detrimental role toward β-cells will be imperative to developing strategies for targeted macrophage depletion and for macrophage immunomodulation. Because macrophages have been identified to contribute, via secretion of proinflammatory factors such as IL-1β and TNF-α [70], to β-cell loss in T1D [25, 71] and T2D [31–33] and during islet graft loss, macrophages represent an interesting target for diabetes therapy. Notably, strategies to prevent the deleterious effects of IL-1β and TNF-α have been only partially successful in slowing the progression of T1D [72]. It could thus be hypothesized that targeting the effector cell per se (i.e., the macrophage) would be more efficient. Administration of M2 polarizing agents could also be considered in (pre)clinical trials aiming to protect β cells from the deleterious effects of M1 macrophages.
Finally, M1-oriented macrophages in adipose tissue, liver, and muscle [50] contribute to insulin resistance in obesity, and M2 macrophages in white and brown adipose tissue enhance insulin sensitivity [38, 41, 45, 73]. A recent report demonstrated that IL-10 promotes macrophage skewing toward an M2 activation state and subsequently abrogated obesity-induced insulin resistance in mice [41]. Administration of factors or compounds that selectively ablate pathogenic M1 macrophages or promote macrophage M2 skewing could potentially combat insulin resistance in patients with T2D. Notably, and underappreciated by many, the current antidiabetic drugs modulate the macrophage activation status. Metformin, a biguanide that activates AMP-activated protein kinase and reduces insulin resistance, has been shown to suppress the lipopolysaccharide (LPS)-induced inflammatory response in murine macrophages through induction of activating transcription factor 3 [74, 75]. Pioglitazone, a PPAR-γ/α agonist that targets both β-cell function and insulin resistance [76], induces apoptosis of macrophages in human adipose tissue [77] and suppresses LPS-induced production of inflammatory factors in mouse macrophages by inactivating NF-κB [78]. Finally, glucagon-like peptide 1 receptor agonists (incretin mimetics) were shown to inhibit adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes [79] and induce M2 polarization in human macrophages via STAT3 activation [80].
Taken together, novel insights in macrophage activation states and their correlation with β-cell failure, proliferation, and peripheral insulin resistance has rendered macrophages interesting therapeutic targets in both preclinical and overt T1D and T2D. Caution is nonetheless warranted for overenthusiasm, because M2 macrophages also contribute to tumor expansion and progression, as well as atherosclerosis, conditions with increased prevalence among patients with diabetes [81, 82].
Author Contributions
N.V.G.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; W.S.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript; E.V.O.: collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; S.D.G.: collection and/or assembly of data, manuscript writing, final approval of manuscript; M.S.: manuscript writing, final approval of manuscript; Y.H.: conception and design, administrative support, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; G.L.: provision of study material or patients, collection and/or assembly of data, final approval of manuscript; M.V.d.C.: manuscript writing, final approval of manuscript; J.A.V.G.: conception and design, financial support, manuscript writing, final approval of manuscript; H.H.: conception and design, financial support, manuscript writing, final approval of manuscript; N.D.L.: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
References
- 1.Geissmann F, Manz MG, Jung S, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulz C, Gomez Perdiguero E, Chorro L, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 4.Ginhoux F, Jung S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto D, Chow A, Noizat C, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat Immunol. 2011;12:1035–1044. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantovani A, Sica A, Sozzani S, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue J, Schmidt SV, Sander J, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40:274–288. doi: 10.1016/j.immuni.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mantovani A, Biswas SK, Galdiero MR, et al. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 11.Movahedi K, Laoui D, Gysemans C, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 12.Ghassabeh GH, De Baetselier P, Brys L, et al. Identification of a common gene signature for type II cytokine-associated myeloid cells elicited in vivo in different pathologic conditions. Blood. 2006;108:575–583. doi: 10.1182/blood-2005-04-1485. [DOI] [PubMed] [Google Scholar]
- 13.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cecchini MG, Dominguez MG, Mocci S, et al. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120:1357–1372. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- 15.Jones CV, Ricardo SD. Macrophages and CSF-1: Implications for development and beyond. Organogenesis. 2013;9:249–260. doi: 10.4161/org.25676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geutskens SB, Otonkoski T, Pulkkinen MA, et al. Macrophages in the murine pancreas and their involvement in fetal endocrine development in vitro. J Leukoc Biol. 2005;78:845–852. doi: 10.1189/jlb.1004624. [DOI] [PubMed] [Google Scholar]
- 17.Banaei-Bouchareb L, Gouon-Evans V, Samara-Boustani D, et al. Insulin cell mass is altered in Csf1op/Csf1op macrophage-deficient mice. J Leukoc Biol. 2004;76:359–367. doi: 10.1189/jlb.1103591. [DOI] [PubMed] [Google Scholar]
- 18.Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- 19.Magenheim J, Ilovich O, Lazarus A, et al. Blood vessels restrain pancreas branching, differentiation and growth. Development. 2011;138:4743–4752. doi: 10.1242/dev.066548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson M, Mattsson G, Andersson A, et al. Islet endothelial cells and pancreatic beta-cell proliferation: Studies in vitro and during pregnancy in adult rats. Endocrinology. 2006;147:2315–2324. doi: 10.1210/en.2005-0997. [DOI] [PubMed] [Google Scholar]
- 21.Nucera S, Biziato D, De Palma M. The interplay between macrophages and angiogenesis in development, tissue injury and regeneration. Int J Dev Biol. 2011;55:495–503. doi: 10.1387/ijdb.103227sn. [DOI] [PubMed] [Google Scholar]
- 22.O’Reilly LA, Hutchings PR, Crocker PR, et al. Characterization of pancreatic islet cell infiltrates in NOD mice: Effect of cell transfer and transgene expression. Eur J Immunol. 1991;21:1171–1180. doi: 10.1002/eji.1830210512. [DOI] [PubMed] [Google Scholar]
- 23.Herold KC, Vignali DA, Cooke A, et al. Type 1 diabetes: Translating mechanistic observations into effective clinical outcomes. Nat Rev Immunol. 2013;13:243–256. doi: 10.1038/nri3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calderon B, Suri A, Unanue ER. In CD4+ T-cell-induced diabetes, macrophages are the final effector cells that mediate islet beta-cell killing: Studies from an acute model. Am J Pathol. 2006;169:2137–2147. doi: 10.2353/ajpath.2006.060539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jun HS, Yoon CS, Zbytnuik L, et al. The role of macrophages in T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Exp Med. 1999;189:347–358. doi: 10.1084/jem.189.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willcox A, Richardson SJ, Bone AJ, et al. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol. 2009;155:173–181. doi: 10.1111/j.1365-2249.2008.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cnop M, Welsh N, Jonas JC, et al. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: Many differences, few similarities. Diabetes. 2005;54(suppl 2):S97–S107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 28.Shao S, He F, Yang Y, et al. Th17 cells in type 1 diabetes. Cell Immunol. 2012;280:16–21. doi: 10.1016/j.cellimm.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Emamaullee JA, Davis J, Merani S, et al. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes. 2009;58:1302–1311. doi: 10.2337/db08-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Honkanen J, Nieminen JK, Gao R, et al. IL-17 immunity in human type 1 diabetes. J Immunol. 2010;185:1959–1967. doi: 10.4049/jimmunol.1000788. [DOI] [PubMed] [Google Scholar]
- 31.Ehses JA, Perren A, Eppler E, et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- 32.Eguchi K, Manabe I, Oishi-Tanaka Y, et al. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab. 2012;15:518–533. doi: 10.1016/j.cmet.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 33.Jourdan T, Godlewski G, Cinar R, et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat Med. 2013;19:1132–1140. doi: 10.1038/nm.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nackiewicz D, Dan M, He W, et al. TLR2/6 and TLR4-activated macrophages contribute to islet inflammation and impair beta cell insulin gene expression via IL-1 and IL-6. Diabetologia. 2014;57:1645–1654. doi: 10.1007/s00125-014-3249-1. [DOI] [PubMed] [Google Scholar]
- 35.Martin AP, Rankin S, Pitchford S, et al. Increased expression of CCL2 in insulin-producing cells of transgenic mice promotes mobilization of myeloid cells from the bone marrow, marked insulitis, and diabetes. Diabetes. 2008;57:3025–3033. doi: 10.2337/db08-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen KD, Qiu Y, Cui X, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chondronikola M, Volpi E, Børsheim E, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089–4099. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wernstedt Asterholm I, Tao C, Morley TS, et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20:103–118. doi: 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim D, Kim J, Yoon JH, et al. CXCL12 secreted from adipose tissue recruits macrophages and induces insulin resistance in mice. Diabetologia. 2014;57:1456–1465. doi: 10.1007/s00125-014-3237-5. [DOI] [PubMed] [Google Scholar]
- 43.Surmi BK, Hasty AH. The role of chemokines in recruitment of immune cells to the artery wall and adipose tissue. Vascul Pharmacol. 2010;52:27–36. doi: 10.1016/j.vph.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeyda M, Stulnig TM. Adipose tissue macrophages. Immunol Lett. 2007;112:61–67. doi: 10.1016/j.imlet.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Jais A, Einwallner E, Sharif O, et al. Heme oxygenase-1 drives metaflammation and insulin resistance in mouse and man. Cell. 2014;158:25–40. doi: 10.1016/j.cell.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagareddy PR, Kraakman M, Masters SL, et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19:821–835. doi: 10.1016/j.cmet.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wentworth JM, Naselli G, Brown WA, et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tordjman J, Poitou C, Hugol D, et al. Association between omental adipose tissue macrophages and liver histopathology in morbid obesity: Influence of glycemic status. J Hepatol. 2009;51:354–362. doi: 10.1016/j.jhep.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 50.Patsouris D, Cao JJ, Vial G, et al. Insulin resistance is associated with MCP1-mediated macrophage accumulation in skeletal muscle in mice and humans. PLoS One. 2014;9:e110653. doi: 10.1371/journal.pone.0110653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanneganti T-D, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012;13:707–712. doi: 10.1038/ni.2343. [DOI] [PubMed] [Google Scholar]
- 52.Lucas T, Waisman A, Ranjan R, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184:3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 53.Duffield JS, Forbes SJ, Constandinou CM, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee S, Huen S, Nishio H, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arnold L, Henry A, Poron F, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stefater JA, III, Ren S, Lang RA, et al. Metchnikoff’s policemen: Macrophages in development, homeostasis and regeneration. Trends Mol Med. 2011;17:743–752. doi: 10.1016/j.molmed.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van den Bossche J, Lamers WH, Koehler ES, et al. Pivotal advance: Arginase-1-independent polyamine production stimulates the expression of IL-4-induced alternatively activated macrophage markers while inhibiting LPS-induced expression of inflammatory genes. J Leukoc Biol. 2012;91:685–699. doi: 10.1189/jlb.0911453. [DOI] [PubMed] [Google Scholar]
- 58.In’t Veld P, De Munck N, Van Belle K, et al. Beta-cell replication is increased in donor organs from young patients after prolonged life support. Diabetes. 2010;59:1702–1708. doi: 10.2337/db09-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tessem JS, Jensen JN, Pelli H, et al. Critical roles for macrophages in islet angiogenesis and maintenance during pancreatic degeneration. Diabetes. 2008;57:1605–1617. doi: 10.2337/db07-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kreider T, Anthony RM, Urban JF, Jr, et al. Alternatively activated macrophages in helminth infections. Curr Opin Immunol. 2007;19:448–453. doi: 10.1016/j.coi.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Q, Sundar K, Mishra PK, et al. Helminth infection can reduce insulitis and type 1 diabetes through CD25- and IL-10-independent mechanisms. Infect Immun. 2009;77:5347–5358. doi: 10.1128/IAI.01170-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Espinoza-Jiménez A, Rivera-Montoya I, Cárdenas-Arreola R, et al. Taenia crassiceps infection attenuates multiple low-dose streptozotocin-induced diabetes. J Biomed Biotechnol. 2010;2010:850541. doi: 10.1155/2010/850541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu D, Molofsky AB, Liang HE, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brissova M, Aamodt K, Brahmachary P, et al. Islet microenvironment, modulated by vascular endothelial growth factor-A signaling, promotes β cell regeneration. Cell Metab. 2014;19:498–511. doi: 10.1016/j.cmet.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiao X, Gaffar I, Guo P, et al. M2 macrophages promote beta-cell proliferation by up-regulation of SMAD7. Proc Natl Acad Sci USA. 2014;111:E1211–E1220. doi: 10.1073/pnas.1321347111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xu X, D’Hoker J, Stangé G, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 67.Van Gassen N, Van Overmeire E, Leuckx G et al. Macrophage dynamics are regulated by local macrophage proliferation and monocyte recruitment in injured pancreas. Eur J Immunol 2015 [Epub ahead of print]. [DOI] [PubMed]
- 68.Lacey DC, Achuthan A, Fleetwood AJ, et al. Defining GM-CSF- and macrophage-CSF-dependent macrophage responses by in vitro models. J Immunol. 2012;188:5752–5765. doi: 10.4049/jimmunol.1103426. [DOI] [PubMed] [Google Scholar]
- 69.Martinez FO, Gordon S, Locati M, et al. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 70.Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: Implications for intrahepatic grafts. J Leukoc Biol. 2005;77:587–597. doi: 10.1189/jlb.1104649. [DOI] [PubMed] [Google Scholar]
- 71.Nikolic T, Geutskens SB, van Rooijen N, et al. Dendritic cells and macrophages are essential for the retention of lymphocytes in (peri)-insulitis of the nonobese diabetic mouse: A phagocyte depletion study. Lab Invest. 2005;85:487–501. doi: 10.1038/labinvest.3700238. [DOI] [PubMed] [Google Scholar]
- 72.Schneider DA, Kretowicz AM, von Herrath MG. Emerging immune therapies in type 1 diabetes and pancreatic islet transplantation. Diabetes Obes Metab. 2013;15:581–592. doi: 10.1111/dom.12046. [DOI] [PubMed] [Google Scholar]
- 73.Vachharajani V, Granger DN. Adipose tissue: A motor for the inflammation associated with obesity. IUBMB Life. 2009;61:424–430. doi: 10.1002/iub.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rena G, Pearson ER, Sakamoto K. Molecular mechanism of action of metformin: Old or new insights? Diabetologia. 2013;56:1898–1906. doi: 10.1007/s00125-013-2991-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim J, Kwak HJ, Cha JY, et al. Metformin suppresses lipopolysaccharide (LPS)-induced inflammatory response in murine macrophages via activating transcription factor-3 (ATF-3) induction. J Biol Chem. 2014;289:23246–23255. doi: 10.1074/jbc.M114.577908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith U.Pioglitazone: Mechanism of action Int J Clin Pract Suppl 2001:13–18. [PubMed]
- 77.Bodles AM, Varma V, Yao-Borengasser A, et al. Pioglitazone induces apoptosis of macrophages in human adipose tissue. J Lipid Res. 2006;47:2080–2088. doi: 10.1194/jlr.M600235-JLR200. [DOI] [PubMed] [Google Scholar]
- 78.Ao C, Huo Y, Qi L, et al. Pioglitazone suppresses the lipopolysaccharide-induced production of inflammatory factors in mouse macrophages by inactivating NF-kappaB. Cell Biol Int. 2010;34:723–730. doi: 10.1042/CBI20090005. [DOI] [PubMed] [Google Scholar]
- 79.Lee YS, Park MS, Choung JS, et al. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia. 2012;55:2456–2468. doi: 10.1007/s00125-012-2592-3. [DOI] [PubMed] [Google Scholar]
- 80.Shiraishi D, Fujiwara Y, Komohara Y, et al. Glucagon-like peptide-1 (GLP-1) induces M2 polarization of human macrophages via STAT3 activation. Biochem Biophys Res Commun. 2012;425:304–308. doi: 10.1016/j.bbrc.2012.07.086. [DOI] [PubMed] [Google Scholar]
- 81.Noto H, Goto A, Tsujimoto T, et al. Latest insights into the risk of cancer in diabetes. J Diabetes Investig. 2013;4:225–232. doi: 10.1111/jdi.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Colwell JA, Lopes-Virella M, Halushka PV. Pathogenesis of atherosclerosis in diabetes mellitus. Diabetes Care. 1981;4:121–133. doi: 10.2337/diacare.4.1.121. [DOI] [PubMed] [Google Scholar]