Shotgun lipidomics affords comprehensive and quantitative analysis of lipid species in cells and tissues at high-throughput 1–5. The methodology is based on direct infusion of lipid extracts by electrospray ionization (ESI) combined with tandem mass spectrometry (MS/MS) and/or high resolution Fourier transform mass spectrometry (FTMS) for identification and quantification of lipid species 6. Shotgun lipidomics affords extensive lipidome coverage by combining the analysis of lipid extracts in positive and negative ion mode 1,3. Notably, sterols such as cholesterol and ergosterol exhibit low ionization efficiency in ESI 7. For this reason, chemical derivatization procedures including acetylation 8 or sulfation 9 are commonly implemented to facilitate ionization, detection and quantification of sterols for global lipidome analysis 1–3,10.
In the course of large-scale lipidomic analyses using an LTQ Orbitrap XL mass spectrometer (Thermo Scientific) equipped with a robotic nanoESI source TriVersa NanoMate (Advion Biosciences), we observed a pronounced decrease in the sensitivity of negative ion mode analysis following repeated injections of samples subjected to chemical sulfation. This decrease in sensitivity was observed only for negative ion mode analysis of lipid species in underivatized lipid extracts. No decrease in sensitivity was observed for negative ion mode analysis of chemically sulfated sterols or for positive ion mode analysis of lipid species in underivatized lipid extracts.
To investigate the decrease in analytical sensitivity in further detail we (i) spiked a yeast lysate with internal standards and executed two-step lipid extraction as previously described 3 for the partition of apolar and polar lipids, (ii) subjected the apolar lipid extract (containing sterols) to chemical sulfation 2,9, and (iii) infused the sulfated sample in batches of ten repeated injections and monitored the intensity of chemically sulfated sterols by negative ion mode FTMS. To monitor the decrease in sensitivity we analyzed the underivatized polar lipid extract [containing phosphatidylinositol (PI) as well as other polar lipids 3] by negative ion mode FTMS before and after each batch of ten repeated injections of the sulfated sample.
This analysis showed a progressive loss of sensitivity for negative ion mode analysis of lipid species in the underivatized polar lipid extract. To exemplify this effect, the intensity of PI 34:1, an abundant glycerophospholipid species in yeast 3,10, was reduced more than 20-fold after 30 injections of the sulfated sample (Fig. 1a). More importantly, since an intensity threshold is routinely used in shotgun lipidomics data processing 11, the decrease in sensitivity translated into a decrease in the number of lipid species identified and quantified (data not shown). Notably, the intensity of chemically sulfated sterols was unaffected (Fig. 1b). As we had previously observed that the sensitivity of positive ion mode analysis was not affected, we transiently switched the polarity to positive for 1 h and subsequently reverted it to evaluate whether the sensitivity of negative ion mode analysis was restored. This polarity switch partially restored the sensitivity (>40%, in the case of PI 34:1) for negative ion mode analysis of lipid species in the underivatized polar lipid extract (Fig. 1a). Based on these results, we recommend that global shotgun lipidomic experiments employing chemical sulfation of sterols use a specific sequence of MS analyses where the analysis of sulfated samples is scheduled at the end of all other analyses in order not to compromise analytical sensitivity (Fig. 2).
Figure 1.
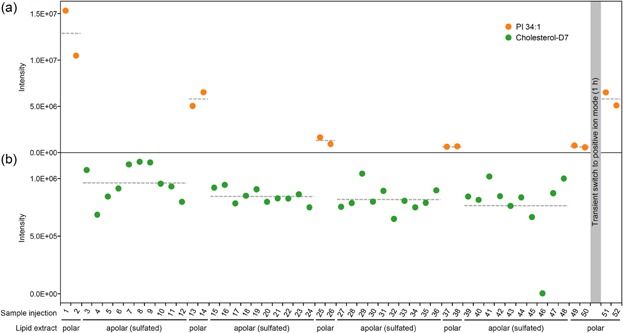
(a) Sensitivity of negative ion mode analysis of lipid species in the underivatized polar lipid extract is reduced after analysis of chemically sulfated sterols. The underivatized polar lipid extract was infused using 0.005% (w/v) methylamine in chloroform/methanol (1:5, v/v), and analyzed for 5 min by negative ion mode FTMS analysis. The intensity of PI 34:1 is shown as a representative lipid species. Note that sensitivity is partially restored after a 1 h polarity switch. (b) Sensitivity of the analysis of chemically sulfated sterols is not affected. The sulfated apolar lipid extract was infused using 0.005% (w/v) methylamine in pyridine/methanol (1:9, v/v), and analyzed for 3 min by negative ion mode FTMS analysis. Dashed grey lines indicate average values.
Figure 2.
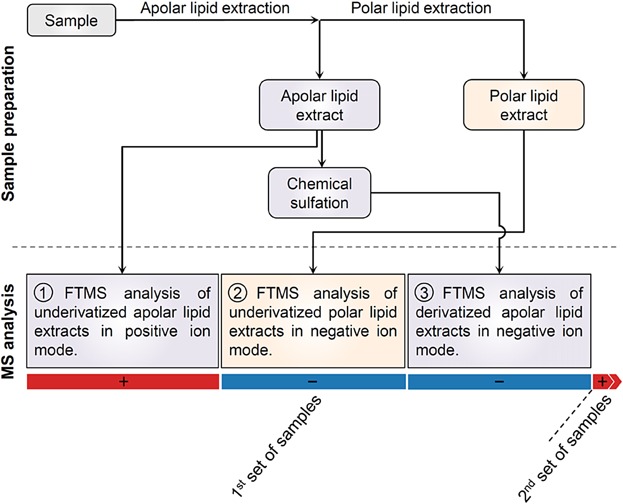
Recommended sequence of MS analyses for global lipidome analysis using shotgun lipidomics. A standard sample preparation routine includes two-step lipid extraction and chemical sulfation of the apolar lipid extract for the quantification of sterols. The recommended sequence of analyses is designed to avoid loss of sensitivity following injection of samples subjected to chemical sulfation. Hence, the analysis of chemically sulfated lipid extracts should be scheduled at the end of all other analyses for a particular set of samples and followed by positive ion mode analysis of the subsequent set of samples.
In conclusion, here we: (i) demonstrate that shotgun lipidomic analysis of sample extracts subjected to chemical sulfation can prompt a pronounced loss of sensitivity for negative ion mode analysis of underivatized lipid extracts; (ii) show that changing polarity is a way to overcome this analytical problem; and (iii) recommend that analysis of chemically sulfated lipid extracts is scheduled at the end of all other analyses in order not to compromise sensitivity and data quality of large-scale global lipidome analyses.
We are grateful to Maria Carvalho for providing the protocol for chemical sulfation of sterol lipids. This work was supported by Lundbeckfonden (R44-A4342, R54-A5858, CSE), the Danish Council for Independent Research | Natural Sciences (10-094213, AC; 09-072484, CSE), and a Marie Curie Intra European Fellowship (AC).
The authors have declared no conflict of interest.
Glossary
- ESI
electrospray ionization
- FTMS
Fourier transform mass spectrometry
- MS/MS
tandem mass spectrometry
- PI
phosphatidylinositol
References
- Sampaio JL, Gerl MJ, Klose C, Ejsing CS, et al. Membrane lipidome of an epithelial cell line. Proc. Natl. Acad. Sci. USA. 2011;108:1903–1907. doi: 10.1073/pnas.1019267108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho M, Sampaio JL, Palm W, Brankatschk M, et al. Effects of diet and development on the Drosophila lipidome. Mol. Syst. Biol. 2012;8:600. doi: 10.1038/msb.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejsing CS, Sampaio JL, Surendranath V, Duchoslav E, et al. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proc. Natl. Acad. Sci. USA. 2009;106:2136–2141. doi: 10.1073/pnas.0811700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamovich Y, Rousso-Noori L, Zwighaft Z, Neufeld-Cohen A, et al. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab. 2014;19:319–330. doi: 10.1016/j.cmet.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasov K, Stefanko A, Casanovas A, Surma MA, et al. High-content screening of yeast mutant libraries by shotgun lipidomics. Mol. Biosyst. 2014;10:1364–1376. doi: 10.1039/c3mb70599d. [DOI] [PubMed] [Google Scholar]
- Han X, Yang K, Gross RW. Multi-dimensional mass spectrometry-based shotgun lipidomics and novel strategies for lipidomic analyses. Mass Spectrom. Rev. 2012;31:134–178. doi: 10.1002/mas.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi T, Shimada K. Derivatization of neutral steroids to enhance their detection characteristics in liquid chromatography–mass spectrometry. Anal. Bioanal. Chem. 2004;378:875–882. doi: 10.1007/s00216-003-2252-z. [DOI] [PubMed] [Google Scholar]
- Liebisch G, Binder M, Schifferer R, Langmann T, et al. High throughput quantification of cholesterol and cholesteryl ester by electrospray ionization tandem mass spectrometry (ESI–MS/MS) Biochim. Biophys. Acta. 2006;1761:121–128. doi: 10.1016/j.bbalip.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Sandhoff R, Brugger B, Jeckel D, Lehmann WD, Wieland FT. Determination of cholesterol at the low picomole level by nano-electrospray ionization tandem mass spectrometry. J. Lipid Res. 1999;40:126–132. [PubMed] [Google Scholar]
- Klose C, Surma MA, Gerl MJ, Meyenhofer F, et al. Flexibility of a eukaryotic lipidome–insights from yeast lipidomics. PLoS ONE. 2012;7:e35063. doi: 10.1371/journal.pone.0035063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husen P, Tarasov K, Katafiasz M, Sokol E, et al. Analysis of lipid experiments (ALEX): A software framework for analysis of high-resolution shotgun lipidomics data. PLoS ONE. 2013;8:e79736. doi: 10.1371/journal.pone.0079736. [DOI] [PMC free article] [PubMed] [Google Scholar]


