Abstract
Objectives
To describe the perspective of research personnel on issues of informed consent in a time-sensitive clinical study under emergency circumstances.
Methods
The authors convened concurrent focus groups of research staff and investigators involved in a pharmacokinetic study of lorazepam for status epilepticus. Moderators led discussion with open-ended questions on selected issues of parental consent, communication and understanding, patient assent, and comparison to other types of studies. Focus group transcripts were analyzed to identify themes and sub-themes from the discussions.
Results
Most themes and sub-themes were identified in both research staff and investigator focus groups. Focus group discussion points were categorized into three main themes: barriers to and enablers of informed consent, barriers to and enablers of actual enrollment, and overall ethical concerns about the research. Many of the issues identified were unique to emergency research.
Conclusions
From the perspectives of research staff and investigators enrolling patients in a time-sensitive emergency department study, the authors identified several areas of concern that should be addressed when planning future emergency studies.
Keywords: informed consent, clinical research, consent documents, research ethics
INTRODUCTION
Research in the emergency setting presents unique ethical challenges as the ability to obtain informed consent is often limited. Examples of emergency research include studies of cardiac arrest, trauma and injury, stroke, seizures, asthma, and other severe acute illnesses that incapacitate patients. The importance of this research is not questioned; however, the mechanisms by which it can be conducted while assuring individual autonomy and respect for persons are challenging.
Pediatric research in the emergency setting is further complicated by the potential unavailability of parents to provide informed consent, or the potential lack of emotional stability to do so. For example, parents of children who have recently experienced cardiac arrest or status epilepticus are often so emotionally distraught as to preclude being approached for enrollment in a clinical study. Little information currently exists regarding the feasibility and acceptability of different methods of obtaining informed consent for research from parents of children with life-threatening emergencies. Previous studies of informed consent for emergency research have focused on ethical and regulatory requirements,1,2,3,4,5,6,7,8,9 administrative aspects,10,11,12 and quantitative descriptions13 of patient enrollment in studies using exception from informed consent (EFIC) for emergency research.14 For example, one study investigated emergency department (ED) patients’ knowledge of an ongoing clinical trial using the exception from informed consent regulations, and found that the community did not accept practices where traditional informed consent was not obtained.15 McClure et al. surveyed adults in EDs in Oregon and Minnesota to determine attitudes toward emergency research conducted under the EFIC regulations, and found that although respondents disagreed with the idea of research without prospective informed consent, many were willing to participate in hypothetical studies using EFIC.16 There have been no studies documenting the experience of research personnel who actually obtain informed consent under emergency circumstances.
To better understand this process, we planned to perform a qualitative assessment of our experiences with informed consent as part of an ongoing pediatric emergency research study investigating the pharmacokinetics of lorazepam used in the treatment of pediatric status epilepticus (SE) (hereafter referred to as the SE study). In the pharmacokinetic study we enrolled children using two different methods of informed consent (detailed in the Methods section). As we neared completion of the pharmacokinetic study, we convened focus groups of investigators and research staff in order to evaluate the process of informed consent under emergency circumstances. This article addresses the results of these focus groups.
METHODS
This focus group study was a planned secondary study of the SE study. A description of the primary SE study is presented first, followed by a description of the focus group study:
Status epilepticus study
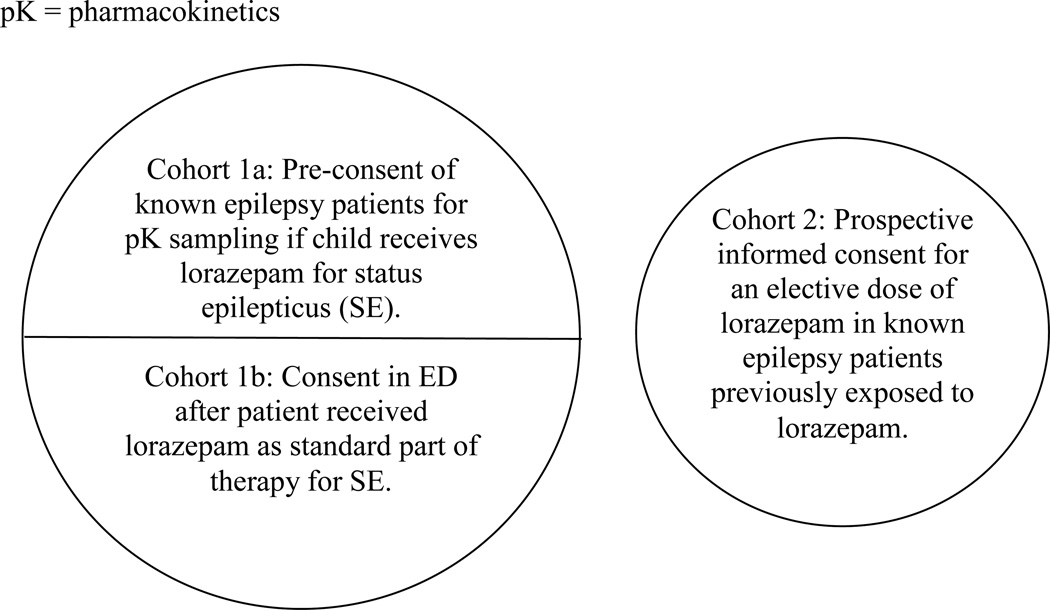
Two cohorts of patients were recruited into the SE study. Cohort 1 consisted of patients actively in status epilepticus who received lorazepam as part of standard clinical care. They were approached for blood sampling to measure the pharmacokinetic properties of lorazepam. Informed consent for Cohort 1 was obtained either prior to the ED visit for SE (Cohort 1A), or in the ED after receiving lorazepam for SE (standard consent, Cohort 1B). Approximately 20% of patients who were approached for enrollment into Cohort 1B under time-sensitive conditions gave consent to participate. Cohort 1A patients were identified in the neurology practices or emergency departments (EDs) of participating sites by having a known previous episode of status epilepticus. They were approached for consent to participate in the event that the child had a future episode of status epilepticus and was brought to the study ED. For Cohort 1 patients, the study protocol required the insertion of a second intravenous line for blood sampling as soon as possible after the patient received lorazepam. The first serum sample for lorazepam concentration was required within 10 minutes after receiving the medication, and thus made the study time-sensitive.
Cohort 2 patients were patients with a previous history of seizures who agreed to be part of a traditional pharmacokinetics study. Cohort 2 patients received an elective dose of lorazepam unrelated to an episode of seizures during an elective admission to a clinical research unit within the study hospital. Although Cohort 2 was not enrolled under time sensitive conditions like Cohort 1, we enrolled these two cohorts to determine whether there are differences in lorazepam pharmacokinetics related to ongoing status epilepticus. The differences in the protocol, and particularly in the informed consent process between the two cohorts, are relevant to the results of the focus group study and are described here for that purpose. Institutional review board (IRB) approval was obtained at all sites, and patient assent was obtained as per local IRB rules where appropriate for patient age, developmental status, and clinical status.
Focus group study
After enrollment of approximately 80% of the planned SE study sample, we invited research staff (research coordinators and research assistants) and site principal investigators (ED attending physician investigators responsible for the study at each site) from the 11 enrollment sites to participate in focus groups to better understand their experiences with the informed consent and enrollment process. We convened one focus group of research staff and one group of investigators, who met concurrently at a previously scheduled SE study protocol meeting. We separated the research staff and investigators to avoid potential professional dominance by the investigators. The two focus group moderators worked together to develop a topic guide and used typical focus group methodology of asking open-ended questions from the topic guide and followed a common script during the sessions. The general areas queried included the overall process of informed consent, barriers and enablers to effective communication about the research, research staff satisfaction with family and patient level of understanding, special considerations around patient assent, interactions with families who refused to participate or withdrew from the SE study, interactions between investigators and treating providers, confirming consent every 90 days, comparison to consent in other trials, suggestions for improving consent processes for emergency research, and adequacy of training for obtaining consent for a clinical trial.
Sample open-ended questions to address these areas of interest included:
“Describe what typically happens during the consent process.”
“What type of questions do the parents ask?”
“How does that neurology clinic compare to the ED?”
“What are some of the challenges to consent?”
Verbal informed consent was obtained from the participants prior to beginning the focus groups, and participants were aware of the purpose of the focus groups. The verbal informed consent disclosed that the focus group proceedings would be recorded and then transcribed for analysis, with results presented without any statements being attributable to individual participants, although anonymity was not guaranteed. For that reason participants were encouraged not to use names or other identifying information during the discussion. Participants were also told that they were not required to contribute to the discussion and were free to leave the discussion at any time.
Moderators pursued discussion along each line of inquiry until no new themes emerged (thematic saturation). Prior to the close of each focus group, participants were given an additional opportunity to contribute new thoughts and to add to any previous discussions. Focus group discussions were recorded and transcribed. Transcribed results were then coded and categorized by the investigators without using a predefined classification scheme. The investigators agreed upon the categorization of themes by consensus achieved during a series of conference calls and meetings. The moderators of the focus groups were included in the consensus process group review of the transcripts. The moderator for the investigator group was also an investigator enrolling patients in the study. The moderator of the non-investigator group was also one of the investigators who was involved in the conception of the focus group study and in the development of the topic guide used to conduct the focus groups. The two moderators were the most knowledgeable and interested parties regarding the time-sensitive nature of consent for this particular study, and therefore were chosen to facilitate the focus groups.
RESULTS
Eleven members of the research staff and seven investigators, plus the investigator-moderator, participated in the focus groups. No invited participants refused consent, but three site investigators in the SE study could not attend because of schedule conflicts. The Table lists themes that were identified more than once by the investigators upon consensus review of the transcripts. We present these data as general themes and sub-themes. The general themes included are 1) enablers of and barriers to the consent process, 2) enablers of and barriers to enrollment, and 3) general ethical issues with the research. In our analysis, there was clearly a distinction between the process of informed consent (Theme 1) and patent enrollment (Theme 2). In discussions of the former, the participants were concerned about how informed consent was obtained and the factors influencing parental understanding of the information presented. In contrast, discussion about patient enrollment centered on the perceived factors that influenced a parent's decision whether or not to enroll his or her child in the study.
Within the sub-themes, there was overlap in that some sub-themes were relevant to more than one major theme. For example, the sub-theme of needing to streamline the information about the study to parents was identified as a sub-theme under barriers to the consent process, barriers to enrollment, and ethical issues. The informed consent document itself was also seen as interfering with the overall informed consent process (e.g. long text that was overwhelming, difficult to understand), interfering with patient enrollment (e.g. listing of low occurrence risks related to lorazepam that were unlikely to present in the study but were required to be listed for regulatory purposes), and causing general ethical concerns (e.g. parents wanting to provide consent when they clearly did not understand the consent documents, documents perceived by study personnel as protecting the hospital rather than protecting the rights of study subjects). Similarly, the clinical environment of the ED was identified as relevant to both barriers to the consent process and barriers to enrollment.
Most themes were identified in both research staff and investigator focus groups. A few sub-themes, however, were identified by investigators but not research staff. These involved the issues of being in the conflicting role of clinician and investigator, but also included perceived difficulties when English was not the primary language. One additional theme that is not displayed in the Table because it did not fit within the three main themes was the scientific concern from the investigator group that failure to enroll a patient related to parental emotional distress could potentially result in selection bias in a clinical trial conducted under emergency circumstances. For example, investigators believed that parents of first-time seizure patients were more likely to be emotionally distraught, and therefore less likely to be approached for enrollment, than parents of children with known epilepsy who had experienced status epilepticus previously. Because patients with known epilepsy are clinically and perhaps pharmacologically different than patients with first episodes of status, this could result in an enrollment bias.
DISCUSSION
The results of this focus group study demonstrate that research personnel identified many important concerns regarding the informed consent process during a clinical trial that took place under emergency circumstances. Perceived and actual difficulties with the process of informed consent are commonly addressed in the medical and bioethics literature, but are typically discussed from a patient or human subjects perspective. Our results indicate that research personnel are sensitive to the same issues. The main issues identified are important for the planning of future studies and raise the following questions.
First, how do we ensure that the essential elements of informed consent required by research regulations are covered in a manner that is understandable and is not overwhelming? Focus group participants identified the ability of parents to understand the informed consent process and corresponding documents as both a significant barrier to consent and as a general ethical issue. In this relatively simple study requiring blood sampling as the only study procedure, informed consent documents were up to 14 pages in length at some sites. Prior investigators have shown promising results with the use of a "short” or “modified” informed consent form, improving literacy, lowering anxiety, and improving satisfaction with the consent process among potential participants.17,18,19 However, none of these studies were conducted with patients or legally authorized representatives who were faced with entering a clinical trial under emergency conditions. Thus, we do not know the validity of modified or simplified forms for emergency research, although this would be an excellent opportunity for future study.
Second, what is the acceptability of eliminating unlikely risks from the informed consent document, or eliminating risks that are part of routine clinical care? For example, focus group participants noted that several IRBs required the inclusion of the risks of lorazepam for patient who had already been given lorazepam as part of standard care prior to enrollment in the study. To our knowledge, no study has compared an informed consent process or document that includes incremental risks to determine what is acceptable from investigator, IRB, or research participant perspectives.
Finally, what is the responsibility of IRBs in assuring that true informed consent occurs in approved studies? Members of the focus groups identified situations in which parents wanted to consent, but clearly did not understand the study. Should a representative of the IRB interview research staff or parents or observe the process of informed consent to assure its validity during the actual conduct of studies? The University of Illinois at Chicago, for example, enacted a policy in October 2008 allowing the IRB to require a third party to observe the informed consent process; the effects of this policy are unknown.20 Given the current burden that IRBs typically bear, this option may be unrealistic at this point in time, but might prove to be one way to help IRBs fulfill their role of human subjects protection.
Many of the issues we identified are undoubtedly present in all human research, and not just in protocols done in an emergency setting. For example, the issues of study complexity and therapeutic misconception are evident for studies such as randomized controlled trials of chemotherapeutic agents for childhood cancers, and studies for research involving mental health emergencies. The conflicting role of the physician as caregiver and scientist has been identified for many years as an ethical issue for most clinical research.21 However, many of these issues may be intensified in a time-sensitive study in the ED setting. For example, our investigator group found that competing clinical demands was one of the biggest barriers to informed consent and patient enrollment. This is not surprising given the nature of EDs, with frequent workflow interruptions and unpredictable surges in patient arrivals. Similarly, the concept of simplifying and shortening study information given to parents is especially important when enrollment is time-sensitive. The earliest we were able to obtain consent from a parent was 35 minutes after medication dosing, despite a very simple study design. Emergency researchers would thus agree that this would preclude the conduct of a study with a very narrow therapeutic window.
One unique aspect of this study was our effort to obtain prospective informed consent significantly in advance of a possible future episode of status epilepticus in high risk patients, which we called “pre-consent.” Although investigators may want to consider this method for future studies of emergency therapies, we identified several concerns with this approach. First, it was clear from both focus groups that parents and patients had difficulty with the hypothetical nature of participation in a study during a possible future episode of status epilepticus. Some felt that parents wished to avoid thinking about such events for psychological reasons. If research subjects cannot truly comprehend the hypothetical future, then informed consent is not truly informed, and other methods of consent must be considered.
Second, the frequency of communication with families needed to confirm ongoing “pre-consent/assent” is unknown. At the suggestion of one of our IRBs, we arbitrarily chose to contact families every 90 days. This may not be frequent enough, as several parents did not recall the study when they arrived with their child in status epilepticus. Some parents recalled hearing about the study but did not recall any meaningful detail. Third, the process of pre-consent implies that one can identify a group of patients at risk for the emergency condition of interest. For status epilepticus, this would lead to a selection bias if we enrolled only patients with known epilepsy. Approximately 35% of status epilepticus cases occur in patients with no prior history of seizures (Chamberlain JM and Singh T, unpublished data). Additionally, for some emergency conditions, such as cardiopulmonary arrest, it would be impossible to identify a high-risk group for pre-consent. Finally, pre-consent was not efficient. We approached over 1,100 families for pre-consent, of whom approximately 500 gave consent for future participation. Only seven such subjects were eventually enrolled with an episode of status epilepticus during the two-year study period.
Many areas of concern raised in the focus groups are crucial to consider for most pediatric research in general. For example, parents had difficulty understanding the concept of off-label use of medications. Our experience showed that it was both enlightening, and frightening, to patients and parents, how often medications are used in off-label fashion. The current study was performed precisely to obtain FDA approval for lorazepam for pediatric use, as the drug is currently approved only in adults; however, this drug is so widely used in children that most clinicians are unaware that it is not currently labeled for use in children. In moving toward a transparent and patient-centered healthcare system,22 it is important for providers to share the uncertainties of current therapies with patients and families. Second, parental age and literacy, as well as the critical nature of the child’s illness, were identified as important barriers to an optimal informed consent process. Simplifying the delivery of the message to suit parental needs is important to ensure comprehension. Several participants in both focus groups suggested the use of some type of assessment tool, such as a post-interview quiz, to test parental comprehension.
LIMITATIONS
We investigated perceived issues with informed consent, patient enrollment, and ethics as viewed through the eyes of research staff and investigators, and therefore we did not include patients and families. This must be kept in perspective as the viewpoints of patients and families should be the ultimate arbiters of change regarding participation in emergency clinical trials and the informed consent process. Second, the retrospective nature of these focus groups raises the possibility of potential recall bias. No prospective data were collected. Nonetheless, the poignant nature of the experiences and anecdotes of the research staff provide important information about their perceptions of the informed consent process, and to our knowledge, have not been previously reported in this context. Furthermore, we performed this study while enrollment was ongoing, which may have generated different opinions than if we explored the issues after the study was completed.
Finally, we chose not to mix the focus group participants to avoid potential professional dominance by investigators. An interaction between investigators and research assistants may have yielded different discussion points had this occurred, although the same themes were identified concurrently by both groups. Given that the two moderators were study investigators and had their own opinions about the consent process, they may have biased the discussion in a particular direction with their follow-up questions and interjections. Personal experience was likely a strong factor in the creation of the questions for the topic guide used during the discussion, and these were also developed by the two moderators. Although we considered the use of a non-study team moderator, we believed that unfamiliarity with the nuances of the consent process and the technical details of the study itself would have reduced the depth and quality of responses that a more knowledgeable moderator was able to elicit. Although the moderators facilitated the discussion itself, the responses from the focus group members were recorded and transcribed verbatim and interpreted by a larger group of individuals, thus reducing any further bias.
CONCLUSIONS
Research coordinators and investigators participating in focus groups raised several concerns about informed consent for patient enrollment in a time-sensitive study of status epilepticus. Concerns centered around three major issues: barriers to and enablers of informed consent, barriers to and enablers of patient enrollment, and ethical issues. With few exceptions, concerns were similar in the research coordinator and investigator focus groups. We recommend the focus group methodology to investigators interested in identifying important ethical considerations or concerns among research personnel in a network or group conducting time-sensitive emergency research.
Figure.
Schematic of study patient cohorts.
Table 1.
Themes and sub-themes identified in investigator (PI) and research coordinator (RC) focus groups. Presented is the number of times each theme was discussed by each focus group.
| Theme/sub-theme | PI Group | RC Group |
|---|---|---|
| 1. Barriers to/enablers of the consent process | ||
| Parent/ Patient factors | ||
| Parental emotional state | ||
| Upset (distraught) | 13 | 5 |
| Inattentive | 1 | 1 |
| Parental knowledge/literacy | 2 | 3 |
| Parental primary language | 2 | 1 |
| Parental age | 1 | 1 |
| Previous experience with seizures | 6 | 4 |
| Previous attitudes/beliefs about research | 4 | 1 |
| Critically ill child | 3 | 1 |
| Ethnicity of family | 2 | |
| Overwhelmed with clinical situation, acute situation versus appointment for chronic care | 6 | 6 |
| Verbal communication with parents | ||
| Should be simplified | 3 | 2 |
| Should be standardized | 5 | 1 |
| Recommend short form | 3 | 2 |
| Recommend bulleted talking points | 4 | 1 |
| Recommend PowerPoint® presentation | 3 | |
| Physicians spoke at too high a level | 2 | |
| Confusion about meaning of off-label/why we can use unapproved medications | 3 | |
| Therapeutic misconception | 6 | 7 |
| Written communication with parents (informed consent documents) | ||
| Unrealistic risks presented | 8 | |
| Some language too complex | 7 | |
| Need for a short form | 2 | 2 |
| Environment | ||
| Need for privacy | 2 | 4 |
| Noise and interruptions in ED | 2 | 3 |
| Clinical investigators uncomfortable with consent process because of competing clinical needs | 4 | |
| Informed consent vs. time-sensitive needs of study | 7 | |
| Availability of invested investigators | 6 | |
| Investigator/Hospital | ||
| Lack of time (Cohort 1a), felt coercive sometimes | 5 | 4 |
| Sense that parents felt more comfortable asking questions of a nurse or social worker or research coordinator rather than the physician for clarification: possible implications for future enrollment studies | 1 | 2 |
| 2. Barriers/enablers to patient enrollment | ||
| Parent/ Patient factors | ||
| Pain of second intravenous stick | 3 | 4 |
| Inconvenience of staying/returning for tests | 5 | 4 |
| Not wanting to think about possible future seizures | 2 | 1 |
| Hypothetical nature of future seizure for pre-consent cohort | 2 | |
| Time between pre-consent and enrollment—some parents forgot that they were consented | 2 | |
| Young age of patient seen as a barrier | 4 | |
| Second parent not available—one parent felt uncomfortable without the other present | 3 | 1 |
| Verbal communication with parents | ||
| Coordination between clinical staff and research staff | 3 | 9 |
| Research coordinators wanted an introduction by the clinical staff | 2 | |
| Written communication with parents (informed consent documents) | ||
| Informed consent document overwhelming and inhibitory | 4 | 7 |
| Unrealistic risks listed in consent document | 8 | |
| Investigator/Hospital | ||
| Trusting relationship with | ||
| Hospital | 2 | 2 |
| ED physician/medical team | 7 | |
| Neurologist | 4 | 2 |
| Research staff | 3 | 1 |
| Personality of person enrolling | 1 | 1 |
| Environment | ||
| Clinical needs vs. needs of study | 5 | |
| 3. Ethical issues | ||
| Families who wanted to consent but didn’t understand | 7 | 4 |
| Financial compensation | ||
| Too much | 4 | 1 |
| Too little | 6 | 2 |
| How to measure understanding during consent process | 2 | 3 |
| Conflicting role as clinician and investigator | 4 |
Acknowledgments
Financial Disclosures: This study was funded by contract # NICHD-2003-10 from The National Institute of Child Health and Development, with support from The Pediatric Emergency Care Applied Research Network (PECARN), supported by cooperative agreements U03MC00001, U03MC00003, U03MC00006, U03MC00007, and U03MC00008 from the Emergency Medical Services for Children (EMSC) program of the Maternal and Child Health Bureau, Health Resources and Services Administration, U.S. Department of Health and Human Services.
APPENDIX
Participating centers and site investigators are listed below in alphabetical order: Children’s Hospital of Buffalo (Kathleen Lillis, MD); Children’s Hospital of Michigan (Prashant Mahajan, MD, MBA); Children’s Hospital of New York – Presbyterian (Stephen Gordon, MD); Children’s Hospital of Philadelphia (Jill Baren, MD); Children’s National Medical Center (James Chamberlain, MD); Johns Hopkins University (Allen Walker, MD); University of California Davis Medical Center (Cheryl Vance, MD); University of Maryland (Richard Lichenstein, MD); University of Michigan (Rachel Stanley, MD); University of Rochester (Colleen Davis, MD)
PECARN Steering Committee: N. Kuppermann, Chair; D. Alexander, E. Alpern, J. Chamberlain, J. M. Dean, M. Gerardi, J. Goepp, M. Gorelick, J. Hoyle, D. Jaffe, C. Johns, N. Levick, P. Mahajan, R. Maio, S. Miller*, D. Monroe, R. Ruddy, R. Stanley, D. Treloar, M. Tunik, A. Walker. MCHB/EMSC liaisons: D. Kavanaugh, H. Park.
Central Data Management and Coordinating Center (CDMCC): M. Dean, R. Holubkov, S. Knight, A. Donaldson.
Data Analysis and Management Subcommittee (DAMS): J. Chamberlain, Chair; M. Brown, H. Corneli, J. Goepp, R. Holubkov, P. Mahajan, K. Melville, E. Stremski, M. Tunik
Grants and Publications Subcommittee (GAPS): M. Gorelick, Chair; E. Alpern, G. Foltin, J. Joseph, N.C. Mann, S. Miller*, F. Moler, O. Soldes, S. Teach
Protocol Concept Review and Development Subcommittee (PCRADS): D. Jaffe, Chair; A. Cooper, J. M. Dean, C. Johns, R. Kanter, R. Maio, N. C. Mann, D. Monroe, K. Shaw, D. Treloar
Quality Assurance Subcommittee (QAS): R. Stanley, Chair; D. Alexander, J, Brown, M. Gerardi, M. Gregor, R. Holubkov, K. Lillis, R. Ruddy, M. Shults, A. Walker
Safety and Regulatory Affairs Subcommittee (SRAS): N. Levick, Chair; J. Brennan, J. Brown, J. M. Dean, J. Hoyle, R. Ruddy, W. Schalick, T. Singh, D. Snowdon, J. Wright
* deceased
Footnotes
Conflict of Interest: None
References
- 1.Biros MH, Lewis RJ, Olson CM, Runge JW, Cummins RO, Fost N. Informed consent in emergency research: consensus statement from the Coalition Conference of Acute Resuscitation and Critical Care Researchers. JAMA. 1995;273:1283–1287. doi: 10.1001/jama.273.16.1283. [DOI] [PubMed] [Google Scholar]
- 2.Salzman JG, Frascone RJ, Godding BK, Provo TA, Gertner E. Implementing emergency research requiring exception from informed consent, community consultation, and public disclosure. Ann Emerg Med. 2007;50:448–455. doi: 10.1016/j.annemergmed.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Vaslef SN, Cairns CB, Falletta JM. Ethical and regulatory challenges associated with the exception from informed consent requirements for emergency research: from experimental design to institutional review board approval. Arch Surg. 2006;141:1019–1023. doi: 10.1001/archsurg.141.10.1019. [DOI] [PubMed] [Google Scholar]
- 4.Baren JM, Fish SS. Resuscitation research involving vulnerable populations: are additional protections needed for emergency exception from informed consent? Acad Emerg Med. 2005;12:1071–1077. doi: 10.1197/j.aem.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 5.DeIorio NM, McClure KB. Does the emergency exception from informed consent process protect research subjects? Acad Emerg Med. 2005;12:1056–1059. doi: 10.1197/j.aem.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Watters D, Sayre MR, Silbergleit R. Research conditions that qualify for emergency exception from informed consent. Acad Emerg Med. 2005;12:1040–1044. doi: 10.1197/j.aem.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt TA, Salo D, Hughes JA, et al. SAEM Ethics Committee. Confronting the ethical challenges to informed consent in emergency medicine research. Acad Emerg Med. 2004;11:1082–1089. doi: 10.1197/j.aem.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 8.Adams JG, Wegener J. Acting without asking: an ethical analysis of the Food and Drug Administration waiver of informed consent for emergency research. Ann Emerg Med. 1999;33:218–223. doi: 10.1016/s0196-0644(99)70398-7. [DOI] [PubMed] [Google Scholar]
- 9.Biros MH, Fish SS, Lewis RJ. Implementing the Food and Drug Administration's final rule for waiver of informed consent in certain emergency research circumstances. Acad Emerg Med. 1999;6:1272–1282. doi: 10.1111/j.1553-2712.1999.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 10.Sloan EP, Nagy K, Barrett J. A proposed consent process in studies that use an exception to informed consent. Acad Emerg Med. 1999;6:1283–1291. doi: 10.1111/j.1553-2712.1999.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 11.Mosesso VN, Jr, Brown LH, Greene HL, et al. PAD Trial Investigators. Conducting research using the emergency exception from informed consent: the Public Access Defibrillation (PAD) Trial experience. Resuscitation. 2004;61:29–36. doi: 10.1016/j.resuscitation.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Dix ES, Esposito D, Spinosa F, Olson N, Chapman S. Implementation of community consultation for waiver of informed consent in emergency research: one Institutional Review Board's experience. J Invest Med. 2004;52:113–116. doi: 10.1136/jim-52-02-20. [DOI] [PubMed] [Google Scholar]
- 13.Sloan EP, Koenigsberg M, Houghton J, et al. The informed consent process and the use of the exception to informed consent in the clinical trial of diaspirin cross-linked hemoglobin (DCLHb) in severe traumatic hemorrhagic shock. DCLHb Traumatic Hemorrhagic Shock study group. Acad Emerg Med. 1999;6:1203–1209. doi: 10.1111/j.1553-2712.1999.tb00134.x. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Food and Drug Administration. Exception from informed consent requirements for emergency research. [Accessed May 7, 2009];Code of Federal Regulations. 21CFR50.24. Available at: http://www.fda.gov/ora/compliance_ref/bimo/default.htm#emer.
- 15.Triner W, Jacoby L, Shelton W, et al. Exception from informed consent enrollment in emergency medical research: attitudes and awareness. Acad Emerg Med. 2007;14:187–191. doi: 10.1197/j.aem.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 16.McClure KB, DeIorio NM, Gunnels MD, Ochsner MJ, Biros MH, Schmidt TA. Attitudes of emergency department patients and visitors regarding emergency exception from informed consent in resuscitation research, community consultation, and public notification. Acad Emerg Med. 2003;10:352–359. doi: 10.1111/j.1553-2712.2003.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 17.Dresden GM, Levitt MA. Modifying a standard industry clinical trial consent form improves patient information retention as part of the informed consent process. Acad Emerg Med. 2001;8:246–252. doi: 10.1111/j.1553-2712.2001.tb01300.x. [DOI] [PubMed] [Google Scholar]
- 18.Coyne CA, Xu R, Raich P, et al. Eastern Cooperative Oncology Group. Randomized, controlled trial of an easy-to-read informed consent statement for clinical trial participation: a study of the Eastern Cooperative Oncology Group. J Clin Oncol. 2003;21:836–842. doi: 10.1200/JCO.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Powers RD. Emergency department patient literacy and the readability of patient-directed materials. Ann Emerg Med. 1988;17(2):124–126. doi: 10.1016/s0196-0644(88)80295-6. [DOI] [PubMed] [Google Scholar]
- 20.University of Illinois at Chicago. Institutional Review Board Policy “IRB Observation: Informed Consent Process/Ombudsman.”. [Accessed Mar 31, 2009];Version 1.0. 2008 Oct 15; Available at: http://tigger.uic.edu/depts/ovcr/research/protocolreview/irb/policies/0869.pdf. [Google Scholar]
- 21.Kimmelman J. The therapeutic misconception at 25: treatment, research, and confusion. Hastings Cent Rep. 2007;37(6):36–42. doi: 10.1353/hcr.2007.0092. [DOI] [PubMed] [Google Scholar]
- 22.Institute of Medicine. Crossing the Quality Chasm: A New Health System for the Twenty-first Century. Washington, DC: National Academy Press; 2001. [Google Scholar]