SYNOPSIS
B-cell receptor (BCR) signaling is essential for chronic lymphocytic leukemia (CLL) cell survival. Many kinases in the BCR signaling pathway are currently being studied as potential therapeutic targets. These include Lyn, Syk, PI3 and Bruton tyrosine (BTK). Ibrutinib (PCI-32765) is a novel first-in-class selective inhibitor of BTK. Preclinical evidence suggests that ibrutinib inhibits CLL cell survival and proliferation. In addition, it also affects CLL cell migration and homing. Early clinical data in CLL and non-Hodgkin’s lymphoma patients is very encouraging. In relapsed-refractory patients with CLL, a 67% response rate was observed (420mg dose cohort) with single-agent ibrutinib. Long-term follow-up of these studies and other ongoing/planned studies of ibrutinib either as single-agent or in combination with monoclonal antibodies and chemoimmunotherapy is eagerly awaited. It is likely that ibrutinib and other drugs targeting the BCR pathway will become an integral component of CLL therapy.
Keywords: B-cell receptor inhibitor, Bruton tyrosine kinase inhibitor, Ibrutinib, PCI-32765, Chronic lymphocytic Leukemia
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults in the western world with approximately 16,060 men and women expected to be diagnosed with CLL in year 2012 in the United States.1 Most patients with CLL do not need treatment at diagnosis; however, the majority of patients will need CLL-directed therapy in their lifetime. Chemoimmunotherapy is the current standard of care for patients with CLL needing treatment.2 One of the commonly employed regimens is FCR (fludarabine, cyclophosphamide, rituximab). Tam and colleagues reported long-term follow-up of the FCR regimen for frontline treatment of CLL with a complete remission (CR) rate 72% and median progression-free survival (PFS) of 80 months.3 Despite these impressive results, certain subgroups of patients treated with chemoimmunotherapy have less than optimal outcomes. These include older adults (>65 years old), those with poor risk cytogenetics [del(17p), del(11q)] and patients with relapsed/refractory disease.2–4 Many approaches have been undertaken to improve the outcome of patients with CLL, including incorporation of drugs such as lenalidomide, ofatumumab, alemtuzumab, and bendamustine.5–11 These treatment strategies offer options after relapse post chemoimmunotherapy but outcomes are still less than satisfactory with approximately 4500 patients expected to die from CLL in the United States in the year 2012.2 This underscores the need to develop better therapeutics for patients with CLL.
B-cell receptor (BCR) activation-signaling plays a crucial role in the pathogenesis in CLL.12–15 The BCR signaling pathway consists of immunoglobulin bound to the cell membrane that attaches to a heterodimer consisting of CD79a and CD79b.15–17 Binding of a ligand to the membrane immunoglobulin leads to recruitment and phosphorylation of spleen tyrosine kinase (Syk) and Src family kinases (Lyn), which in turn recruit and phosphorylate many kinases and adapter proteins including Bruton tyrosine kinase (BTK). BTK is a non-receptor tyrosine kinase of the Tec kinase family and plays a crucial role in BCR signaling.18,19 BTK is expressed in non-T cell hematopoietic cell lineages.20,21 The BTK gene is located on chromosome Xq21.33-q22 and mutations in this gene result in X-linked agammaglobulinemia, a condition characterized by marked reduction in mature B-cells, severe hypogammaglobulinemia, and increased susceptibility to infections.22 BTK activates downstream molecules such as nuclear-factor-kappa B and MEK/ERK, which are involved in many cellular processes including proliferation, survival, differentiation, apoptosis, and metabolism. Gene expression profiling has shown that BCR signaling is the most expressed signaling pathway in patients with CLL.14 BCR signaling is enhanced in patients with poor prognostic markers such as ZAP-70 overexpression and those with unmutated immunoglobulin heavy chain gene (IGHV) rearrangement.23,24 Activated BCR signaling has also been shown to be required for cell survival in the activated B-cell (ABC) subtype of diffuse large B-cell lymphoma (DLBCL).25 Thus, multiple lines of data point to the crucial role of BCR signaling in CLL and other B-cell lymphoid malignancies. Many kinases in the BCR signaling pathway are now being pursued as therapeutic targets in CLL including Lyn,26 Syk,27–29 PI3K,30–32 and BTK.16,17
Ibrutinib (formerly PCI-32765, Pharmacyclics, Sunnyvale, California) (Figure 1) is an oral, selective and irreversible inhibitor of BTK and is the focus of this paper. Ibrutinib was initially developed by Celera Genomics (now Quest Diagnostics, Madison, New Jersey) and acquired by Pharmacyclics in 2006. Ibrutinib forms a specific bond with the cysteine-481 of BTK.33 It leads to highly potent BTK inhibition with an IC50 0.5nM (Table 1).34 Ibrutinib is orally administered with daily dosing and has no cytotoxic effect on T-cells.35
FIGURE 1.
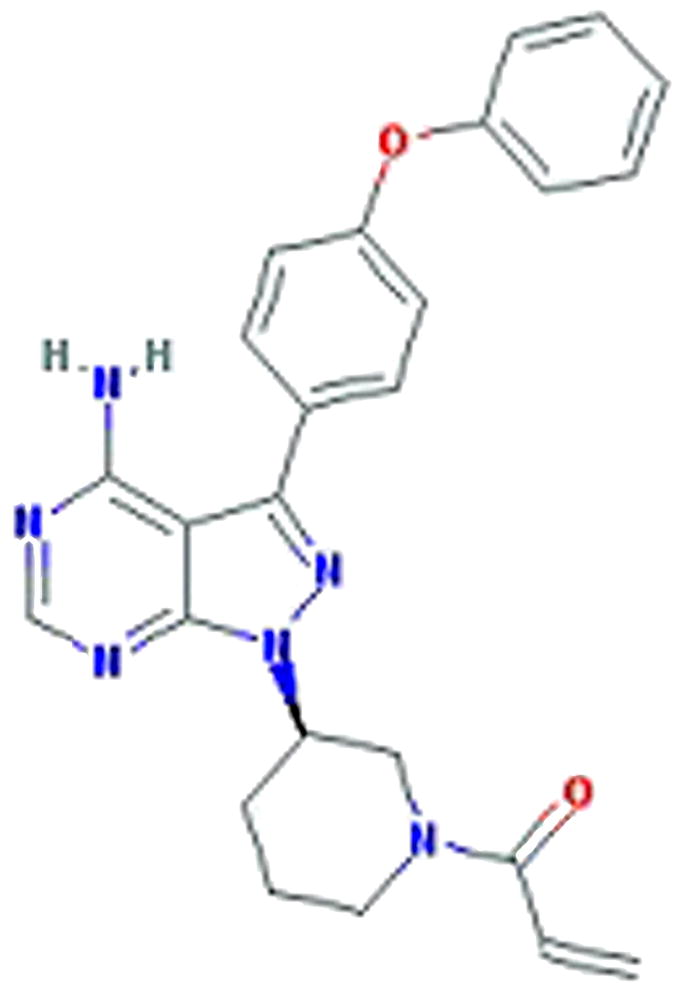
Chemical structure of PCI-32765 (Ibrutinib), a BTK inhibitor
TABLE 1. Inhibition of selected kinases by Ibrutinib.
(Data from Honigberg LA et al. Proceedings of the National Academy of Sciences USA, 2010, 107(29):13075–13080)34
| Kinase | IC50 (nM) | Fold selectivity for BTK inhibition |
|---|---|---|
| BTK | 0.5 | — |
| BLK | 0.5 | 1 |
| BMX | 0.8 | 1.6 |
| CSK | 2.3 | 4.6 |
| FGR | 2.3 | 4.6 |
| EGFR | 5.6 | 11.2 |
| ErbB2 | 9.4 | 18.8 |
| ITK | 10.7 | 21.4 |
| JAK3 | 16.1 | 32.2 |
| RET | 36.5 | 73 |
| FLT3 | 73 | 146 |
| TEC | 78 | 156 |
| ABL | 86 | 172 |
| c-SRC | 171 | 342 |
| LYN | 200 | 400 |
| PDGFRα | 718 | 1436 |
| JAK1 | >10,000 | >10,000 |
| JAK2 | >10,000 | >10,000 |
| PI3K | >10,000 | >10,000 |
| PLK1 | >10,000 | >10,000 |
| SYK | >10,000 | >10,000 |
PRECLINICAL STUDIES
Honigberg and colleagues showed that in a B-cell lymphoma cell line (DOHH2), ibrutinib irreversibly inhibited autophosphorylation of BTK (IC50 11nM) and phosphorylation of downstream kinases such as ERK.34 Phosphorylation of upstream kinases such as SYK was not affected. They also showed that ibrutinib blocked the transcriptional upregulation of B-cell activation genes in primary cultures of human peripheral B-cells.34 In a mouse model for lupus nephritis, ibrutinib reduced proteinuria, lowered anti-dsDNA antibody levels and improved glomerular function, indicating the potential role for ibrutinib in autoimmune diseases.34 Clinical activity of ibrutinib with once daily oral dosing was also seen in naturally occurring B-cell lymphoma in dogs.34
Herman and colleagues reported that BTK mRNA expression was significantly higher in CLL (CD19+) cells compared to normal B-cells.35 They also noted that baseline BTK protein expression was highly variable in the CLL cells and protein expression did not correlate with known prognostic markers such as age, cytogenetics, IGHV status and ZAP-70 expression. Treatment of CLL cells with ibrutinib induced apoptosis in a dose- and time- dependent manner which was independent of baseline cytogenetics, IGHV mutational status or baseline BTK protein expression but dependent on caspase-pathway activation.35 Ibrutinib also induced apoptosis in normal B cells, but this was significantly less than that seen in CLL cells, indicating that CLL cells are more sensitive to ibrutinib than normal B cells. Ibrutinib treatment of CLL cells inhibited downstream signaling pathways including ERK1/2 phosphorylation, CD40L induced AKT phosphorylation and CD40L induced NF-kB DNA binding.35
Ponader and colleagues evaluated the role of the tissue microenvironment of CLL cells and its effect on treatment with ibrutinib.36 They reported that ibrutinib treatment significantly inhibited CLL cell migration and survival in a nurselike cell (NLC) coculture assay. In this model, ibrutinib treatment significantly decreased the levels of CCL3 and CCL4 and inhibited chemotaxis towards CXCL12 and CXCL13. In an adoptive transfer TCL1 mouse model, ibrutinib treatment was reported to delay CLL disease progression.36 Overall the preclinical data suggests that ibrutinib is a selective, irreversible BTK inhibitor with effect on both CLL cell survival/proliferation and CLL cell migration/homing.15
CLINICAL STUDIES
Clinical studies with ibrutinib have only been published in abstract form thus far. Ibrutinib was evaluated in a phase I study in CLL and lymphoma [small lymphocytic lymphoma (SLL), follicular lymphoma, mantle cell lymphoma (MCL), DLBCL, marginal zone lymphoma, Waldenstrom macroglobulinemia] patients with a 28-day on / 7-day off schedule in 5 dose-cohorts (1.25–12.5 mg/kg orally daily) and once daily continuous dose in 2 dose-cohorts (8.3 mg/kg and 560-mg fixed dose).37 Fifty-six patients with relapsed/refractory disease [median 3 prior regimens (range 1–10)] were enrolled. No dose-limiting toxicity was observed. MTD was not reached. Of the 50 evaluable patients, 30 (60%) patients achieved an objective response rate (ORR) [23% CR, 77% partial response (PR)]. Responses were seen in all NHL subtypes and irrespective of the dose levels. A unique pattern of response was noted, with a transient lymphocytosis lasting a few months. Transient lymphocytosis was also noted by Ponader and colleagues in an adoptive transfer TCL1 mouse model after treatment with ibrutinib.36 This is postulated to be due to an initial compartment shift of CLL cells from lymphatic tissues into the peripheral blood.
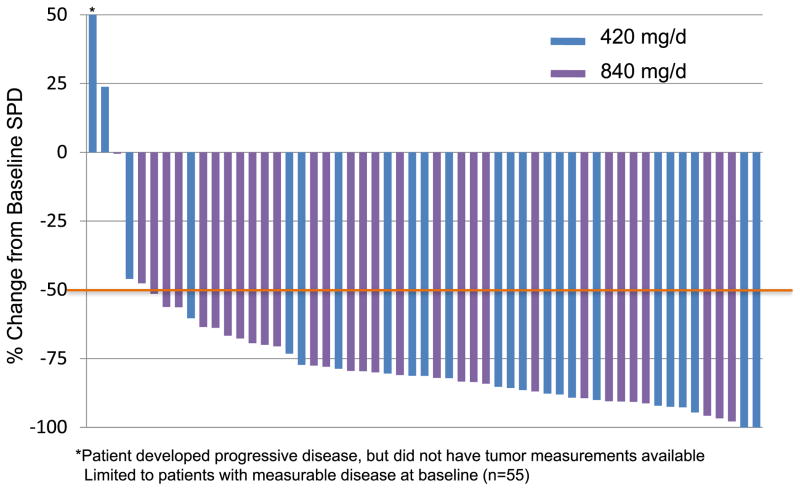
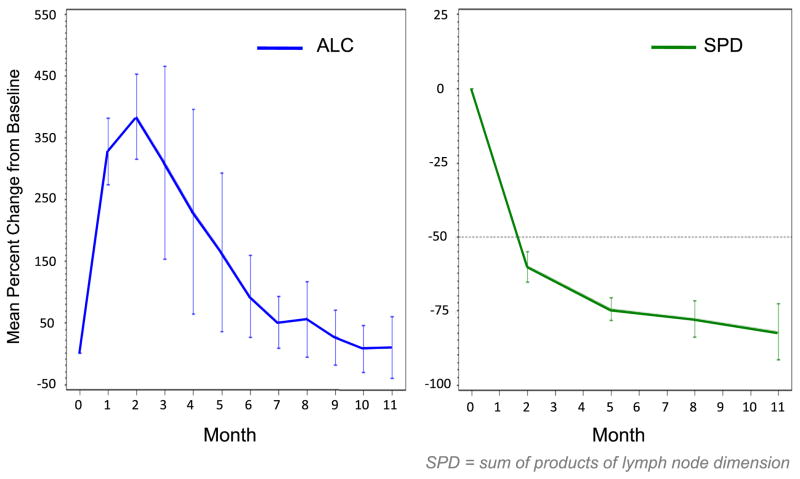
In a phase IB/II study (PCYC-1102), patients with relapsed/refractory CLL and older adults (≥65 years) with untreated CLL were treated with 2 fixed doses of ibrutinib (420mg daily and 840mg daily).38 Ibrutinib was given orally once daily for 28-day cycles until disease progression. Patient enrollment occurred from May 2010 to July 2011. Sixty-one patients were enrolled in the relapsed/refractory cohort (420mg cohort n=27, 840mg cohort n=34). The median age was 64 years (range, 40–81). The median number of prior therapies for the 420mg cohort was 3 (2–10) and for the 840mg cohort was 5 (1–12). High-risk molecular features were present in the majority of the patients [unmutated IGHV:79%; del(17p):36%; del(11q):39%]. The median follow-up for the 420mg cohort was 12.6 months and for the 840mg cohort was 9.3 months. Seventy-five percent of the patients were still on the study at the time of last follow-up. Treatment was well tolerated with the majority of adverse-events being grade I/II (diarrhea, fatigue, and nausea). Six patients needed dose reduction (2 in the 420mg cohort, 4 in the 840mg cohort). Grade 3/4 hematologic toxicity (neutropenia, anemia, thrombocytopenia), irrespective of attribution, was seen in 8%, 7%, 7% (420mg cohort) and 21%, 12%, 9% (840mg cohort), respectively. ORR was noted to be 67% (63% PR, 4% CR) in the 420mg cohort and 68% (all PR) in the 840mg cohort. An additional 22% (420mg cohort) and 24% (840mg cohort) of patients achieved nodal PR (>50% reduction in aggregate lymph node size) with residual lymphocytosis. Maximum change in tumor burden is shown in figure 2. Importantly, clinical responses were independent of the high-risk molecular features. Seventy-four percent of the patients with unmutated IGHV, 65% with del(17p), and 73% with del(11q) responded. Most clinical responses were nodal responses in the first 4–5 months of therapy which then improved to PR/CR with continued treatment over the next few months. Estimated 18-month PFS was 87.7% in the 420 mg cohort.39 A transient lymphocytosis typically peaking within the first 2 months of treatment, followed by gradual resolution over the next 6–8 months was noted (Figure 3).
FIGURE 2.
Maximum change in tumor burden in relapsed/refractory CLL patients treated with single-agent ibrutinib (PCYC-1102 trial) (From data presented at American Society of Hematology Annual Meeting, San Diego, California, December, 2011).
FIGURE 3.
Characteristic response pattern with transient lymphocytosis typically peaking within the first 2 months of treatment, followed by gradual resolution over the next 6–8 months. Decrease in lymphadenopathy was consistent from the beginning. Data derived from relapsed/refractory CLL patients treated with single-agent ibrutinib (PCYC-1102 trial) (From data presented at American Society of Hematology Annual Meeting, San Diego, California, December, 2011).
In the update of the data presented at the European Hematology Association meeting in June 2012, O’Brien and colleagues reported the outcomes of 31 treatment naïve older patients (420mg, n=26; 840mg, n=5).39 Enrollment in the 840mg cohort was terminated after similar results were noted between the 420mg and 840mg cohort in the relapsed/refractory cohort. Median age was 71 years (range, 65–84) with 75% of patients being older than 70 years. Forty-three percent patients had unmutated IGHV and 6% had del(17p). The majority of the adverse events (AE) were mild (grade I–II) and included diarrhea, nausea, and fatigue. Grade III non-hematological AE potentially related to the drug was seen in 6 (19%) patients (diarrhea 4 patients, hyponatremia 2 patients, hemorrhagic enterocolitis 1 patient). No grade 4 non-hematological toxicity was seen. Grade ≥3 hematological toxicity was seen in 4 (12%) patients and included 2 patients each with anemia and thrombocytopenia. Neutropenia was not observed. In the 420mg cohort, only 4 of the 26 patients have discontinued therapy, and only one patient for disease progression. With a median follow-up of 14.4 months on the 420 mg cohort, 81% achieved a response (69% partial response, 12% complete response) by IWCLL criteria. An additional 12% of patients achieved a nodal response. Fifty percent of patients with baseline thrombocytopenia or anemia noted sustained improvement in blood counts. The responses were independent of high-risk features. Ninety-two percent of patients with unmutated IGHV responded. There were 2 patients with del(17p) and both responded. The estimated 15-month median PFS for the 420 mg cohort was 96%.
Given the impressive single-agent activity of ibrutinib in patients with CLL, trials exploring combinations of ibrutinib with either monoclonal antibodies (rituximab or ofatumumab), or with chemotherapy (bendamustine or fludarabine/cyclophosphamide/rituximab) have been initiated. Some of these trials have been reported in abstract form. Preliminary data has been reported for ibrutinib in combination with ofatumumab (PCYC-1109-CA trial).40 Patients with relapsed/refractory CLL following ≥2 prior therapies, including a purine-nucleoside analog, were treated with ibrutinib 420 mg daily with addition of ofatumumab from cycle 2 onwards. Twenty-four patients with CLL/ pro-lymphocytic leukemia (PLL) and three with Richter’s transformation were treated. The median age was 66 (range 51–85). High-risk cytogenetics were seen in the majority patients [10 patients with del(17p) and 9 patients with del(11q)]. The majority of AE were grade 1–2. All CLL/PLL patients and 2 of the 3 patients with Richter’s transformation achieved PR.
Brown and colleagues reported preliminary data on the combination of ibrutinib with bendamustine/rituximab (PCYC-1108-CA) in relapsed/refractory patients with CLL.41 Thirty patients were enrolled with a median age of 62 years (range 41–82). The median number of prior therapies was 2 (range 1–4). Twenty-three percent had deletion 17p and 43% had deletion 11q. No added toxicity was observed with the addition of ibrutinib. With a median follow-up of 8.1 months, 23 of the 30 patients were still on study, with only 2 patients coming off-protocol for disease progression. The ORR was 93% (13% CR, 80% PR) which is higher than the 59% seen with bendamustine-rituximab in historical controls. As with single-agent ibrutinib, responses were independent of high-risk genetic and molecular features.
Ibrutinib has also been evaluated in other lymphoid malignancies including MCL, DLBCL, Waldenstrom macroglobulinemia, and multiple myeloma. In the preliminary results of a phase II study (PCYC-1104), Wang and colleagues reported outcomes of 48 relapsed/refractory patients with MCL (29 bortezomib-naïve; 19 bortezomib-exposed) who were treated with single-agent ibrutinib.42 Ibrutinib was administered orally at 560mg daily until disease progression. The median age was 67 years (range, 62–72). Therapy was well tolerated with most frequently reported adverse events being grade 1 or 2 diarrhea, fatigue, and nausea (similar to the CLL study). The ORR was 67% (16 of the 24 evaluable patients). Responses were seen in both bortezomib-naïve and bortezomib-exposed cohorts.
Many ongoing/planned trials are exploring the role of ibrutinib in hematologic malignancies. Some examples include ibrutinib as a single agent in Waldenstrom macroglobulinemia and multiple myeloma, ibrutinib with rituximab in CLL, ibrutinib with bendamustine/rituximab in relapsed DLBCL/MCL, ibrutinib with R-CHOP chemotherapy in newly-diagnosed DLBCL. A phase III randomized, open-label registration trial of ibrutinib versus ofatumumab in patients with relapsed or refractory CLL (RESONATE trial) has been initiated.43 The primary end-point of this trial is PFS with key secondary endpoints being overall response rate, overall survival, and quality of life measures. Another planned phase III study includes a randomized study of bendamustine/rituximab plus ibrutinib versus bendamustine/rituximab plus placebo in relapsed or refractory patients with CLL/SLL.
SUMMARY
It is clear from the preclinical and preliminary clinical data (as stated above) that BTK inhibitors (along with other BCR-signaling pathway inhibitors) are going to revolutionize the treatment of patients with CLL. Besides ibrutinib, there are other BTK inhibitors in clinical development such as AVL-292 (Avila Therapeutics, now part of Celgene Corporation, Summit, New Jersey) and ONO-WG-307 (Ono Pharmaceutical, Osaka, Japan). In the coming few years, there will be a barrage of preclinical and clinical data with these drugs. Thus far the clinical responses with ibrutinib have been impressive with manageable toxicities. It is likely that ibrutinib and other drugs targeting the BCR pathway will become an integral component of CLL and NHL therapy.
KEY POINTS.
B-cell receptor (BCR) signaling plays a crucial role in pathogenesis of chronic lymphocytic leukemia (CLL).
Many kinases in the BCR signaling pathway are being explored as therapeutic targets such as Lyn, Syk, PI3 kinase and Bruton tyrosine kinase (BTK).
Ibrutinib (PCI-32765) is a selective, irreversible and oral inhibitor of BTK.
Preclinical data suggests that ibrutinib affects both CLL cell survival/proliferation as well as CLL cell migration/homing.
Preliminary clinical data in patients with CLL is very encouraging with 67% response rate in the 420mg dose cohort in the relapsed/refractory CLL setting.
Combination of ibrutinib with monoclonal antibodies and chemoimmunotherapy has also shown favorable early results.
BCR inhibitors will likely become an important component of CLL therapeutics in the near future.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) National Cancer Institute; Bethesda, MD: 2012. http://seer.cancer.gov/csr/1975_2009_pops09/, based on November 2011 SEER data submission, posted to the SEER web site. [Google Scholar]
- 2.Gribben JG, O’Brien S. Update on therapy of chronic lymphocytic leukemia. J Clin Oncol. 2011 Feb 10;29(5):544–550. doi: 10.1200/JCO.2010.32.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tam CS, O’Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008 Aug 15;112(4):975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000 Dec 28;343(26):1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 5.Badoux XC, Keating MJ, Wen S, et al. Lenalidomide as initial therapy of elderly patients with chronic lymphocytic leukemia. Blood. 2011 Sep 29;118(13):3489–3498. doi: 10.1182/blood-2011-03-339077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrajoli A, Lee BN, Schlette EJ, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008 Jun 1;111(11):5291–5297. doi: 10.1182/blood-2007-12-130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wierda WG, Kipps TJ, Durig J, et al. Chemoimmunotherapy with O-FC in previously untreated patients with chronic lymphocytic leukemia. Blood. 2011 Jun 16;117(24):6450–6458. doi: 10.1182/blood-2010-12-323980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Brien SM, Kantarjian HM, Thomas DA, et al. Alemtuzumab as treatment for residual disease after chemotherapy in patients with chronic lymphocytic leukemia. Cancer. 2003 Dec 15;98(12):2657–2663. doi: 10.1002/cncr.11871. [DOI] [PubMed] [Google Scholar]
- 9.Elter T, Gercheva-Kyuchukova L, Pylylpenko H, et al. Fludarabine plus alemtuzumab versus fludarabine alone in patients with previously treated chronic lymphocytic leukaemia: a randomised phase 3 trial. Lancet Oncol. 2011 Dec;12(13):1204–1213. doi: 10.1016/S1470-2045(11)70242-X. [DOI] [PubMed] [Google Scholar]
- 10.Fischer K, Cramer P, Busch R, et al. Bendamustine combined with rituximab in patients with relapsed and/or refractory chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2011 Sep 10;29(26):3559–3566. doi: 10.1200/JCO.2010.33.8061. [DOI] [PubMed] [Google Scholar]
- 11.Fischer K, Cramer P, Busch R, et al. Bendamustine in Combination With Rituximab for Previously Untreated Patients With Chronic Lymphocytic Leukemia: A Multicenter Phase II Trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2012 Aug 6; doi: 10.1200/JCO.2011.39.2688. [DOI] [PubMed] [Google Scholar]
- 12.Chiorazzi N, Ferrarini M. B cell chronic lymphocytic leukemia: lessons learned from studies of the B cell antigen receptor. Annual review of immunology. 2003;21:841–894. doi: 10.1146/annurev.immunol.21.120601.141018. [DOI] [PubMed] [Google Scholar]
- 13.Stevenson FK, Caligaris-Cappio F. Chronic lymphocytic leukemia: revelations from the B-cell receptor. Blood. 2004 Jun 15;103(12):4389–4395. doi: 10.1182/blood-2003-12-4312. [DOI] [PubMed] [Google Scholar]
- 14.Herishanu Y, Perez-Galan P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011 Jan 13;117(2):563–574. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burger JA. Nurture versus nature: the microenvironment in chronic lymphocytic leukemia. Hematology / the Education Program of the American Society of Hematology. American Society of Hematology. Education Program. 2011;2011:96–103. doi: 10.1182/asheducation-2011.1.96. [DOI] [PubMed] [Google Scholar]
- 16.Woyach JA, Johnson AJ, Byrd JC. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood. 2012 Aug 9;120(6):1175–1184. doi: 10.1182/blood-2012-02-362624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiestner A. Emerging role of kinase targeted strategies in chronic lymphocytic leukemia. Blood. 2012 Aug 8; doi: 10.1182/blood-2012-05-423194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan WN. Regulation of B lymphocyte development and activation by Bruton’s tyrosine kinase. Immunologic research. 2001;23(2–3):147–156. doi: 10.1385/IR:23:2-3:147. [DOI] [PubMed] [Google Scholar]
- 19.Satterthwaite AB, Witte ON. The role of Bruton’s tyrosine kinase in B-cell development and function: a genetic perspective. Immunological reviews. 2000 Jun;175:120–127. [PubMed] [Google Scholar]
- 20.Smith CI, Baskin B, Humire-Greiff P, et al. Expression of Bruton’s agammaglobulinemia tyrosine kinase gene, BTK, is selectively down-regulated in T lymphocytes and plasma cells. Journal of immunology. 1994 Jan 15;152(2):557–565. [PubMed] [Google Scholar]
- 21.Genevier HC, Hinshelwood S, Gaspar HB, et al. Expression of Bruton’s tyrosine kinase protein within the B cell lineage. European journal of immunology. 1994 Dec;24(12):3100–3105. doi: 10.1002/eji.1830241228. [DOI] [PubMed] [Google Scholar]
- 22.Conley ME, Dobbs AK, Farmer DM, et al. Primary B cell immunodeficiencies: comparisons and contrasts. Annual review of immunology. 2009;27:199–227. doi: 10.1146/annurev.immunol.021908.132649. [DOI] [PubMed] [Google Scholar]
- 23.Chen L, Widhopf G, Huynh L, et al. Expression of ZAP-70 is associated with increased B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2002 Dec 15;100(13):4609–4614. doi: 10.1182/blood-2002-06-1683. [DOI] [PubMed] [Google Scholar]
- 24.Rosenwald A, Alizadeh AA, Widhopf G, et al. Relation of gene expression phenotype to immunoglobulin mutation genotype in B cell chronic lymphocytic leukemia. The Journal of experimental medicine. 2001 Dec 3;194(11):1639–1647. doi: 10.1084/jem.194.11.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010 Jan 7;463(7277):88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Contri A, Brunati AM, Trentin L, et al. Chronic lymphocytic leukemia B cells contain anomalous Lyn tyrosine kinase, a putative contribution to defective apoptosis. The Journal of clinical investigation. 2005 Feb;115(2):369–378. doi: 10.1172/JCI22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoellenriegel J, Coffey GP, Sinha U, et al. Selective, novel spleen tyrosine kinase (Syk) inhibitors suppress chronic lymphocytic leukemia B-cell activation and migration. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2012 Jul;26(7):1576–1583. doi: 10.1038/leu.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010 Apr 1;115(13):2578–2585. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchner M, Baer C, Prinz G, et al. Spleen tyrosine kinase inhibition prevents chemokine- and integrin-mediated stromal protective effects in chronic lymphocytic leukemia. Blood. 2010 Jun 3;115(22):4497–4506. doi: 10.1182/blood-2009-07-233692. [DOI] [PubMed] [Google Scholar]
- 30.Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010 Sep 23;116(12):2078–2088. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoellenriegel J, Meadows SA, Sivina M, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011 Sep 29;118(13):3603–3612. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011 Jan 13;117(2):591–594. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan Z, Scheerens H, Li SJ, et al. Discovery of selective irreversible inhibitors for Bruton’s tyrosine kinase. ChemMedChem. 2007 Jan;2(1):58–61. doi: 10.1002/cmdc.200600221. [DOI] [PubMed] [Google Scholar]
- 34.Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proceedings of the National Academy of Sciences of the United States of America. 2010 Jul 20;107(29):13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011 Jun 9;117(23):6287–6296. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012 Feb 2;119(5):1182–1189. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Advani RH, Sharman JP, Smith SM, et al. The Btk Inhibitor PCI-32765 Is Highly Active and Well Tolerated in Patients with Relapsed/Refractory B Cell Malignancies: Final Results from a Phase I Study. 11th International Conference on Malignant Lymphoma Meeting Abstract.; 2011; p. 153a. [Google Scholar]
- 38.O’Brien S, Burger JA, Blum KA, et al. The Bruton’s Tyrosine Kinase (BTK) Inhibitor PCI-32765 Induces Durable Responses in Relapsed or Refractory (R/R) Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL): Follow-up of a Phase Ib/II Study. American Society of Hematology Annual Meeting Abstracts; 2011; p. 983a. [Google Scholar]
- 39.O’Brien S, Furman R, Coutre S, et al. The Bruton’s Tyrosine Kinase Inhibitor Ibrutinib is Highly Active and Tolerable in Relapsed or Refractory and Treatment Naïve CLL Patients, Updated Results of a Phase IB/II Study. European Hematology Meeting Annual Abstracts; 2012; p. 542a. [Google Scholar]
- 40.Jaglowski SM, Jones JA, Flynn JM, et al. A phase Ib/II study evaluating activity and tolerability of BTK inhibitor PCI-32765 and ofatumumab in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and related diseases. American Society of Clinical Oncology Annual Meeting Abstracts; 2012; p. 6508a. [Google Scholar]
- 41.Brown J, Barrientos J, Flinn I, et al. The Bruton’s Tyrosine Kinase (Btk) Inhibitor Ibrutinib Combined with Bendamustine and Rituximab is Active and Tolerable in Patients with Relapsed/Refractory CLL, Interim Results of A Phase Ib/II Study. European Hematology Meeting Annual Abstracts; 2012; p. 543a. [Google Scholar]
- 42.Wang L, Martin P, Blum KA, et al. The Bruton’s Tyrosine Kinase Inhibitor PCI-32765 Is Highly Active As Single-Agent Therapy in Previously-Treated Mantle Cell Lymphoma (MCL): Preliminary Results of a Phase II Trial. American Society of Hematology Annual Meeting Abstracts; 2011; p. 442a. [Google Scholar]
- 43. [Accessed 08/25/2012]; http://clinicaltrials.gov/ct2/show/NCT01578707?term=ibrutinib&rank=6.