Abstract
Objective
The definite prognostic role of p-STAT3 has not been well defined. We performed a meta-analysis evaluating the prognostic role of p-STAT3 expression in patients with digestive system cancers.
Methods
We searched the available articles reporting the prognostic value of p-STAT3 in patients with cancers of the digestive system, mainly including colorectal cancer, gastric cancer, hepatocellular carcinoma, esophagus cancer and pancreatic cancer. The pooled hazard ratios (HRs) with 95 % confidence intervals (95 % CIs) of overall survival (OS) and disease-free survival (DFS) were used to assess the prognostic role of p-STAT3 expression level in cancer tissues. And the association between p-STAT3 expression and clinicopathological characteristics was evaluated.
Results
A total of 22 studies with 3585 patients were finally enrolled in the meta-analysis. The results showed that elevated p-STAT3 expression level predicted inferior OS (HR=1.809, 95% CI: 1.442-2.270, P<0.001) and DFS (HR=1.481, 95% CI: 1.028-2.133, P= 0.035) in patients with malignant cancers of the digestive system. Increased expression of p-STAT3 is significantly related with tumor cell differentiation (Odds ratio (OR) =1.895, 95% CI: 1.364-2.632, P<0.001) and lymph node metastases (OR=2.108, 95% CI: 1.104-4.024, P=0.024). Sensitivity analysis suggested that the pooled HR was stable and omitting a single study did not change the significance of the pooled HR. Funnel plots and Egger’s tests revealed there was no significant publication bias in the meta-analysis.
Conclusion
Phospho-STAT3 might be a prognostic factor of patients with digestive system cancers. More well designed studies with adequate follow-up are needed to gain a thorough understanding of the prognostic role of p-STAT3.
Introduction
Cancers derived from the digestive system, mainly including esophagus cancer, colorectal cancer, gastric cancer, hepatocellular carcinoma, pancreatic cancer, et al., account for a majority portion of the most killing malignant cancers worldwide [1, 2]. Digestive system cancers are featured by the aggressive biological behavior and unfavorable clinical outcome [3]. Despite the improvement of diagnostic and therapeutic approaches in the past decades, the prognosis of digestive system cancers remains to be dismal mainly due to local recurrence and distal metastases [1]. Currently, the designation of treatment strategy mainly depends on the TNM stage of tumor. It is common to observe that patients at the same TNM stage may have various clinical outcomes [4]. Molecular based prognostic factors could act as an implement of the current staging system. Thus, it is essential to identify molecular prognostic factors of digestive system cancers which aids in rational stratification of the patients according to the clinical prognosis as well as provides us with the potential therapeutic targets.
Signal transducer and activator of transcription proteins (STATs) are often activated through tyrosine phosphorylation and are then converted into its active form as phosphorylated STATs (p-STATs) [5]. Among the STATs family members, STAT3 is a prominent molecular hub connecting multiple vital molecular pathways involved in cancer progression. p-STAT3 can trigger gene expression by interacting with STAT cognate sequence in the DNA or numerous regulatory proteins [6], which further modulate the cellular biological behaviors including the cellular cycle [7], epithelial-mesenchymal transition (EMT) [8], the inflammatory responses [9] and angiogenesis [10].
Numerous studies indicated that alternation of p-STAT3 expression in tumor samples was associated with prognosis of various human malignancies such as breast cancer [11], lung cancer [12], lymphoma [13]. However, the exact prognostic role of p-STAT3 in cancers with the digestive systems remains to be unsettled. Several studies showed that p-STAT3 overexpression could significantly predict unfavorable outcome in patients with colorectal cancer [14], gastric cancer [15], hepatocellular carcinoma [16], esophagus cancer [17] and pancreatic cancer [18]; whereas the study by Monnien et al. [19] revealed that elevated expression of p-STAT3 was significantly related with advantageous clinical prognosis in colorectal cancer patients; and some other studies unveiled no significant association between p-STAT3 expression and the survival outcome in patients with gastric cancer [20], hepatocellular carcinoma [21], esophagus cancer [22] and pancreatic cancer [23]. Therefore we searched the available articles and performed the present meta-analysis in order to explore the prognostic value of p-STAT3 in patients with digestive system cancers. Besides, the association between p-STAT3 expression and the clinicopathological characteristics was assessed.
Materials and Methods
Search strategy
Literature search of MEDLINE, Web of Science, Cochrane library, EMBASE from their inception to September, 2014 was carefully performed. The following retrieval strategy was used: (‘cancer’ OR ‘tumor’ OR ‘tumour’ OR ‘neoplasm’ OR ‘carcinoma’ OR ‘adenocarcinoma’) AND (‘phosphorylated signal transducer and activator of transcription3’ OR ‘phosphate STAT3’ OR ‘phospho-STAT3’ OR ‘p-STAT3’) AND (‘prognosis’ OR ‘prognostic’ OR ‘outcome’). The reference list of each manuscript was manually screened in order to gain the potential related articles. No advanced limitations were additionally set. The written language was limited to English. Only articles published in peer-review journals were admitted into our further analysis.
Study inclusion/exclusion criteria
Li MX and Bi XY independently scrutinized the initially identified articles. Studies were considered eligible if the following criteria were fulfilled: (1) they studied patients with digestive system cancers (i.e. gastric cancer, hepatocellular carcinoma, colorectal cancer, esophagus cancer and pancreatic cancer). (2) Expressions of p-STAT3 were measured in the tumor tissue samples. (3) studies presenting data regarding association between p-STAT3 expression and survival outcome or clinicopathological information such as tumor differentiation, TNM stages, and lymph node metastasis; (4) HRs (ORs) and 95% CI were directly extracted or synthesized by the relevant published data [24]. And HRs and 95% CI in terms of the survival outcome should be produced by the multivariate analysis; (5) for articles with duplicated or overlapping study population; only the most complete ones were enrolled. Agreement was reached by discussion.
The following items were defined as the exclusion criteria: (1) literature published as abstracts, letters, editorials, expert opinions, reviews and case reports; (2) articles without sufficient data to obtain the HRs (ORs) and CI; (3) researches based on cancer cells or animal models but not based on patients.
Data extraction
The following data of each article was extracted: (1) general information including first author, publication year, country (area) of origin, age and gender of the study patients, sample size, and the follow-up duration; (2) clinicopathological characteristics including TNM stage, differential grade and lymph node metastasis; (3) HRs and 95% CIs produced by multivariate analysis investigating the relationship between elevated level of p-STAT3 and OS or DFS; (4) antibodies used for IHC; (5) scoring method and cut-off value defining “elevated expression” of p-STAT3. The index of scoring method in immunohistochemical staining (IHC) mainly included staining extent (E), intensity (I) and staining extent& intensity (EI). As the cut-off score for positive expression of p-STAT3 was not uniform among the studies, positive p-STAT3 expression was defined with respect to the original contents. Since half of the enrolled studies (11/22) used rabbit polyclonal antibody as the primary antibody in IHC, primary antibodies were classified as rabbit polyclonal antibody and other antibodies. Tumor cell differentiation grade was dichotomized as well/moderate and poor differentiation. TNM stage was subdivided as I/II and III/IV. Status of lymph node metastasis (N-status) was categorized as positive lymph node metastasis and negative lymph node metastasis.
Quality assessment
Two authors (Li MX and Bi XY) independently conducted the quality assessment with the Newcastle–Ottawa Quality Assessment Scale (NOS) [25]. The assessment of the scale mainly includes selection of cases, comparability of populations, and ascertainment of exposure to risks. NOS scores of ≥ 6 were assigned as high-quality studies. Consensus was reached by discussion when disparity occurred.
Statistical analysis
For each meta-analysis, the Cochrane’s Q statistic was undertaken to assess the heterogeneity of the trials. When combining the data, the random effect model or the fixed effect model was selected according the level of between study heterogeneity. A random effect model was used if severe between study heterogeneity was observed (I2≥50%); the fixed effect model was applied if there was no remarkable inter-study heterogeneity (I2<50%). All statistical tests were two-sided and P<0.05 was considered statistical significant. Publication bias was assessed by Begg’s funnel plot and Egger’s linear regression test [26]. All the analyses were conducted using STATA statistical software package version 12.0 (STATA Corp., College Station, Texas, USA).
Results
The description of the included studies
The initial search yielded a total of 462 articles. After meticulous inspection of the primary search results, 22 studies [14–23, 27–38] published between 2006 and 2014 were finally enrolled in our meta-analysis (details in Table 1). The flowchart of the selection of enrolled studies was illustrated in Fig 1. We found that Deng was the first author of two included articles [30, 33] which studied patients from the same institution but from separated time period. We marked them as Deng1 and Deng2 respectively.
Table 1. Main characteristics of all the studies included in the meta-analysis.
| First author | Year | Study region | Primary site | Treatment | NO. (M/F) | Age | Follow-up(months) | N status(-/+) |
|---|---|---|---|---|---|---|---|---|
| Kusaba [27] | 2006 | Japan | colorectal | surgery | 66/42 | 65.6ys range:44–86ys | Median:43.7ms | 74/34 |
| Range:0.71–60ms | ||||||||
| Monnien [19] | 2010 | France | colorectal | surgery | 76/28 | Median: 66ys | >60ms | 67/31 |
| Morikawa [28] | 2011 | USA | colorectal | surgery | 266/458 | ≥65ys:454, <65ys:270 | Median: 129ms | NR |
| Dobi [14] | 2012 | France | colorectal | chemotherapy | 50/44 | ≤70ys:60, >70ys:34 | >120ms | NR |
| Yakata [29] | 2006 | Japan | gastric | surgery | 63/48 | Median:68.9ys | NR | 79/32 |
| Range:38–89ys | ||||||||
| Lee [15] | 2009 | Korea | gastric | surgery | 210/101 | Median: 53ys | >60ms | NR |
| Deng [30] | 2010 | China Mainland | gastric | surgery | 37/16 | Median:55ys | Median:35ms | NR |
| range:31–78ys | Range:4–85ms | |||||||
| Inokuchi [20] | 2011 | Japan | gastric | surgery | 88/38 | ≥70ys:41, <70ys:85 | 73ms | 59/76 |
| Woo [31] | 2011 | Korea | gastric | surgery | 193/92 | NR | Mean:51ms | 102/183 |
| Xiong [32] | 2012 | China Mainland | gastric | NR | 176/86 | Mean 59.3ys | Mean: 39.7ms | NR |
| Deng [33] | 2013 | China Mainland | gastric | surgery | 76/38 | Median:56.7ys | Median:38ms | 42/68 |
| Range:29–83ys | Range:2–108ms | |||||||
| Song [34] | 2014 | China Mainland | gastric | surgery | 46/14 | Median:60ys | Range:6–72ms | 0/60 |
| Wu [35] | 2014 | China Mainland | gastric | surgery | 43/17 | Mean:62ys | Median:23ms | NR |
| Range:37–90ys | Range:1–79ms | |||||||
| Zhang [16] | 2012 | Austria | hepatocellular | surgery | 80/20 | Mean:55.1ys | NR | NR |
| Range:28–77ys | ||||||||
| Wu [36] | 2011 | China Mainland | hepatocellular | surgery | 115/23 | ≤55ys:86,>55ys:52 | NR | NR |
| Mano [21] | 2013 | Japan | hepatocellular | surgery | 81/20 | positive 63.9±7.3ys | Median:1391days | NR |
| negative 63.6±9.5ys | Range:36–3289days | |||||||
| You [17] | 2011 | China Mainland | esophagus | surgery | 68/32 | 62.2±6.6ys | Median:16.8ms | NR |
| Range:0.8–69.2ms | ||||||||
| Schoppmann [37] | 2012 | Austria | esophagus | surgery | 253/72 | 63±10ys | 52ms | NR |
| Chen [22] | 2013 | Taiwan | esophagus | radiotherapy | 173 | NR | NR | 48/125 |
| Denley [18] | 2013 | UK | pancreatic | surgery | 43/43 | Median:64ys | NR | NR |
| Range:38–77ys | ||||||||
| Huang [23] | 2013 | China Mainland | pancreatic | surgery | 50/21 | Median:67ys | Mean:15.9ms | NR |
| Range:40–80ys | Range:1–101ms | |||||||
| Koperek [38] | 2013 | Caucasian | pancreatic | surgery | 48/31 | 66±11ys | 629±594days | NR |
| Study Cohort | Tumor differentiation (well or moderate/poor) | TNM(I+II/III+IV) | Antibody | Scoring Method | Cut-off | NO.of positive expression (%) | Survival analysis | HR |
| Kusaba [27] | 100/4 | NR | goat polyclonal antibody | E | 15% | 62(57.4%) | NR | R(M) |
| Monnien [19] | 97/1 | 0/104 | rabbit polyclonal antibody | E | 15% | 39(37.5%) | OS | R(M) |
| Morikawa [28] | NR | 384/301 | rabbit polyclonal antibody | I | NR | 131(18%,) | OS | R(M) |
| Dobi [14] | 362/0 | 19/75 | rabbit polyclonal antibody | E | 15% | 23(24.5%) | OS,DFS | R(M) |
| Yakata [29] | NR | NR | goat polyclonal antibody | E | 10% | 55(49.5%) | NR | R(M) |
| Lee [15] | 101/166 | 144/167 | rabbit polyclonal antibody | E | 1% | 79(26.1%) | OS,DFS | R(M) |
| Deng [30] | NR | NR | rabbit polyclonal antibody | E | 10% | 26(49.1%) | OS | R(M) |
| Inokuchi [20] | NR | NR | rabbit polyclonal antibody | E | 10% | 52(41%) | OS | R(M) |
| Woo [31] | NR | NR | NS | E | 1% | 103(36%) | NR | R(M) |
| Xiong [32] | 80/182 1+2/3+4 | 93/169 | NS | E | 15% | 136(51.9%) | OS | R(M) |
| Deng [33] | NR | NR | rabbit polyclonal antibody | EI | 25% | 89(78.07%) | OS | E(M) |
| Song [34] | 26/34 1+2/3 | 26/34 | rabbit polyclonal antibody | EI | 4 | 35(58.3%) | OS | R(M) |
| Wu [35] | NR | NR | NS | E | 5 | 26(43.3%) | NR | R(M) |
| Zhang [16] | 68/32 1+2/3+4 | 60/40 | mouse monoclonal antibody | E | 15% | 58(58%) | OS | R(M) |
| Wu [36] | NR | 106/31 | NS | E | 25% | 75(54.3%) | NR | R(M) |
| Mano [21] | 66/35 1+2/3 | NR | rabbit monoclonal antibody | E | 10.7% | 36(36%) | OS,DFS | R(M) |
| You [17] | 73/27 | 20/80 | rabbit polyclonal antibody | EI | 2 | 76(76%) | OS | R(M) |
| Schoppmann [37] | 183/141 | NR | rabbit monoclonal antibody | EI | 10 | 144(44.4%) | OS,DFS | R(M) |
| Chen [22] | NR | 45/128 | NS | EI | 2 | 82(47.4%) | OS,DFS | R(M) |
| Denley [18] | NR | NR | rabbit polyclonal antibody | EI | NR | 29(33.7%) | OS | R(M) |
| Huang [23] | 59/10 | 66/5 | rabbit polyclonal antibody | E | 5% | 39(54.9%) | OS | R(M) |
| Koperek [38] | 53/26 | NR | rabbit monoclonal antibody | E | 5% | 33 (41.8%) | NR | R(M) |
ms: months; ys: years; OS: overall survival; DFS: progression-free survival; HR: hazard ratio, obtained by reporting in text (R) or estimating (E). ‘‘M” means the HR come from multivariate analysis; E: extent; I: intensity; NR: not reported; NS: non-specific; Rabbit pAb: rabbit polyclonal antibody.
Fig 1. Flow chart for the selection of articles to include.
The sample sizes of the included cohorts ranged from 53 to 724. Colorectal cancer, gastric cancer, hepatocellular carcinoma, esophagus cancer and pancreatic cancer were studied in 4, 9, 3, 3 and 3 articles, respectively. All of these studies evaluated the expression of p-STAT3 in tumor samples by IHC. Different antibodies including rabbit polyclonal antibody (11 studies), rabbit monoclonal antibody (3 studies), mouse monoclonal antibody (1 study), and non-specific antibody (5 studies), goat polyclonal antibody (2 studies) were used. Study patients in 20 of the 22 included studies received surgical operation as the main treatment. Quality assessment revealed that only 3 [31, 36, 38]of the 22 studies gained a NOS<6 (details in Table 2).
Table 2. Quality assessment of eligible studies with Newcastle-Ottawa Scale.
| Author | Year | Selection | Comparability | Outcome | NOS |
|---|---|---|---|---|---|
| Kusaba [27] | 2006 | ★★* | ★★* | ★★* | 6 |
| Monnien [19] | 2010 | ★★★* | ★★* | ★★* | 7 |
| Morikawa [28] | 2011 | ★★★* | ★★* | ★★★* | 8 |
| Dobi [14] | 2012 | ★★★* | ★* | ★★* | 6 |
| Yakata [29] | 2006 | ★★★* | ★* | ★★* | 6 |
| Lee [15] | 2009 | ★★* | ★★* | ★★* | 6 |
| Deng [30] | 2010 | ★★ Δ | ★★* | ★★* | 6 |
| Inokuchi [20] | 2011 | ★★* | ★★* | ★★* | 6 |
| Woo [31] | 2011 | ★★* | ★* | ★★* | 5 |
| Xiong [32] | 2012 | ★★* | ★★* | ★★* | 6 |
| Deng [33] | 2013 | ★★* | ★★* | ★★* | 6 |
| Song [34] | 2014 | ★★* | ★★* | ★★* | 6 |
| Wu [35] | 2014 | ★★★* | ★* | ★★* | 6 |
| Zhang [16] | 2012 | ★★* | ★★* | ★★* | 6 |
| Wu [36] | 2011 | ★★* | ★* | ★★ Δ | 5 |
| Mano [21] | 2013 | ★★* | ★★* | ★★* | 6 |
| You [17] | 2011 | ★★* | ★★* | ★★* | 6 |
| Schoppmann [37] | 2012 | ★★* | ★★ Δ | ★★* | 6 |
| Chen [22] | 2013 | ★★* | ★★* | ★★* | 6 |
| Denley [18] | 2013 | ★★* | ★★* | ★★* | 6 |
| Huang [23] | 2013 | ★★* | ★★* | ★★* | 6 |
| Koperek [38] | 2013 | ★★* | ★* | ★★ Δ | 5 |
The table presented the final quality assessment score of the enrolled studies by the authors.
* The score was consistent in the initially separate assessment by Li MX and Bi XY.
Δ The score was produced by the joint discussion.
p-STAT3 and OS
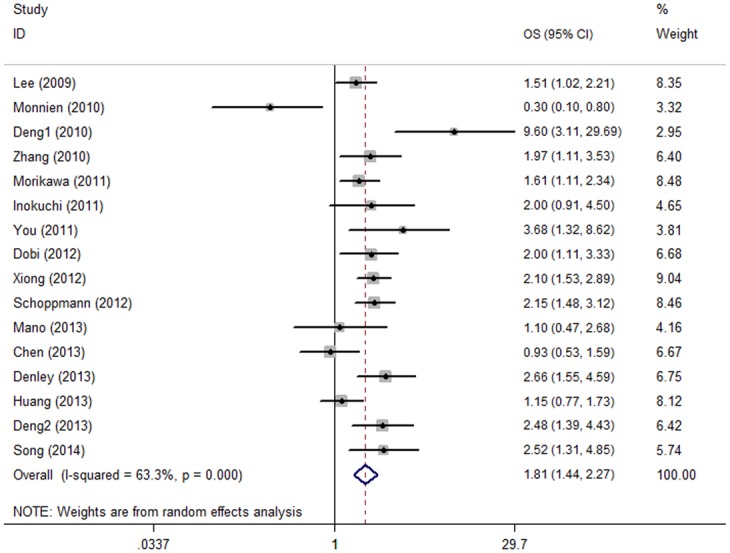
There were 16 cohorts presented the data of p-STAT3 expression and overall survival of the patients. Though with heterogeneity (I2 = 63.3%, P value of Q test for heterogeneity test (Ph) <0.001), pooled estimates showed that elevated p-STAT3 expression predicted poor OS with a pooling HR being 1.809 (95% CI: 1.442–2.270, P<0.001. Table 3, Fig 2).
Table 3. Meta-analysis results for OS and DFS.
| Analysis | OS | DFS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | HR(95% CI) | I2 | Ph | P | N | HR(95% CI) | I2 | Ph | P | |
| 16 | 1.809(1.442–2.270) | 63.3% | <0.001 | <0.001 | 5 | 1.481(1.028–2.133) | 71.4% | 0.007 | 0.035 | |
| Subgroup1: digestive tract | 12 | 1.875(1.426–2.464) | 66.5% | 0.001 | <0.001 | |||||
| Digestive gland | 4 | 1.636(1.056–2.536) | 57.4% | 0.070 | 0.028 | |||||
| Subgroup2: surgery | 13 | 1.871(1.425–2.458) | 65.0% | 0.052 | <0.001 | |||||
| Non-surgical | 2 | 1.361(0.640–2.895) | 73.4% | 0.001 | 0.423 | |||||
| Subgroup3: Caucasian | 5 | 1.656(1.067–2.571) | 72.8% | 0.005 | 0.025 | |||||
| Asian | 11 | 1.873(1.415–2.481) | 61.7% | 0.004 | <0.001 | |||||
| Subgroup4: E | 8 | 1.490(1.101–2.016) | 60.0% | 0.015 | 0.010 | |||||
| I | 1 | 1.610(1.109–2.338) | - | - | 0.012 | |||||
| EI | 7 | 2.430(1.622–3.641) | 66.7% | 0.006 | <0.001 | |||||
| Subgroup5: Sample size≥200 | 4 | 1.846(1.542–2.209) | 0 | 0.416 | <0.001 | |||||
| Sample size<200 | 12 | 1.818(1.276–2.591) | 70.9% | <0.001 | 0.001 | |||||
| Subgroup6: Rabbit pAb | 11 | 1.918(1.394–2.640) | 69.0% | <0.001 | <0.001 | |||||
| others | 3 | 1.947(1.448–2.617) | 0 | 0.392 | <0.001 | |||||
Ph: p value of Q test for heterogeneity test; N: number of studies (cohorts); HR: hazard ratio; 95% CI: 95% confidence interval; OS: overall survival; DFS: progression-free survival; E: extent; I: intensity; Rabbit pAb: rabbit polyclonal antibody
Fig 2. Forest plot of hazard ratio (HR) for the association between p-STAT3 overexpression and overall survival (OS) in patients with cancers of the digestive system with random effects model.
We stratified the pooled data by tumor site (digestive tract vs. digestive gland), main treatment (surgical vs. non-surgical), study region (Caucasian vs. Asian), scoring methods (E vs. I vs. EI), sample size (≥200 vs. <200) and the primary antibody (rabbit polyclonal antibody vs. others) used in IHC. Majority of the results of subgroup analyses were consistent with the overall result in the total study population (data shown in Table 3).
Of note, when performing the subgroup analyses stratified by sample size, we found that studies with sample size ≥ 200 gained an I2 as 0.0%; while the subgroup with sample size < 200 had an I2 as 70.9%. It suggested that sample size may explain the source of heterogeneity to some extent. We further performed the meta-regression analysis by tumor site, main treatment, study region, scoring methods, sample size and the primary antibody used in IHC. To our disappointment, we did not figure out a single factor that was responsible for the source of heterogeneity (data not shown).
p-STAT3 and DFS
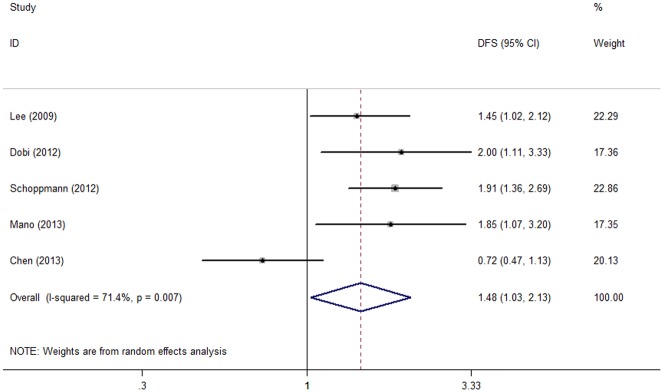
Five cohorts reported the data concerning the association between p-STAT3 expression and disease-free survival of the enrolled patients. Meta-analysis adopting the random effect model revealed that elevated p-STAT3 expression was significantly associated with shorter DFS (HR = 1.481, 95% CI: 1.028–2.133, P = 0.035, Table 3, Fig 3) with observed heterogeneity (I2 = 71.4%, Ph = 0.007).
Fig 3. Forest plot of hazard ratio (HR) for the association between p-STAT3 overexpression and disease-free survival (DFS) in patients with cancers of the digestive system with random effects model.
p-STAT3 and clinical pathological factors
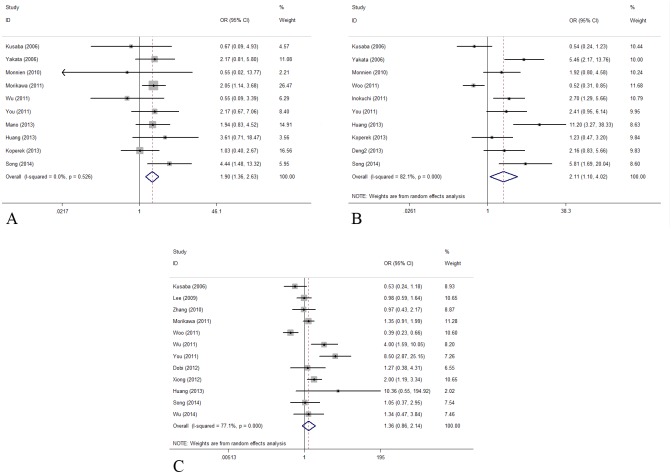
The secondary results of the present meta-analysis came as the relationship between p-STAT3 expression and the clinicopathological factors. Without observable heterogeneity, pooled estimates of 12 cohorts discovered that elevated p-STAT3 expression was significantly associated with poor tumor differentiation (OR = 1.895, 95% CI: 1.364–2.632, P<0.001, I2 = 0, Ph = 0.526, Fig 4A). Ten studies presented data about p-STAT3 expression and lymph node metastases, a combined OR of 2.108 revealed the positive relationship between increased p-STAT3 expression and positive N status (OR = 2.108, 95% CI: 1.104–4.024, P = 0.024, I2 = 82.1%, Ph<0.001, Fig 4B). In the meta-analysis assessing the association between p-STAT3 expression and TNM stage, the relationship failed to obtain the statistical significance (OR = 1.355, 95% CI: 0.859–2.139, P = 0.192, I2 = 77.1%, Ph<0.001, Fig 4C).
Fig 4. Forest plots of odds ratios (OR) for the association between p-STAT3 overexpression and clinicopathological features in digestive system cancer patients.
(A) The relationship between p-STAT3 overexpression and tumor cell differentiation with fixed effects model (OR = 1.895, 95% CI: 1.364–2.632, P<0.001, I2 = 0, Ph = 0.526); (B) The relationship between p-STAT3 overexpression and lymph node metastases with random effects model (OR = 2.108, 95% CI: 1.104–4.024, P = 0.024, I2 = 82.1%, Ph<0.001); (C) OR for TNM stage with random effects model (OR = 1.355, 95% CI: 0.859–2.139, P = 0.192, I2 = 77.1%, Ph<0.001).
Sensitivity analyses
To test the strength of our results, we removed an individual study each time and calculated the pooled HRs (or ORs) of the remaining studies. No significant differences were observed between the corresponding results and the overall results (data not shown), which indicated that our results were robust.
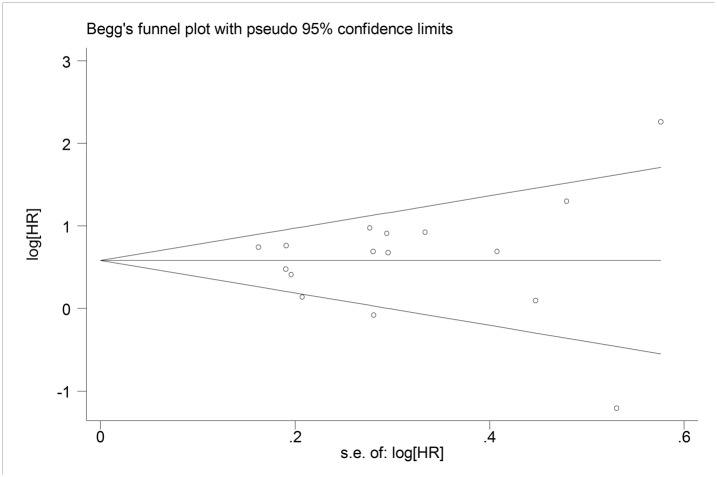
Publication bias
No obvious asymmetry was observed in the funnel plot of the meta-analysis evaluating the relationship between p-STAT3 expression and overall survival (Fig 5). And the P value of Egger’s test (P = 0.144) also indicated that there was no obvious publication bias.
Fig 5. Funnel plot for p-STAT3 expression and overall survival (OS) in patients with cancers of the digestive system.
Discussion
To our knowledge, the present meta-analysis, involving a total 22 studies and 3585 patients, was the first meta-analysis systematically evaluating the prognostic value of p-STAT3 in patients with digestive system cancers. The results showed that elevated p-STAT3 expression level was a strong predictor of inferior OS and DFS in patients with malignant cancers of the digestive system. Majority of the results from subgroup analyses were similar with those from the overall study population, which indicated that the pooled results were robust. Additionally, increased p-STAT3 expression was also significantly interrelated with positive lymph node metastases status and poorer differentiation of tumor cells.
Among the tumor types evaluated, gastric cancer was the tumor type most linked with a worse outcome for patients who expressed high level of p-STAT3 (HR = 2.264, 95% CI: 1.629–3.147, P<0.001, I2 = 52.2%, S2 Table). As pooled estimates with limited number of enrolled studies are inclined to have insufficient statistical power, we dichotomized the enrolled studies as “digestive tract cancer” and “digestive gland cancer” in order to gain more statistical sound results. The respective results suggested that significant relationship between overexpression of p-STAT3 in tumor samples was detected both in the digestive tract cancer and digestive gland cancer subgroups.
The molecular functions of p-STAT3 in malignant tumors, mainly including its influence on cell cycle, inflammatory process and angiogenesis, have been extensively discussed in the recent years [39]. It has long been held that cytokines produced by inflammatory reaction contributed a lot in cancer progression. JAK/STAT3 pathway activation, which may be stimulated by the carcinogenetic inflammatory cytokine IL-6 through gp130, can lead to cell proliferation and antagonize cellular apoptosis [40]. In addition, EGFR activation can function through STAT3 phosphorylation. The activated downstream cytokines, such as TWIST, are able to fascinate the process of epithelial-mesenchymal transition (EMT), which is widely recognized as a critical step in lymph node/vascular metastasis [41]. Moreover, the interaction between VEGF expression and p-STAT3 plays an important role in the regulation of transcription of genes which are involved in angiogenesis [8]. These molecular mechanisms related with p-STAT3 may partially account for the molecular basis of reduced survival benefits and the unfavorable clinicopathological features.
Molecular targeted therapy has drawn cumulating attention theses years. Monoclonal antibodies or small molecule anti-tumor agents targeting the critical carcinogenic molecular hubs, such as VEGF, EGFR and HER-2, have earned appreciation in the anti-cancer treatment and have been adopted by the evidence based clinical guidelines. The growing number of preclinical studies in numerous malignances along with the clinical trials testing STAT3 inhibitors suggest that STAT3 (p-STAT3) remains a valid target for the treatment of malignant cancers [42]. Our results also suggest that molecular therapy countering p-STAT3 may shed light on the future development of molecular targeted therapy[43]. Several clinical trials assessing the therapeutic effects of STAT3 (p-STAT3) antagonists in patients with solid cancers (NCT01563302, NCT02058017, etc. http://www.clinicaltrials.gov/, Table 4) are underway. For cancers derived from the digestive system, a Phase I/Ib Study evaluating AZD9150 (a STAT3 inhibitor, ISIS-STAT3Rx) in Patients with Advanced/Metastatic Hepatocellular Carcinoma (NCT01839604, http://www.clinicaltrials.gov/, Table 4) is in process. We are looking forward that the results of the clinical trials will further unveil the value of p-STAT3 in oncological therapy.
Table 4. Ongoing studies evaluating STAT3 (p-STAT3) therapeutic strategies (from http://www.clinicaltrials.gov/).
| Study | sponsor | Phase/setting | Experimental arm(s) |
|---|---|---|---|
| NCT01563302 | Isis Pharmaceuticals | Phase 1/2, Open-label, Dose-escalation Study, Advanced Cancers | Three-hour IV infusions on Cycle 0 Days 1, 3, 5, and weekly three-hour IV infusions in Cycles 1 and beyond, on Days 1, 8, and 15 of each cycle. |
| NCT01568996 | John Kirkwood | Phase 0, Atypical Nevi Melanoma | Low dose BSE-SFN: |
| BSE-SFN will be orally administered at 50 μmol SFN for 28 days. | |||
| Mid dose BSE-SFN: | |||
| BSE-SFN will be orally administered at 100 μmol SFN for 28 days. | |||
| High dose BSE-SFN: | |||
| BSE-SFN will be orally administered at 200 μmol SFN for 28 days. | |||
| NCT02058017 | National University Hospital, Singapore | Phase 1, Nasopharyngeal Carcinoma | Experimental: Part I & Part II |
| Part I- This is a lead-in dose-finding, open-label, non-randomised arm of the study: Using a starting dose of 10mg per week, an accelerated dose titration escalation followed by a 3+3 design will be employed until MTD and recommended weekly dose are determined. | |||
| Part II- This is a single-centre, open-label non-randomised phase II study evaluating OPB-51602 in stage III-IVB NPC conducted in the window period prior to definitive chemoradiotherapy. Eligible patients will receive OPB-51602 on a weekly basis (Day 1, 8, 15) at the recommended dose determined in part I for a total of 15 days prior to definitive chemoradiotherapy. | |||
| NCT01839604 | AstraZeneca | Phase 1, Advanced Adult Hepatocellular Carcinoma, Hepatocellular Carcinoma Metastatic | Experimental: AZD9150, Intravenous infusion over 3 hours. There are two parts, dose escalation phase (Part A) and dose expansion phase (Part B). |
| NCT01066663 | Dana-Farber Cancer Institute | Phase 1& Phase 2, Chronic Lymphocytic Leukemia, Small Lymphocytic Leukemia | Experimental: Pyrimethamine, Single daily oral 50 mg dose. |
Furthermore, p-STAT3 expression in all of the enrolled articles was determined by IHC. IHC is a widely available method, but IHC is not strictly quantitative and there is no uniformly complied scoring system; therefore, interpretation of the staining results varies from person to person [44], which might potentially engender a certain degree of heterogeneity. Cut-off value defining elevated expression of p-STAT3 differed among the included cohorts: some authors arbitrarily defined it as 10%, 25% by staining extent or defined it as 2, 4, or 5 by the specific scoring system; in some studies, the cut-off value was determined as the mean/median value of the results by the specific scoring protocol in the respective article. Besides, diversities existed in the primary antibodies used in IHC, and the dilutions of the antibodies were not uniform, leading to a potential heterogeneity, because the sensitivity of the IHC may depend on the antibody concentration, fixation method and storage time [44–46]. The predicative value of elevated p-STAT3 expression in OS was not undermined by the subgroup analysis according to the primary antibody used. Of note, it was not possible to perform subgroup analysis stratified by all these technical issues, because limited number of studies shared the same laboratory procedure. Therefore a consistent and reproducible method to evaluate p-STAT3 expression is warranted.
Admittedly, there were some limitations in the present meta-analysis. First, the majority of the enrolled studies were retrospective. Thus some bias, such as selection bias, misclassification bias and information bias, may be present in the meta-analysis; Secondly, in order to gain more statistically robust data and sound accuracy of the pooled estimates, we only incorporated HRs and 95% CIs that were produced by the multivariate analysis in the present meta-analysis. We did not take studies that just present the Kaplan-Meier curves or studies that only present the HRs (95% CIs) from the univariate analysis. Therefore the number of the enrolled cohorts investigating the impaction of p-STAT3 expression on survival outcome is relatively limited. Thirdly, as the tumor samples used for IHC were commonly from the surgical resection, surgical operation remained to be the dominant treatment in majority of the enrolled population. It is possible that the results of our meta-analysis may have more hints to patients who underwent operation. Fourthly, though we did not detect obvious asymmetry in the funnel plots and evidence of publication bias in the Egger’s test, the combined outcomes may be relatively overestimated. Since small scale studies with negative results are prone to remain unpublished [26]. What is more, the subgroup analysis showed that smaller sample size may partially explain the source of heterogeneity. It could be explained by the fact that studies with smaller sample size are featured by the drawback of remarkable statistic instability. In the further meta-regression test, we did not find a single factor that was responsible for the sources of heterogeneity. We assumed that complex varieties in study design, study population, follow-up period, scoring system and laboratory protocol might contribute to the heterogeneity. In addition, we only searched limited online databases and only took English written articles into account. Though we tried our best to identify the relevant articles, the terms and algorithm adopted in the literature search may not be the best. The scope of the identified studies was somehow narrow.
In conclusion, the current meta-analysis suggests that p-STAT3 expression in the tumor sample of digestive system was a promising predictor of both OS and DFS. And level of p-STAT3 expression is closely related with lymph node metastasis and tumor cell differentiation. Future well designed studies with adequate follow up are needed. The promising therapeutic role of STAT3 (p-STAT3) targeted therapy will be further revealed by the ongoing clinical trials.
Supporting Information
(DOC)
(DOC)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by The State Key Project on Infection Diseases of China (Grant No.2012ZX10002016). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64(1):9–29. Epub 2014/01/09. 10.3322/caac.21208 . [DOI] [PubMed] [Google Scholar]
- 2. Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179–1187 e1171–1173. Epub 2012/08/14. 10.1053/j.gastro.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herszenyi L, Tulassay Z. Epidemiology of gastrointestinal and liver tumors. European review for medical and pharmacological sciences. 2010;14(4):249–258. Epub 2010/05/26. . [PubMed] [Google Scholar]
- 4. Hari DM, Leung AM, Lee JH, Sim MS, Vuong B, Chiu CG, et al. AJCC Cancer Staging Manual 7th edition criteria for colon cancer: do the complex modifications improve prognostic assessment? Journal of the American College of Surgeons. 2013;217(2):181–190. Epub 2013/06/19. 10.1016/j.jamcollsurg.2013.04.018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Darnell JE Jr. STATs and gene regulation. Science. 1997;277(5332):1630–1635. Epub 1997/09/12. . [DOI] [PubMed] [Google Scholar]
- 6. Stark GR, Darnell JE Jr. The JAK-STAT pathway at twenty. Immunity. 2012;36(4):503–514. Epub 2012/04/24. 10.1016/j.immuni.2012.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer cell. 2009;15(2):91–102. Epub 2009/02/03. 10.1016/j.ccr.2009.01.002 . [DOI] [PubMed] [Google Scholar]
- 8. Dodd KM, Yang J, Shen MH, Sampson JR, Tee AR. mTORC1 drives HIF-1alpha and VEGF-A signalling via multiple mechanisms involving 4E-BP1, S6K1 and STAT3. Oncogene. 2014;. 10.1038/onc.2014.164. Epub 2014/06/17. 10.1038/onc.2014.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tye H, Kennedy CL, Najdovska M, McLeod L, McCormack W, Hughes N, et al. STAT3-driven upregulation of TLR2 promotes gastric tumorigenesis independent of tumor inflammation. Cancer cell. 2012;22(4):466–478. Epub 2012/10/20. 10.1016/j.ccr.2012.08.010 . [DOI] [PubMed] [Google Scholar]
- 10. Chen SH, Murphy DA, Lassoued W, Thurston G, Feldman MD, Lee WM. Activated STAT3 is a mediator and biomarker of VEGF endothelial activation. Cancer biology & therapy. 2008;7(12):1994–2003. Epub 2008/11/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sonnenblick A, Shriki A, Galun E, Axelrod JH, Daum H, Rottenberg Y, et al. Tissue microarray-based study of patients with lymph node-positive breast cancer shows tyrosine phosphorylation of signal transducer and activator of transcription 3 (tyrosine705-STAT3) is a marker of good prognosis. Clinical & translational oncology: official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2012;14(3):232–236. Epub 2012/03/01. 10.1007/s12094-012-0789-z . [DOI] [PubMed] [Google Scholar]
- 12. Wang M, Chen GY, Song HT, Hong X, Yang ZY, Sui GJ. Significance of CXCR4, phosphorylated STAT3 and VEGF-A expression in resected non-small cell lung cancer. Experimental and therapeutic medicine. 2011;2(3):517–522. Epub 2011/05/01. 10.3892/etm.2011.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clinical cancer research: an official journal of the American Association for Cancer Research. 2002;8(4):945–954. Epub 2002/04/12. . [PubMed] [Google Scholar]
- 14. Dobi E, Monnien F, Kim S, Ivanaj A, N'Guyen T, Demarchi M, et al. Impact of STAT3 phosphorylation on the clinical effectiveness of anti-EGFR-based therapy in patients with metastatic colorectal cancer. Clinical colorectal cancer. 2013;12(1):28–36. Epub 2012/10/23. 10.1016/j.clcc.2012.09.002 . [DOI] [PubMed] [Google Scholar]
- 15. Lee J, Kang WK, Park JO, Park SH, Park YS, Lim HY, et al. Expression of activated signal transducer and activator of transcription 3 predicts poor clinical outcome in gastric adenocarcinoma. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica. 2009;117(8):598–606. Epub 2009/08/12. 10.1111/j.1600-0463.2009.02512.x . [DOI] [PubMed] [Google Scholar]
- 16. Zhang CH, Xu GL, Jia WD, Li JS, Ma JL, Ren WH, et al. Activation of STAT3 signal pathway correlates with twist and E-cadherin expression in hepatocellular carcinoma and their clinical significance. The Journal of surgical research. 2012;174(1):120–129. Epub 2011/02/15. 10.1016/j.jss.2010.10.030 . [DOI] [PubMed] [Google Scholar]
- 17. You Z, Xu D, Ji J, Guo W, Zhu W, He J. JAK/STAT signal pathway activation promotes progression and survival of human oesophageal squamous cell carcinoma. Clinical & translational oncology: official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2012;14(2):143–149. Epub 2012/02/04. 10.1007/s12094-012-0774-6 . [DOI] [PubMed] [Google Scholar]
- 18. Denley SM, Jamieson NB, McCall P, Oien KA, Morton JP, Carter CR, et al. Activation of the IL-6R/Jak/stat pathway is associated with a poor outcome in resected pancreatic ductal adenocarcinoma. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2013;17(5):887–898. Epub 2013/02/26. 10.1007/s11605-013-2168-7 . [DOI] [PubMed] [Google Scholar]
- 19. Monnien F, Zaki H, Borg C, Mougin C, Bosset JF, Mercier M, et al. Prognostic value of phosphorylated STAT3 in advanced rectal cancer: a study from 104 French patients included in the EORTC 22921 trial. Journal of clinical pathology. 2010;63(10):873–878. Epub 2010/09/30. 10.1136/jcp.2010.076414 . [DOI] [PubMed] [Google Scholar]
- 20. Inokuchi M, Murayama T, Hayashi M, Takagi Y, Kato K, Enjoji M, et al. Prognostic value of co-expression of STAT3, mTOR and EGFR in gastric cancer. Experimental and therapeutic medicine. 2011;2(2):251–256. Epub 2011/03/01. 10.3892/etm.2011.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mano Y, Aishima S, Fujita N, Tanaka Y, Kubo Y, Motomura T, et al. Tumor-associated macrophage promotes tumor progression via STAT3 signaling in hepatocellular carcinoma. Pathobiology: journal of immunopathology, molecular and cellular biology. 2013;80(3):146–154. Epub 2013/02/01. 10.1159/000346196 . [DOI] [PubMed] [Google Scholar]
- 22. Chen MF, Chen PT, Lu MS, Lin PY, Chen WC, Lee KD. IL-6 expression predicts treatment response and outcome in squamous cell carcinoma of the esophagus. Molecular cancer. 2013;12:26 Epub 2013/04/09. 10.1186/1476-4598-12-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang C, Huang R, Chang W, Jiang T, Huang K, Cao J, et al. The expression and clinical significance of pSTAT3, VEGF and VEGF-C in pancreatic adenocarcinoma. Neoplasma. 2012;59(1):52–61. Epub 2011/11/16. 10.4149/neo_2012_007 . [DOI] [PubMed] [Google Scholar]
- 24. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in medicine. 1998;17(24):2815–2834. Epub 1999/01/28. . [DOI] [PubMed] [Google Scholar]
- 25. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010;25(9):603–605. Epub 2010/07/24. 10.1007/s10654-010-9491-z . [DOI] [PubMed] [Google Scholar]
- 26. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. Epub 1997/10/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kusaba T, Nakayama T, Yamazumi K, Yakata Y, Yoshizaki A, Inoue K, et al. Activation of STAT3 is a marker of poor prognosis in human colorectal cancer. Oncology reports. 2006;15(6):1445–1451. Epub 2006/05/11. . [PubMed] [Google Scholar]
- 28. Morikawa T, Baba Y, Yamauchi M, Kuchiba A, Nosho K, Shima K, et al. STAT3 expression, molecular features, inflammation patterns, and prognosis in a database of 724 colorectal cancers. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17(6):1452–1462. Epub 2011/02/12. 10.1158/1078-0432.CCR-10-2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yakata Y, Nakayama T, Yoshizaki A, Kusaba T, Inoue K, Sekine I. Expression of p-STAT3 in human gastric carcinoma: significant correlation in tumour invasion and prognosis. International journal of oncology. 2007;30(2):437–442. Epub 2007/01/05. . [PubMed] [Google Scholar]
- 30. Deng JY, Sun D, Liu XY, Pan Y, Liang H. STAT-3 correlates with lymph node metastasis and cell survival in gastric cancer. World journal of gastroenterology: WJG. 2010;16(42):5380–5387. Epub 2010/11/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Woo S, Lee BL, Yoon J, Cho SJ, Baik TK, Chang MS, et al. Constitutive activation of signal transducers and activators of transcription 3 correlates with better prognosis, cell proliferation and hypoxia-inducible factor-1alpha in human gastric cancer. Pathobiology: journal of immunopathology, molecular and cellular biology. 2011;78(6):295–301. Epub 2011/11/23. 10.1159/000321696 . [DOI] [PubMed] [Google Scholar]
- 32. Xiong H, Du W, Wang JL, Wang YC, Tang JT, Hong J, et al. Constitutive activation of STAT3 is predictive of poor prognosis in human gastric cancer. J Mol Med (Berl). 2012;90(9):1037–1046. Epub 2012/02/14. 10.1007/s00109-012-0869-0 . [DOI] [PubMed] [Google Scholar]
- 33. Deng J, Liang H, Zhang R, Sun D, Pan Y, Liu Y, et al. STAT3 is associated with lymph node metastasis in gastric cancer. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine. 2013;34(5):2791–2800. Epub 2013/07/05. 10.1007/s13277-013-0837-5 . [DOI] [PubMed] [Google Scholar]
- 34. Song YY, Sun LD, Liu ML, Liu ZL, Chen F, Zhang YZ, et al. STAT3, p-STAT3 and HIF-1alpha are associated with vasculogenic mimicry and impact on survival in gastric adenocarcinoma. Oncology letters. 2014;8(1):431–437. Epub 2014/06/25. 10.3892/ol.2014.2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu LJ, Li HX, Luo XT, Lu RZ, Ma YF, Wang R, et al. STAT3 activation in tumor cell-free lymph nodes predicts a poor prognosis for gastric cancer. International journal of clinical and experimental pathology. 2014;7(3):1140–1146. Epub 2014/04/04. [PMC free article] [PubMed] [Google Scholar]
- 36. Wu WY, Li J, Wu ZS, Zhang CL, Meng XL, Lobie PE. Prognostic significance of phosphorylated signal transducer and activator of transcription 3 and suppressor of cytokine signaling 3 expression in hepatocellular carcinoma. Experimental and therapeutic medicine. 2011;2(4):647–653. Epub 2011/07/01. 10.3892/etm.2011.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schoppmann SF, Jesch B, Friedrich J, Jomrich G, Maroske F, Birner P. Phosphorylation of signal transducer and activator of transcription 3 (STAT3) correlates with Her-2 status, carbonic anhydrase 9 expression and prognosis in esophageal cancer. Clinical & experimental metastasis. 2012;29(6):615–624. Epub 2012/04/10. 10.1007/s10585-012-9475-3 . [DOI] [PubMed] [Google Scholar]
- 38. Koperek O, Aumayr K, Schindl M, Werba G, Soleiman A, Schoppmann S, et al. Phosphorylation of STAT3 correlates with HER2 status, but not with survival in pancreatic ductal adenocarcinoma. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica. 2014;122(6):476–481. Epub 2013/10/30. 10.1111/apm.12194 . [DOI] [PubMed] [Google Scholar]
- 39. Zhang HF, Lai R. STAT3 in Cancer-Friend or Foe? Cancers. 2014;6(3):1408–1440. Epub 2014/07/06. 10.3390/cancers6031408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. The Biochemical journal. 1998;334 (Pt 2):297–314. Epub 1998/08/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei Y, et al. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer research. 2007;67(19):9066–9076. Epub 2007/10/03. 10.1158/0008-5472.CAN-07-0575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lavecchia A, Di Giovanni C, Cerchia C. Novel inhibitors of signal transducer and activator of transcription 3 signaling pathway: an update on the recent patent literature. Expert opinion on therapeutic patents. 2014;24(4):383–400. Epub 2014/01/18. 10.1517/13543776.2014.877443 . [DOI] [PubMed] [Google Scholar]
- 43. Xiong A, Yang Z, Shen Y, Zhou J, Shen Q. Transcription Factor STAT3 as a Novel Molecular Target for Cancer Prevention. Cancers. 2014;6(2):926–957. Epub 2014/04/20. 10.3390/cancers6020926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Taylor CR, Levenson RM. Quantification of immunohistochemistry--issues concerning methods, utility and semiquantitative assessment II. Histopathology. 2006;49(4):411–424. Epub 2006/09/19. 10.1111/j.1365-2559.2006.02513.x . [DOI] [PubMed] [Google Scholar]
- 45. Idikio HA. Immunohistochemistry in diagnostic surgical pathology: contributions of protein life-cycle, use of evidence-based methods and data normalization on interpretation of immunohistochemical stains. International journal of clinical and experimental pathology. 2009;3(2):169–176. Epub 2010/02/04. [PMC free article] [PubMed] [Google Scholar]
- 46. Kothmaier H, Rohrer D, Stacher E, Quehenberger F, Becker KF, Popper HH. Comparison of formalin-free tissue fixatives: a proteomic study testing their application for routine pathology and research. Archives of pathology & laboratory medicine. 2011;135(6):744–752. Epub 2011/06/03. 10.1043/2009-0676-OA.1 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.