Abstract
Many health conditions, ranging from psychiatric disorders to cardiovascular disease, display notable seasonal variation in severity and onset. In order to understand the molecular processes underlying this phenomenon, we have examined seasonal variation in the transcriptome of 606 healthy individuals. We show that 74 transcripts associated with a 12-month seasonal cycle were enriched for processes involved in DNA repair and binding. An additional 94 transcripts demonstrated significant seasonal variability that was largely influenced by blood cell count levels. These transcripts were enriched for immune function, protein production, and specific cellular markers for lymphocytes. Accordingly, cell counts for erythrocytes, platelets, neutrophils, monocytes, and CD19 cells demonstrated significant association with a 12-month seasonal cycle. These results demonstrate that seasonal variation is an important environmental regulator of gene expression and blood cell composition. Notable changes in leukocyte counts and genes involved in immune function indicate that immune cell physiology varies throughout the year in healthy individuals.
Introduction
The variation of RNA transcription levels within a population (P), is driven by both genetic (G) and environmental (E) factors (Eq (1)):
| (1) |
Gene expression studies in humans have aimed to quantify the total contribution of genetic variation underlying RNA level variation within a population and to identify genetic loci that contribute to that variation [1] [2]. The proportion of phenotypic variance explained by genetic variance is termed heritability (H 2) and can be estimated using information from related [3] and unrelated [4] [5] individuals. The RNA levels of transcripts measured using high-throughput arrays have moderate to high heritability estimates, with 42% of transcripts having additive genetic variance h 2 > 0.3 and 5% with h2 > 0.5 [6] [7]. Expression quantitative trait loci (eQTL) mapping studies have been extremely successful in identifying many loci that contribute to the heritable variation [8] [9] [10] [11]. However, environmental variation, which can be considered as 1- H 2, makes the largest contribution to variation in RNA expression levels [12]. Identifying and quantifying the influence of the environmental factors can provide a more thorough understanding of the differences in expression levels between individuals and between populations. Knowledge of environmental effects will also provide information on gene function and potentially gene-environment interactions, which will aid in understanding how expression profiles are affected by certain environmental conditions, such as geographical location [13].
One environmental factor that has a well-documented effect is seasonal variation. Changes in gene regulation have been associated with seasonal effects such as photoperiod in animals [14] [15] and plants [16] [17]. Previous research into the effect of seasonal variation in humans has focused only on a subset of genes [18] or cluster of genes [19] [20] and failed to identify many genes whose expression levels change in response to the season.
Seasonal changes between months of the year include but are not limited to differences in day length [21], ultraviolet (UV) index [22], humidity [23] and temperature [24], all of which could potentially influence expression levels either independently or interactively. Several health conditions are affected by seasonal changes, including asthma [25], cardiovascular disease [26], depression [27], diabetes [28], bipolar disorder [29], schizophrenia [30], migraine [31] and multiple sclerosis [32] [33]. Environmental changes between seasons also influence infection rates of influenza and respiratory syncytial virus [34] and vitamin D deficiency has been attributed to seasonal UV changes [35] [36]. Identifying genes, the expression levels of which change in response to the season could potentially shed light on some of the mechanisms that might be driving these health conditions. Here we report results from a systematic, genome-wide analysis of the effect of season on gene expression levels in a human population. We identified significant blood cell count changes in erythrocytes, leukocytes and platelets associated with seasonality and enrichment for expressed cellular gene markers for lymphocytes. Furthermore, after correcting for blood cell counts, we identified 135 probes whose expression levels were significantly associated with 12-month seasonal cycle.
Materials and Methods
Ethics Statement
This study used previously published data, deposited in GEO under accession number GSE33321. The research and study design were approved by the University of Queensland Human Ethics Review Board and the QIMR Berghofer Medical Research Institute Institutional Review Board for Research on Human Subjects. All participants gave informed written consent.
Samples
This study comprised of 606 individuals from 246 families in the Brisbane System Genetics Study (BSGS) [37]. Genotype, gene expression and cell counts were measured for each individual.
Genotyping
All individuals were genotyped on an Illumina 610-Quad Beadchip (Illumina Inc, San Diego, CA) by the Scientific Services Division at deCODE Genetics, Iceland. Full details of the genotyping procedure are given in [37].
Gene expression
RNA levels were measured from whole blood collected with a PAXgeneTM tube (QIAGEN, Valencia, CA). The expression levels were quantified on an Illumina HumanHT-12 v4.0 Beadchip. Samples were randomized across the chip to minimize batch effects due to family, sex and age. Full details of the procedures used to generate the expression levels are given in [37]. Pre-processing of the microarray data, including calculation of average bead signal, removal of outliers and the calculation of detection p-values, was performed in the Illumina software Genome studio. Probes were considered significantly expressed above background noise at a p < 0.05. All probes falling below this threshold were considered non-expressed and denoted as such for further analysis. Probes that did not map to characterized Ref-Seq genes were removed. Probes with non-expression in > 50 of samples were excluded, leaving 13,311 probes for further analysis.
Cell counts
Cell counts measured in BSGS include individual measures of single cell types, along with measures representing a composite of multiple cell types. For example, total white blood count includes measures of several cell types such as monocytes, lymphocytes, basophils, neutrophils and eosinophils. We chose to correct for the individual blood cell types, rather than composite measures. The cell types that were selected for correction were red blood (RBC), platelets (PLT), monocytes (MONO), basophils (BASO), neutrophils (NEUT), eosinophils (EOS), B-cells (CD19), Two subtypes of T-cells (CD4, CD8) and NK cells (CD56). Cell counts were log transformed and converted to z-scores. Linear regression was used to correct expression levels for effects due to cellular composition.
Normalization
A rank-based inverse normal transformation (INT) was used to transform probe expression to a normal distribution. The normalization was done using the R package GenABEL [38]. As the BSGS contains related individuals, the polygenetic (cryptic and family) effects were removed by fitting the relationship matrix (A), determined using an identity-by-state (IBS) Genomic Relationship Matrix (GRM) in software package, Genome-wide Complex Trait Analysis (GCTA) [39]:
| (2) |
Where and . Variation in expression levels can be attributed to batch effects such as chip and chip processing. Corrections were made for batch effects using Eq (3).
| (3) |
Where y2 = e1, Z is the incidence matrix for the chip ID fitted as a random effect (b) with . Fixed effect covariates (X) were selected from a list of recorded batch effects using forward step-wise regression with model selection based on the lowest Akaike information criterion (AIC). Covariates that demonstrated significant association with gene expression levels included chip position, age, sex and length of sample storage, which is the difference between the date that the tissue sample was collected and the date that the mRNA was extracted. The values calculated from e2 (Eq (3)) were used for further analysis and referred to as the “uncorrected” dataset.
Cell count correction
The expression dataset was corrected for cell count using Eq (4):
| (4) |
Where and X is the fixed effect cell count covariates selected previously. The values obtained in e3 (Eq (4)) were used for further analysis and referred to as the “corrected” dataset.
Conversion to time series
BSGS tissue samples were collected over a six-year period, between February 2005 and March 2010. Expression levels and cell counts were averaged by the month of sample collection, creating a monthly time series for each probe. Of the 606 samples, 597 were collected between February 2005 and February 2008. The remaining 9 samples, which were collected between March 2008 and March 2010, were excluded from further time series analysis due to the low number of samples per month.
Seasonal decomposition
The gene expression and cell count time series data were decomposed into seasonal (s), trend (t) and error components (ϵ) using loess function [40].
| (5) |
Where y 4 are the residuals from e2 from Eq (2) for the uncorrected analysis or e3, from Eq (3) for the corrected anlaysis. g(s) and g(t) are estimated by loess smoothing functions, which allow the estimation of repeating periodic variation without any constraint to a particular cyclical pattern. The trend component represents the overall changes that occur over the whole time series.
Autocorrelation
Autocorrelation within time series data indicates the presence of periodic repeating patterns. A Ljung-Box test [41] was used to test for significant levels of autocorrelation in the g(s) estimates:
| (6) |
Where n is the sample size, k is the lag, is the autocorrelation, and h is the number of lags [41]. The test statistic (Q) follows a chi-square distribution with h degrees of freedom.
Cosinor regression
Cyclic seasonal patterns, which have periodical cycles repeating over set time frames, can be modelled by the cosine function:
| (7) |
Where t = month (1–12 for January to December), T = time period (in months) over which the cycle repeats, a = amplitude and θ = horizontal shift or phase of the cosine function [42]. This transformation creates the cosinor regression model [43]:
| (8) |
Where y 4 = s, the seasonal component from Eq (5). Cosine and sine curves with repeating cycles (T) of 12 months were fitted. Significance was determined with ANOVA F-statistic and multiple testing accounted for using Bonferroni correction.
Measured weather variables
The cosinor model was applied to 12 measured environmental variables collected for each month: mean maximum temperature, mean minimum temperature, mean daily ground minimum temperature, mean rainfall, mean number of rainy days, maximum wind gust speed, mean daily sunshine, mean daily solar exposure (MJ/m2)), mean number of sunny days, mean number of cloudy days, and mean daily evaporation (all obtained from the Australian Bureau of Meteorology—http://www.bom.gov.au/) and mean UV level (obtained from the Australian Radiation Protection and Nuclear Safety Agency—http://www.arpansa.gov.au/). The weather values for each individual used in this study can be found in S1 Table. The association of weather variables to a 12-month repeating cosine curve was determined with a Kendall tau rank correlation test. Kendall tau rank correlation is a non-parametric test that determines dependence between two ordinal variables.
Biological enrichment analysis
Probes that demonstrated significant association to cyclic seasonal variation were tested for biological enrichment using DAVID (v6.7) [44] [45]. Functional annotation clustering was used to identify groups with shared annotation. Statistical significance of the clusters is given by an enrichment scores, where a score > = 1.3 is equivalent to a p-value of 0.05. Molecular pathways were identified from the Kyoto Encyclopaedia of Genes and Genomes (KEGG) pathway enrichment implemented by DAVID functional annotation tool. Significance of Gene Ontology (GO)-terms obtained from KEGG pathway enrichment and the functional annotation chart was determined using modified the Fisher’s exact test [44] [45] and corrected for multiple testing using the Benjamini-Hochberg false discovery rate (FDR) [46].
Blood cell specific markers
Enrichment for blood cell-specific markers was determined using the userListEnrichment() function in the WGCNA R package [47]. This function compares the gene list obtained from the seasonal analysis to 11 published gene lists that are representative markers for blood cells including red blood cells, lymphocytes, leukocytes and platelets. The function tests for significant overlap between each list of genes and the cell markers using a hypergeometric test. Significant enrichment was determined by a study-wide significance threshold of p < 0.05/11 [48].
Results
Decomposition of time series data
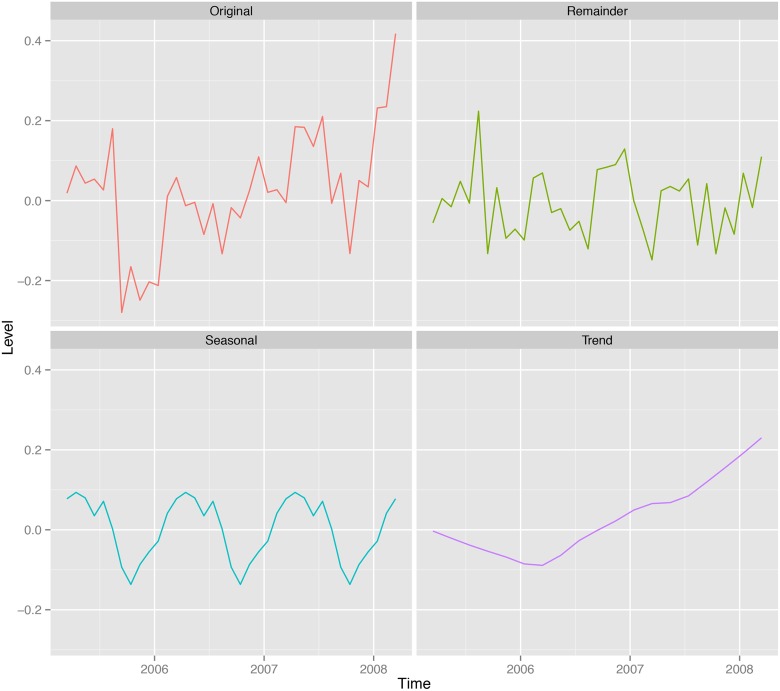
The Brisbane Systems Genetics Study (BSGS) dataset [37], comprising gene expression levels for 606 individuals and 13,311 probes, were decomposed into seasonal, trend and irregular (remainder) components using the loess smoothing function (see Fig 1 and Methods). This enables regular cyclic components for each probe to be isolated from residual or background noise.
Fig 1. Time series decomposition for TRIM23 (ILMN_1752741) using loess decomposition.
Original = The raw time-series data for the probe. Seasonal = The regular cyclic component. Trend = The linear drift over time. Remainder = The irregular (error) component that is not explained by the seasonal and trend components.
Effect of season on gene expression
Cosinor regression was used to test for effect of season (based on when the expression levels were sampled) for each of the time series adjusted probes. Cosinor regression is a linear model with sine and cosine terms that estimate the parameters of repeating cyclic variation across multiple phases (see Methods). To investigate the effect of season on blood cell counts, we performed the cosinor regression analysis on expression levels that had been adjusted for cell counts (“corrected”, see Methods) and unadjusted (“uncorrected”).
Significant associations with season at study-wide threshold of p < 0.05/13,311 were identified for 169 (uncorrected) and 135 (corrected) probes (Table 1). The significant probes from these models also demonstrated significant autocorrelation, an alternative statistical test for repeating patterns, in 160 (uncorrected) and 121 (corrected) probes (Table 1). Of these probes, 75 (approximately 50% of the significant seasonal probes) were shared between the uncorrected and corrected datasets.
Table 1. Significant probes for cosinor regression.
| Dataset | Significant probes | Mean R2 for significant probes | Significant autocorrelation |
|---|---|---|---|
| Uncorrected | 169 | 0.59 | 160 |
| Corrected | 135 | 0.59 | 121 |
The mean variance of gene expression explained by seasonal variation for probe significant at the Bonferroni corrected thresholds.
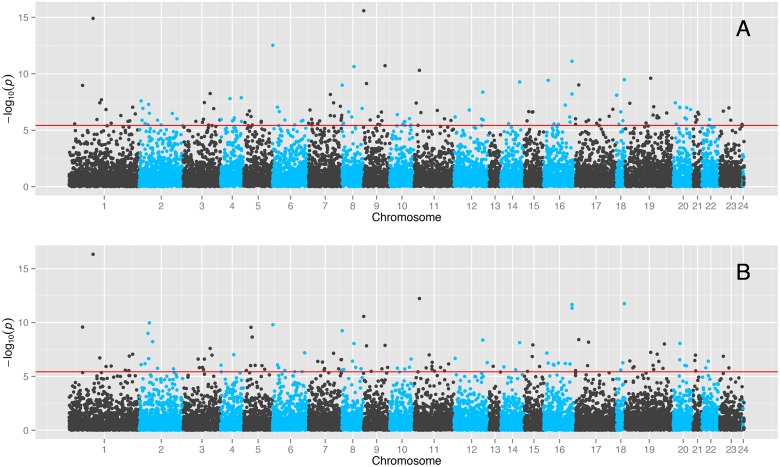
The probes that were significantly associated with season, were located throughout the genome (Fig 2), indicating a diverse range of probes affected by seasonality. The mean proportion of phenotypic variation in expression levels explained by the seasonal effect was 0.13 (uncorrected) 0.12 (corrected) (S1 Fig). The variance explained by the models (R 2) values for probes with significant levels of association were much higher with both corrected and uncorrected datasets having and R 2 of 0.59 for (Table 1).
Fig 2. Manhattan plot of the cosinor seasonal analysis.
The −log10(p) of each cosinor regression model is plotted against the chromosomal location of each probe. Bonferroni correction significance line is added. A) Not corrected for cell count B) Corrected for cell count. Includes autosomal chromosomes 1–22, X(23), Y(24) and Mitochondrial(25).
Cell count seasonality
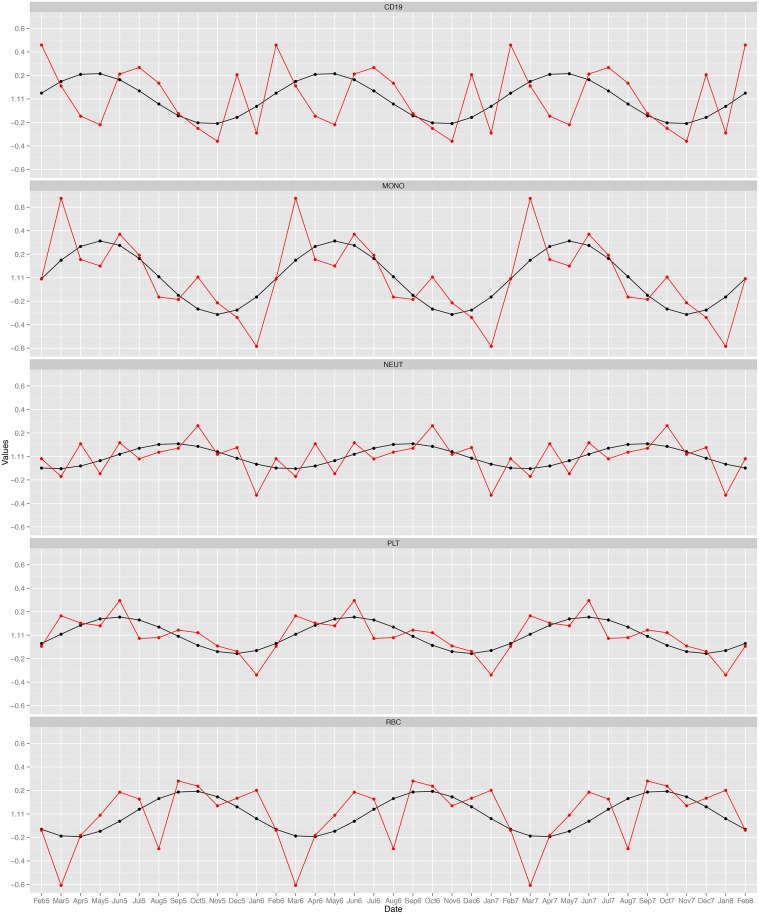
Cell counts for 10 blood cell types representing distinct subgrouping in erythrocytes, platelets, granulocytes, monocular cells and lymphocytes were selected for association to seasonal variation using cosinor regression with a 12-month repeating cycle. Five cell types demonstrated a significant association with season: Erythrocytes (p = 1.78e −3, R 2 = 0.3), Platelets (p = 5.46e −6, R 2 = 0.51), Neutrophils (p = 4.41e −3, R 2 = 0.27), Monocytes (p = 1.58e −5, R 2 = 0.48) and CD19 cells (p = 4.89e −6, R 2 = 0.51). The R 2 value denotes how much variance in the cell counts the linear model explains. The fitted 12-month seasonal cycle explained between 30–51% of variance in the cell counts. The change in cell count levels throughout the year demonstrates differing seasonal highs and lows (Fig 3). The CD19 cells, Monocytes and Platelets share a similar seasonal cycle that peaks in autumn and drops in spring. Red blood cells and Neutrophils demonstrate a slightly shifted pattern peaking in late winter/early spring and dropping in summer. These seasonal patterns have been reported before for platelets [49] and red blood cells [50].
Fig 3. Seasonal variation in cell count.
The seasonal variation of five cells that demonstrate significant seasonal variation. The black lines represent the fitted values in a cosinor regression. The red lines represent the actual cell values. From these figures it is evident that the cells follow complex repeating patterns of peaks and troughs throughout the year. However, it can be observed that they show a consistent seasonal trend following one clear peak and trough per year. These values were collected over a three year period and are plotted in sequential order. The year of collection is labeled in the axis as a number (5–8) after the month. This corresponds to the years 2005, 2006, 2007 and 2008 respectively.
Environmental variables
The cosinor regression models a regular cyclic wave that represents natural seasonal variation. For 12 measured environmental conditions ranging from temperature to UV exposure, there was a significant association with a 12 month repeating cosine curve (S2 Fig) with tau-rank correlation coefficient of τ ∼ 1 for temperature (ground, maximum and minimum), τ ∼ 0.7 for UV, clear days, cloudy days, evaporation, rainy days, rainfall and solar exposure and τ ∼ 0.16 for wind speed and hours of sunshine (S2 Fig). The 12-month cycle therefore is a good seasonal surrogate variable that represents various seasonal environmental conditions, in particular temperature, in Brisbane (S2 Fig).
Enrichment analysis
We next sought to identify a shared biological function of genes exhibiting significant levels of cyclic seasonal variation by performing an enrichment analysis using the Database for Annotation, Visualization and Integrated Discovery (DAVID) [44].
DAVID Functional annotation of the significant seasonal probes for the uncorrected dataset showed enrichment for several Kyoto Encyclopaedia of Genes and Genomes (KEGG) pathways and GO terms related to immune function. This included a number of autoimmune disorders (autoimmune thyroid disease (p = 2.3e −3), type 1 diabetes (p = 4.77e −4)), chronic inflammatory diseases (asthma (p = 2.1e −4)), antigen process and presentation (p = 1.97e −3) (S3 Fig) cellular activation, differentiation and development (cluster enrichment score 1.8) as well as MHC class 2 immune response (cluster enrichment score 2.28) (Table 2). There was also enrichment for protein production and modification including translation, post-translation modifications and localizations (cluster enrichment score 1.4). Cellular components involved in protein production, the endoplasmic reticulum (p = 1.24e −3) and the Golgi apparatus membrane (cluster enrichment score 1.44) were also enriched (Table 2). The significant seasonal probes for the corrected dataset, however only showed enrichment for DNA repair and binding (Table 2), a pathway that was also identified for the uncorrected seasonal probes.
Table 2. Biological enrichment for the 12 month seasonal cycle.
| Seasonal cycle | General process | Terms | Significance |
|---|---|---|---|
| Corrected | DNA | DNA repair | CER 1.68 |
| DNA binding | CER 1.5 | ||
| Uncorrected | Protein production and modification | Acetylation | p = 3.21e −5 |
| Ubiquitin | CER 1.64 | ||
| Ribosome biogenesis | CER 1.48 | ||
| Protein localization | CER 1.46 | ||
| Translation | CER 1.44 | ||
| Peptidase activity | CER 1.42 | ||
| Uncorrected | Cellular component | Endoplasmic reticulum | p = 1.24e −3 |
| Golgi apparatus membrane | CER 1.44 | ||
| Immune response | Allograph rejection | p = 4.19e−4 | |
| Asthma | p = 2.1e−4 | ||
| Intestinal immune network for IgA production | p = 4.29e−4 | ||
| Type 1 diabetes mellitus | p = 4.77e−4 | ||
| Autoimmune thyroid disease | p = 2.3e−3 | ||
| Antigen processing and presentation | p = 1.97e−3 | ||
| Lymphocytes differentiation | CER 1.6 | ||
| Antigen processing and presentation | CER 1.5 | ||
| Immune cell activation differentiation and development | CER 1.8 | ||
| MHC class two immune response pathway | CER 2.28 | ||
| Uncorrected | DNA | DNA binding | CER 1.75 |
| Nucleotide metabolism | CER 1.67 | ||
| Uncorrected | Cellular function | Apoptosis | CER 1.93 |
CER = cluster enrichment score
Cell-specific mRNA markers
As the expression levels were measured in whole blood, which is composed of many cell types, we attempted to determine whether a specific cell type drove the seasonal expression patterns. Using the userListEnrichment() function incorporated in the WGCNA R package, we tested 11 lists of different blood cell markers for enrichment. This analysis revealed that the non-corrected seasonal probes were significantly enriched for lymphocyte markers (Table 3) after Bonferroni correction (0.05/11 test sets). After correction for cell count, no association to any cell markers were observed, indicating that cellular markers can reflect the cell counts present for the individuals. This demonstrates that fluctuations in cell count can be observed within the transcriptome through the presence of specific cellular markers and also through the enrichment of seasonal genes involved in immune function.
Table 3. Gene list enrichment analysis for blood cells.
| User Defined Categories | Type | Number of Genes | Corrected p-values | Genes |
|---|---|---|---|---|
| Bcell Blood (composite) | Blood | 31 | 3.52E-06 | BANK1, BCL11A, C22ORF13, C4ORF34, CCDC106, CCR6, CD24, CD79A, CD79B, CXXC5, CYBASC3, EIF2AK3, GJB6, GNB5, HLA-DOA, HVCN1, ITPR1, IVD, MEF2C, NOC3L, P2RY10, PACAP, PNOC, SMARCB1, SP100, SPIB, TLR10, TPD52, TTC21A, ZDHHC23, ZNF165 |
| Lymphcytes genesCorrelatedAcrossIndividuals Whitney | Blood | 11 | 3.2e-02 | BTG1, CD74, CD79A, CSF1R, HLA-DMB, HLA-DPA1, HLA-DRA, HLA-DRB4, MS4A1, SPIB, TCL1A |
Enrichment for blood cell signature was found using the userListEnrichment function in the WGCNA R package.
Discussion
There are seasonal differences in the prevalence and severity of conditions such as psychiatric disorders [27] [51] [30] [29], inflammatory [25] and cardiovascular diseases [52] [26]. The effect of seasonal variability has also been recorded for several molecular phenotypes such as homovanillic acid [53], serotonin [54] [55], monoamine neurotransmitters [56], 25-hydroxyvitamin D3 [57] [58] [59] [60] and N-3 poly-unsaturated fatty acid [61]. Gene expression provides an intermediate phenotype between the genome and higher order phenotypes such as metabolites and can provide clues as to the underlying biological functions that are being altered in response to seasonal changes.
We investigated whole blood gene expression for seasonal variability in a cohort of 606 individuals. Using cosinor regression models we were able to identify 135 probes (1% of all probes tested) that showed significant seasonal variation after being corrected for blood cell composition. Significant autocorrelation, an alternative statistical technique, was also identified for 90% of these probes further confirming the presence of repeating cyclic trends in expression levels of numerous transcripts. In order to examine how this impacts seasonal gene expression, we examined the expression levels with and without corrections for cell counts.
Transcripts showing seasonal variation in the uncorrected data were enriched for immune pathways and protein production. The enriched KEGG immune pathways included (Table 2) allograph rejection, antigen processing and presentation (S3 Fig), lymphocyte differentiation, immune cell activation, differentiation and development, MHC class two immune response and autoimmune diseases; type 1 diabetes, autoimmune thyroid disease and asthma. Asthma is a chromic inflammatory disease condition, which has been observed to exhibit seasonal variability [25], potentially through the cellular mechanisms identified here. Other cellular function, such as protein modification and apoptosis, were found in the uncorrected gene expression dataset (Table 2). There were 74 seasonally associated genes shared between the corrected and uncorrected datasets and these were enriched with terms for DNA binding. This suggests that transcripts encoding genes involved with DNA binding experience significant seasonal variation, independent of seasonal fluctuations in cell count.
A previous study by De Jong et al. [20] identified three modules, comprising 5,062 probes (mapping to 1,458 unique genes), that were associated with cyclic seasonal patterns. However, these probes were primarily driven by changes in red blood cells and platelets [20]. Here, we did not identify cell type specific gene signatures for red blood cells, but instead identified enrichment for leukocytes markers. This difference could be attributed to the single-gene approach we employed and that we only shared 2,406 genes (18% of our dataset) with the De Jong et al. analysis [20].
We demonstrate in this study that the 12-month cosinor regression has perfect correlation (τ ∼ 1) with temperature, a high correlation (τ ∼ 0.7) with UV index, number of clear, cloudy and rainy days, evaporation, rainfall and solar exposure, and a low correlation (τ ∼ 0.16) for wind speed and hours of sunshine. This relationship suggests that temperature could be a major factor driving the seasonal variation in gene expression levels identified with the 12-month cosinor regression model.
A limitation of this study is that each time point represents the mean expression levels of a group of samples collected during the same time period. Therefore, estimates of effects represent population variation, rather than intra-individual variation. To more accurately assess the impact of seasonal environmental factors of gene expression, repeat measures should be collected for samples throughout the year.
Conclusion
Our results demonstrate the effect of seasonality on cell count and gene expression levels. We observe that the cellular composition of erythrocytes, platelets and leukocytes varies throughout the year, following seasonal patters. This trend was also evident in gene expression levels, with significant seasonal changes in the expression of genes involved in immune function and protein translation. However, we are unable to determine the direct route of causation for these transcriptional changes. DNA binding however, a key component of transcription control, demonstrated significant seasonal variation independent of cell counts, indicating that gene expression could be pervasively influenced by seasonality in a regulatory manner. Collectively, our results show that seasonality is an important environmental regulator of physiological processes, which can be identified through transcriptional variation.
Supporting Information
Blue denotes probes with statistically levels of association between gene expression levels and cyclic variation A) Not corrected for cell count B) Corrected for cell count.
(TIFF)
Measured weather variables that exhibit seasonal variation in Brisbane (black dots and connecting lines). The red dots represent the cosine curve with a 12-month repeating cycle.
(TIF)
Significant seasonal genes in our study are highlighted with red stars.
(TIF)
Sample IDs match those of the expression data deposed in GEO GSE33321
(TXT)
Acknowledgments
We gratefully acknowledge the participation of the individuals sampled in this work. We would like to thank Sara Smith and Anthony Caracella for their technical assistance with the microarray hybridizations, Alison Mackenzie, Marlene Grace and Ann Eldridge for data collection and Dale Nyholt, David Smyth and Scott Gordon for data management. Thanks to Proffessor Peter M. Visscher for his input on statistical methods. Anita Goldinger wrote the manuscript and carried out the data analysis. Anita Goldinger and Joseph E. Powell conceived and supervised the study design. Anjali K. Henders, Allan F McRae and Grant W. Montogomery were involved in the generation of the data used for the study. Joseph E. Powell, Allan F. McRae and Konstatin Shakhbazov provided input on statistical analysis. All authors read, revised, and approved the final manuscript.
Data Availability
All gene expression and corresponding batch, age and sex covariate data, cell count data and family-corrected gene expression data have been deposited in GEO (accession number: GSE531195). All seasonal data was obtained from the publically accessible Australian Bureau of Meteorology (http://www.bom.gov.au/) and the Australian Radiation Protection and Nuclear Safety Agency (http://www.arpansa.gov.au/) and are available in the supplementary information of the paper.
Funding Statement
This research was supported by Australian National Health and Medical Research Council (NHMRC) grants 389892, 496667, 613601, 1010374, and 1046880 and National Institutes of Health (NIH) grant GM057091. Dr. Montgomery is supported by the NHMRC Fellowship Scheme. Dr. Powell is supported by the Australia Research Council (ARC) Fellowship Scheme. Anita Goldinger is supported by an Australian Postgraduate Award. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Zeller T, Wild P, Szymczak S, Rotival M, Schillert A, Castagne R, et al. Genetics and beyond-the transcriptome of human monocytes and disease susceptibility. PloS one. 2010. January;5(5):e10693 10.1371/journal.pone.0010693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sieberts SK, Schadt EE. Moving toward a system genetics view of disease. Mammalian Genome. 2007;18(6–7):389–401. 10.1007/s00335-007-9040-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falconer DS, Mackay TFC, Frankham R. Introduction to Quantitative Genetics (4th edn). Trends in Genetics. 1996;12(7):280 10.1016/0168-9525(96)81458-2 [DOI] [Google Scholar]
- 4. Yang J, Benyamin B, McEvoy BP, Gordon S, Henders AK, Nyholt DR, et al. Common SNPs explain a large proportion of the heritability for human height. Nature genetics. 2010;42(7):565–569. 10.1038/ng.608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Powell JE, Visscher PM, Goddard ME. Reconciling the analysis of IBD and IBS in complex trait studies. Nature reviews Genetics. 2010. November;11(11):800–5. Available from: 10.1038/nrg2865 [DOI] [PubMed] [Google Scholar]
- 6. Dixon AL, Liang L, Moffatt MF, Chen W, Heath S, Wong KCC, et al. A genome-wide association study of global gene expression. Nature genetics. 2007;39(10):1202–1207. 10.1038/ng2109 [DOI] [PubMed] [Google Scholar]
- 7. Goldinger A, Henders AK, McRae AF, Martin NG, Gibson G, Montgomery GW, et al. Genetic and nongenetic variation revealed for the principal components of human gene expression. Genetics. 2013. November;195(3):1117–28. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24026092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Göring HHH, Curran JE, Johnson MP, Dyer TD, Charlesworth J, Cole SA, et al. Discovery of expression QTLs using large-scale transcriptional profiling in human lymphocytes. Nature genetics. 2007;39(10):1208–1216. 10.1038/ng2119 [DOI] [PubMed] [Google Scholar]
- 9. Cheung VG, Spielman RS. Genetics of human gene expression: mapping DNA variants that influence gene expression. Nature Reviews Genetics. 2009;10(9):595–604. 10.1038/nrg2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Powell JE, Henders AK, McRae AF, Wright MJ, Martin NG, Dermitzakis ET, et al. Genetic control of gene expression in whole blood and lymphoblastoid cell lines is largely independent. Genome Res. 2012;22(3):456–466. 10.1101/gr.126540.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Q, Seo JH, Stranger B, McKenna A, Pe’er I, Laframboise T, et al. Integrative eQTL-based analyses reveal the biology of breast cancer risk loci. Cell. 2013. January;152(3):633–41. Available from: 10.1016/j.cell.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Powell JE, Henders AK, McRae AF, Kim J, Hemani G, Martin NG, et al. Congruence of additive and non-additive effects on gene expression estimated from pedigree and SNP data. PLoS genetics. 2013. May;9(5):e1003502 10.1371/journal.pgen.1003502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Idaghdour Y, Czika W, Shianna KV, Lee SH, Visscher PM, Martin HC, et al. Geographical genomics of human leukocyte gene expression variation in southern Morocco. Nature genetics. 2010. January;42;(1):62–7. Available from: 10.1038/ng.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tournier BB, Dardente H, Simonneaux V, Vivien-Roels B, P’vet P, Masson-P’vet M, et al. Seasonal variations of clock gene expression in the suprachiasmatic nuclei and pars tuberalis of the European hamster (Cricetus cricetus). European Journal of Neuroscience. 2007;25(5):1529–1536. 10.1111/j.1460-9568.2007.05421.x [DOI] [PubMed] [Google Scholar]
- 15. Davie A, Minghetti M, Migaud H. Seasonal variations in clock-gene expression in Atlantic salmon (Salmo salar). Chronobiology international. 2009;26(3):379–395. 10.1080/07420520902820947 [DOI] [PubMed] [Google Scholar]
- 16. Yang SH, Loopstra CA. Seasonal variation in gene expression for loblolly pines (Pinus taeda) from different geographical regions. Tree physiology. 2005;25(8):1063–1073. 10.1093/treephys/25.8.1063 [DOI] [PubMed] [Google Scholar]
- 17. Landi L, Romanazzi G. Seasonal variation of defense-related gene expression in leaves from Bois noir affected and recovered grapevines. Journal of Agricultural and Food chemistry. 2011;59(12):6628–6637. 10.1021/jf104297n [DOI] [PubMed] [Google Scholar]
- 18. Vogel U, MØ ller P, Dragsted L, Loft S, Pedersen A, Sandström B. Inter-individual variation, seasonal variation and close correlation of OGG1 and ERCC1 mRNA levels in full blood from healthy volunteers. Carcinogenesis. 2002;23(9):1505–1509. 10.1093/carcin/23.9.1505 [DOI] [PubMed] [Google Scholar]
- 19. Xiao X, Moreno-Moral A, Rotival M, Bottolo L, Petretto E. Multi-tissue Analysis of Co-expression Networks by Higher-Order Generalized Singular Value Decomposition Identifies Functionally Coherent Transcriptional Modules. PLoS genetics. 2014. January;10(1):e1004006 10.1371/journal.pgen.1004006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Jong S, Neeleman M, Luykx JJ, Ten Berg MJ, Strengman E, den Breeijen HH, et al. Seasonal changes in gene expression represent cell type compostion in whole blood. Human molecular genetics. 2014. January;p. ddt665–. Available from: http://hmg.oxfordjournals.org/content/early/2014/01/07/hmg.ddt665.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Azzi A, Dallmann R, Casserly A, Rehrauer H, Patrignani A, Maier B, et al. Circadian behavior is light-reprogrammed by plastic DNA methylation. Nature neuroscience. 2014. March;17;(3):377–82. Available from: http://www.nature.com/neuro/journal/v17/n3/fig_tab/nn.3651_F1.html. [DOI] [PubMed] [Google Scholar]
- 22. Weinkauf B, Rukwied R, Quiding H, Dahllund L, Johansson P, Schmelz M. Local gene expression changes after UV-irradiation of human skin. PloS one. 2012. January;7(6):e39411 10.1371/journal.pone.0039411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Okamoto M, Tanaka Y, Abrams SR, Kamiya Y, Seki M, Nambara E. High humidity induces abscisic acid 8’-hydroxylase in stomata and vasculature to regulate local and systemic abscisic acid responses in Arabidopsis. Plant physiology. 2009. February;149;(2):825–34. Available from: http://www.plantphysiol.org/content/149/2/825.full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kiyohara T, Miyata S, Nakamura T, Shido O, Nakashima T, Shibata M. Differences in Fos expression in the rat brains between cold and warm ambient exposures. Brain Research Bulletin. 1995. January;38(2):193–201. Available from: http://www.sciencedirect.com/science/article/pii/036192309500093T. [DOI] [PubMed] [Google Scholar]
- 25. Kao CC, Huang JL, Ou LS, See LC. The prevalence, severity and seasonal variations of asthma, rhinitis and eczema in Taiwanese schoolchildren. Pediatric allergy and immunology: official publication of the European Society of Pediatric Allergy and Immunology. 2005. August;16(5):408–15. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16101933. [DOI] [PubMed] [Google Scholar]
- 26. Barnett AG, de Looper M, Fraser JF. The seasonality in heart failure deaths and total cardiovascular deaths. Australian and New Zealand journal of public health. 2008. October;32(5):408–13. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18959541. [DOI] [PubMed] [Google Scholar]
- 27. Kasper S, Wehr TA, Rosenthal NE. Season-related forms of depression. I. Principles and clinical description of the syndrome. Der Nervenarzt. 1988. April;59(4):191–9. Available from: http://europepmc.org/abstract/MED/3290693/reload=0. [PubMed] [Google Scholar]
- 28. Green A, Patterson CC. Trends in the incidence of childhood-onset diabetes in Europe 1989–1998. Diabetologia. 2001. October;44(S3):B3–B8. Available from: http://link.springer.com/10.1007/PL00002950. 10.1007/PL00002950 [DOI] [PubMed] [Google Scholar]
- 29. Friedman E, Gyulai L, Bhargava M, Landen M, Wisniewski S, Foris J, et al. Seasonal changes in clinical status in bipolar disorder: a prospective study in 1000 STEP-BD patients. Acta psychiatrica Scandinavica. 2006. June;113(6):510–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16677228. [DOI] [PubMed] [Google Scholar]
- 30. Messias E, Kirkpatrick B, Bromet E, Ross D, Buchanan RW, Carpenter WT, et al. Summer birth and deficit schizophrenia: a pooled analysis from 6 countries. Archives of general psychiatry. 2004. October;61(10):985–9. Available from: http://archpsyc.jamanetwork.com/article.aspx?articleid=482066. [DOI] [PubMed] [Google Scholar]
- 31. Alstadhaug KB, Salvesen R, Bekkelund SI. Seasonal variation in migraine. Cephalalgia: an international journal of headache. 2005. October;25(10):811–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16162258. [DOI] [PubMed] [Google Scholar]
- 32. Iuliano G, Boz C, Cristiano E, Duquette P, Lugaresi A, Oreja-Guevara C, et al. Historical changes of seasonal differences in the frequency of multiple sclerosis clinical attacks: a multicenter study. Journal of neurology. 2012;p. 1–5. [DOI] [PubMed] [Google Scholar]
- 33. Parnell GP, Gatt PN, McKay FC, Schibeci S, Krupa M, Powell JE, et al. Ribosomal protein S6 mRNA is a biomarker upregulated in multiple sclerosis, downregulated by interferon treatment, and affected by season. Multiple sclerosis (Houndmills, Basingstoke, England). 2014. May;20(6):675–85. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24126065. [DOI] [PubMed] [Google Scholar]
- 34. Bloom-Feshbach K, Alonso WJ, Charu V, Tamerius J, Simonsen L, Miller MA, et al. Latitudinal variations in seasonal activity of influenza and respiratory syncytial virus (RSV): a global comparative review. PloS one. 2013. January;8(2):e54445 Available from: http://www.plosone.org/article/info:doi/10.1371/journal.pone.0054445#pone-0054445-g006. 10.1371/journal.pone.0054445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. The American journal of medicine. 2002;112(8):659 10.1016/S0002-9343(02)01091-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Levis S, Gomez A, Jimenez C, Veras L, Ma F, Lai S, et al. Vitamin D deficiency and seasonal variation in an adult South Florida population. Journal of Clinical Endocrinology & Metabolism. 2005;90(3):1557–1562. 10.1210/jc.2004-0746 [DOI] [PubMed] [Google Scholar]
- 37. Powell JE, Henders AK, McRae AF, Caracella A, Smith S, Wright MJ, et al. The Brisbane Systems Genetics Study: Genetical Genomics Meets Complex Trait Genetics. PLoS ONE. 2012;7(4):e35430 10.1371/journal.pone.0035430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aulchenko YS, Ripke S, Isaacs A, van Duijn CM. GenABEL: an R library for genome-wide association analysis. Bioinformatics (Oxford, England). 2007. May;23(10):1294–6. Available from: http://bioinformatics.oxfordjournals.org/content/23/10/1294.full. [DOI] [PubMed] [Google Scholar]
- 39. Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. American journal of human genetics. 2011. January;88(1):76–82. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3014363&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cleveland RB, Cleveland WS, McRae JE, Terpenning I. STL: A seasonal-trend decomposition procedure based on loess. Journal of Official Statistics. 1990;6(1):3–73. [Google Scholar]
- 41. Ljung GM, Box GEP. On a measure of lack of fit in time series models. Biometrika. 1978. August;65(2):297–303. Available from: http://biomet.oxfordjournals.org/content/65/2/297.abstract. [Google Scholar]
- 42. Stolwijk AM, Straatman H, Zielhuis GA. Studying seasonality by using sine and cosine functions in regression analysis. Journal of Epidemiology & Community Health. 1999. April;53(4):235–238. Available from: http://jech.bmj.com/content/53/4/235.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mikulich SK, Zerbe GO, Jones RH, Crowley TJ. Comparing linear and nonlinear mixed model approaches to cosinor analysis. Statistics in medicine. 2003. October;22(20):3195–211. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14518023. [DOI] [PubMed] [Google Scholar]
- 44. Sherman BT, Tan Q, Kir J, Liu D, Bryant D, Guo Y, et al. DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic acids research. 2007;35(suppl 2):W169–W175. 10.1093/nar/gkm415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols. 2008;4(1):44–57. 10.1038/nprot.2008.211 [DOI] [PubMed] [Google Scholar]
- 46. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological). 1995;p. 289–300. [Google Scholar]
- 47. Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC bioinformatics. 2008. January;9(1):559 Available from: http://www.biomedcentral.com/1471-2105/9/559. 10.1186/1471-2105-9-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Miller JA, Cai C, Langfelder P, Geschwind DH, Kurian SM, Salomon DR, et al. Strategies for aggregating gene expression data: the collapseRows R function. BMC bioinformatics. 2011. January;12(1):322 Available from: http://www.biomedcentral.com/1471-2105/12/322. 10.1186/1471-2105-12-322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gallerani M, Reverberi R, Salmi R, Smolensky MH, Manfredini R. Seasonal variation of platelets in a cohort of Italian blood donors: a preliminary report. European journal of medical research. 2013. January;18(1):31 Available from: http://www.eurjmedres.com/content/18/1/31. 10.1186/2047-783X-18-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kristal-Boneh E, Froom P, Harari G, Shapiro Y, Green MS. Seasonal changes in red blood cell parameters. British Journal of Haematology. 2008. March;85(3):603–607. Available from: http://doi.wiley.com/10.1111/j.1365-2141.1993.tb03354.x. [DOI] [PubMed] [Google Scholar]
- 51. Faedda GL, Tondo L, Teicher MH, Baldessarini RJ, Gelbard HA, Floris GF. Seasonal mood disorders. Patterns of seasonal recurrence in mania and depression. Archives of general psychiatry. 1993. January;50(1):17–23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8422217. [DOI] [PubMed] [Google Scholar]
- 52. Scragg R. Seasonality of Cardiovascular Disease Mortality and the Possible Protective Effect of Ultra-violet Radiation. International Journal of Epidemiology. 1981. December;10(4):337–341. Available from: http://ije.oxfordjournals.org/content/10/4/337.short. [DOI] [PubMed] [Google Scholar]
- 53. Losonczy MF, Mohs RC, Davis KL. Seasonal variations of human lumbar CSF neurotransmitter metabolite concentrations. Psychiatry Research. 1984. May;12(1):79–87. Available from: http://www.sciencedirect.com/science/article/pii/0165178184901409. [DOI] [PubMed] [Google Scholar]
- 54. Lambert G, Reid C, Kaye D, Jennings G, Esler M. Effect of sunlight and season on serotonin turnover in the brain. The Lancet. 2002. December;360(9348):1840–1842. Available from: http://www.sciencedirect.com/science/article/pii/S0140673602117375. [DOI] [PubMed] [Google Scholar]
- 55. Luykx JJ, Bakker SC, Lentjes E, Boks MPM, van Geloven N, Eijkemans MJC, et al. Season of sampling and season of birth influence serotonin metabolite levels in human cerebrospinal fluid. PloS one. 2012. January;7(2):e30497 10.1371/journal.pone.0030497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kruesi MJP. Cerebrospinal Fluid Monoamine Metabolites, Aggression, and Impulsivity in Disruptive Behavior Disorders of Children and Adolescents. Archives of General Psychiatry. 1990. May;47(5):419 Available from: http://archpsyc.jamanetwork.com/article.aspx?articleid=495018. 10.1001/archpsyc.1990.01810170019003 [DOI] [PubMed] [Google Scholar]
- 57. Stamp TCB, Round JM. Seasonal Changes in Human Plasma Levels of 25-Hydroxyvitamin D. Nature. 1974. February;247(5442):563–565. Available from: 10.1038/247563a0 [DOI] [PubMed] [Google Scholar]
- 58. Stryd RP, Gilbertson TJ, Brunden MN. A seasonal variation study of 25-hydroxyvitamin D3 serum levels in normal humans. The Journal of clinical endocrinology and metabolism. 1979. May;48(5):771–5. Available from: http://press.endocrine.org/doi/abs/10.1210/jcem-48-5-771. [DOI] [PubMed] [Google Scholar]
- 59. Juttmann JR, Visser TJ, Buurman C, de Kam E, Birkenhäger JC. Seasonal fluctuations in serum concentrations of vitamin D metabolites in normal subjects. British medical journal (Clinical research ed). 1981. April;282(6273):1349–52. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1504994&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Harris S, Dawson-Hughes B. Seasonal changes in plasma 25-hydroxyvitamin D concentrations of young American black and white women. Am J Clin Nutr. 1998. June;67(6):1232–1236. Available from: http://ajcn.nutrition.org/content/67/6/1232.short. [DOI] [PubMed] [Google Scholar]
- 61. De Vriese SR, Christophe AB, Maes M. In humans, the seasonal variation in poly-unsaturated fatty acids is related to the seasonal variation in violent suicide and serotonergic markers of violent suicide. Prostaglandins, leukotrienes, and essential fatty acids. 2004. July;71(1):13–8. Available from: http://www.sciencedirect.com/science/article/pii/S0952327803002564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Blue denotes probes with statistically levels of association between gene expression levels and cyclic variation A) Not corrected for cell count B) Corrected for cell count.
(TIFF)
Measured weather variables that exhibit seasonal variation in Brisbane (black dots and connecting lines). The red dots represent the cosine curve with a 12-month repeating cycle.
(TIF)
Significant seasonal genes in our study are highlighted with red stars.
(TIF)
Sample IDs match those of the expression data deposed in GEO GSE33321
(TXT)
Data Availability Statement
All gene expression and corresponding batch, age and sex covariate data, cell count data and family-corrected gene expression data have been deposited in GEO (accession number: GSE531195). All seasonal data was obtained from the publically accessible Australian Bureau of Meteorology (http://www.bom.gov.au/) and the Australian Radiation Protection and Nuclear Safety Agency (http://www.arpansa.gov.au/) and are available in the supplementary information of the paper.