Abstract
Background
The associations between toll-like receptor 2 (TLR2) and toll-like receptor 4(TLR4) polymorphisms and inflammatory bowel disease (IBD) susceptibility remain controversial. A meta-analysis was performed to assess these associations.
Methods
A systematic search was performed to identify all relevant studies relating TLR2 and TLR4 polymorphisms and IBD susceptibility. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Subgroup analyses were performed by ethnicity and publication quality.
Results
Thirty-eight eligible studies, assessing 10970 cases and 7061 controls were included. No TLR2 Arg677Trp polymorphism was found. No significant association was observed between TLR2 Arg753Gln polymorphism and Crohn’s disease (CD) or ulcerative colitis (UC) in all genetic models. Interestingly, TLR4 Asp299Gly polymorphism was significantly associated with increased risk of CD and UC in all genetic models, except for the additive one in CD. In addition, a statistically significant association between TLR4 Asp299Gly polymorphism and IBD was observed among high quality studies evaluating Caucasians, but not Asians. Associations between TLR4 Thr399Ile polymorphisms and CD risk were found only in the allele and dominant models. The TLR4 Thr399Ile polymorphism was associated with UC risk in pooled results as well as subgroup analysis of high quality publications assessing Caucasians, in allele and dominant models.
Conclusions
The meta-analysis provides evidence that TLR2 Arg753Gln is not associated with CD and UC susceptibility in Asians; TLR4 Asp299Gly is associated with CD and UC susceptibility in Caucasians, but not Asians. TLR4 Thr399Ile may be associated with IBD susceptibility in Caucasians only. Additional well-powered studies of Asp299Gly and other TLR4 variants are warranted.
Introduction
Inflammatory bowel disease (IBD), which mainly consists of ulcerative colitis (UC) and Crohn's disease (CD), is a group of chronic non-specific gastrointestinal inflammatory conditions. IncreasedIBD incidence and prevalence havebeen observed in different regions of the world[1]. IBD is an autoimmune disease that results from an aberrant immune response to intestinal bacteria or other foreign substances as well as genetic factors[2]. Previous studies have demonstrated that genetic polymorphisms contribute to individual variations in the genetic susceptibility to IBD[3]. Among the genetically predisposing alleles tightly linked to IBD, Toll-like receptor (TLR) polymorphisms have attracted increasing attention in recent years[4].
Toll-like receptors (TLRs) are transmembrane proteins usually expressed by antigen presenting cells; they are important immune receptors that participate in the recognition of pathogen-associated molecular patterns and activation of signal transduction pathways of antimicrobial genes, by identifying and binding to small molecular components on pathogens[5]. TLRs also play an important role in the digestive system. In addition, they can recognize invading microbes in the intestinal barrier and activate the immune response. However, sustained hyper-activation of TLRs may lead to chronic inflammation in IBD. At present, at least 13 TLR family members recognizing different pathogens independently or together in various internal organs have been described, of which TLR2 and TLR4 are most commonly studied for their association with risk of IBD[6].
TLR2, located at 4q31.3, recognizes bacterial lipopeptides and lipoteichoic acid found abundantly in the cell wall of Gram positive bacteria[7]. TLR4, located at 9q33.1, serves as a surface receptor for lipopolysaccharides (LPS), the main endotoxins derived from Gram-negative bacteria[8]. In the normal intestine, TLR2 and TLR4 are expressed at low levels in intestinal epithelial cells (IECs), thus minimizing the recognition of luminal bacteria[9]. However, TLR2 and TLR4 are up-regulated in primary IECs throughout the lower gastrointestinal tract in IBD patients, which may cause excessive immune response[10–12].
Population-based case-control show an association between TLR4 polymorphism and susceptibility to CD and UC. The association between TLR2 gene variantss and extensive colonic disease in UC and CD has also been discribed[13]. TLR2 Arg677Trp (R677W, rs12191786) and Arg753Gln (R753Q, rs5743708), and TLR4 Asp299Gly (D299G, rs4986790) and Thr399Ile (T399I, rs4986791) polymorphisms are the most widely discussed SNPs in the investigation of the association between polymorphisms of TLR family and susceptibility to IBD. The association between TLR4 Asp299Gly and Thr399Ile polymorphisms and IBD is controversial. TLR2 single studies did not found the association of TLR2 Arg677Trp and Arg753Gln polymorphisms and IBD. However, these studies has relatively sample size and might be underpowered to reveal a small effect of the polymorphisms of TLR2 on IBD susceptibility. Meta-analysis can combine results from different studies to produce an estimate of the major effect with enhanced precision.The aim of this meta-analysis was to investigate the associations between TLR2 (Arg677Trp, Arg753Gln) and TLR4 (Asp299Gly, Thr399Ile) genetic polymorphisms and susceptibility to IBD.
Methods
Literature search
A systemic search was conducted on PubMed, Embase, Biosis Preview and China National Knowledge Infrastructure databases up to August 31, 2014 using the following keywords: (1) “toll-like receptor” or “TLR”; (2) “Crohn’s disease” or “CD” or “ulcerative colitis” or “UC” or “inflammatory bowel disease” or “IBD”; (3) “polymorphism” or “variant” or “genotype”. There was no language restriction and species were limited to human. References in the reviews and retrieved articles were hand-searched as well. For articles by the same author using the same case series, the study with the largest sample size was selected.
Inclusion criteria
The inclusion criteria were: (1) case-control study; (2) investigation evaluating the relationship between TLR2 (Arg677Trp, Arg753Gln) and TLR4 (Asp299Gly, Thr399Ile) genetic polymorphisms and IBD (CD or UC) susceptibility; (3) sufficient available published data for odds ratio (OR) estimation with 95% confidence interval (CI); (4) human study; (5) data not republished.
Data extraction and quality assessment
Missing data were requested by contacting study authors through email. Data were blindly extracted from all selected publications by two investigators (Cheng and Zhu) separately. For each of the included articles, the first author name, publication year, study population (ethnicity), source of controls, total numbers of patients and controls, and polymorphism frequencies in patients and controls were extracted. For studies that included subjects of different ethnic groups, data were extracted for each one. Any disagreement on a given item of the extracted data was fully discussed to reach a consensus.
Predefined criteria (Table 1) based on the scale of Thakkinstian[14] were used to assess the methodological quality of eligible studies. The revised criteria cover the representativeness of cases and controls, assessment of IBD, genotyping examination, Hardy-Weinberg equilibrium (HWE) in the control population, and association assessment. Scores ranged from 0 (lowest) to 11 (highest). Articles with scores of less than 6 were considered to be low-quality studies, whereas those with scores equal to or higher than 6 were considered high-quality reports. Quality assessment was also performed by two authors separately (Yang and Yun). Disagreements were resolved by consensus as well.
Table 1. Scale for methodologic Quality Assessment of the Single Nucleotide Polymorphism association studies of IBD.
| Criteria | Score | |
|---|---|---|
| A | Representativeness of cases | |
| Consecutive/randomly selected from case population with clearly defined sampling frame | 2 | |
| Consecutive/randomly selected from case population without clearly defined sampling frame or with extensive inclusion/exclusion criteria | 1 | |
| No method of selection described | 0 | |
| B | Representativeness of controls | |
| Controls were consecutive/randomly drawn from the same sampling frame (ward/community) as cases | 2 | |
| Controls were consecutive/randomly drawn from a different sampling frame as cases | 1 | |
| Not described | 0 | |
| C | Ascertainment of IBD | |
| Clearly described objective criteria for diagnosis of IBD | 2 | |
| Diagnosis of IBD by patient self-report or by patient history | 1 | |
| Not described | 0 | |
| D | Genotyping examination | |
| Genotyping done under “blinded” condition | 1 | |
| Unblinded or not mentioned | 0 | |
| E | Hardy-Weinberg equilibrium | |
| Hardy-Weinberg equilibrium in control group | 2 | |
| Hardy-Weinberg disequilibrium in control group | 1 | |
| No checking for Hardy-Weinberg equilibrium | 0 | |
| F | Association assessment | |
| Assess association between genotypes and IBD with appropriate statistics and adjustment for confounders | 2 | |
| Assess association between genotypes and IBD with appropriate statistics without adjustment for confounders | 1 | |
| Inappropriate statistics used | 0 |
Statistical analysis
Pooled crude odds ratios (ORs) and 95% confidence intervals (95% CIs) were determined to assess the associations between TLR2 (Arg677Trp, Arg753Gln) and TLR4 (Asp299Gly, Thr399Ile) genetic polymorphisms and the risk of IBD under dominant, recessive, additive and allele models, based on the extracted data. The fixed-effects (random-effects) model was used depending on the heterogeneity among studies[15, 16]. Subgroup analysis was performed to assess the ethnic-specific effects. Potential heterogeneity was examined by the chi-square based Q-test and I2. A P value for heterogeneity < 0.10 or I2 > 50% was considered statistically significant. Sensitivity analysis was performed by sequentially excluding each single study to assess the stability of the results[17]. Galbraith plots were performed to identify possible distinct articles, which might contribute to the heterogeneity[18]. Hardy-Weinberg equilibrium (HWE) in the control group was assessed by the chi-square test[19]. Potential publication bias was estimated by the funnel plot of the ORs versus their standard errors[20]. Funnel plot asymmetry was assessed by the Egger’s test (linear regression test) when the number of studies included was more than 10. P value > 0.10 indicated no significant publication bias[21]. Studies with P value <0.1 were corrected using the Duval’s trim and fill method[22]. All statistical analyses were performed with STATA 10.0 (StataCorp LP, College Station, TX).
Results
Literature search
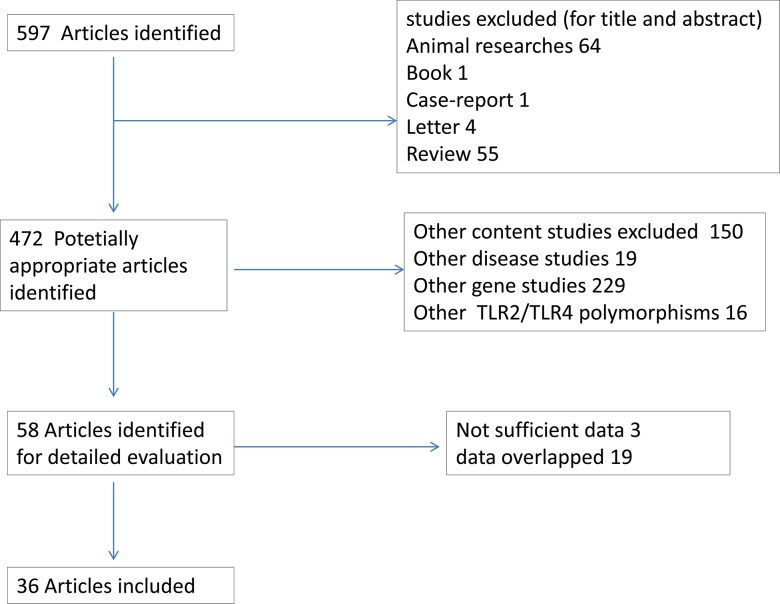
This meta-analysis was performed and reported according to the PRISMA guidelines. The search of PubMed, Biosis Previews, Embase and two Chinese databases (Chinese National Knowledge Infrastructure and Wanfang databases) for relevant articles published up to July 2014 yielded 597 articles. A total of 36 articles[23–58][23–58] met the inclusion criteria and were selected. Three article contained 2 separated studies each, as each of them involved two different populations. Overall, 39 studies, assessing 10970 cases and 7061 controls were included in the analysis. A flow chart demonstrating the selection process of relevant studies is represented in Fig 1.
Fig 1. Flow chart showing literature search for studies of TLR2 and TLR4 polymorphism in relation to risk of CD and UC.
Study characteristics and quality assessment
The basic information for each study, including authors and publication years, ethnicity, numbers of cases and controls, frequencies of various genotypes in IBD patients and healthy controls, and Hardy-Weinberg equilibrium (HWE) in healthy controls, are summarized in Table 2. Of the 39 qualifying studies, 23 were conducted among Caucasian populations, 10carried out among Asians and 6 performed in other ethnicities. All studies were published between 2002 and 2013, and were case-control designed. IBD patients and controls were age and gender matched in 6 studies, while the other 33 studies did not specifically mention this detail in their reports. Allelic distribution for TLR2 and TLR4 is shown in S1 Table.
Table 2. Characteristics of the included studies on TLR polymorphism and susceptibility of CD and UC.
| Year of | Author | Region | TLR | Phenotype | Cases | Controls | HWE in | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Publication | Variant | studied | Number | Males(%) | Age | Number | Males(%) | Age | controls | |||
| 1 | 2002 | Okayama[23] | Japanese | TLR4 299 | UC | UC: 86 | nr | nr | 107 | nr | nr | equilibrium |
| 2 | 2004 | Arnott[24] | Scotland | TLR4 299 | CD&UC | CD: 234 | 43.7 | 28(21–41) | 189 | 52.2 | 38(27–50) | equilibrium |
| UC: 246 | 53.4 | 33(25–49) | equilibrium | |||||||||
| 3 | 2004 | Franchimont(1)[25] | Belgium | TLR4 299 | CDs&UC | CD: 334 | 40.7 | 26.6±10.3 | 139 | nr | nr | equilibrium |
| UC: 163 | 52.2 | 29.78±12.8 | ||||||||||
| 4 | 2004 | Franchimont(2)[25] | Belgium | TLR4 299 | CD | CD: 114 | 56.2 | 28.9±12.4 | 139 | nr | nr | equilibrium |
| 5 | 2004 | Torok[26] | Germany | TLR4 299 | CD&UC | CD: 102 | 36.3 | 40.9±13.7 | 145 | 49 | 44.6±12.5 | equilibrium |
| TLR4 399 | UC: 98 | 45.9 | 42.7±13.3 | |||||||||
| 6 | 2005 | Brand [27] | Germany | TLR4 299 | CD | CD: 204 | 47.1 | 37.8±11.8 | 199 | 49.8 | 46.4±15.3 | equilibrium |
| TLR4 399 | ||||||||||||
| 7 | 2005 | Fries[28] | Italy | TLR4 299 | CD | CD:23 | 56.5 | 43(15–75) | 59 | 47.5 | 38(18–68) | nr/ equilibrium |
| 8 | 2005 | Gazouli[29] | Greek | TLR4 299 | CD&UC | CD: 120 | nr | nr | 100 | nr | nr | nr/disequilibrium |
| TLR4 399 | UC: 85 | nr | nr | |||||||||
| 9 | 2005 | Ouburg[30] | The Netherlands | TLR4 299 | CD | CD:112 | nr | nr | 170 | nr | nr | nr/equilibrium |
| 10 | 2005 | Braat[31] | The Netherlands | TLR4 299 | CD&UC | CD: 441 | 35% | mean 40.7 | 137 | nr | nr | equilibrium |
| UC: 226 | 53% | mean 44.4 | ||||||||||
| 11 | 2005 | Oostenbrug[32] | The Netherlands | TLR4 299 | CD&UC | CD: 393 | nr | nr | 296 | nr | nr | equilibrium |
| TLR4 399 | UC: 179 | nr | nr | |||||||||
| 12 | 2005 | Lakatos[33] | Hungary | TLR4 299 | CD | CD:527 | 50.3 | 37.1±7.6 | 200 | 0.51 | 38.05±10.7 | nr/ equilibrium |
| 13 | 2006 | Figueroa[34] | Chile | TLR4 299 | CD&UC | CD: 22 | 36.4 | 46.8(16–65) | 20 | nr | nr | nr/imponderable |
| UC: 22 | 40.9 | 37.8(19–67) | ||||||||||
| 14 | 2006 | Pierik[35] | Belgium | TLR2 753 | CD&UC | CD:179 | 41.1 | 25.0±10.1 | 191 | nr | nr | equilibrium |
| TLR4 299 | UC:106 | 54.3 | 28.9±13.3 | |||||||||
| 15 | 2007 | Xiong[36] | China | TLR2 677 | IBD | 120 | nr | nr | 110 | nr | nr | nr/imponderable |
| TLR2 753 | ||||||||||||
| TLR4 299 | ||||||||||||
| TLR4 399 | ||||||||||||
| 16 | 2007 | Jiang[37] | China | TLR4 299 | UC | UC:68 | 57.4 | 37.58±12.37 | 152 | 57.9 | 46.90±12.73 | equilibrium |
| 17 | 2007 | Xue[38] | China | TLR2 677 | CD&UC | CD: 41 | 60.7 | 34. 54±14. 21 | 135 | 55.6 | 41. 85±10. 82 | nr/imponderable |
| TLR2 753 | UC: 43 | 45. 95±17. 11 | ||||||||||
| TLR4 299 | ||||||||||||
| TLR4 399 | ||||||||||||
| 18 | 2007 | Henckaerts[39] | Belgium | TLR2 753 | CD&UC | CD: 874 | 41 | 24 (18–31) | 312 | 45 | 39(30–57) | equilibrium |
| TLR4 299 | UC: 259 | 52 | 26 (21–36) | |||||||||
| 19 | 2007 | Hong[40] | New Zealand | TLR2 753 | CD | CD:182 | nr | nr | 188 | nr | nr | equilibrium |
| TLR4 299 | ||||||||||||
| TLR4 399 | ||||||||||||
| 20 | 2007 | Baumgart(1)[41] | Hungary | TLR4 299 | CD&UC | CD: 144 | 43.1 | 24±11.2 | 202 | 46.5 | (18–54) | nr/ equilibrium |
| UC: 118 | 37.3 | 31±10.6 | ||||||||||
| 21 | 2007 | Baumgart(2)[41] | Germany | TLR4 299 | CD&UC | CD: 235 | 38.3 | 26±10.3 | 403 | 42.4 | (21–61) | nr/ equilibrium |
| UC: 145 | 46.2 | 31±13.6 | ||||||||||
| 22 | 2007 | Browning[42] | New Zealand | TLR4 299 | CD&UC | CD: 389 | 36 | nr | 416 | 44 | nr | equilibrium |
| TLR4 399 | UC: 405 | 47 | nr | |||||||||
| 23 | 2008 | Rigoli[43] | Italy | TLR4 299 | CD&UC | CD:133 | 52.6 | 43.5 ± 10.7 | 103 | 66 | 46.6 ± 9.8 | equilibrium |
| TLR4 399 | UC:45 | 60 | 43.2 ± 11.0 | |||||||||
| 24 | 2008 | Hume[44] | Australia | TLR4 299 | CD&UC | CD:619 | nr | nr | 360 | nr | nr | equilibrium |
| UC:300 | nr | nr | ||||||||||
| 25 | 2008 | Akin[45] | Turkey | TLR4 299 | CD&UC | CD:108 | nr | nr | 191 | 52.4 | 35.2 ±11.2 | nr/ equilibrium |
| TLR4 399 | UC:120 | nr | nr | |||||||||
| 26 | 2008 | Lappalainen[46] | Finland | TLR4 299 | CD&UC | CD: 240 | nr | nr | 190 | nr | nr | equilibrium |
| TLR4 399 | UC: 459 | nr | nr | |||||||||
| 27 | 2009 | Ye[47] | Korea | TLR4 299 | CD | CD: 380 | 62.6 | 27.2±7.7 | 380 | 52.3 | 36.6±13.8 | nr/ disequilibrium |
| 28 | 2009 | Zouiten-Mekki[48] | Tunisia | TLR4 299 | CD&UC | CD:90 | nr | nr | 80 | nr | nr | nr/ disequilibrium |
| TLR4 399 | UC:30 | nr | nr | |||||||||
| 29 | 2009 | Queiroz[49] | Brazil | TLR2 677 | CD&UC | CD:43 | 46.51 | 40.88±14.16 | 541 | 75.6 | 33.87±9.96 | equilibrium |
| TLR2 753 | UC:42 | 14.29 | 38.93±14.73 | |||||||||
| TLR4 299 | ||||||||||||
| 30 | 2009 | Bueno[50] | Belgium | TLR4 299 | CD&UC | CD: 80 | 60 | 22.86±7.4 | 79 | nr | nr | equilibrium |
| UC: 15 | 58.8 | 18.3±5.3 | ||||||||||
| 31 | 2010 | Wagner[51] | Australia | TLR4 299 | CD | CD: 72 | 63.9 | 11.6(2.2–17.2) | 98 | 45.9 | 11.9 (1.7–19.8) | equilibrium |
| 32 | 2010 | Shen[52] | China | TLR2 677 | CD&UC | CD:30 | 60 | 32.5(14–64) | 120 | equilibrium | ||
| TLR2 753 | UC:83 | 60.2 | 46.0(19–72) | |||||||||
| TLR4 299 | ||||||||||||
| TLR4 399 | ||||||||||||
| 33 | 2011 | Chen(1)[53] | China | TLR2 677 | CD&UC | CD:30 | nr | nr | 60 | 49 | 36.8 ± 12.2 | equilibrium |
| TLR2 753 | UC:40 | nr | nr | |||||||||
| TLR4 299 | ||||||||||||
| 34 | 2011 | Chen(2)[53] | China | TLR2 677 | CD&UC | CD:30 | nr | nr | 84 | 48.5 | 33.2 ± 12.0 | equilibrium |
| TLR2 753 | UC:46 | nr | nr | |||||||||
| TLR4 399 | ||||||||||||
| 35 | 2012 | Sivaram[54] | India | TLR4 299 | UC | UC: 139 | nr | nr | 176 | nr | nr | nr/ equilibrium |
| 36 | 2012 | Azzam[55] | Saudi | TLR4399 | CD | CD:46 | 67.4 | 30.43 ± 10.20 | 50 | nr | nr | nr/ equilibrium |
| 37 | 2012 | Kim[56] | Korea | TLR2 677 | CD&UC | CD: 45 | 56 | 32.1±11.4 | 178 | 49 | 47.2±13.0 | equilibrium |
| TLR2 753 | ||||||||||||
| TLR4 299 | UC: 99 | 46 | 49.8±14.7 | |||||||||
| TLR4 399 | ||||||||||||
| 38 | 2012 | Guagnozzi[57] | Italia | TLR4 299 | CD&UC | CD: 84 | nr | nr | 227 | nr | nr | nr/ equilibrium |
| UC: 133 | nr | nr | ||||||||||
| 39 | 2013 | Manolakis[58] | Greece | TLR4 299 | CD&UC | CD: 187 | 47.8 | 43.23±22.9 | 274 | 55.2 | 46.9±22.4 | equilibrium |
| TLR4 399 | UC: 163 | 63.1 | 50.1±18.6 | |||||||||
Of the 39 studies, 7 assessed the TLR2 Arg677Trp polymorphism, 10 studied the TLR2 Arg753Gln polymorphism, 37 evaluated the TLR4 Asp299Gly polymorphism and 17 studied the TLR4Thr399Ile polymorphism. There were 30 high quality and 9 low quality studies, respectively according to the set quality criteria (Table 3). All low quality studies encompassed TLR4 polymorphism analyses.
Table 3. Quality assessment of studies included.
| Year of | Aurthor | Representativeness | Representativeness | Ascertainment | Genotyping | Hardy-Weinberg | Association | Total | |
|---|---|---|---|---|---|---|---|---|---|
| Publication | of cases | of controls | of IBD | examination | equilibrium | assessment | |||
| 1 | 2002 | Okayama | 0 | 0 | 0 | 0 | 2 | 1 | 3 |
| 2 | 2004 | Torok | 0 | 0 | 2 | 0 | 2 | 1 | 5 |
| 3 | 2004 | Arnott | 2 | 0 | 2 | 0 | 2 | 1 | 7 |
| 4 | 2004 | Franchimont(1) | 2 | 2 | 2 | 0 | 2 | 1 | 7 |
| 5 | 2004 | Franchimont(2) | 2 | 2 | 2 | 0 | 2 | 1 | 7 |
| 6 | 2005 | Brand | 0 | 0 | 2 | 0 | 2 | 1 | 5 |
| 7 | 2005 | Fries | 2 | 2 | 1 | 0 | 0 | 1 | 6 |
| 8 | 2005 | Gazouli | 2 | 2 | 2 | 0 | 0 | 1 | 7 |
| 9 | 2005 | Ouburg | 2 | 2 | 2 | 0 | 0 | 1 | 7 |
| 10 | 2005 | Braat | 2 | 2 | 2 | 0 | 2 | 1 | 9 |
| 11 | 2005 | Oostenbrug | 2 | 2 | 2 | 0 | 2 | 1 | 9 |
| 12 | 2005 | Lakatos | 2 | 0 | 2 | 0 | 0 | 1 | 5 |
| 13 | 2006 | Figueroa | 2 | 2 | 2 | 0 | 0 | 1 | 7 |
| 14 | 2006 | Pierik | 2 | 2 | 1 | 0 | 2 | 0 | 7 |
| 15 | 2007 | Xiong | 2 | 2 | 2 | 0 | 0 | 1 | 7 |
| 16 | 2007 | Jiang | 2 | 2 | 2 | 0 | 2 | 1 | 9 |
| 17 | 2007 | Xue | 2 | 2 | 2 | 0 | 2 | 1 | 9 |
| 18 | 2007 | Henckaerts | 2 | 2 | 2 | 0 | 2 | 0 | 8 |
| 19 | 2007 | Hong | 1 | 1 | 2 | 0 | 2 | 1 | 7 |
| 20 | 2007 | Baumgart(1) | 1 | 1 | 2 | 0 | 0 | 1 | 5 |
| 21 | 2007 | Baumgart(2) | 1 | 1 | 2 | 0 | 0 | 1 | 5 |
| 22 | 2008 | Lappalainen | 1 | 1 | 2 | 0 | 2 | 1 | 7 |
| 23 | 2007 | Browning | 2 | 1 | 2 | 0 | 2 | 1 | 8 |
| 24 | 2008 | Rigoli | 2 | 2 | 2 | 0 | 2 | 1 | 9 |
| 25 | 2008 | Hume | 2 | 1 | 2 | 0 | 2 | 1 | 8 |
| 26 | 2008 | Akin | 2 | 2 | 1 | 0 | 0 | 1 | 6 |
| 27 | 2009 | Ye | 2 | 2 | 2 | 0 | 0 | 1 | 7 |
| 28 | 2009 | Zouiten-Mekki | 0 | 0 | 2 | 0 | 0 | 1 | 3 |
| 29 | 2009 | Queiroz | 2 | 2 | 2 | 0 | 2 | 2 | 8 |
| 30 | 2009 | Bueno | 2 | 2 | 2 | 0 | 2 | 1 | 9 |
| 31 | 2010 | Wagner | 2 | 1 | 2 | 0 | 2 | 1 | 8 |
| 32 | 2010 | Shen | 2 | 2 | 2 | 0 | 2 | 1 | 9 |
| 33 | 2011 | Chen(1) | 2 | 2 | 2 | 0 | 2 | 1 | 9 |
| 34 | 2011 | Chen(2) | 2 | 2 | 2 | 0 | 2 | 1 | 9 |
| 35 | 2012 | Sivaram | 0 | 0 | 2 | 0 | 0 | 1 | 3 |
| 36 | 2012 | Azzam | 2 | 2 | 2 | 0 | 0 | 1 | 7 |
| 37 | 2012 | Kim | 2 | 2 | 2 | 0 | 2 | 1 | 9 |
| 38 | 2012 | Guagnozzi | 0 | 0 | 1 | 0 | 0 | 1 | 2 |
| 39 | 2013 | Manolakis | 2 | 1 | 2 | 0 | 2 | 1 | 8 |
Main meta-analysis results
An estimation of the association between TLR2 Arg677Trp, Arg753Gln and TLR4Asp299Gly, Thr399Ile polymorphisms and susceptibility to CD and UC is presented in Table 4. The corresponding forest plots are shown in Fig 2.
Table 4. Results of the meta-analysis of the relationship of TLR2 and TLR4 polymorphism with CD or UC risk.
| Study | Genetic model | OR | 95% CI | I2(%) | P value | No. of study | Egger |
|---|---|---|---|---|---|---|---|
| TLR2 677 vs CD | dominant model | 3.54 | 0.71–17.71 | 0.00 | 0.99 | 6 | / |
| additive model | 3.54 | 0.71–17.71 | 0.00 | 0.99 | 6 | / | |
| recessive model | 3.54 | 0.71–17.71 | 0.00 | 0.99 | 6 | / | |
| TLR2 677 vs UC | dominant model | 1.93 | 0.39–9.59 | 0.00 | 1.00 | 6 | / |
| additive model | 1.93 | 0.39–9.59 | 0.00 | 1.00 | 6 | / | |
| recessive model | 1.93 | 0.39–9.59 | 0.00 | 1.00 | 6 | / | |
| TLR2 753 vs CD | dominant model | 0.84 | 0.53–1.33 | 0.00 | 0.84 | 9 | / |
| allele model | 2.69 | 0.77–9.44 | 0.00 | 1.00 | 9 | / | |
| TLR2 753 vs UC | dominant model | 1.14 | 0.63–2.05 | 0.00 | 1.00 | 8 | / |
| allele model | 1.14 | 0.63–2.05 | 0.00 | 1.00 | 8 | / | |
| TLR4 299 vs CD | dominant model | 1.44 | 1.27–1.63 | 20.50 | 0.15 | 33 | 0.50 |
| additive model | 1.62 | 0.98–2.67 | 0.00 | 1.00 | 32 | 0.39 | |
| recessive model | 1.82 | 1.11–3.01 | 0.00 | 1.00 | 32 | 0.74 | |
| allele model | 1.40 | 1.24–1.57 | 25.00 | 0.10 | 33 | 0.49 | |
| TLR4 299 vs UC | dominant model | 1.50 | 1.28–1.76 | 44.90 | 0.01 | 26 | 0.82 |
| additive model | 2.37 | 1.29–4.35 | 0.00 | 1.00 | 26 | 0.97 | |
| recessive model | 2.25 | 1.22–4.12 | 0.00 | 1.00 | 26 | 0.91 | |
| allele model | 1.40 | 1.22–1.62 | 43.60 | 0.01 | 27 | 0.62 | |
| TLR4 399 vs CD | dominant model | 1.26 | 1.03–1.54 | 0.00 | 0.97 | 16 | 0.04 |
| additive model | 1.45 | 0.66–3.18 | 0.00 | 0.98 | 16 | 0.59 | |
| recessive model | 1.35 | 0.62–2.95 | 0.00 | 0.98 | 16 | 0.41 | |
| allele model | 1.21 | 1.01–1.44 | 0.00 | 0.95 | 17 | 0.10 | |
| TLR4 399 vs UC | dominant model | 1.41 | 1.09–1.82 | 0.00 | 0.61 | 13 | 0.56 |
| additive model | 1.89 | 0.70–5.13 | 0.00 | 1.00 | 13 | 0.74 | |
| recessive model | 1.84 | 0.68–5.00 | 0.00 | 1.00 | 13 | 0.65 | |
| allele model | 1.26 | 1.02–1.56 | 0.00 | 0.52 | 14 | 0.36 | |
| High quality studies | |||||||
| TLR4 299 vs CD | dominant model | 1.56 | 1.35–1.80 | 3.00 | 0.42 | 26 | 0.47 |
| additive model | 1.72 | 0.98–3.02 | 0.00 | 1.00 | 25 | 0.44 | |
| recessive | 1.94 | 1.10–3.40 | 0.00 | 1.00 | 25 | 0.60 | |
| allele model | 1.50 | 1.31–1.72 | 12.20 | 0.29 | 26 | 0.47 | |
| TLR4 299 vs UC | dominant model | 1.55 | 1.28–1.87 | 49.10 | 0.01 | 20 | 0.66 |
| additive model | 2.49 | 1.25–4.95 | 0.00 | 0.99 | 20 | 0.97 | |
| recessive | 2.35 | 1.18–4.67 | 0.00 | 0.99 | 20 | 0.88 | |
| allele model | 1.51 | 1.16–1.98 | 47.10 | 0.01 | 21 | 0.46 | |
| TLR4 399 vs CD | dominant model | 1.16 | 0.92–1.46 | 0.00 | 0.98 | 13 | 0.03 |
| additive model | 1.50 | 0.65–3.46 | 0.00 | 0.92 | 13 | 0.53 | |
| recessive | 1.40 | 0.61–3.21 | 0.00 | 0.92 | 13 | 0.37 | |
| allele model | 1.13 | 0.93–1.37 | 0.00 | 0.96 | 14 | 0.14 | |
| TLR4 399 vs UC | dominant model | 1.32 | 1.00–1.75 | 0.00 | 0.78 | 11 | 0.38 |
| additive model | 1.65 | 0.55–4.90 | 0.00 | 1.00 | 11 | 0.43 | |
| recessive | 1.62 | 0.54–4.81 | 0.00 | 1.00 | 11 | 0.41 | |
| allele model | 1.19 | 0.95–1.49 | 0.00 | 0.76 | 12 | 0.24 | |
| Group by ethinicity | |||||||
| TLR2 753 vs CD | |||||||
| Asia | dominant model | 3.54 | 0.71–17.71 | 0.00 | 0.99 | 6 | / |
| allele model | 3.54 | 0.71–17.71 | 0.00 | 0.99 | 6 | / | |
| Caucasian | dominant model | 0.73 | 0.36–1.47 | 0.00 | 0.48 | 2 | / |
| allele model | 1.05 | 0.07–16.82 | 0.00 | 0.99 | 2 | / | |
| TLR2 753 vs UC | |||||||
| Asian | dominant model | 1.93 | 0.39–9.59 | 0.00 | 1.00 | 6 | / |
| allele model | 1.93 | 0.39–9.59 | 0.00 | 1.00 | 6 | / | |
| Caucasian | dominant model | 1.05 | 0.56–1.97 | 0.00 | 0.77 | 2 | / |
| allele model | 1.50 | 0.09–24.07 | 0.00 | 0.87 | 2 | / | |
| TLR4 299 vs CD | |||||||
| Asian | dominant model | 2.17 | 0.52–9.14 | 0.00 | 0.92 | 7 | / |
| additive model | 2.17 | 0.52–9.14 | 0.00 | 0.92 | 7 | / | |
| recessive | 2.17 | 0.52–9.14 | 0.92 | 7 | / | ||
| allele model | 1.87 | 0.45–7.74 | 0.00 | 0.80 | 7 | / | |
| Caucasian | dominant model | 1.45 | 1.28–1.64 | 37.90 | 0.04 | 23 | / |
| additive model | 1.72 | 1.00–2.96 | 0.00 | 1.00 | 23 | / | |
| recessive | 1.74 | 1.01–2.99 | 0.00 | 1.00 | 23 | / | |
| allele model | 1.43 | 1.26–1.62 | 42.50 | 0.02 | 22 | / | |
| Others | dominant model | 0.99 | 0.46–2.10 | 0.00 | 0.46 | 3 | / |
| additive model | 0.98 | 0.10–9.61 | 0.00 | 1.00 | 3 | / | |
| recessive | 2.17 | 0.22–21.12 | 0.00 | 0.57 | 3 | / | |
| allele model | 1.14 | 0.78–1.66 | 0.00 | 0.64 | 4 | / | |
| TLR4 299 vs UC | |||||||
| Asian | dominant model | 1.86 | 0.46–7.46 | 0.00 | 1.00 | 8 | / |
| additive model | 1.86 | 0.46–7.46 | 0.00 | 1.00 | 8 | / | |
| recessive | 1.86 | 0.46–7.46 | 0.00 | 1.00 | 8 | / | |
| allele model | 1.86 | 0.46–7.46 | 0.00 | 1.00 | 8 | / | |
| Caucasian | dominant model | 1.51 | 1.01–2.07 | 67.90 | 0.00 | 15 | 0.93 |
| additive model | 2.14 | 1.04–4.39 | 0.00 | 0.98 | 15 | 0.86 | |
| recessive | 1.99 | 0.97–4.08 | 0.00 | 0.99 | 15 | 0.82 | |
| allele model | 1.48 | 1.11–1.96 | 64.10 | 0.00 | 15 | 0.94 | |
| Others | dominant model | 1.73 | 1.04–2.88 | 0.00 | 0.55 | 3 | / |
| additive model | 8.10 | 1.17–55.93 | 0.80 | 0.37 | 3 | / | |
| recessive | 7.74 | 1.12–53.40 | 4.90 | 0.35 | 3 | / | |
| allele model | 1.20 | 0.88–1.64 | 47.60 | 0.13 | 4 | / | |
| TLR4 399 vs CD | |||||||
| Asian | dominant model | 3.54 | 0.71–17.71 | 0.00 | 1.00 | 6 | / |
| additive model | 3.54 | 0.71–17.71 | 0.00 | 1.00 | 6 | / | |
| recessive | 3.54 | 0.71–17.71 | 0.00 | 1.00 | 6 | / | |
| allele model | 3.57 | 0.72–17.75 | 0.00 | 1.00 | 6 | / | |
| Caucasian | dominant model | 1.20 | 0.97–1.49 | 0.00 | 0.78 | 8 | / |
| additive model | 0.98 | 0.30–3.21 | 0.00 | 0.78 | 8 | / | |
| recessive | 0.96 | 0.29–3.14 | 0.00 | 0.78 | 8 | / | |
| allele model | 1.19 | 0.96–1.46 | 0.00 | 0.62 | 8 | / | |
| Others | dominant model | 1.58 | 0.85–2.93 | 0.00 | 0.91 | 2 | / |
| additive model | 1.28 | 0.32–5.11 | 0.00 | 0.87 | 2 | / | |
| recessive | 1.07 | 0.28–4.16 | 0.00 | 0.92 | 2 | / | |
| allele model | 1.20 | 0.85–1.69 | 0.00 | 0.70 | 3 | / | |
| TLR4 399 vs UC | |||||||
| Asian | dominant model | 1.93 | 0.39–9.59 | 0.00 | 1.00 | 6 | / |
| additive model | 1.93 | 0.39–9.59 | 0.00 | 1.00 | 6 | / | |
| recessive | 1.93 | 0.39–9.59 | 0.00 | 1.00 | 6 | / | |
| allele model | 1.93 | 0.39–9.59 | 0.00 | 1.00 | 7 | / | |
| Caucasian | dominant model | 1.42 | 1.09–1.85 | 43.70 | 0.11 | 6 | / |
| additive model | 1.80 | 0.47–6.92 | 0.00 | 0.85 | 6 | / | |
| recessive | 1.71 | 0.44–6.59 | 0.00 | 0.84 | 6 | / | |
| allele model | 1.42 | 1.11–1.83 | 38.10 | 0.15 | 6 | / | |
| Others | allele model | 0.91 | 0.62–1.35 | 0.00 | 0.67 | 2 | / |
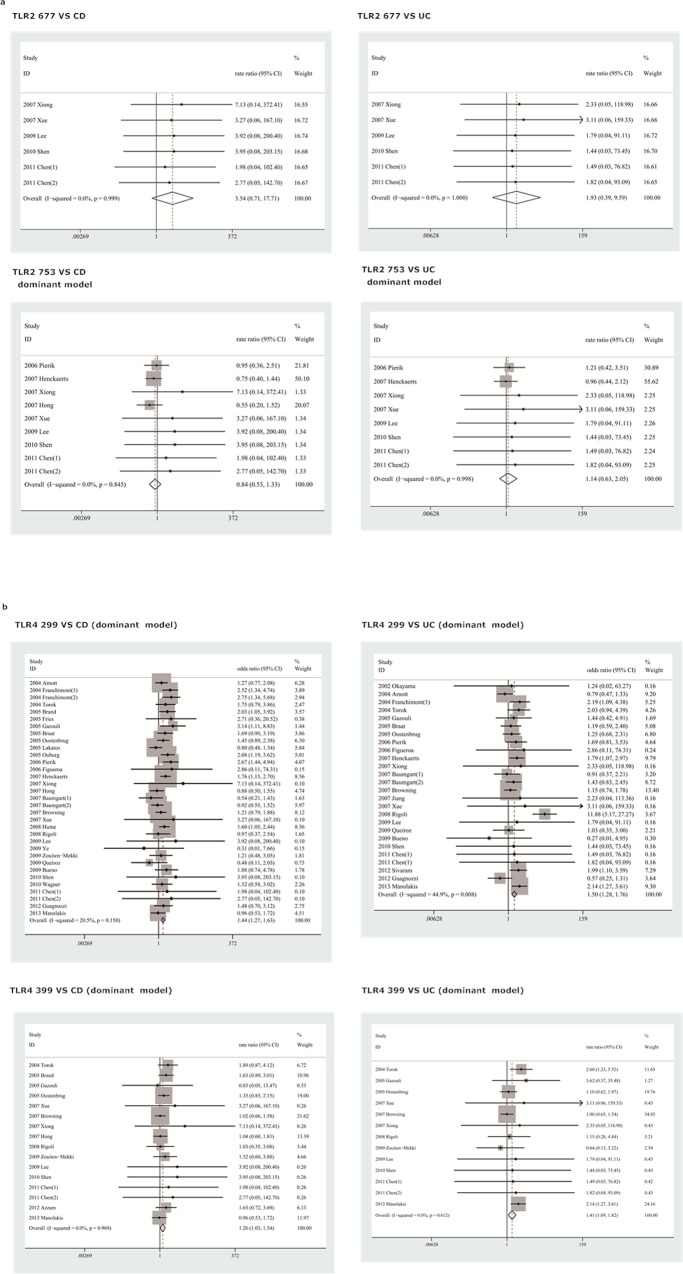
Fig 2. Forest plot showing the association between TLR2 and TLR4 polymorphisms and CD and UC risk.
Squares represent the effect size for the odds ratios of CD or UC risk among subjects. Error bars represent 95% confidence intervals (CI). Diamonds represent pooled estimates within each analysis. (a) TLR2 polymorphisms and CD/UC in dominant model; (b) TLR4 polymorphisms and CD/UC in dominant model.
TLR2 Arg677Trp
All studies evaluating the TLR2 Arg677Trp polymorphism were conducted among Asians. No variant allele A carrier or mutant homozygous was found in either the IBD patients or control population in the included studies. In addition, TLR2 Arg677Trp polymorphism did not show any association with CD (OR = 3.54, 95%CI = 0.71–17.71, P = 0.99) or UC (OR = 1.93, 95%CI = 0.39–9.60, P = 1.00) in Asian populations.
TLR2 Arg753Gln
Due to the rarity of the TLR2 Arg753Gln mutant homozygous genotype in the included studies, the data could only be pooled in the allele and dominant models. In the allele model, no association was found between the A allele and CD(A vs G: OR = 2.69, 95%CI = 0.77–9.44, P = 1.00)or UC(A vs G: OR = 1.81, 95%CI = 0.45–7.26, P = 1.00)susceptibility. Similarly, the AA genotype was not associated with risk of CD (AA vs GG: OR = 0.84, 95%CI = 0.53–1.82, P = 1.00) or UC (AA vs GG: OR = 1.14, 95%CI = 0.63–2.05, P = 1.00).
Subgroup analyses based on ethnic were performed to assess CD and UC susceptibility in Asians and Caucasians. No significant association was identified in both Asians(for CD, A vs G: OR = 3.54, 95%CI = 0.71–17.71, P = 0.99; AA vs GG: OR = 3.54, 95%CI = 0.71–17.71, P = 0.99; for UC, A vs G: OR = 1.93, 95%CI = 0.39–9.59, P = 1.00; AA vs GG: OR = 1.93, 95%CI = 0.39–9.59, P = 1.00) and Caucasians (G vs. A: OR = 1.05, 95% CI: 0.07–16.82, P = 0.99; AA vs. GG: OR = 0.73, 95% CI: 0.36–1.47, P = 0.48).
TLR4 Asp299Gly
A significantly increased susceptibility was found between TLR4 D299G and CD in the allele model (A vs G: OR = 1.40, 95%CI = 1.24–1.57, P = 0.1), dominant model (AA+GA vs GG: OR = 1.44, 95%CI = 1.27–1.63, P = 0.15), recessive model (AA vs GA+GG: OR = 1.82, 95%CI = 1.11–3.01, P = 1.00) and additive model (AA vs GG: OR = 1.62, 95%CI = 0.98–2.67, P = 1). Similar results were also found between TLR4 D299G and UC in the allele model (A vs G: OR = 1.40, 95%CI = 1.22–1.62, P = 0.01), dominant model (AA+GA vs GG: OR = 1.50, 95%CI = 1.28–1.76, P = 0.01), recessive model (AA vs GA+GG: OR = 2.25, 95%CI = 1.22–4.12, P = 1.00) and additive model (AA vs GG: OR = 2.37, 95%CI = 1.29–4.35, P = 1.00).
Stratified analyses by study quality and ethnicity were conducted to further explore the actual effect of TLR4 D299G polymorphism on the risk of CD and UC. Similar results were obtained with subgroup analyses by study quality. When ethnicity was restricted to Asians, no TLR4 D299G polymorphism was found in patients with CD or UC. However, when only studies with Caucasians were considered, a significant association with CD was obtained in all contrast models (A vs G: OR = 1.43, 95%CI = 1.26–1.62, P = 0.02; AA vs GG: OR = 1.72, 95%CI = 1.00–2.99, P = 1; AA+GA vs GG: OR = 1.45, 95%CI = 1.28–1.64, P = 0.04; AA vs GA+GG: OR = 1.74, 95%CI = 1.01–2.99, P = 1.00). Furthermore, significant associations with UC were found in the allele model (A vs G: OR = 1.48, 95%CI = 1.11–1.96, P<0.01), dominant model (AA+GA vs GG: OR = 1.51, 95%CI = 1.10–2.07, P = P<0.01) and additive model (AA vs GG: OR = 2.14, 95%CI = 1.04–4.39, P = 0.98). However, in the recessive model, only a marginal association (AA vs GA+GG: OR = 1.99, 95%CI = 0.97–4.08, P = 0.99) was found. Similar results were found in UC in Caucasians (A vs G: OR = 1.48, 95%CI = 1.11–1.96, P<0.01; AA vs GG: OR = 2.14, 95%CI = 1.04–4.39, P = 0.98; AA+GA vs GG: OR = 1.51, 95%CI = 1.10–2.07, P<0.01; AA vs GA+GG: OR = 1.99, 95%CI = 0.97–4.08, P = 0.99).
TLR4 Thr399Ile
The pooled results of all studies suggested that TLR4 T399I polymorphism was significantly associated with CD susceptibility in the dominant (TT+CT vs CC: OR = 1.26, 95%CI = 1.03–1.54, P = 0.97) and allele (T vs C: OR = 1.21, 95%CI = 1.01–1.44, P = 0.95) models, whereas no significant association was found in the recessive (TT vs CT+CC: OR = 1.35, 95%CI = 0.62–2.95, P = 0.98) and additive (TT vs CC: OR = 1.45, 95%CI = 0.66–3.18, P = 0.98) models. Similarly, the CC genotype significantly increased UC susceptibility in the dominant model (TT+CT vs CC: OR = 1.41, 95%CI = 1.09–1.82, P = 0.61) and the C allele was found associated with a higher UC susceptibility in the allele model (T vs C: OR = 1.26, 95%CI = 1.02–1.56, P = 0.52); no significant association was found in the recessive (TT vs CT+CC: OR = 1.84, 95%CI = 0.68–5.00, P = 1.00) and additive (TT vs CC: OR = 1.89, 95%CI = 0.70–5.13, P = 1.00) models.
Meta-analyses of high quality studies showed that TLR4 T399I polymorphism was not associated with the risk of CD in any genetic model and only the dominant model showed a significant association with the risk of UC.
Stratified by ethnicity, neither Asians nor Caucasians were associated with CD susceptibility in all four genetic models. With respect to UC, a significant association was found for Caucasians in dominant (TT+CT vs CC: OR = 1.42, 95%CI = 1.09–1.85, P = 0.11) and allele (T vs C: OR = 1.42, 95%CI = 1.11–1.83, P = 0.15) models, but not in recessive (TT vs CT+CC: OR = 1.71, 95%CI = 0.44–6.59, P = 0.84) and additive (TT vs CC: OR = 1.80, 95%CI = 0.47–6.92, P = 0.85) models.
Heterogeneity analysis
For the TLR4 299 polymorphism versus UC, a statistically significant heterogeneity among studies was found in the dominant and allele models with the I2 values of heterogeneity> and P values< 0.10. To further investigate the heterogeneity in studies assessing TLR4 299 polymorphism in Caucasians, Galbraith plots were generated to identify the outliers which might contribute to this observation. Our results showed that the study published in 2008 by Rigoli was an outlier in both dominant and allele models (Fig 3). All I2 and P values decreased overtly after excluding 2008 Rigoli in dominant and allele models with Caucasians. The heterogeneity of the remainingmeta-analyses was acceptable.
Fig 3. Galbraith plot of the association between TLR4 299 polymorphism and UC risk in Caucasians.
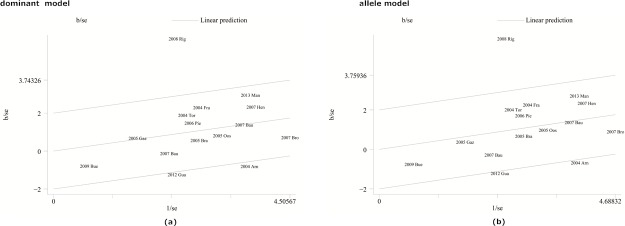
Each figure represents a unique article in this meta-analysis. The figures outside the three lines were spotted as the outlier and the possible source of heterogeneity in the analysis pooled from the total available numbers. (a) Galbraith plot results of TLR4 299 polymorphisms and UC risk in the dominant model; (b) Galbraith plot results of TLR4 299 polymorphisms and UC risk in the allele model.
Sensitivity analysis
Sensitivity analysis was performed by sequentially excluding individual studies. For analyses pooling more than three individual studies, the summary ORs were not influenced by excluding any single study (data not shown), indicating that our results were statistically robust.
Publication bias
There was no evidence of obvious asymmetry in the funnel plots. The Egger’s test was performed to access publication bias in the articles included in this meta-analysis, when the number of included studies was greater than 10. All p values obtained in the Egger’s test were more than 0.1 except for the dominant model of TLR4 399 in CD for both overall analysis and the assessment including only high quality studies. There were five unreported studies according to the Duval’s trim and full method (Fig 4). After possibly unpublished studies were imputed, the pooled OR and 95%CI were slightly shifted toward null (Overall study: OR = 1.24, 95%CI = 1.02–1.51; High quality study: OR = 1.13, 95%CI = 0.91–1.42). However, no change was observed in the meta-analysis results.
Fig 4. Funnel plots for studies evaluating TLR4 399 polymorphisms and risk of CD included in the meta-analysis.
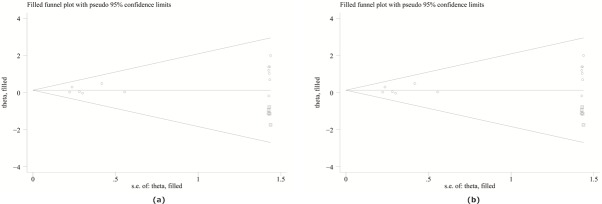
(a) Trim and fill data for all studies on TLR4 299 polymorphisms and UC risk in the dominant model; (b) Trim and fill data for high quality studies on TLR4 299 polymorphisms and UC risk in the dominant model. Imputed data (squares) are imaginary values to compensate for non-symmetric funnel plot.
Discussion
Genome-wide association studies(GWAS) has improved our knowledge of many common variants and molecular pathways leading to IBD[59]. Recently, a meta-analyses of GWAS conducted by Jostins et al. have identified 163 loci that are significantly associated with IBD[60]. Such discoveries are limited to studies in North America, Oceania and Europe. Yang, S.K., et al. conducted a GWAS and two validation studies in the Korean population and revealed three new susceptibility loci for CD[61]. Till now, most GWAS were conducted in Caucasians with limited studies in other populations. Despite the success of GWAS in identifying IBD susceptibility loci, it explains only a minority of(<25%) the variance in IBD risk[62]-. The advent of GWAS also prompt mechanistic research aimed at exploring the complex interplay between genes, immune networks, and microbiome. Functional studies to assess the in vivo impact of the genetic variants involved in IBD also emerges. Recently, Coelho, T et al. performed a unique systematic review of literature with mechanistic studies in assessing the functional impact of the a selected panel of gene variants implicated in IBD through GWAS and other genetic studies. However, they limited to only 71 genes and TLR is not included in the study. They did not make a meta-analysis due to the lack of functional studies and more functional studies is needed[63].
Both TLR4 and TLR2 was not detected to be associated with IBD in the previous GWAS. Not surprisingly, uncommon genetic variation which may contribute significantly toward the heritability of IBD may not be captured by GWAS[64]. Population-based studies have also provided compelling evidence for genetic factors contribute to the IBD for these rarer variants are more likely to be population specific. We performed a meta-analysis of population based case-control studis for TLR2 and TLR4 polymorphism and IBD susceptibility.
All studies assessing the TLR2 Arg677Trp polymorphism and IBD were carried out in Asia, and no TLR2 Arg677Trp polymorphism was found in Asians, as described above. This is the first meta-analysis evaluating TLR2 Arg677Trp polymorphism and IBD.
In the case of TLR2 Arg753Gln, we only pooled data in the allele and dominant models due to the rarity of the TLR2 Arg753Gln mutant homozygous genotype in the included studies. All studies were of high quality. Our meta-analysis showed no association between the Arg753Gln polymorphism and UC or CD. We then restricted to ethnicity-specific data for subgroup analyses, and found that the Arg753Gln polymorphism was not associated with UC or CD susceptibility in Asians or Caucasians. These findings indicated that Arg753Gln with the mutant allele does not significantly increase IBD susceptibility.To our knowledge, this is the first meta-analysis evaluating TLR2 Arg753Gln polymorphism and IBD.
Interestingly, this meta-analysis revealed a modest association between the TLR4 Asp299Gly polymorphism and IBD (CD and UC). This result was very well supported: sensitivity analyses excluding low quality studies did not significantly change the magnitude of the gene effect or genetic model. Next, we restricted to race-specific assessments to perform a subgroup analysis. Consistent with meta-studies reported by Hume and Shen[44, 52], our study suggested that TLR4 Asp299Gly polymorphism might be associated with UC and CD susceptibility in Caucasians. Browning et al found no relationship between TLR4 Asp299Gly and UC in Caucasians, which contradicts our findings[42]. However, only 12 articles were included in their study. Some differences exist between our study and Hume and Shen’s reports. First, the included studies were updated. Then, quality assessment and publication bias assessment of the included studies were performed. TLR4 Asp299Gly polymorphism was not associated with UC or CD in Asians in our study. Ng et al. has made a systematic review and meta-analysis of genetics of IBD in Asia and they also found no association of TLR4 Asp299Gly and risk of CD[65].However, they only included two studies (Xiong 2006 and Ye 2009) conducted in Chinese and Korean patients. We included 7 more studies with 1 conducted in Japanese, 1 in Korean and 5 in Chinese. Our study might further confirm that population differences, such as genetic heterogeneity, play a vital role in IBD susceptibility.
The current study indicates that the TLR4 399T allele might increase the risk of UC and CD. It is plausible that TLR4 Thr399Ile’s T allele affect TLR4 transcription and expression, further impacting TLR4 protein function. Further studies should focus on how the variant might impact gene expression and function. Subgroup analysis of high quality studies showed no association between TLR4 Thr 399Ile polymorphism and CD, and marginal association between TLR4 Thr399Ile polymorphism and UC. In the meta-analysis performed by Shen et al[52], TLR4 399 Ile polymorphism is associated with both UC and CD susceptibility in Caucasians. Our race-specific subgroup analyses found that the TLR4 Thr 399Ile polymorphism was associated with UC susceptibility in Caucasians while no association between TLR4 Thr399Ile polymorphism and CD susceptibility was observed. For Asians, there was no association observed in any genetic model for TLR4 Thr399Ile and CD or UC susceptibility.
The overall meta-analysis has little heterogeneity. When ethnicity sub-stratification was performed, heterogeneity was decreased or even removed among Asians while significant heterogeneity existed in study for TLR4 Asp299Gly polymorphism and UC in the dominant and allele models in Caucasions. Galbraith plot was used to identify heterogeneous records. One heterogeneous article for TLR Asp299Gly vs UC was detected by the Galbraith plot[43]. The potential bias of the article might result from the elderly population assessed or unknown reasons. After omitting this article, heterogeneity decreased substantially and the association was still significant. The Egger’s test suggested that there was no significant publication bias except for the meta-analysis of TLR4 Thr399Ile and CD in the dominant model. This publication bias was then corrected using the Duval’s trim and fill method. Publication bias is caused by the tendency of researchers and editors to publish reports with positive results, while those showing inconclusive results are likely not considered for publication. Several original studies has controls depart from the HWE which may cause bias in estimates of genetic effects results. Currently, there is no consensus on whether to pool studies that are not in HWE for meta-analysis of genetic association studies. We performed a quality assessment of the studies based on the HWE as well as other criteria such as representativeness of cases and controls. Sensitive analysis quality were also performed by excluding low quality studies in our study which is the merit of our study.
A few limitations of this study need to be mentioned. First, a variety of confounding factors may be associated with increased damage to IBD, such as gender, age, smoking status, clinical phenotype, et al. Unfortunately, we were unable to obtain sufficient data to perform appropriate stratified analyses due to the limited information in the included studies. In addition, the number of cases and controls included was relatively small and most were included in Asians as far as studies on TLR2 Arg677Trp and Arg753Gln are concerned. Thus, the association between in different populations need to be confirmed by further studies. Finally, we could not identify the gene-gene and gene-environment interactions in this study. In conclusion, TLR2 Arg677Trp and Arg753Gln is not associated with the risk of UC or CD. The TLR4 Asp299Gly and Thr399Thr genotypes seem to be more susceptible to UC and CD in Caucasian populations but not in Asians. This finding needs to be further confirmed in future well-designed studies including different ethnicities.
Supporting Information
(DOC)
(DOCX)
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54 e42; quiz e30. Epub 2011/10/18. 10.1053/j.gastro.2011.10.001 . [DOI] [PubMed] [Google Scholar]
- 2. Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nature reviews Immunology. 2008;8(6):458–66. Epub 2008/05/27. 10.1038/nri2340 . [DOI] [PubMed] [Google Scholar]
- 3. Weterman IT, Pena AS. Familial incidence of Crohn's disease in The Netherlands and a review of the literature. Gastroenterology. 1984;86(3):449–52. Epub 1984/03/01. . [PubMed] [Google Scholar]
- 4. Rodriguez-Bores L, Fonseca GC, Villeda MA, Yamamoto-Furusho JK. Novel genetic markers in inflammatory bowel disease. World journal of gastroenterology: WJG. 2007;13(42):5560–70. Epub 2007/10/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seitz M. Toll-like receptors: sensors of the innate immune system. Allergy. 2003;58(12):1247–9. Epub 2003/11/18. . [DOI] [PubMed] [Google Scholar]
- 6. Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(2):588–93. Epub 1998/01/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, et al. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11(4):443–51. Epub 1999/11/05. . [DOI] [PubMed] [Google Scholar]
- 8. Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406(6797):782–7. Epub 2000/08/30. 10.1038/35021228 . [DOI] [PubMed] [Google Scholar]
- 9. Singh JC, Cruickshank SM, Newton DJ, Wakenshaw L, Graham A, Lan J, et al. Toll-like receptor-mediated responses of primary intestinal epithelial cells during the development of colitis. American journal of physiology Gastrointestinal and liver physiology. 2005;288(3):G514–24. Epub 2004/10/23. 10.1152/ajpgi.00377.2004 . [DOI] [PubMed] [Google Scholar]
- 10. Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infection and immunity. 2000;68(12):7010–7. Epub 2000/11/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Erridge C, Stewart J, Poxton IR. Monocytes heterozygous for the Asp299Gly and Thr399Ile mutations in the Toll-like receptor 4 gene show no deficit in lipopolysaccharide signalling. The Journal of experimental medicine. 2003;197(12):1787–91. Epub 2003/06/11. 10.1084/jem.20022078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hausmann M, Kiessling S, Mestermann S, Webb G, Spottl T, Andus T, et al. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122(7):1987–2000. Epub 2002/06/11. . [DOI] [PubMed] [Google Scholar]
- 13. Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin—rapid analysis of dense genetic maps using sparse gene flow trees. Nature genetics. 2002;30(1):97–101. Epub 2001/12/04. 10.1038/ng786 . [DOI] [PubMed] [Google Scholar]
- 14. Thakkinstian A, D'Este C, Eisman J, Nguyen T, Attia J. Meta-analysis of molecular association studies: vitamin D receptor gene polymorphisms and BMD as a case study. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2004;19(3):419–28. Epub 2004/03/26. 10.1359/jbmr.0301265 . [DOI] [PubMed] [Google Scholar]
- 15. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed). 2003;327(7414):557–60. Epub 2003/09/06. 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brittain EH, Fay MP, Follmann DA. A valid formulation of the analysis of noninferiority trials under random effects meta-analysis. Biostatistics (Oxford, England). 2012;13(4):637–49. Epub 2012/04/03. 10.1093/biostatistics/kxs006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chootrakool H, Shi JQ, Yue R. Meta-analysis and sensitivity analysis for multi-arm trials with selection bias. Statistics in medicine. 2011;30(11):1183–98. Epub 2011/05/04. 10.1002/sim.4143 . [DOI] [PubMed] [Google Scholar]
- 18. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62(1):10–29. Epub 2012/01/13. 10.3322/caac.20138 . [DOI] [PubMed] [Google Scholar]
- 19. Munafo MR, Clark TG, Flint J. Assessing publication bias in genetic association studies: evidence from a recent meta-analysis. Psychiatry research. 2004;129(1):39–44. Epub 2004/12/02. 10.1016/j.psychres.2004.06.011 . [DOI] [PubMed] [Google Scholar]
- 20. Song F, Gilbody S. Bias in meta-analysis detected by a simple, graphical test. Increase in studies of publication bias coincided with increasing use of meta-analysis. BMJ (Clinical research ed). 1998;316(7129):471 Epub 1998/03/11. [PMC free article] [PubMed] [Google Scholar]
- 21. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed). 1997;315(7109):629–34. Epub 1997/10/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63. Epub 2000/07/06. . [DOI] [PubMed] [Google Scholar]
- 23. Okayama N, Fujimura K, Suehiro Y, Hamanaka Y, Fujiwara M, Matsubara T, et al. Simple genotype analysis of the Asp299Gly polymorphism of the Toll-like receptor-4 gene that is associated with lipopolysaccharide hyporesponsiveness. Journal of clinical laboratory analysis. 2002;16(1):56–8. Epub 2002/02/09. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arnott ID, Nimmo ER, Drummond HE, Fennell J, Smith BR, MacKinlay E, et al. NOD2/CARD15, TLR4 and CD14 mutations in Scottish and Irish Crohn's disease patients: evidence for genetic heterogeneity within Europe? Genes and immunity. 2004;5(5):417–25. Epub 2004/06/11. 10.1038/sj.gene.6364111 . [DOI] [PubMed] [Google Scholar]
- 25. Franchimont D, Vermeire S, El Housni H, Pierik M, Van Steen K, Gustot T, et al. Deficient host-bacteria interactions in inflammatory bowel disease? The toll-like receptor (TLR)-4 Asp299gly polymorphism is associated with Crohn's disease and ulcerative colitis. Gut. 2004;53(7):987–92. Epub 2004/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brand S, Staudinger T, Schnitzler F, Pfennig S, Hofbauer K, Dambacher J, et al. The role of Toll-like receptor 4 Asp299Gly and Thr399Ile polymorphisms and CARD15/NOD2 mutations in the susceptibility and phenotype of Crohn's disease. Inflammatory bowel diseases. 2005;11(7):645–52. Epub 2005/06/24. . [DOI] [PubMed] [Google Scholar]
- 27. Fries W, Renda MC, Lo Presti MA, Raso A, Orlando A, Oliva L, et al. Intestinal permeability and genetic determinants in patients, first-degree relatives, and controls in a high-incidence area of Crohn's disease in Southern Italy. The American journal of gastroenterology. 2005;100(12):2730–6. Epub 2006/01/06. 10.1111/j.1572-0241.2005.00325.x . [DOI] [PubMed] [Google Scholar]
- 28. Gazouli M, Mantzaris G, Kotsinas A, Zacharatos P, Papalambros E, Archimandritis A, et al. Association between polymorphisms in the Toll-like receptor 4, CD14, and CARD15/NOD2 and inflammatory bowel disease in the Greek population. World journal of gastroenterology: WJG. 2005;11(5):681–5. Epub 2005/01/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ouburg S, Mallant-Hent R, Crusius JB, van Bodegraven AA, Mulder CJ, Linskens R, et al. The toll-like receptor 4 (TLR4) Asp299Gly polymorphism is associated with colonic localisation of Crohn's disease without a major role for the Saccharomyces cerevisiae mannan-LBP-CD14-TLR4 pathway. Gut. 2005;54(3):439–40. Epub 2005/02/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Torok HP, Glas J, Tonenchi L, Mussack T, Folwaczny C. Polymorphisms of the lipopolysaccharide-signaling complex in inflammatory bowel disease: association of a mutation in the Toll-like receptor 4 gene with ulcerative colitis. Clinical immunology (Orlando, Fla). 2004;112(1):85–91. Epub 2004/06/23. 10.1016/j.clim.2004.03.002 . [DOI] [PubMed] [Google Scholar]
- 31. Braat H, Stokkers P, Hommes T, Cohn D, Vogels E, Pronk I, et al. Consequence of functional Nod2 and Tlr4 mutations on gene transcription in Crohn's disease patients. Journal of molecular medicine (Berlin, Germany). 2005;83(8):601–9. Epub 2005/07/13. 10.1007/s00109-005-0685-x. . [DOI] [PubMed] [Google Scholar]
- 32. Oostenbrug LE, Drenth JP, de Jong DJ, Nolte IM, Oosterom E, van Dullemen HM, et al. Association between Toll-like receptor 4 and inflammatory bowel disease. Inflammatory bowel diseases. 2005;11(6):567–75. Epub 2005/05/21. . [DOI] [PubMed] [Google Scholar]
- 33. Lakatos PL, Lakatos L, Szalay F, Willheim-Polli C, Osterreicher C, Tulassay Z, et al. Toll-like receptor 4 and NOD2/CARD15 mutations in Hungarian patients with Crohn's disease: phenotype-genotype correlations. World journal of gastroenterology: WJG. 2005;11(10):1489–95. Epub 2005/03/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Figueroa C, Peralta A, Herrera L, Castro P, Gutierrez A, Valenzuela J, et al. NOD2/CARD15 and Toll-like 4 receptor gene polymorphism in Chilean patients with inflammatory bowel disease. European cytokine network. 2006;17(2):125–30. Epub 2006/07/15. . [PubMed] [Google Scholar]
- 35. Pierik M, Joossens S, Van Steen K, Van Schuerbeek N, Vlietinck R, Rutgeerts P, et al. Toll-like receptor-1, -2, and -6 polymorphisms influence disease extension in inflammatory bowel diseases. Inflammatory bowel diseases. 2006;12(1):1–8. Epub 2005/12/24. . [DOI] [PubMed] [Google Scholar]
- 36. Xiong L- F, Xia B, Jiang L, Guo Q- S, Sun Z- Q. No association of TLR4 gene Asp299Gly, TLR2 gene Arg753Glu and Arg677Trp polymorphisms with inflammatiory bowel disease in Chinese Han Population of Hubei Province(Chinese). World Chinese Journal of Digestology. 2006;14(2):212–25. [Google Scholar]
- 37. Yi Jiang X- YM, Ding-Liang Z, Wen-Xing W, Xuan-Ping X. The association between Toll-like receptor-4 gene Asp299Gly polymorphism and ulcerativecolitis and colorectal adenocarcinoma(Chinese). Chin J Intern. 2006;45(7):585–6. [Google Scholar]
- 38. Hui-Ping Xue P-HN, Wu J-M, Tong J-F. Gene polymorphisms of Toll like receptors in inflammatory bowel disease and their distributionin different populations(Chinese). Journal of Shanghai Jiaotong University(Medical Science). 2007;27(10):1226–31. [Google Scholar]
- 39. Henckaerts L, Pierik M, Joossens M, Ferrante M, Rutgeerts P, Vermeire S. Mutations in pattern recognition receptor genes modulate seroreactivity to microbial antigens in patients with inflammatory bowel disease. Gut. 2007;56(11):1536–42. Epub 2007/06/28. 10.1136/gut.2007.125468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hong J, Leung E, Fraser AG, Merriman TR, Vishnu P, Krissansen GW. TLR2, TLR4 and TLR9 polymorphisms and Crohn's disease in a New Zealand Caucasian cohort. Journal of gastroenterology and hepatology. 2007;22(11):1760–6. Epub 2007/10/05. 10.1111/j.1440-1746.2006.04727.x . [DOI] [PubMed] [Google Scholar]
- 41. Baumgart DC, Buning C, Geerdts L, Schmidt HH, Genschel J, Fiedler T, et al. The c.1-260C>T promoter variant of CD14 but not the c.896A>G (p.D299G) variant of toll-like receptor 4 (TLR4) genes is associated with inflammatory bowel disease. Digestion. 2007;76(3–4):196–202. Epub 2008/01/05. 10.1159/000112646 . [DOI] [PubMed] [Google Scholar]
- 42. Browning BL, Huebner C, Petermann I, Gearry RB, Barclay ML, Shelling AN, et al. Has toll-like receptor 4 been prematurely dismissed as an inflammatory bowel disease gene? Association study combined with meta-analysis shows strong evidence for association. The American journal of gastroenterology. 2007;102(11):2504–12. Epub 2007/09/14. 10.1111/j.1572-0241.2007.01463.x . [DOI] [PubMed] [Google Scholar]
- 43. Rigoli L, Romano C, Caruso RA, Lo Presti MA, Di Bella C, Procopio V, et al. Clinical significance of NOD2/CARD15 and Toll-like receptor 4 gene single nucleotide polymorphisms in inflammatory bowel disease. World journal of gastroenterology: WJG. 2008;14(28):4454–61. Epub 2008/08/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hume GE, Fowler EV, Doecke J, Simms LA, Huang N, Palmieri O, et al. Novel NOD2 haplotype strengthens the association between TLR4 Asp299gly and Crohn's disease in an Australian population. Inflammatory bowel diseases. 2008;14(5):585–90. Epub 2008/01/24. 10.1002/ibd.20362 . [DOI] [PubMed] [Google Scholar]
- 45. Akin H, Ozdemir FT, Atug O, Tahan G, Eren F, Baca B, et al. Association between Toll-like 4, cd14 polymorphisms and inflammatory bowel disease in Turkish population. Gastroenterology. 2008;134(4):A465–A. . [Google Scholar]
- 46. Lappalainen M, Halme L, Turunen U, Saavalainen P, Einarsdottir E, Farkkila M, et al. Association of IL23R, TNFRSF1A, and HLA-DRB1*0103 allele variants with inflammatory bowel disease phenotypes in the Finnish population. Inflammatory bowel diseases. 2008;14(8):1118–24. Epub 2008/03/15. 10.1002/ibd.20431 . [DOI] [PubMed] [Google Scholar]
- 47. Ye BD, Yang SK, Song K, Yang DH, Yoon SM, Kim KJ, et al. [Association of Toll-like receptor gene with Crohn's disease in Koreans]. The Korean journal of gastroenterology = Taehan Sohwagi Hakhoe chi. 2009;54(6):377–83. Epub 2009/12/23. . [DOI] [PubMed] [Google Scholar]
- 48. Zouiten-Mekki L, Kharrat M, Karoui S, Serghimi M, Fekih M, Matri S, et al. Tolllike receptor 4 (TLR4) polymorphisms in Tunisian patients with Crohn's disease: genotype-phenotype correlation. BMC gastroenterology. 2009;9:62 Epub 2009/08/12. 10.1186/1471-230x-9-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Queiroz DM, Oliveira AG, Saraiva IE, Rocha GA, Rocha AM, das Gracas Pimenta Sanna M, et al. Immune response and gene polymorphism profiles in Crohn's disease and ulcerative colitis. Inflammatory bowel diseases. 2009;15(3):353–8. Epub 2008/10/24. 10.1002/ibd.20757 . [DOI] [PubMed] [Google Scholar]
- 50. Bueno de Mesquita M, Ferrante M, Henckaerts L, Joossens M, Janssens V, Hlavaty T, et al. Clustering of (auto)immune diseases with early-onset and complicated inflammatory bowel disease. European journal of pediatrics. 2009;168(5):575–83. Epub 2008/08/02. 10.1007/s00431-008-0798-7 . [DOI] [PubMed] [Google Scholar]
- 51. Wagner J, Sim WH, Ellis JA, Ong EK, Catto-Smith AG, Cameron DJ, et al. Interaction of Crohn's disease susceptibility genes in an Australian paediatric cohort. PloS one. 2010;5(11):e15376 Epub 2010/11/17. 10.1371/journal.pone.0015376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shen XY, Shi RH, Wang Y, Zhang HJ, Zhou XQ, Shen FC, et al. [Toll-like receptor gene polymorphisms and susceptibility to inflammatory bowel disease in Chinese Han and Caucasian populations]. Zhonghua yi xue za zhi. 2010;90(20):1416–20. Epub 2010/07/22. . [PubMed] [Google Scholar]
- 53. Chen L. Correlation of ATG16L1, TLR4 and TLR2 gene polymorphisms with inflammatory bowel disease in Zhuang population in GUANGXI province: Guangxi Medical University; 2011. [Google Scholar]
- 54. Sivaram G, Tiwari SK, Bardia A, Anjum F, Vishnupriya S, Habeeb A, et al. Macrophage migration inhibitory factor, Toll-like receptor 4, and CD14 polymorphisms with altered expression levels in patients with ulcerative colitis. Human immunology. 2012;73(2):201–5. Epub 2011/12/24. 10.1016/j.humimm.2011.12.006 . [DOI] [PubMed] [Google Scholar]
- 55. Azzam N, Nounou H, Alharbi O, Aljebreen A, Shalaby M. CARD15/NOD2, CD14 and toll-like 4 receptor gene polymorphisms in Saudi patients with Crohn's Disease. International journal of molecular sciences. 2012;13(4):4268–80. Epub 2012/05/19. 10.3390/ijms13044268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim EJ, Chung WC, Lee KM, Paik CN, Jung SH, Lee BI, et al. Association between toll-like receptors/CD14 gene polymorphisms and inflammatory bowel disease in Korean population. Journal of Korean medical science. 2012;27(1):72–7. Epub 2012/01/06. 10.3346/jkms.2012.27.1.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Guagnozzi D, Pagnini C, Delle Fave G, Corleto VD. CARD15 and Toll-like receptor 4 mutations in Italian patients with inflammatory bowel disease. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2012;44(9):800–1. Epub 2012/05/11. 10.1016/j.dld.2012.03.024 . [DOI] [PubMed] [Google Scholar]
- 58. Manolakis AC, Kapsoritakis AN, Kapsoritaki A, Tiaka EK, Oikonomou KA, Lotis V, et al. Readressing the role of Toll-like receptor-4 alleles in inflammatory bowel disease: colitis, smoking, and seroreactivity. Digestive diseases and sciences. 2013;58(2):371–80. Epub 2012/08/25. 10.1007/s10620-012-2348-4 . [DOI] [PubMed] [Google Scholar]
- 59. Budarf ML, Labbe C, David G, Rioux JD. GWA studies: rewriting the story of IBD. Trends in genetics: TIG. 2009;25(3):137–46. Epub 2009/02/17. 10.1016/j.tig.2009.01.001 . [DOI] [PubMed] [Google Scholar]
- 60. Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–24. Epub 2012/11/07. 10.1038/nature11582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yang SK, Hong M, Zhao W, Jung Y, Baek J, Tayebi N, et al. Genome-wide association study of Crohn's disease in Koreans revealed three new susceptibility loci and common attributes of genetic susceptibility across ethnic populations. Gut. 2014;63(1):80–7. Epub 2013/07/16. 10.1136/gutjnl-2013-305193 . [DOI] [PubMed] [Google Scholar]
- 62. Cho JH, Brant SR. Recent insights into the genetics of inflammatory bowel disease. Gastroenterology. 2011;140(6):1704–12. Epub 2011/05/03. 10.1053/j.gastro.2011.02.046 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Coelho T, Andreoletti G, Ashton JJ, Pengelly RJ, Gao Y, RamaKrishnan A, et al. Immuno-genomic profiling of patients with inflammatory bowel disease: a systematic review of genetic and functional in vivo studies of implicated genes. Inflammatory bowel diseases. 2014;20(10):1813–9. Epub 2014/08/30. 10.1097/mib.0000000000000174 . [DOI] [PubMed] [Google Scholar]
- 64. Mathew CG. New links to the pathogenesis of Crohn disease provided by genome-wide association scans. Nature reviews Genetics. 2008;9(1):9–14. Epub 2007/10/31. 10.1038/nrg2203 . [DOI] [PubMed] [Google Scholar]
- 65. Ng SC, Tsoi KK, Kamm MA, Xia B, Wu J, Chan FK, et al. Genetics of inflammatory bowel disease in Asia: systematic review and meta-analysis. Inflammatory bowel diseases. 2012;18(6):1164–76. Epub 2011/09/03. 10.1002/ibd.21845 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.