Abstract Abstract
There are few data on the epidemiology of pulmonary hypertension (PH)–related hospitalizations in children in the United States. Our aim was to determine hospital mortality, length of hospitalization, and hospital charges pertaining to PH-related hospitalizations and also the effects of codiagnoses and comorbidities. A retrospective review of the Kids’ Inpatient Database during the years 2000, 2003, 2006, and 2009 was analyzed for patients ≤20 years of age with a diagnosis of PH by ICD-9 (International Classification of Diseases, Ninth Revision) codes, along with associated diagnoses and comorbidities. Descriptive statistics, including Rao-Scott χ2, ANOVA, and logistic regression models, were utilized on weighted values with survey analysis procedures. The number of PH-related hospital admissions is rising, from an estimated 7,331 (95% confidence interval [CI]: 5,556–9,106) in 2000 to 10,792 (95% CI: 8,568–13,016) in 2009. While infant age and congenital heart disease were most commonly associated with PH-related hospitalizations, they were not associated with mortality. Overall mortality for PH-related hospitalizations was greater than that for hospitalizations not associated with PH, 5.7% versus 0.4% (odds ratio: 16.22 [95% CI: 14.78%–17.8%], P < 0.001), but mortality is decreasing over time. Sepsis, respiratory failure, acute renal failure, hepatic insufficiency, arrhythmias, and the use of extracorporeal membrane oxygenation are associated with mortality. The number of PH-related hospitalizations is increasing in the United States. The demographics of PH in this study are evolving. Despite the increasing prevalence, mortality is improving.
Keywords: pulmonary hypertension, pediatrics, epidemiology, survival
Pulmonary hypertension (PH) is a result of developmental lung abnormalities or aberrant remodeling, leading to both reversible and irreversible lesions of the pulmonary vasculature that result in elevated pulmonary artery pressures.1 Over time, the disease can propagate to right heart failure and death. PH is defined by the World Health Organization (WHO) as a persistently elevated mean pulmonary arterial pressure of ≥25 mmHg and includes an additional requirement of a pulmonary capillary wedge pressure of ≤15 mmHg for precapillary PH.1-7 Although excluded in the newer definition of PH, a pulmonary vascular resistance of ≥3 indexed Wood units is still considered in the diagnosis of children with PH.8,9 Currently, PH in adults and children is classified into 5 groups: pulmonary arterial hypertension (PAH); PH associated with left-sided heart disease; PH associated with lung disease and/or hypoxia; chronic thromboembolic disease; and PH of unclear etiology or related to multiple factors.7,10
PH is an uncommon but serious disease in children, with few data on its prevalence, morbidity, and mortality. Recent PH registries have revealed some information on the epidemiology of PH.2,4-7 Previous results estimated the incidence of PAH in adults and children at 1–2 new cases per million each year.8,11-15 However, several recent studies have shown varying incidence and prevalence. The annual incidence rates in the Netherlands for all PH diagnoses averaged 63.7 cases per million children per year, with transient PAH accounting for nearly 52 cases per million children per year.7,16 Progressive PAH incidence rates in this study are smaller and include about 3 new cases per million children per year, with congenital heart disease (CHD)–related cases constituting a majority of new cases. The prevalence varies in registries, ranging from 3.7 cases per million in France to 20 million cases per million in the Dutch registry.2,5-7,17 Importantly, data from population-based studies on hospital admissions among children with PH are scarce, and most existing data on PH-related hospitalizations pertain to transient forms of PH, such as persistent pulmonary hypertension of the newborn (PPHN).11-15,18
Morbidity and mortality remain high despite improvements in the treatment of PH in the past 20 years. Before the advancements in treatment of PH, survival rates for 1, 3, and 5 years were estimated at 66%, 52%, and 35%, respectively.16,19,20 Modern-day treatment has improved survival for PH, with 1-, 3-, and 5-year survival rates of 73%–96%, 63%–88%, and 60%–81%, respectively.2,5-7,17,21
Data on the epidemiology of pediatric PH in the United States are scarce, with very little analysis of inpatient mortality, length of stay, and comorbidities associated with PH-related hospitalizations. Given this lack of data, the National Heart, Lung, and Blood Institute has listed defining the natural history, epidemiology, and course of pediatric pulmonary vascular disease as a priority.18,22 Therefore, this study was designed to examine prevalence and outcomes in PH-related hospitalizations in children to test the hypothesis that there has been an increase in PH-related hospitalizations. We also hypothesized that with advancements in medical therapy, mortality and morbidity have decreased.
Methods
Data
The study was designed to retrospectively analyze a public database of nationwide pediatric hospital admissions over the past decade. Details of the database and modalities used for analysis have been previously described.4-7,17,19,20,23 Briefly, the Kids’ Inpatient Database (KID) is part of the Healthcare Cost and Utilization Projection run by the Agency for Healthcare Research and Quality in the Department of Health and Human Services.20,21,24-27 The data have been collected every 3 years since 1997 and arise from specialty hospital, public hospital, and academic medical center admissions from 27 states with 2,874 hospitals in 2000, 36 states with 3,438 hospitals in 2003, 38 states with 3,739 hospitals in 2006, and 44 states with 4,121 hospitals in 2009. Patient data included are primary and secondary diagnoses, admission and discharge status, patient demographics, hospital characteristics, total charges, length of stay, and severity and comorbidity measures.
Data can be weighted to provide national estimates. The KID was analyzed for pediatric patients ≤20 years old. We reviewed discharge data from the years 2000, 2003, 2006, and 2009. PH was designated the primary diagnosis and identified by the ICD-9 (International Classification of Diseases, Ninth Revision) codes 415.0 (acute cor pulmonale), 416.0 (primary pulmonary hypertension), 416.8 (other chronic pulmonary heart disease), and 416.9 (chronic pulmonary heart disease, unspecified). The ICD-9 codes used for other secondary diagnoses are listed in Table S1. Data collected included associated diagnoses, hospitalization duration, hospital mortality, and hospital charges, which are not corrected for inflation. Hospitals were identified as children’s hospitals if they were identified as such by the National Association of Children’s Hospitals and Related Institutions. The details and results of procedures, imaging, or laboratory studies are not included.
Statistical analysis
Baseline characteristics of PH-related admissions were analyzed with descriptive statistics. Calculations of prevalence rates for PH-related hospital admissions used age-related number estimates from US Census data for the years 2000, 2003, 2006, and 2009.22,28 Rao-Scott χ2 and ANOVA were used to test for a lack of association between characteristics and years. For univariable analysis, a contingency table analysis was performed for categorical variables and ANOVA for continuous variables. For multivariable analysis, logistic regression models were utilized. The variables in the model included urban location, children’s hospital, large hospital, teaching hospital, female, nonwhite, age at admission, CHD, chronic respiratory disease arising in the perinatal period or bronchopulmonary dysplasia (BPD), congenital diaphragmatic hernia (CDH), chromosomal abnormalities, systemic lupus erythematosus (SLE), sickle cell disease (SCD), sepsis, respiratory failure, acute renal failure, hepatic insufficiency, extracorporeal membrane oxygenation (ECMO), arrhythmias, and cardiomyopathy. The calendar year 2009 was compared against all other years, as it was the year with lowest mortality. Only age <1 year was used as a variable in the final model, because of multicollinearity. All analyses were performed on weighted values, with survey analysis procedures from SAS, version 9.3 (SAS, Cary, NC). Statistical significance was defined as P < 0.05.
Results
Baseline characteristics
The number of hospital admissions related to a diagnosis of PH increased significantly over the past decade (Table 1). In 2000, there were an estimated 7,331 (95% confidence interval [95% CI]: 5,556–9,106) PH-related admissions, which corresponds to 8.7 admissions for every 100,000 children. The number of admissions briefly decreased in 2003, but it steadily increased over the next 6 years, with an estimated 10,792 admissions (95% CI: 8,568–13,016), or 12.3 admissions for every 100,000 children, in 2009 (P < 0.001). While infants accounted for more admissions than any other age group, the proportion of admissions from infants decreased from 55.2% in 2000 to 44.7% in 2009 (P < 0.001). Conversely, from the year 2000 to 2009, the percentages of children aged 1–12 and 13–20 years steadily increased, from 26.4% to 34.8% and from 18.3% to 20.4%, respectively (P < 0.001 for ages 1–12 years and P = 0.045 for ages 13–20 years). Nonwhite patients now account for a majority of PH-related hospital admissions, rising from 40.3% in 2000 to 54.8% in 2009 (P = 0.010). Although the increase was not statistically significant, the number of PH-related hospitalizations for the female sex rose from 48.7% in 2000 to 55.7% in 2009 (P = 0.640). Almost all of the PH-related hospital admissions occurred in hospitals in urban locations, and a significant portion of admissions were at teaching hospitals and large hospitals. Likewise, a majority of PH-related hospital admissions were at children’s hospitals, and the proportion rose from 59.6% in 2000 to 74.4% in 2009 (P < 0.001).
Table 1.
Baseline characteristics of pulmonary hypertension–related hospitalizations
| 2000 | 2003 | 2006 | 2009 | Pa | |
|---|---|---|---|---|---|
| Hospitalizations, n | 7,331 (5,556–9,106) | 6,359 (4,898–7,821) | 9,097 (7,013–11,170) | 10,792 (8,568–13,016) | <0.001 |
| Hospitalization rateb | 8.7 (6.6–10.8) | 7.4 (5.7–9.1) | 10.5 (8.1–12.9) | 12.3 (9.8–14.8) | <0.001 |
| Urban location | 95.5 (94.1–96.4) | 96.7 (95.7–97.2) | 98 (97.6–98.5) | 97.8 (97.2–98.2) | 0.003 |
| Children’s hospital | 59.6 (52.6–63.8) | 70.2 (65.2–73.3) | 72.5 (67.3–75.9) | 74.4 (70.3–77.2) | <0.001 |
| Large hospital | 54.6 (47.4–59.0) | 53.5 (43.8–59.6) | 59.4 (52.1–64.0) | 64.0 (56.6–68.9) | 0.093 |
| Teaching hospital | 83.6 (78.9–86.4) | 87.0 (83.6–89.2) | 85.6 (83.2–87.2) | 86.5 (83.3–88.6) | 0.424 |
| Female | 48.7 (47.5–49.4) | 51.5 (50.8–51.9) | 49.1 (49.1–49.1) | 55.7 (55.4–56.0) | 0.640 |
| Nonwhite | 40.3 (37.4–42.1) | 39.8 (36.4–41.9) | 44.6 (39.7–47.7) | 54.8 (52.1–56.5) | 0.010 |
| Age at admission, years | |||||
| <1 | 55.2 (42.9–67.7) | 43.9 (35.1–52.8) | 46.4 (37.0–55.9) | 44.7 (36.2–53.2) | <0.001 |
| 1–12 | 26.4 (17.8–35.0) | 34.3 (24.9–43.7) | 31.9 (22.6–41.2) | 34.8 (26.5–43.1) | <0.001 |
| 13–20 | 18.3 (13.6–23.0) | 21.8 (15.2–26.9) | 21.7 (17.1–26.3) | 20.4 (16.3–24.6) | 0.045 |
Unless otherwise specified, data are percentage of hospitalizations; 95% confidence intervals are presented in parentheses.
Comparing characteristic across years.
Hospital admissions per 100,000 children.
Codiagnoses and comorbidities associated with PH-related hospitalizations
Among examined comorbidities, CHD was the most common, being associated with nearly 50% of PH-related hospitalizations in all years (P = 0.025; Table 2). While not as common, PH-related admissions with BPD rose more than 2-fold, from 4.2% in 2000 to 13.7% in 2009 (P < 0.001). Other less commonly associated comorbidities that grew in incidence include sepsis, respiratory failure, acute renal failure, hepatic insufficiency, arrhythmias, cardiomyopathy, and the use of ECMO. Diagnoses of CDH, SLE/connective-tissue disorder (CTD), and SCD were uncommon in all years, at 1%–2% of patients, and this did not change significantly over time.
Table 2.
Associated comorbidities in pulmonary hypertension–related hospitalizations
| 2000 | 2003 | 2006 | 2009 | Pa | |
|---|---|---|---|---|---|
| CHD | 49.8 (48.8–50.4) | 52.6 (52.0–53.0) | 47.9 (47.4–48.3) | 52.8 (52.5–52.9) | 0.025 |
| BPD | 4.2 (3.9–4.4) | 8.6 (8.3–8.8) | 10.5 (9.7–11.1) | 13.7 (13.2–14.0) | <0.001 |
| CDH | 2.4 (1.8–3.0) | 1.7 (1.3–2.1) | 1.7 (9.7–11.1) | 1.9 (1.5–2.3) | 0.1868 |
| Chromosomal abnormalities | 15.5 (13.8–17.2) | 19.0 (16.8–21.2) | 17.6 (15.6–19.6) | 17.6 (15.8–19.4) | 0.0561 |
| SLE/CTD | 1.4 (0.8–1.7) | 1.4 (1.2–1.5) | 1.8 (1.1–2.3) | 1.8 (1.5–2.1) | …b |
| SCD | 1.6 (0.9–2.0) | 2.8 (1.9–3.4) | 3.5 (3.2–3.6) | 5.1 (4.2–5.7) | …b |
| Sepsis | 7.6 (7.4–7.7) | 10.4 (10.0–10.6) | 11.8 (11.7–11.9) | 13.3 (13.2–13.4) | <0.001 |
| Respiratory failure | 8.6 (7.9–9.0) | 11.7 (11.4–11.9) | 13.0 (12.3–13.5) | 19.2 (18.4–19.8) | <0.001 |
| Acute renal failure | 1.8 (1.5–2.0) | 1.7 (1.5–1.8) | 2.4 (2.3–2.5) | 3.7 (3.5–3.9) | <0.001 |
| ECMO | 2.3 (1.6–2.8) | 1.4 (1.2–1.6) | 1.5 (1.3–1.7) | 2.0 (1.8–2.2) | 0.045 |
| Hepatic insufficiency | 1.5 (1.2–1.6) | 2.0 (1.7–2.2) | 2.2 (2.0–2.3) | 2.9 (2.8–3.1) | 0.003 |
| Arrhythmias | 8.5 (7.9–8.9) | 9.4 (9.3–9.6) | 10.0 (9.7–10.2) | 12.7 (12.5–12.8) | 0.001 |
| Cardiomyopathy | 5.1 (4.9–5.3) | 5.4 (5.1–5.7) | 4.2 (3.9–4.4) | 4.4 (4.2–4.6) | 0.016 |
Data are percent of hospitalizations (95% confidence interval). BPD: bronchopulmonary dysplasia; CDH: congenital diaphragmatic hernia; CHD: congenital heart disease; CTD: connective tissue disease; ECMO: extracorporeal membrane oxygenation; PH: pulmonary hypertension; SCD: sickle cell disease; SLE: systemic lupus erythematosus.
Comparing associated comorbidities across years.
P value could not be calculated because of rarity of the event.
Factors associated with length of PH-related hospitalization
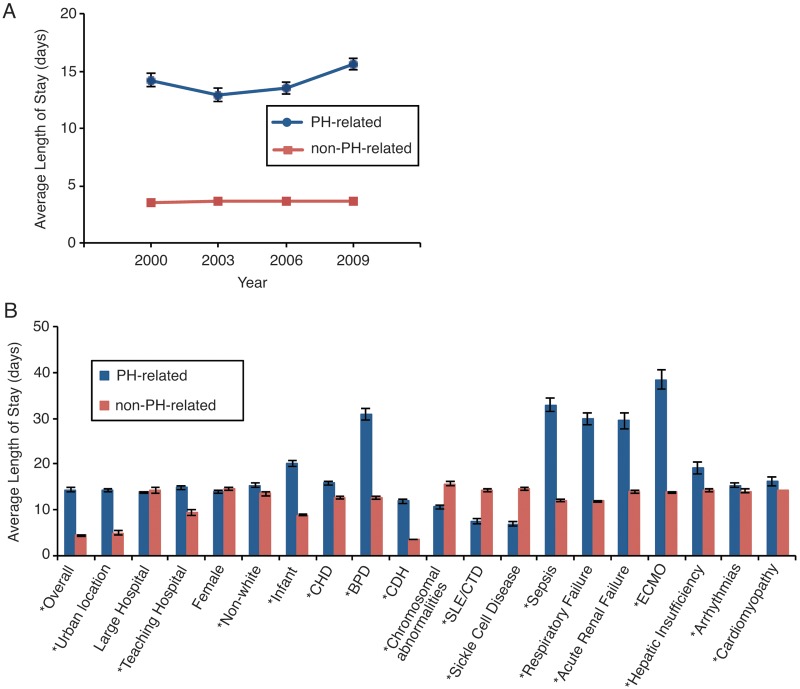
In comparison with non-PH hospitalizations, length of stay for a PH-related hospital admission averaged 14.3 days (P < 0.001), and it increased from 2000 to 2009 (from 14.2 ± 0.6 to 15.6 ± 0.5 days, P = 0.001; Fig. 1). The shortest length of stay was found in 2003, at 12.9 ± 0.6 days, and the longest mean length of stay was in 2009, at 15.6 ± 0.5 days (P = 0.001, 2003 vs. 2009). The most significant comorbidities associated with increased duration of hospitalization included ECMO use (38.4 ± 2.1 days, P < 0.001), sepsis (32.9 ± 1.5 days, P < 0.001), BPD (30.9 ± 1.3 days, P < 0.001), respiratory failure (29.8 ± 1.2 days, P < 0.001), hepatic insufficiency (19.1 ± 1.4 days, P = 0.001), and age <1 year (20.1 ± 0.6 days, P < 0.001). Of note, sex and hospitalization in a large hospital were not significantly associated with increased length of stay.
Figure 1.
A, Mean length of stay, in days, for pulmonary hypertension (PH)–related hospitalizations in children across the years studied; P < 0.001 from ANOVA across years. B, Associated diagnoses’ effect on mean length of stay, in days, for pulmonary hypertension–related hospitalizations. An asterisk indicates P < 0.05 for diagnoses associated with a change in length of stay compared to non–pulmonary hypertension–related hospitalizations. BPD: bronchopulmonary dysplasia; CDH: congenital diaphragmatic hernia; CHD: congenital heart disease; ECMO: extracorporeal membrane oxygenation; SLE/CTD: systemic lupus erythematosus/connective-tissue disorder.
Mortality and factors associated with PH-related hospitalizations
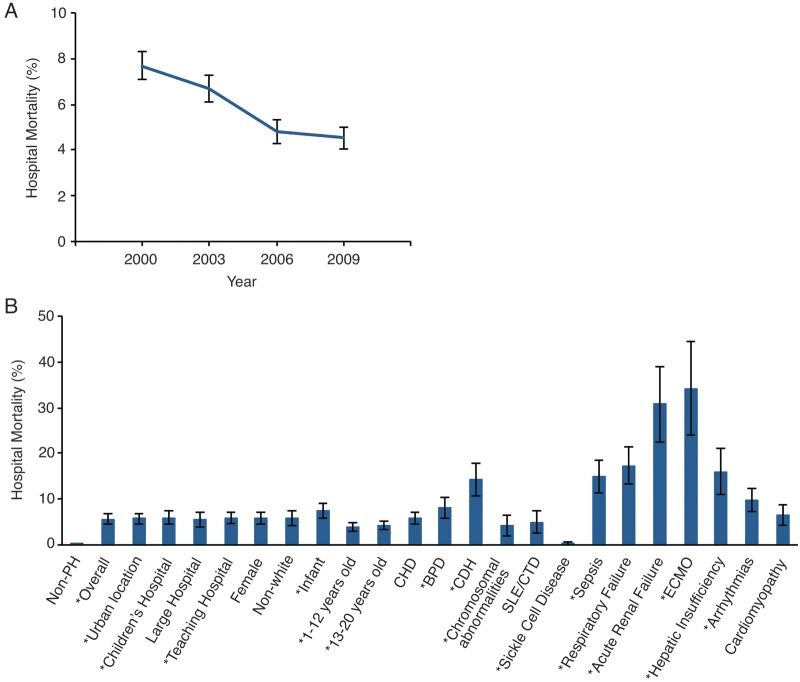
The overall mortality for PH-related hospitalizations was significantly greater than that for hospitalizations not associated with PH, 5.7% versus 0.4% (odds ratio: 16.22 [95% CI: 14.78–17.8]). However, mortality during the study years decreased over time, ranging from 7.7% in 2000 to 4.5% in 2009 (P < 0.001; Fig. 2). Mortality in association with other comorbidities varied from 0.3% with SCD to as high as 34.3% in association with the use of ECMO (P < 0.001 for both). Although most comorbidities were associated with increased mortality in PH-related hospitalizations, compared to that in non-PH-related hospitalizations, mortality in association with a large hospital, female sex, nonwhite race, ages 1–12 and 13–20 years, CHD, chromosomal abnormalities, SLE/CTD, SCD, and cardiomyopathy were not associated with increased mortality. On univariable analysis, urban location, teaching hospitals, age <1 year, BPD, CDH, sepsis, respiratory failure, acute renal failure, ECMO, hepatic insufficiency, and arrhythmias were all associated with increased mortality (Table 3). Factors associated with reduced mortality include a later calendar year, older age (1–12 years: odds ratio: 0.593 [95% CI: 0.494–0.711]; 13–20 years: odds ratio: 0.707 [95% CI: 0.600–0.833]), and chromosomal abnormalities. On multivariable analysis, only urban location, teaching hospital, CDH, sepsis, respiratory failure, acute renal failure, ECMO, hepatic insufficiency, and arrhythmias retained statistical significance.
Figure 2.
A, Hospital mortality, in percent, for pulmonary hypertension–related hospitalization in children across the years studied; P < 0.001 from ANOVA across years. B, Association of codiagnoses and mortality. An asterisk indicates P < 0.05 for factors significantly associated with increased or decreased mortality. BPD: bronchopulmonary dysplasia; CDH: congenital diaphragmatic hernia; CHD: congenital heart disease; ECMO: extracorporeal membrane oxygenation; SLE/CTD: systemic lupus erythematosus/connective tissue disorder.
Table 3.
Factors associated with mortality in pulmonary hypertension–related hospitalizations
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Factor | Odds ratio | 95% CI | Odds ratio | 95% CI |
| Year 2009 vs. other years | 0.716 | 0.621–0.825 | 0.507 | 0.421–0.611 |
| Urban location | 10.862 | 3.448–34.222 | 5.659 | 1.281–24.997 |
| Children’s hospital | 1.356 | 1.126–1.634 | 0.915 | 0.730–1.146 |
| Large hospital | 0.946 | 0.806–1.111 | 1.013 | 0.819–1.254 |
| Teaching hospital | 1.894 | 1.500–2.390 | 1.491 | 1.131–1.966 |
| Female | 1.038 | 0.917–1.174 | 1.118 | 0.941–1.328 |
| Nonwhite | 1.106 | 0.946–1.294 | 1.103 | 0.919–1.323 |
| <1 year of age | 1.878 | 1.595–2.211 | 1.180 | 0.879–1.585 |
| CHD | 1.086 | 0.942–1.252 | 1.044 | 0.848–1.286 |
| BPD | 1.524 | 1.268–1.831 | 1.142 | 0.901–1.447 |
| CDH | 2.774 | 2.047–3.759 | 1.937 | 1.250–3.001 |
| Chromosomal abnormalities | 0.683 | 0.568–0.823 | 0.882 | 0.680–1.144 |
| Sepsis | 3.670 | 3.145–4.284 | 2.421 | 1.987–2.950 |
| Respiratory failure | 5.158 | 4.549–5.848 | 4.446 | 3.736–5.290 |
| Acute renal failure | 8.390 | 6.779–10.384 | 4.851 | 3.460–6.801 |
| ECMO | 9.599 | 7.823–11.779 | 5.113 | 3.730–7.009 |
| Hepatic insufficiency | 3.306 | 2.534–4.312 | 1.967 | 1.295–2.987 |
| Arrhythmias | 1.969 | 1.647–2.354 | 1.941 | 1.573–2.395 |
| Cardiomyopathy | 1.183 | 0.867–1.614 | 1.171 | 0.744–1.844 |
BPD: bronchopulmonary dysplasia; CDH: congenital diaphragmatic hernia; CHD: congenital heart disease; CI: confidence interval; CTD: connective tissue disease, ECMO: extracorporeal membrane oxygenation; SLE: systemic lupus erythematosus.
Hospital charges for PH-related hospitalizations
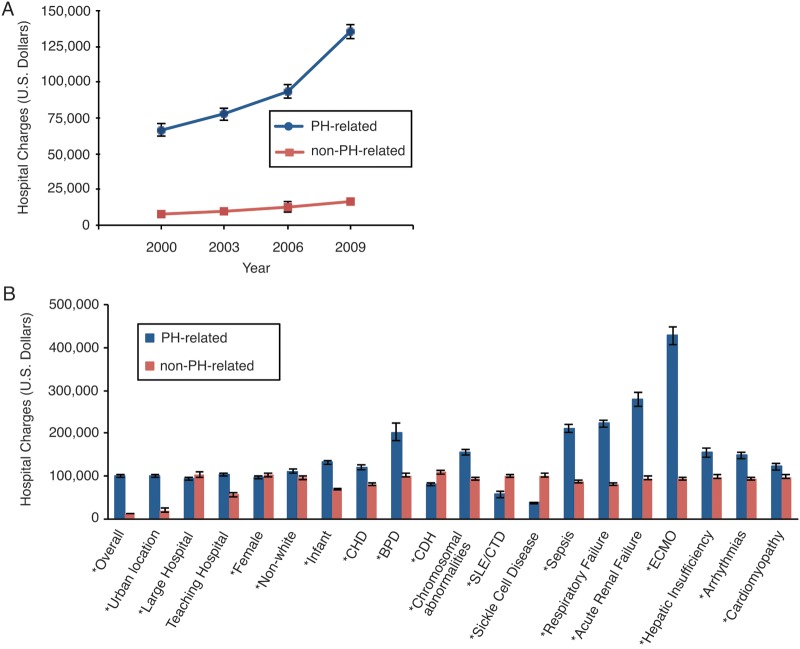
Hospital charges for PH-related hospitalizations rose from 2000 to 2009. In 2000 the average charge for a PH-related hospitalization was $66,457 ± $4,487, and in 2009 it was $135,572 ± $5,144 (P < 0.001; Fig. 3). Compared to those for non-PH hospitalizations, comorbidities associated with increased charges included CHD, BPD, CDH, sepsis, respiratory failure, acute renal failure, ECMO, hepatic insufficiency, and arrhythmias (P < 0.001 for all). Conversely, a large hospital, female sex, chromosomal abnormalities, SLE/CTD, and SCD were associated with a reduction in charges.
Figure 3.
A, Hospital charges, in US dollars, for pulmonary hypertension–related hospitalizations in children across the years studied; P < 0.001 from ANOVA across years. B, Associated diagnoses effect on hospital charges for pulmonary hypertension–related hospitalizations. An asterisk indicates P < 0.05 for diagnoses associated with a change in hospital charges compared to non–pulmonary hypertension–related hospitalizations. BPD: bronchopulmonary dysplasia; CDH: congenital diaphragmatic hernia; CHD: congenital heart disease; ECMO: extracorporeal membrane oxygenation; SLE/CTD: systemic lupus erythematosus/connective tissue disorder.
Discussion
This is one of the first studies to determine prevalence, cost, and outcomes for most forms of PH in hospitalizations of children in the United States. The data collected were obtained from hospital records of patient discharges from more than 4,000 hospitals in the United States. Unlike PH registries, this study does not derive prevalence of PH in a population on individual records but rather on the number of all hospitalizations due to PH of all types.4-7,17,23,27 In addition, these data are representative of the entire United States and include admissions from all types of hospitals. As a result, the study provides important information on outcomes of PH-related hospitalizations as well as on morbidities and diagnoses associated with them. Likewise, this study is the first to identify hospital-related morbidities contributing to poor outcomes in PH-related mortality. Similar studies have been performed in children with heart failure, adults with right heart failure, and neonates.20,24-27,29,30
These data demonstrate an increasing prevalence of PH-related hospitalizations over the past decade. While it is certainly possible that the number of children being diagnosed with PH has increased during this time period, this study is limited by a large national database based on hospital admissions and not on individuals. Consequently, increasing prevalence can be related to alternative reasons, including an improved ability to diagnose less severe disease, an increased awareness of PH associated with other diseases, and an improved ability to recognize the disease earlier, with resultant hospitalization for more rapid and earlier treatment of PH. Nonetheless, this study adds important information to the very few data on hospitalizations related to PH as well as to longitudinal epidemiological data on PH in children. Likewise, there are only a few similar studies in adult PH. Data from a surveillance report published in 2005 by the Centers for Disease Control and Prevention revealed that hospitalization rates for adult Medicare patients with PH in the United States increased from 1980 to 2002.7,28 Likewise, a study examining the National Inpatient Sampling database and the US National Center for Health Statistics database published similar findings on the number of individual diagnoses of adult PH.2,5,6,16,23,27,31,32 Furthermore, a recent study on data from the ASPIRE registry (Assessing the Spectrum of Pulmonary hypertension Identified at a REferral centre) and a study examining hospitalization and mortality data on adults with PH from several referral centers in the United Kingdom and Ireland both demonstrated an increase in the number of new cases of PH per year from 2001 to 2009.3,29,30,33 In contrast, the number of new patient diagnoses for all forms of PH in children in the Netherlands PH registry declined from 2000 to 2006.2,4,7 However, yearly prevalence data were not examined for pediatric patients with all forms of PH. Given the improvement in survival with today’s therapies, prevalence is likely to have at least remained stable, if not increased.2,5,6,16,23,31,32,34-36
The patient characteristics of PH-related hospitalizations are changing. As the treatment of PH has become more specialized and is done mostly at PH-specific centers, the increase in the percentage of PH-related hospitalizations at children’s hospitals is not unexpected. The diagnosis and treatment of the disease require a team of specialists most often found at children’s hospitals. In addition, guidelines today suggest that care be given at PH centers.3,4,33 Interestingly, the percentage of nonwhite patients associated with PH-related hospitalizations rose over the study period, and nonwhite races accounted for the majority of PH-related hospitalizations. Again, the study by Hyduk et al.28 observed similar increasing prevalence of nonwhite Medicare patients hospitalized because of PH. Whether the actual number of individual PH diagnoses in nonwhites has increased cannot be ascertained by this study. Nonetheless, these observations would be in contrast to those from several PH registries, in which 67%–70% of PH patients are of the white race.2,4,5,7
Hospitalizations for all forms of PH were most often associated with CHD, a factor in nearly 50% of the admissions in this study. Interestingly, these numbers contrast with those from studies in adults, where CHD is less commonly associated with PH and CTD-related PH is more common.34-37 CHD is associated with nearly 50% of patients with all forms of PH in the Tracking Outcomes and Practice in Pediatric Pulmonary Hypertension (TOPP) registry as well,4,7 and it also represents anywhere from 24% to 72% of patients in PAH registries.2,5,7,38 Our study does not delineate between simple and complex cardiac lesions and may overestimate the number of patients with PH due to CHD. This study is also limited in the ability to decipher whether CHD caused the PH. In addition, many patients with CHD will have undergone a cardiac procedure for repair or palliation, both of which can be associated with the development of postprocedural or postsurgical PH, as the risk of acidemia, hypercarbia, alveolar hypoxia, and acute pulmonary endothelial injury can lead to acute postoperative PH crises.4,37,39-41 Nonetheless, despite being commonly associated with PH-related hospitalizations, CHD did not affect mortality in our study, and it had only a minor effect on hospital length of stay and charges.
While not as common as CHD-associated PH, BPD was codiagnosed in up to 13.7% of PH-related hospitalizations, a finding that increased with time. In the TOPP and Dutch PH registries, 1.8%–3.7% of patients with PH had coexisting BPD.4,7,41 BPD is a developmental lung disease that is associated with a reduction in the pulmonary vascular area of the lung due to arrest of growth and injury, leading to the development of PH.38-41 Therefore, it is little surprise that studies examining the prevalence of PH in patients with BPD reveal that the codiagnoses are not uncommon, occurring in 15%–25% of these patients.4,38-43 These results may be partially explained by the increasing incidence of diagnosis of BPD as premature and very-low-birth-weight neonates receive improved care and survival in the surfactant era.2,6,7,17,23,41 Similar reasoning may explain the higher prevalence of infants associated with PH-related hospitalizations. However, the benefit of survival came with an increase in the length of stay, thereby compounding to the cost of each hospitalization, a finding generally observed in BPD admissions with and without codiagnosed PH.39-41,44-46 And despite the improved care and survival in BPD patients, BPD associated with PH increased mortality up to 4-fold, as seen in previous studies.20,38-40,42,43
The overall mortality for all years of PH-related hospitalizations was markedly high. There was a more than 15-fold increase in hospital mortality in patients with PH, compared to those patients without PH. Fortunately, mortality is declining. A reduction in mortality has been observed in almost all pediatric studies in the modern treatment era, including several patient registries, such as the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL), the UK Pulmonary Hypertension Service for Children, and the Netherlands PH registry, as well as studies done at individual institutions.2,6,7,17,23,25,47,48 Improvement in mortality is most likely a product of improved inpatient care, new therapies derived from large adult trials and smaller pediatric studies, and the development of standardized algorithms to treat PH.44-46,49,50 However, it also possible that some component of the improved mortality seen may be secondary to increases in the diagnosing of less severe disease, improvement in recognizing the disease, and earlier hospitalization for treatment.
Comorbidities with increasing frequency in PH-related hospitalizations included sepsis, respiratory failure, acute renal failure, and arrhythmias. Concomitant with increasing frequency, these comorbidities were associated with increased duration of hospitalization, cost of hospitalization, and mortality. These findings are in congruence with those of a previous study of heart failure in children,20,51,52 and similar comorbidities are found associated in PH-related hospitalizations in adults.25,47,48,53 On the other hand, the use of ECMO is declining. But despite its declining use, its influence on increased hospitalization duration, charges associated with hospitalization, and mortality was greatest among the comorbidities examined. Outcomes with the use of ECMO in the treatment of PH in children vary. Most success has been accomplished in children with PH associated with CHD or PPHN, where survival has been quite high in several studies involving smaller population sizes, with up to nearly 100% survival.49,50 However, in pre–lung transplant patients with severe PH, mortality is as high as 60%.51,52 Our study revealed a hospital mortality of 34% and is a reflection of results obtained from all forms of PH rather than a single subgroup.
The charges for hospitalizations associated with PH are increasing over time. Our results indicate that this is not a result of increasing duration of hospitalization, because that measure has not changed significantly over the past decade. Some component of these increased charges is likely from newer pharmacological therapies for PH.53 It should be noted that, whereas in 2000 there was only one FDA-approved medication for PH, by 2009 there were 6 different medications approved by the FDA in 3 separate pharmacologic classes. The use of these medications likely has resulted in greater longevity and higher cost of care for pediatric patients. In one cost-analysis study, the use of prostacyclins can be more than $100,000 each year.54
This study has several limitations. It is a retrospective study, and the findings may certainly differ from those for a prospectively enrolled cohort. There are also notable limitations of this administrative database. Admissions are identified by an ICD-9 code and not by the WHO classification of PH, and the nature of coding can change over the years.55 This is true for idiopathic PAH (IPAH), as there was a change in the default code in 2000 that corresponded to the reduction in IPAH hospital discharges for that year.27 Therefore, we elected to include all codes for PH in this study. It is also not known whether an expert in PH made the diagnosis, and the standards used to diagnose patients are not identified in this database. Furthermore, there is no information for lab values, catheterization data, echocardiograms, and other imaging studies to help determine the severity of the disease. As mentioned above, the database does not identify individual patients, as do registries, and for that reason multiple admissions from the same patient cannot be ascertained. Only hospital data are available, and thus it is not possible to determine the burden of prior hospitalizations or hospital readmissions for a given patient. Alternatively, registries may suffer from a referral bias, as registries are often associated with specialized PH centers, which are mostly found in urban areas. Therefore, large databases like the KID allow a larger and more diverse sampling of a population. Finally, the 10-year time period of the study is relatively brief and thus may not accurately reflect trends that may be evident over longer time periods.
Conclusions. This is the first longitudinal study to examine prevalence and outcomes in pediatric PH-related hospitalizations. Our analysis demonstrates that prevalence is increasing, with a changing demographic of patients. Despite comprising the majority of PH-related hospitalizations, CHD is not associated with increased mortality. While hospital mortality is improving, it is still significantly higher than that for hospitalizations due to non-PH etiologies. The charges associated with PH-related hospitalizations continue to rise, with non-PH-related comorbidities being associated with these increased charges.
Acknowledgments
This work was presented at the 2011 American Heart Association Scientific Sessions, Orlando, Florida, November 14–16.
Supplement.
Table S1.
Diagnostic codes from the International Classification of Diseases, Ninth Revision (ICD-9)
| Code | Description |
|---|---|
| Pulmonary hypertension | |
| 415.0 | Acute cor pulmonale |
| 416.0 | Primary pulmonary hypertension |
| 416.8 | Other chronic pulmonary heart diseases |
| 416.9 | Unspecified chronic pulmonary heart disease |
| Congenital heart disease | |
| 745.0 | Common truncus |
| 745.10 | Complete transposition of the great vessels |
| 745.11 | Double-outlet right ventricle |
| 745.12 | Corrected transposition of the great vessels |
| 745.19 | Other transposition of the great vessels |
| 745.2 | Tetralogy of Fallot |
| 745.3 | Common ventricle |
| 745.4 | Ventricular septal defect |
| 745.5 | Ostium secundum type atrial septal defect |
| 745.60 | Unspecified endocardial cushion defect |
| 745.61 | Ostium primum defect |
| 745.69 | Other endocardial cushion defect |
| 746.00 | Unspecified pulmonary valve anomaly |
| 746.01 | Congenital pulmonary valve atresia |
| 746.02 | Congenital pulmonary valve stenosis |
| 746.09 | Other pulmonary valve anomaly |
| 746.1 | Congenital tricuspid atresia and stenosis |
| 746.2 | Ebstein anomaly |
| 746.3 | Congenital stenosis of the aortic valve |
| 746.6 | Congenital insufficiency of aortic valve |
| 746.6 | Congenital mitral stenosis |
| 746.7 | Hypoplastic left heart syndrome |
| 746.81 | Subaortic stenosis |
| 746.82 | Cor triatriatum |
| 746.83 | Infundibular pulmonic stenosis |
| 746.84 | Obstructive anomalies of heart, not elsewhere classified |
| 746.85 | Coronary artery anomaly |
| 746.86 | Congenital heart block |
| 746.87 | Malposition of heart and cardiac apex |
| 746.89 | Other specified anomalies of heart |
| 746.9 | Unspecified anomalies of heart |
| 747.0 | Patent ductus arteriosus |
| 747.10 | Coarctation of aorta |
| 747.11 | Interruption of aortic arch |
| 747.20 | Unspecified anomaly of aorta |
| 747.11 | Anomalies of aortic arch |
| 747.22 | Atresia and stenosis of aorta |
| 747.29 | Other anomalies of aorta |
| 747.3 | Anomalies of pulmonary artery |
| 747.40 | Unspecified anomaly of great veins |
| 747.41 | Total anomalous pulmonary venous connection |
| 747.42 | Partial anomalous pulmonary venous connection |
| 747.49 | Other anomalies of great veins |
| Arrhythmias | |
| 426.0 | Third-degree atrioventricular block |
| 426.54 | Trifascicular block |
| 426.7 | Anomalous atrioventricular excitation |
| 426.81 | Lown-Ganong-Levine syndrome |
| 426.82 | Long QT syndrome |
| 427.0 | Paroxysmal supraventricular tachycardia |
| 427.1 | Paroxysmal ventricular tachycardia |
| 427.2 | Unspecified paroxysmal tachycardia |
| 427.31 | Atrial fibrillation |
| 427.32 | Atrial flutter |
| 427.41 | Ventricular fibrillation |
| 427.42 | Ventricular flutter |
| 427.81 | SA node dysfunction |
| 427.89 | Other specified cardiac dysrhythmias |
| 427.9 | Unspecified cardiac dysrhythmia |
| 785.0 | Unspecified tachycardia |
| Chromosomal abnormalities | |
| 758.0 | Down’s syndrome |
| 758.1 | Patau’s syndrome |
| 758.2 | Edward’s syndrome |
| 758.3 | Autosomal deletion syndromes |
| 758.31 | Cri-du-chat syndrome |
| 758.32 | Velo-cardio-facial syndrome |
| 758.33 | Other microdeletions |
| 758.39 | Other autosomal deletions |
| 758.4 | Balanced autosomal translocation in normal individual |
| 758.5 | Other conditions due to autosomal anomalies |
| 758.6 | Gonadal dysgenesis |
| 758.7 | Klinefelter’s syndrome |
| 758.8 | Other conditions due to chromosome anomalies |
| 758.81 | Other conditions due to sex chromosome anomalies |
| 758.89 | Other conditions due to chromosome anomalies |
| 758.9 | Conditions due to anomaly of unspecified chromosome |
| Acute renal failure (ARF) | |
| 584.5 | ARF with tubular necrosis |
| 584.6 | ARF with lesion of renal cortical necrosis |
| 584.7 | ARF with lesion of renal medullary necrosis |
| 584.8 | ARF with other specified pathologic lesion in kidney |
| 584.9 | Unspecified ARF |
| Lung disease and respiratory failure | |
| 799.1 | Respiratory arrest |
| 770.7 | Chronic respiratory disease arising in the perinatal period (bronchopulmonary dysplasia) |
| 756.6 | Anomalies of diaphragm |
| 553.3 | Diaphragmatic hernia without mention of obstruction or gangrene |
| 552.3 | Diaphragmatic hernia with obstruction |
| 518.81 | Acute respiratory failure |
| 518.83 | Chronic respiratory failure |
| 518.84 | Acute and chronic respiratory failure |
| Hepatic impairment | |
| 570 | Acute and subacute necrosis of liver |
| 571.0 | Alcoholic fatty liver |
| 571.1 | Acute alcoholic hepatitis |
| 571.2 | Alcoholic liver cirrhosis |
| 571.3 | Unspecified alcoholic liver damage |
| 571.40 | Unspecified chronic hepatitis |
| 571.41 | Chronic persistent hepatitis |
| 571.42 | Autoimmune hepatitis |
| 571.49 | Other chronic hepatitis |
| 571.5 | Cirrhosis, not otherwise specified |
| 571.6 | Primary biliary cirrhosis |
| 571.8 | Other chronic nonalcoholic liver disease |
| 571.9 | Unspecified chronic liver disease |
| 572.0 | Liver abscess and sequelae of chronic liver disease |
| 572.2 | Hepatic coma |
| 572.3 | Portal hypertension |
| 572.4 | Hepatorenal syndrome |
| 572.8 | Other sequelae of chronic liver disease |
| 573.0 | Chronic passive congestion of liver |
| 573.3 | Hepatitis, unspecified |
| 573.4 | Hepatic infarction |
| 573.8 | Other specified disorders of liver |
| 573.9 | Unspecified disorder of liver |
| Sepsis/systemic inflammatory response syndrome (SIRS) | |
| 995.90 | Unspecified SIRS |
| 995.91 | Sepsis |
| 995.92 | Severe sepsis |
| 995.93 | SIRS due to noninfectious process without acute organ dysfunction |
| 995.94 | SIRS due to noninfectious process with acute organ dysfunction |
| 038.0 | Streptococcal septicemia |
| 038.10 | Unspecified staphylococcal septicemia |
| 038.11 | Methicillin-sensitive staphylococcal septicemia |
| 038.12 | Methicillin-resistant staphylococcal septicemia |
| 038.19 | Other staphylococcal septicemia |
| 038.2 | Pneumococcal septicemia |
| 038.3 | Septicemia due to anaerobes |
| 038.40 | Unspecified gram-negative septicemia |
| 038.41 | Haemophilus influenzae septicemia |
| 038.42 | Escherichia coli septicemia |
| 038.43 | Pseudomonas septicemia |
| 038.44 | Serratia septicemia |
| 038.49 | Other septicemia |
| 038.8 | Other specified septicemias |
| 038.9 | Septicemia, not otherwise specified |
| 054.5 | Herpetic septicemia |
| 790.7 | Bacteremia |
| 771.81 | Septicemia of the newborn |
| 771.83 | Bacteremia of the newborn |
| Extracorporeal membrane oxygenation (ECMO) | |
| 39.65 | ECMO |
| Cardomyopathy | |
| 425.00 | Endomyocardial fibrosis |
| 425.10 | Hypertrophic obstructive cardiomyopathy |
| 425.20 | Obscure cardiomyopathy of Africa |
| 425.30 | Endocardial fibroelastosis |
| 425.40 | Other primary cardiomyopathies |
| 425.50 | Alcoholic cardiomyopathy |
| 425.70 | Nutritional and metabolic cardiomyopathy |
| 425.80 | Cardiomyopathy in other diseases classified |
| 425.90 | Secondary cardiomyopathy, unspecified |
| Others | |
| 282.60 | Sickle cell disease |
| 710.00 | Systemic lupus erythematosus |
| 710.90 | Unspecified diffuse connective tissue disease |
Source of Support: Support for DBF is from NIH T32 HL007915.
Conflict of Interest: None declared.
Supplements
SupplementPulmCirc-005-339.s001.pdf (435.1KB, pdf)
References
- 1.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62(25 suppl.):D34–D41. [DOI] [PubMed]
- 2.Barst RJ, McGoon MD, Elliott CG, Foreman AJ, Miller DP, Ivy DD. Survival in childhood pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management. Circulation 2012;125(1):113–122. [DOI] [PubMed]
- 3.Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiéry JL, Barbera JA, Beghetti M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009;30(20):2493–2537. [DOI] [PubMed]
- 4.Berger RM, Beghetti M, Humpl T, Raskob GE, Ivy DD, Jing ZC, Bonnet D, Schulze-Neick I, Barst RJ. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet 2012;379(9815):537–546. [DOI] [PMC free article] [PubMed]
- 5.Fraisse A, Jaïs X, Schleich JM, Filippo S, Maragnès P, Beghetti M, Gressin V, et al. Characteristics and prospective 2-year follow-up of children with pulmonary arterial hypertension in France. Arch Cardiovasc Dis 2010;103(2):66–74. [DOI] [PubMed]
- 6.Haworth SG, Hislop AA. Treatment and survival in children with pulmonary arterial hypertension: the UK Pulmonary Hypertension Service for Children 2001–2006. Heart 2009;95(4):312–317. [DOI] [PubMed]
- 7.van Loon RL, Roofthooft MT, Hillege HL, ten Harkel ADJ, van Osch-Gevers M, Delhaas T, Kapusta L, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation 2011;124(16):1755–1764. [DOI] [PubMed]
- 8.Rubin LJ. Primary pulmonary hypertension. N Engl J Med 1997;336(2):111–117. [DOI] [PubMed]
- 9.Abman SH, Ivy DD. Recent progress in understanding pediatric pulmonary hypertension. Curr Opin Pediatr 2011;23(3):298–304. [DOI] [PMC free article] [PubMed]
- 10.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009;54(1 suppl.):S43–S54. [DOI] [PubMed]
- 11.Walsh-Sukys MC, Tyson JE, Wright LL, Bauer CB, Korones SB, Stevenson DK, Verter J, et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics 2000;105(1):14–20. [DOI] [PubMed]
- 12.Roberts JD Jr., Fineman JR, Morin FC, Shaul PW, Rimar S, Schreiber MD, Polin RA, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. N Engl J Med 1997;336(9):605–610. [DOI] [PubMed]
- 13.Kinsella JP, Truog WE, Walsh WF, Goldberg RN, Bancalari E, Mayock DE, Redding GJ, et al. Randomized, multicenter trial of inhaled nitric oxide and high-frequency oscillatory ventilation in severe, persistent pulmonary hypertension of the newborn. J Pediatr 1997;131(1):55–62. [DOI] [PubMed]
- 14.Christou H, Van Marter LJ, Wessel DL, Allred EN, Kane JW, Thompson JE, Stark AR, et al. Inhaled nitric oxide reduces the need for extracorporeal membrane oxygenation in infants with persistent pulmonary hypertension of the newborn. Crit Care Med 2000;28(11):3722–3727. [DOI] [PubMed]
- 15.Roofthooft MTR, Elema A, Bergman KA, Berger RMF. Patient characteristics in persistent pulmonary hypertension of the newborn. Pulm Med 2011;2011(3):1–8. [DOI] [PMC free article] [PubMed]
- 16.Barst RJ, Maislin G, Fishman AP. Vasodilator therapy for primary pulmonary hypertension in children. Circulation 1999;99(9):1197–1208. [DOI] [PubMed]
- 17.Yung D, Widlitz AC, Rosenzweig EB, Kerstein D, Maislin G, Barst RJ. Outcomes in children with idiopathic pulmonary arterial hypertension. Circulation 2004;110(6):660–665. [DOI] [PubMed]
- 18.Castro M, Ramirez MI, Gern JE, Cutting G, Redding G, Hagood JS, Whitsett J, et al. Strategic plan for pediatric respiratory diseases research: an NHLBI working group report. Proc Am Thorac Soc 2009;6(1):1–10. [DOI] [PMC free article] [PubMed]
- 19.Knudson JD, Neish SR, Cabrera AG, Lowry AW, Shamszad P, Morales DLS, Graves DE, Williams EA, Rossano JW. Prevalence and outcomes of pediatric in-hospital cardiopulmonary resuscitation in the united States: an analysis of the Kids’ Inpatient Database. Crit Care Med 2012;40(11):2940–2944. [DOI] [PubMed]
- 20.Rossano JW, Kim JJ, Decker JA, Price JF, Zafar F, Graves DE, Morales DLS, et al. Prevalence, morbidity, and mortality of heart failure-related hospitalizations in children in the United States: a population-based study. J Card Fail 2012;18(6):459–470. [DOI] [PubMed]
- 21.Healthcare Cost and Utilization Project. Overview of the Kids’ Inpatient Database (KID). https://www.hcup-us.ahrq.gov/kidoverview.jsp.
- 22.United States Census Bureau. Population estimates: vintage 2009: national tables. www.census.gov/popest/data/historical/2000s/vintage_2009/index.html.
- 23.Barst RJ, Ertel SI, Beghetti M, Ivy DD. Pulmonary arterial hypertension: a comparison between children and adults. Eur Respir J 2011;37(3):665–677. [DOI] [PMC free article] [PubMed]
- 24.Haddad F, Fuh E, Peterson T, Skhiri M, Kudelko KT, de Jesus Perez V, Winkelmayer WC, Doyle RL, Chertow GM, Zamanian RT. Incidence, correlates, and consequences of acute kidney injury in patients with pulmonary arterial hypertension hospitalized with acute right-side heart failure. J Card Fail 2011;17(7):533–539. [DOI] [PubMed]
- 25.Haddad F, Peterson T, Fuh E, Kudelko KT, de Jesus Perez V, Skhiri M, Vagelos R, et al. Characteristics and outcome after hospitalization for acute right heart failure in patients with pulmonary arterial hypertension. Circ Heart Fail 2011;4(6):692–699. [DOI] [PubMed]
- 26.Ford JB, Roberts CL, Algert CS, Bowen JR, Bajuk B, Henderson-Smart DJ. Using hospital discharge data for determining neonatal morbidity and mortality: a validation study. BMC Health Serv Res 2007;7:188. doi:10.1186/1472-6963-7-188. [DOI] [PMC free article] [PubMed]
- 27.Link J, Glazer C, Torres F, Chin K. International Classification of Diseases coding changes lead to profound declines in reported idiopathic pulmonary arterial hypertension mortality and hospitalizations: implications for database studies. Chest 2011;139(3):497–504. [DOI] [PMC free article] [PubMed]
- 28.Hyduk A, Croft JB, Ayala C, Zheng K, Zheng ZJ, Mensah GA. Pulmonary hypertension surveillance—United States, 1980–2002. MMWR 2005;54(SS05):1–28. [PubMed]
- 29.Ling Y, Johnson MK, Kiely DG, Condliffe R, Elliot CA, Gibbs JSR, Howard LS, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med 2012;186(8):790–796. [DOI] [PubMed]
- 30.Hurdman J, Condliffe R, Elliot CA, Davies C, Hill C, Wild JM, Capener D, et al. ASPIRE registry: Assessing the Spectrum of Pulmonary hypertension Identified at a REferral centre. Eur Respir J 2012;39(4):945–955. [DOI] [PubMed]
- 31.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, et al. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Ann Intern Med 1991;115(5):343–349. [DOI] [PubMed]
- 32.van Loon RLE, Roofthooft MTR, Delhaas T, van Osch-Gevers M, ten Harkel ADJ, Strengers JLM, Backx A, Hillege HL, Berger RMF. Outcome of pediatric patients with pulmonary arterial hypertension in the era of new medical therapies. Am J Cardiol 2010;106(1):117–124. [DOI] [PubMed]
- 33.National Pulmonary Hypertension Centres of the UK and Ireland. Consensus statement on the management of pulmonary hypertension in clinical practice in the UK and Ireland. Thorax 2008;63(suppl. 2):ii1–ii41. doi:10.1136/thx.2007.090480. [DOI] [PubMed]
- 34.Peacock AJ, Murphy NF, McMurray JJ, Caballero L, Stewart S. An epidemiological study of pulmonary arterial hypertension. Eur Respir J 2007;30(1):104–109. [DOI] [PubMed]
- 35.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 2010;137(2):376–387. [DOI] [PubMed]
- 36.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaïci A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006;173(9):1023–1030. [DOI] [PubMed]
- 37.Kyle WB. Pulmonary hypertension associated with congenital heart disease: a practical review for the pediatric cardiologist. Congenital Heart Dis 2012; [DOI] [PubMed]
- 38.Mourani PM, Mullen M, Abman SH. Pulmonary hypertension in bronchopulmonary dysplasia. Prog Pediatr Cardiol 2009;27(1–2):43–48.
- 39.An HS, Bae EJ, Kim GB, Kwon BS, Beak JS, Kim EK, Kim HS, Choi JH, Noh CI, Yun YS. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ J 2010;40(3):131–136. [DOI] [PMC free article] [PubMed]
- 40.Bhat R, Salas AA, Foster C, Carlo WA, Ambalavanan N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics 2012;129(3):e682–e689. [DOI] [PMC free article] [PubMed]
- 41.Stroustrup A, Trasande L. Epidemiological characteristics and resource use in neonates with bronchopulmonary dysplasia: 1993–2006. Pediatrics 2010;126(2):291–297. [DOI] [PubMed]
- 42.Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, Mullen MP. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics 2007;120(6):1260–1269. [DOI] [PubMed]
- 43.Slaughter JL, Pakrashi T, Jones DE, South AP, Shah TA. Echocardiographic detection of pulmonary hypertension in extremely low birth weight infants with bronchopulmonary dysplasia requiring prolonged positive pressure ventilation. J Perinatol 2011;31(10):1–6. [DOI] [PubMed]
- 44.Ivy D. Advances in pediatric pulmonary arterial hypertension. Curr Opin Cardiol 2012;27(2):70–81. [DOI] [PMC free article] [PubMed]
- 45.Tissot C, Ivy DD, Beghetti M. Medical therapy for pediatric pulmonary arterial hypertension. J Pediatr 2010;157(4):528–532. [DOI] [PMC free article] [PubMed]
- 46.Ivy DD, Calderbank M, Wagner BD, Dolan S, Nyquist AC, Wade M, Nickels WM, Doran AK. Closed-hub systems with protected connections and the reduction of risk of catheter-related bloodstream infection in pediatric patients receiving intravenous prostanoid therapy for pulmonary hypertension. Infect Control Hosp Epidemiol 2009;30(9):823–829. [DOI] [PMC free article] [PubMed]
- 47.Mielniczuk LM, Chandy G, Stewart D, Contreras-Dominguez V, Haddad H, Pugliese C, Davies RA. Worsening renal function and prognosis in pulmonary hypertension patients hospitalized for right heart failure. Congest Heart Fail 2012;18(3):151–157. [DOI] [PubMed]
- 48.Campo A, Mathai SC, Le Pavec J, Zaiman AL, Hummers LK, Boyce D, Housten T, et al. Outcomes of hospitalisation for right heart failure in pulmonary arterial hypertension. Eur Respir J 2011;38(2):359–367. [DOI] [PubMed]
- 49.Dhillon R, Pearson GA, Firmin RK, Chan KC, Leanage R. Extracorporeal membrane oxygenation and the treatment of critical pulmonary hypertension in congenital heart disease. Eur J Cardiothorac Surg 1995;9(10):553–556. [DOI] [PubMed]
- 50.Karimova A, Brown K, Ridout D, Beierlein W, Cassidy J, Smith J, Pandya H, et al. Neonatal extracorporeal membrane oxygenation: practice patterns and predictors of outcome in the UK. Arch Dis Child Fetal Neonat Ed 2009;94(2):F129–F132. [DOI] [PubMed]
- 51.Puri V, Epstein D, Raithel SC, Gandhi SK, Sweet SC, Faro A, Huddleston CB. Extracorporeal membrane oxygenation in pediatric lung transplantation. J Thorac Cardiovasc Surg 2010;140(2):427–432. [DOI] [PubMed]
- 52.Gazit AZ, Sweet SC, Grady RM, Huddleston CB. First experience with a paracorporeal artificial lung in a small child with pulmonary hypertension. J Thorac Cardiovasc Surg 2011;141(6):e48–e50. [DOI] [PubMed]
- 53.Berger A, Edelsberg J, Teal S, Mychaskiw MA, Oster G. Changes in healthcare utilization and costs associated with sildenafil therapy for pulmonary arterial hypertension: a retrospective cohort study. BMC Pulm Med 2012;12:75. doi:10.1186/1471-2466-12-75. [DOI] [PMC free article] [PubMed]
- 54.Narine L, Hague LK, Walker JH, Vicente C, Schilz R, Desjardins O, Einarson TR, Iskedjian M. Cost-minimization analysis of treprostinil vs. epoprostenol as an alternate to oral therapy non-responders for the treatment of pulmonary arterial hypertension. Curr Med Res Opin 2005;21(12):2007–2016. [DOI] [PubMed]
- 55.Lindenauer PK, Lagu T, Shieh M-S, Pekow PS, Rothberg MB. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003–2009. JAMA 2012;307(13):1405–1413. [DOI] [PubMed]