Abstract Abstract
Left ventricular diastolic dysfunction is a well-described complication of systemic hypertension. However, less is known regarding the effect of chronic pressure overload on right ventricular (RV) diastolic function. We hypothesized that pulmonary hypertension (PHT) is associated with abnormal RV early relaxation and that this would be best shown by invasive pressure measurement. Twenty-five patients undergoing right heart catheterization for investigation of breathlessness and/or suspected PHT were studied. In addition to standard measurements, RV pressure was sampled with a high-fidelity micromanometer, and RV pressure/time curves were analyzed. Patients were divided into a PHT group and a non-PHT group on the basis of a derived mean pulmonary artery systolic pressure of 25 mmHg. Eleven patients were classified to the PHT group. This group had significantly higher RV minimum diastolic pressure ( vs.
vs. 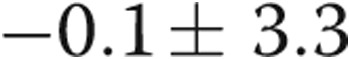 mmHg,
mmHg, 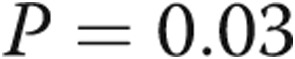 ) and RV end-diastolic pressure (RVEDP;
) and RV end-diastolic pressure (RVEDP; 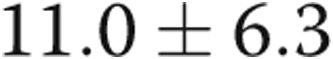 vs.
vs.  mmHg,
mmHg, 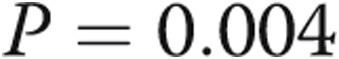 ), and RV τ was significantly prolonged (
), and RV τ was significantly prolonged (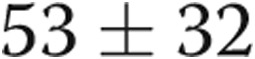 vs.
vs. 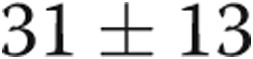 ms,
ms, 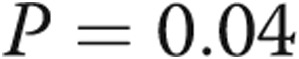 ). There were strong correlations between RV τ and RV minimum diastolic pressure (
). There were strong correlations between RV τ and RV minimum diastolic pressure ( ,
, 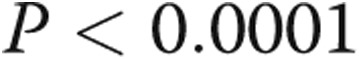 ) and between RV τ and RVEDP (
) and between RV τ and RVEDP (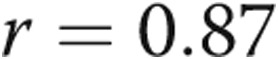 ,
, 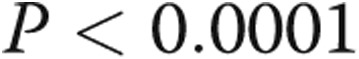 ). There was a trend toward increased RV contractility (end-systolic elastance) in the PHT group (
). There was a trend toward increased RV contractility (end-systolic elastance) in the PHT group (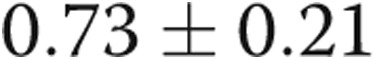 vs.
vs. 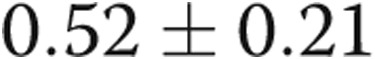 mmHg/mL,
mmHg/mL, 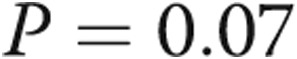 ) and a correlation between RV systolic pressure and first derivative of maximum pressure change (
) and a correlation between RV systolic pressure and first derivative of maximum pressure change (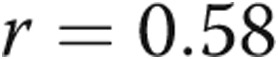 ,
, 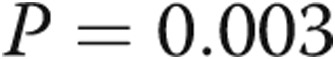 ). Stroke volumes were similar. Invasive measures of RV early relaxation are abnormal in patients with PHT, whereas measured contractility is static or increasing, which suggests that diastolic dysfunction may precede systolic dysfunction. Furthermore, there is a strong association between measures of RV relaxation and RV filling pressures.
). Stroke volumes were similar. Invasive measures of RV early relaxation are abnormal in patients with PHT, whereas measured contractility is static or increasing, which suggests that diastolic dysfunction may precede systolic dysfunction. Furthermore, there is a strong association between measures of RV relaxation and RV filling pressures.
Keywords: right ventricle, right ventricular function, diastolic function, pulmonary hypertension, heart failure
Systemic hypertension is recognized to be one of the major causes of diastolic dysfunction in the left ventricle.1 The hypertrophied myocardium becomes stiff, and abnormalities in both active myocardial relaxation and passive elastance are observed, eventually leading to an increase in left ventricular filling pressures and left atrial pressure.2,3
Evidence for a similar phenomenon occurring in the right ventricle (RV) has been sparse. Although it is a thinner-walled structure that is exposed to much lower absolute afterload, the law of Laplace dictates that the RV is subjected to increased wall stress under conditions of pulmonary hypertension (PHT).4 Compensatory RV hypertrophy is often seen.5 A recent study involving humans also demonstrated RV hypertrophy and collagen deposition, increased sarcomeric stiffness, and reduced titin phosphorylation in patients with pulmonary arterial hypertension compared with controls.6
In 1967, Burstin first described the association between PHT and the postsystolic isovolumic period in the RV, suggesting the presence of diastolic dysfunction.7 Previously, this time period has been referred to as the isovolumic relaxation period, and multiple studies have confirmed that there is a linear relationship between the length of this period and the pulmonary arterial pressure.8-10 However, recent cardiac magnetic resonance studies have demonstrated that this may be related to continued postsystolic contraction rather than a prolonged relaxation period.11,12 Nonetheless, echocardiographic studies suggest the presence of abnormal active myocardial relaxation with decreased diastolic tissue Doppler velocities in the RV free wall of patients with scleroderma and PHT.13,14
We hypothesized that PHT is associated with abnormal RV active relaxation, and in light of discrepant noninvasive data, this could be better demonstrated by an invasive gold standard. We chose the time constant of ventricular pressure decay (τ) as our primary measure of active relaxation, which is well validated in the left ventricle as a relatively preload-independent measure of early diastolic ventricular function.15
Methods
Patient selection
Patients referred to St. Vincent’s Hospital Melbourne for right heart catheterization for the investigation of breathlessness and/or suspected PHT were invited to participate. Patients with pulmonary valve stenosis, atrial fibrillation, permanent pacemaker, or congenital heart disease were excluded. This study was conducted with the approval of the human research ethics committee of St. Vincent’s Hospital Melbourne, and all patients provided written informed consent.
Study protocol
Studies were undertaken in the cardiac catheterization laboratory with subjects in the supine position. All patients underwent routine right heart catheterization using a Swan-Ganz catheter from the right femoral venous approach, and hemodynamic data were obtained in a standard fashion. A high-fidelity micromanometer mounted at the distal tip of a guide wire 0.014 inches in diameter (Radi PressureWire, St. Jude Medical) was calibrated and advanced to a stable position in the RV via a 7-Fr multipurpose guiding catheter. Continuous pressure data were obtained at 100 Hz for at least 5 cardiac cycles. Measurements for RV peak systolic pressure (RVSP) and RV minimum diastolic pressure (RVDP) were obtained. The first derivatives of maximum (RV dP/dtmax) and minimum (RV dP/dtmin) pressure change were calculated offline using raw pressure/time data imported into GraphPad Prism 6 (GraphPad Software). RV end-diastolic pressure (RVEDP) was defined as the pressure at 10% RV dP/dtmax on the upstroke of the pressure curve of the subsequent cardiac cycle. RV τ was determined using the method initially described by Weiss et al.16 RV isovolumic relaxation time was defined as the time between RV dP/dtmin and the pressure at RVEDP during initial pressure decay. Contractility and right ventriculo-arterial coupling were estimated using the single-beat method described by Brimioulle et al.17 Briefly, peak systolic pressure (Pmax) of an isovolumic beat was estimated by curve fitting time/pressure points obtained before dP/dtmax and after dP/dtmin to the expression 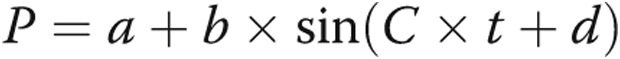 . Pmax was then obtained as
. Pmax was then obtained as 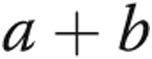 . End-systolic elastance (Ees) was then estimated as
. End-systolic elastance (Ees) was then estimated as 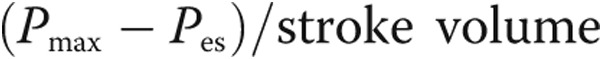 , where mean pulmonary artery pressure (PAP) was substituted for end-systolic RV pressure (Pes).18 Arterial elastance (Ea) was estimated as mean PAP/stroke volume. The coupling ratio Ees/Ea was then calculated. All measurements were averaged over 3–5 cardiac cycles.
, where mean pulmonary artery pressure (PAP) was substituted for end-systolic RV pressure (Pes).18 Arterial elastance (Ea) was estimated as mean PAP/stroke volume. The coupling ratio Ees/Ea was then calculated. All measurements were averaged over 3–5 cardiac cycles.
Patients were divided into a PHT group and a non-PHT group on the basis of a derived mean PAP (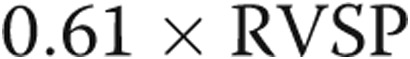 ) of 25 mmHg, consistent with current European Society of Cardiology criteria for the diagnosis of PHT.19 This was obtained from our invasive measurement of RVSP.
) of 25 mmHg, consistent with current European Society of Cardiology criteria for the diagnosis of PHT.19 This was obtained from our invasive measurement of RVSP.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6. On the basis of earlier data,20 we hypothesized a mean RV τ of 30 ms in patients without PHT with a standard deviation of 10 ms. With an α error of 0.05 and a power of 80%, we estimated a minimum sample size of 24 patients would be required to demonstrate a difference in τ of 10 ms. Results are expressed as mean ± standard deviation. Statistical significance was set at a value of 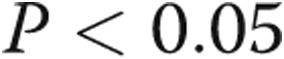 . Comparisons between patient groups were made with unpaired t tests, without correction for multiple comparisons. Correlations of RV pressure data were examined with Pearson correlations.
. Comparisons between patient groups were made with unpaired t tests, without correction for multiple comparisons. Correlations of RV pressure data were examined with Pearson correlations.
Results
Twenty-five patients were studied in total. The baseline characteristics of the two groups are presented in Table 1. Fourteen patients were classified as belonging to the non-PHT group, and 11 patients were classified as belonging to the PHT group. There were no significant differences with respect to age, sex, or body mass index. Of the patients in the PHT group, 5 were classified as having group 1 PHT (1 patient with idiopathic PHT, 4 patients with PHT associated with scleroderma), 5 had group 2 PHT (4 patients with left ventricular diastolic dysfunction, 1 patient with mitral stenosis), and 1 had group 3 PHT (interstitial lung disease). The majority of patients in the non-PHT group were in New York Heart Association functional class II, whereas the PHT group patients were split between functional classes II and III. No patient was receiving specific pulmonary vasodilator therapy for PHT, reflecting that most of the patients included in the study were in the early phase of diagnosis and treatment. With respect to hemodynamic data, the PHT group had a significantly higher PAP and pulmonary capillary wedge pressure, and there was a trend toward higher PVR. Heart rate, cardiac output, and stroke volume were similar between the two groups (Table 1).
Table 1.
Demographic characteristics and hemodynamic variables of patients with and without pulmonary hypertension (PHT)
| Variable | Non-PHT group (n = 14) |
PHT group (n = 11) |
P |
|---|---|---|---|
| Age, years | 58 ± 8 | 62 ± 13 | 0.37 |
| Male sex, no. (%) of patients | 3 (21.4) | 4 (36.4) | |
| BMI | 29.1 ± 5.9 | 28.5 ± 6.5 | 0.8 |
| Functional status | |||
| NYHA class 1∶2∶3∶4 | 1∶12∶1∶0 | 0∶6∶5∶0 | |
| Medication use | |||
| Calcium channel antagonist | 4 | 6 | |
| Hemodynamic data | |||
| PASP, mmHg | 35.4 ± 8.8 | 60.2 ± 22.8 | 0.001 |
| PADP, mmHg | 14.4 ± 5.8 | 22.4 ± 10.1 | 0.02 |
| mPAP, mmHg | 23.9 ± 7.5 | 38.5 ± 14.5 | 0.003 |
| PCWP, mmHg | 12.1 ± 5.7 | 17.5 ± 7.5 | 0.05 |
| PVR, Wood units | 2.0 ± 0.8 | 3.4 ± 3.0 | 0.11 |
| HR, beats/min | 73 ± 14 | 74 ± 14 | 0.81 |
| CO, L/min | 6.0 ± 1.7 | 6.5 ± 1.9 | 0.52 |
| SV, mL | 83.3 ± 17.7 | 89.2 ± 21.1 | 0.45 |
Data are mean value ± standard deviation, unless otherwise indicated. BMI: body mass index; NYHA: New York Heart Association; PASP: pulmonary artery systolic pressure; PADP: pulmonary artery diastolic pressure; mPAP: mean pulmonary artery pressure; PCWP: pulmonary capillary wedge pressure; PVR: pulmonary vascular resistance; HR: heart rate; CO: cardiac output; SV: stroke volume.
There was a trend toward increased RV contractility and Ea in the PHT group, but coupling ratios were preserved. The PHT group had significantly higher RVDP and RVEDP. RV τ was also significantly prolonged (Table 2). RV dP/dtmax was not significantly different between the groups, whereas the magnitude of RV dP/dtmin was significantly greater in the PHT group. There was a strong linear correlation between RV τ and RVDP (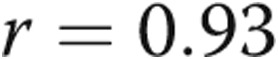 ,
, 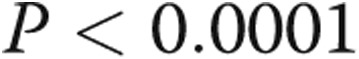 ) and between RV τ and RVEDP (
) and between RV τ and RVEDP (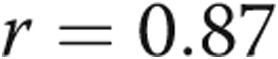 ,
, 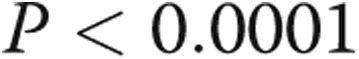 ; fig. 1). There was a strong linear correlation between RVSP and RV dP/dtmax (
; fig. 1). There was a strong linear correlation between RVSP and RV dP/dtmax (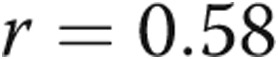 ,
, 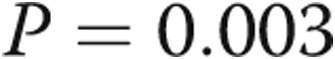 ) and a strong negative linear correlation between RVSP and RV dP/dtmin (
) and a strong negative linear correlation between RVSP and RV dP/dtmin (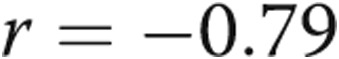 ,
, 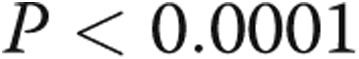 ; fig. 2). These correlations remained when each patient group was analyzed individually.
; fig. 2). These correlations remained when each patient group was analyzed individually.
Table 2.
Micromanometer and coupling data
| Variable | Non-PHT | PHT | P |
|---|---|---|---|
| Ees, mmHg/mL | 0.52 ± 0.21 | 0.73 ± 0.37 | 0.07 |
| Ea, mmHg/mL | 0.29 ± 0.08 | 0.49 ± 0.40 | 0.07 |
| Ees/Ea | 1.88 ± 0.77 | 1.69 ± 0.68 | 0.52 |
| RVEDP, mmHg | 3.8 ± 3.7 | 11.0 ± 6.3 | 0.004 |
| RVDP, mmHg | −0.1 ± 3.3 | 5.1 ± 6.6 | 0.03 |
| RVSP, mmHg | 30.0 ± 5.2 | 56.0 ± 21.5 | 0.002 |
| RV dP/dtmax, mmHg/s | 436 ± 146 | 521 ± 209 | 0.27 |
| RV dP/dtmin, mmHg/s | −304 ± 78 | −530 ± 219 | 0.006 |
| RV IVRT, ms | 38 ± 16 | 52 ± 25 | 0.13 |
| RV τ, ms | 31 ± 13 | 53 ± 32 | 0.04 |
Data are mean value ± standard deviation. PHT: pulmonary hypertension; Ees: end-systolic elastance; Ea: arterial elastance; RVEDP: right ventricular end-diastolic pressure; RVDP: right ventricle minimum diastolic pressure; RVSP: right ventricular peak systolic pressure; RV dP/dtmax: right ventricular first derivative of maximum pressure change; RV dP/dtmin: right ventricular first derivative of minimum pressure change; RV IVRT: right ventricular isovolumic relaxation time.
Figure 1.
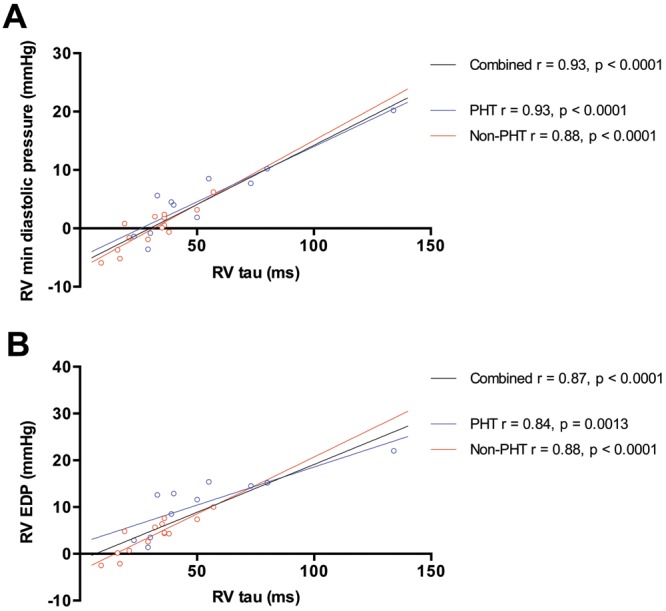
Relationship between right ventricle (RV) τ and RV minimum diastolic pressure (A) and RV end-diastolic pressure (EDP; B). Patients with pulmonary hypertension (PHT) are represented by open blue circles, and patients without PHT are represented by open red circles. Regression lines are shown for patients with PHT (blue), patients without PHT (red), and both groups combined (black).
Figure 2.
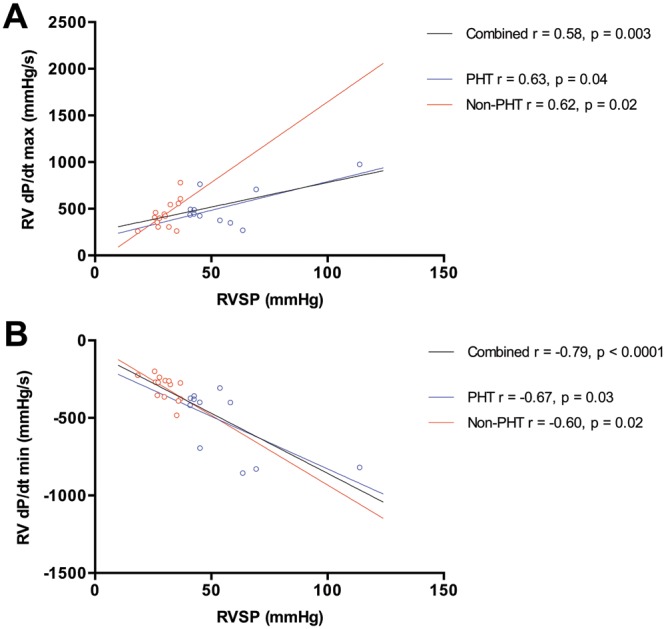
Relationship between right ventricle systolic pressure (RVSP) and RV first derivative of maximum pressure change (dP/dtmax; A) and relationship between RVSP and RV first derivative of minimum pressure change (dP/dtmin; B). Patients with pulmonary hypertension (PHT) are represented by open blue circles, and patients without PHT are represented by open red circles. Regression lines are shown for patients with PHT (blue), patients without PHT (red), and both groups combined (black).
Individual patient characteristics are presented in Table 3. Patient numbers were considered too small to undertake further subgroup analysis on the basis of etiology of PHT.
Table 3.
Individual patient characteristics
| Patient | PHT etiology | mPAP, mmHg | RVDP, mmHg | RVEDP, mmHg | RV dP/dtmin, mmHg/s | RV τ, ms |
|---|---|---|---|---|---|---|
| 1 | Non-PHT | 22.4 | 1.8 | 4.4 | −377 | 36 |
| 2 | Non-PHT | 18.2 | −1.6 | 0.7 | −364 | 21 |
| 3 | Non-PHT | 16.9 | 0.5 | 4.5 | −238 | 36 |
| 4 | Non-PHT | 15.9 | −5.2 | −2.1 | −270 | 17 |
| 5 | Non-PHT | 21.9 | −3.7 | 0.2 | −391 | 16 |
| 6 | Non-PHT | 22.4 | 2.0 | 5.7 | −274 | 32 |
| 7 | Non-PHT | 16.5 | −0.6 | 4.3 | −271 | 38 |
| 8 | Non-PHT | 18.4 | 0.1 | 6.4 | −260 | 35 |
| 9 | Non-PHT | 11.2 | −5.9 | −2.5 | −224 | 9 |
| 10 | Non-PHT | 15.6 | 3.2 | 7.4 | −200 | 50 |
| 11 | Non-PHT | 19.8 | −1.9 | 2.6 | −284 | 29 |
| 12 | Non-PHT | 16.4 | 0.8 | 4.8 | −355 | 19 |
| 13 | Non-PHT | 21.4 | 2.4 | 7.6 | −483 | 36 |
| 14 | Non-PHT | 19.4 | 6.2 | 10 | −262 | 57 |
| 15 | LVDD | 26.0 | 4.0 | 12.9 | −358 | 40 |
| 16 | LVDD | 35.5 | 10.2 | 15.2 | −401 | 80 |
| 17 | LVDD | 27.5 | 7.7 | 14.5 | −400 | 73 |
| 18 | LVDD | 25.0 | 5.6 | 12.6 | −418 | 33 |
| 19 | MS | 69.4 | 8.5 | 15.4 | −819 | 55 |
| 20 | ILD | 32.8 | 20.2 | 22.0 | −307 | 134 |
| 21 | Scleroderma | 25.0 | −0.8 | 3.5 | −373 | 30 |
| 22 | Scleroderma | 27.5 | −1.4 | 2.9 | −694 | 23 |
| 23 | Scleroderma | 42.3 | −3.6 | 1.4 | −830 | 29 |
| 24 | Scleroderma | 25.9 | 1.9 | 11.6 | −379 | 50 |
| 25 | Idiopathic | 32.8 | 4.5 | 8.5 | −856 | 39 |
PHT: pulmonary hypertension; mPAP: mean pulmonary artery pressure; RVDP: right ventricular minimum diastolic pressure; RVEDP: right ventricular end-diastolic pressure; RV dP/dtmin: right ventricular first derivative of minimum pressure change; LVDD: left ventricular diastolic dysfunction; MS: mitral stenosis; ILD: interstitial lung disease.
Discussion
We have demonstrated that an invasive measure of early RV relaxation, τ, is abnormal in patients with PHT. This finding strongly suggests that RV diastolic dysfunction does exist in this setting, despite recent data showing that the prolonged postsystolic isovolumic period in patients with PHT is related to a continued postsystolic contraction. We contend that both of these disturbances can exist simultaneously and may each contribute to increased RVDP.
In the left ventricle, τ has been validated as a relatively load-independent measure of active relaxation,16 as opposed to dP/dtmin and dP/dtmax, which have greater load dependence.21 Although active relaxation has been studied in some detail in the left ventricle, human data from the RV are sparse. Some questions exist in relation to the appropriateness of the use of τ in the RV. The time period over which τ is measured, the isovolumic relaxation period, begins with dP/dtmin. Data from a canine model have indicated that, in contrast to the left ventricle, where dP/dtmin occurs shortly after aortic valve closure, RV dP/dtmin occurs substantially before the end of RV ejection, and the normal cardiac cycle does not include an isovolumic relaxation period.22 Early human data also questioned whether an isovolumic relaxation period exists in the normal RV, given that RV dP/dtmin can occur late on the downstroke of pressure decay.23 However, the presence of an isovolumic relaxation period in healthy humans has clearly been shown in a recent study.12 Furthermore, RV τ has been shown to be prolonged in patients with hypertrophic cardiomyopathy20 and in lambs exposed to chronic pressure overload.24 We were able to measure RV τ in all of our patients, and the average time periods that τ was measured over were 38 and 52 ms in the non-PHT and PHT groups, respectively. Although shorter than similar time periods in the left ventricle, these are still measurable with high-fidelity micromanometers, and our values for τ compare favorably to other recent studies.25 Moreover, one could argue that, even if there are some patients in whom τ cannot be measured, this would be a reassuring finding suggestive of normal pulmonary pressures, and τ could still function as a useful hemodynamic index in this situation.
We found that prolonged RV τ is strongly associated with a higher RVDP and RVEDP, suggesting that impaired relaxation may impact on RV filling pressures and that RVDP was higher in patients with PHT. This phenomenon is well documented in the left ventricle26 and is responsible for considerable clinical sequelae. The systemic effect of chronically elevated right atrial pressure is no doubt also important, as shown by its impact on renal function in heart failure27 and its prognostic impact in pulmonary arterial hypertension.28-30 Moreover, left ventricular minimum diastolic pressure is important in the creation of a suction gradient to augment left ventricular filling and is an accepted index of diastolic suction in clinical studies.21,31 Negative RVDPs are frequently seen in healthy humans,23 and a canine model suggests that the RV is capable of generating an early diastolic suction gradient independent of changes in intrathoracic pressure.32 A noninvasive study has also suggested the presence of a diastolic filling gradient in humans.33 A major caveat to the above is that we do not have data on diastolic stiffness or RV volume for our patients. Further study is needed to clarify the relative impact on RV filling pressures of changes in these factors versus active relaxation.
It is plausible that abnormal relaxation precedes the development of overt systolic dysfunction in the RV. RV systolic dysfunction tends to be associated with very high PAP and is known to be a marker for poor prognosis in the disease.5 Routinely available clinical measures of RV systolic function, such as tricuspid annular plane systolic excursion and RV fractional area change measured by echocardiography, may remain normal until late in disease progression. Our data indicated a trend toward increasing contractility in the PHT group as evidenced by Ees and dP/dtmax, with preserved overall systolic function, as demonstrated by similar stroke volumes. Ventriculo-arterial coupling ratios were also preserved in the PHT group. On the other hand, RV τ is clearly impaired. Given that the patients in the PHT group had relatively mild elevations in pulmonary pressure, this finding suggests that measured RV systolic function may be falsely reassuring in the early stages of disease, whereas relaxation is abnormal. This deserves further study, because there is significant clinical need for novel approaches to the initial diagnosis and novel markers of early disease progression to help guide management of this challenging condition.
Study limitations
The relaxation phase is just one component of ventricular diastole. The other major component, filling, can best be described by a pressure/volume loop, with stiffness described by the slope of the end-diastolic pressure volume relation.34 Although this relation is curvilinear, several methods have been proposed to approximate this with as few as 2 data points.35 However, this is still not trivial, given the challenges in obtaining accurate volume data simultaneously with pressure data in the geometrically complex RV.36,37 In light of these issues and the paucity of previous data, our study has focused on the active relaxation phase of RV diastole. We believe that interrogation of individual parameters of diastole may still be useful if they are able to independently identify disease. Furthermore, our methods are easily reproducible with equipment already available in many cardiac catheter laboratories (e.g., Radi PressureWire, which is used for fractional flow reserve determination in coronary arteries to guide percutaneous coronary intervention).
We adopted an “all-comers” approach to patient recruitment, rather than use a defined control group of healthy subjects, because of the invasive nature of our study. This may have led to an underestimation of the magnitude of difference in τ, because, although these patients did not meet the formal mean PAP cut-off value for diagnosis of PHT, some of these patients had higher PAPs than would have been anticipated.
There is a possibility that different underlying etiologies of PHT result in different changes in RV adaptation. Given the relatively small number of patients studied, our study was underpowered to address this issue. There is some animal evidence that the type of PHT and location of obstruction influences changes in RV diastolic function.38 Examination of RV diastolic function in patients with different types of PHT would therefore be interesting.
Conclusion
Invasive measures of early active relaxation in the RV are abnormal in patients with PHT, even while measures of contractility are static or increasing, compared with patients with normal pulmonary pressures. This suggests that RV diastolic dysfunction may precede overt systolic dysfunction in this setting. Moreover, abnormal relaxation is associated with higher RV filling pressures, which could theoretically be clinically important. This deserves further study as a potential novel marker of early disease or disease progression, and it also provides a framework for future noninvasive study of RV diastolic function.
Acknowledgments
Craig Murch assisted in the Matlab computation of Pmax. All authors take responsibility for the reliability and freedom from bias of the data presented and their discussed interpretation.
Source of Support: This study was supported by a grant from Actelion Pharmaceuticals.
Conflict of Interest: None declared.
References
- 1.Aurigemma GP, Gaasch WH. Diastolic heart failure. N Engl J Med 2004;351(11):1097–1105. [DOI] [PubMed]
- 2.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J 2011;32(6):670–679. [DOI] [PMC free article] [PubMed]
- 3.Hay I, Rich J, Ferber P, Burkhoff D, Maurer MS. Role of impaired myocardial relaxation in the production of elevated left ventricular filling pressure. Am J Physiol Heart Circ Physiol 2005;288(3):H1203–H1208. [DOI] [PubMed]
- 4.La Gerche A, Heidbüchel H, Burns AT, et al. Disproportionate exercise load and remodeling of the athlete’s right ventricle. Med Sci Sports Exerc 2011;43(6):974–981. [DOI] [PubMed]
- 5.Champion HC, Michelakis ED, Hassoun PM. Comprehensive invasive and noninvasive approach to the right ventricle-pulmonary circulation unit: state of the art and clinical and research implications. Circulation 2009;120(11):992–1007. [DOI] [PubMed]
- 6.Rain S, Handoko ML, Trip P, et al. Right ventricular diastolic impairment in patients with pulmonary arterial hypertension. Circulation 2013;128(18):2016–2025. [DOI] [PubMed]
- 7.Burstin L. Determination of pressure in the pulmonary artery by external graphic recordings. Br Heart J 1967;29(3):396–404. [DOI] [PMC free article] [PubMed]
- 8.Gan CTJ, Holverda S, Marcus JT, et al. Right ventricular diastolic dysfunction and the acute effects of sildenafil in pulmonary hypertension patients. Chest 2007;132(1):11–17. [DOI] [PubMed]
- 9.Lindqvist P, Caidahl K, Neuman-Andersen G, et al. Disturbed right ventricular diastolic function in patients with systemic sclerosis: a Doppler tissue imaging study. Chest 2005;128(2):755–763. [DOI] [PubMed]
- 10.Dambrauskaite V, Delcroix M, Claus P, et al. Regional right ventricular dysfunction in chronic pulmonary hypertension. J Am Soc Echocardiogr 2007;20(10):1172–1180. [DOI] [PubMed]
- 11.Marcus JT, Gan CT-J, Zwanenburg JJM, et al. Interventricular mechanical asynchrony in pulmonary arterial hypertension: left-to-right delay in peak shortening is related to right ventricular overload and left ventricular underfilling. J Am Coll Cardiol 2008;51(7):750–757. [DOI] [PubMed]
- 12.Mauritz G-J, Marcus JT, Westerhof N, Postmus PE, Vonk-Noordegraaf A. Prolonged right ventricular post-systolic isovolumic period in pulmonary arterial hypertension is not a reflection of diastolic dysfunction. Heart 2011;97(6):473–478. [DOI] [PubMed]
- 13.Faludi R, Komócsi A, Bozó J, et al. Isolated diastolic dysfunction of right ventricle: stress-induced pulmonary hypertension. Eur Respir J 2008;31(2):475–476. [DOI] [PubMed]
- 14.Huez S, Roufosse F, Vachiéry J-L, et al. Isolated right ventricular dysfunction in systemic sclerosis: latent pulmonary hypertension? Eur Respir J 2007;30(5):928–936. [DOI] [PubMed]
- 15.Maurer MS, Spevack D, Burkhoff D, Kronzon I. Diastolic dysfunction: can it be diagnosed by Doppler echocardiography? J Am Coll Cardiol 2004;44(8):1543–1549. [DOI] [PubMed]
- 16.Weiss JL, Frederiksen JW, Weisfeldt ML. Hemodynamic determinants of the time-course of fall in canine left ventricular pressure. J Clin Invest 1976;58(3):751–760. [DOI] [PMC free article] [PubMed]
- 17.Brimioulle S, Wauthy P, Ewalenko P, et al. Single-beat estimation of right ventricular end-systolic pressure-volume relationship. Am J Physiol Heart Circ Physiol 2003;284(5):H1625–H1630. [DOI] [PubMed]
- 18.Chemla D, Hebert JL, Coirault C, Salmeron S, Zamani K, Lecarpentier Y. Matching dicrotic notch and mean pulmonary artery pressures: implications for effective arterial elastance. Am J Physiol 1996;271(4 Pt. 2):H1287–H1295. [DOI] [PubMed]
- 19.Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009;30(20):2493–2537. [DOI] [PubMed]
- 20.Maeda M, Yamakado T, Nakano T. Right ventricular diastolic function in patients with hypertrophic cardiomyopathy—an invasive study. Jpn Circ J 1999;63(9):681–687. [DOI] [PubMed]
- 21.Burns AT, La Gerche A, Prior DL, MacIsaac AI. Left ventricular untwisting is an important determinant of early diastolic function. JACC Cardiovasc Imaging 2009;2(6):709–716. [DOI] [PubMed]
- 22.Myhre ESE, Slinker BKB, LeWinter MMM. Absence of right ventricular isovolumic relaxation in open-chest anesthetized dogs. Am J Physiol 1992;263(5 Pt. 2):H1587–H1590. [DOI] [PubMed]
- 23.Stein PD, Sabbah HN, Mazilli M, Anbe DT. Effect of chronic pressure overload on the maximal rate of pressure fall of the right ventricle. Chest 1980;78(1):10–15. [DOI] [PubMed]
- 24.Leeuwenburgh BPJ, Steendijk P, Helbing WA, Baan J. Indexes of diastolic RV function: load dependence and changes after chronic RV pressure overload in lambs. Am J Physiol Heart Circ Physiol 2002;282(4):H1350–H1358. [DOI] [PubMed]
- 25.Okumura K, Slorach C, Mroczek D, et al. Right ventricular diastolic performance in children with pulmonary arterial hypertension associated with congenital heart disease: correlation of echocardiographic parameters with invasive reference standards by high-fidelity micro-manometer catheter. Circ Cardiovasc Imaging 2014;7:491–501. [DOI] [PubMed]
- 26.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure—abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 2004;350(19):1953–1959. [DOI] [PubMed]
- 27.Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009;53(7):589–596. [DOI] [PMC free article] [PubMed]
- 28.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Ann Intern Med 1991;115(5):343–349. [DOI] [PubMed]
- 29.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010;122(2):156–163. [DOI] [PubMed]
- 30.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010;122(2):164–172. [DOI] [PubMed]
- 31.Udelson JE, Bacharach SL, Cannon RO, Bonow RO. Minimum left ventricular pressure during beta-adrenergic stimulation in human subjects: evidence for elastic recoil and diastolic “suction” in the normal heart. Circulation 1990;82(4):1174–1182. [DOI] [PubMed]
- 32.Sabbah HN, Stein PD. Negative diastolic pressure in the intact canine right ventricle: evidence of diastolic suction. Circ Res 1981;49(1):108–113. [DOI] [PubMed]
- 33.Cortina C, Bermejo J, Yotti R, et al. Noninvasive assessment of the right ventricular filling pressure gradient. Circulation 2007;116(9):1015–1023. [DOI] [PubMed]
- 34.Vonk-Noordegraaf A, Westerhof N. Describing right ventricular function. Eur Respir J 2013;41:1419–1423. [DOI] [PubMed]
- 35.Gaasch WH, Cole JS, Quinones MA, Alexander JK. Dynamic determinants of left ventricular diastolic pressure-volume relations in man. Circulation 1975;51(2):317–323. [DOI] [PubMed]
- 36.Danton M, Greil GF, Byrne JG. Right ventricular volume measurement by conductance catheter. Am J Physiol Heart Circ Physiol 2003;285:H1774–H1785. [DOI] [PubMed]
- 37.Maughan WL, Shoukas AA, Sagawa K, Weisfeldt ML. Instantaneous pressure-volume relationship of the canine right ventricle. Circ Res 1979;44(3):309–315. [DOI] [PubMed]
- 38.Borgdorff MAJ, Bartelds B, Dickinson MG, Steendijk P, de Vroomen M, Berger RMF. Distinct loading conditions reveal various patterns of right ventricular adaptation. Am J Physiol Heart Circ Physiol 2013;305(3):H354–H364. [DOI] [PubMed]


