Abstract Abstract
Patients with end-stage renal disease (ESRD) with arteriovenous dialysis access (AVDA) can develop symptoms of heart failure and pulmonary hypertension (PH). We report on 5 patients with ESRD and AVDA who presented with shortness of breath, heart failure, and PH. All patients had partial or complete closure of AVDA and were reevaluated after AVDA revision. All 5 subjects had clinical and echocardiographic evidence of heart failure, hypertensive heart disease, left ventricular diastolic dysfunction, and PH at baseline. After complete closure (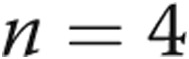 ) or partial banding (
) or partial banding (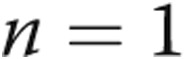 ) of AVDA, mean New York Heart Association class improved from
) of AVDA, mean New York Heart Association class improved from  to
to 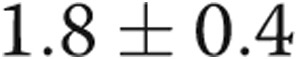 (
( ). Mean 6-minute walk distance improved from
). Mean 6-minute walk distance improved from 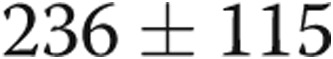 to
to 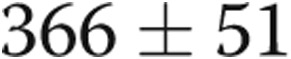 m (
m (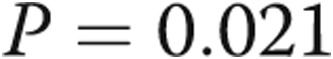 ). Serial echocardiography revealed a decrease in the right ventricle∶left ventricle ratio from
). Serial echocardiography revealed a decrease in the right ventricle∶left ventricle ratio from  to
to 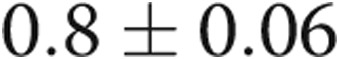 (
(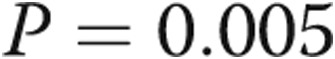 ) and improved diastolic dysfunction parameters. On right heart catheterization before definitive AVDA revision, acute manual fistula or graft occlusion led to an average decrease in cardiac output of 1.1 L/min with no other changes in hemodynamics:
) and improved diastolic dysfunction parameters. On right heart catheterization before definitive AVDA revision, acute manual fistula or graft occlusion led to an average decrease in cardiac output of 1.1 L/min with no other changes in hemodynamics:  to
to  L/min (
L/min (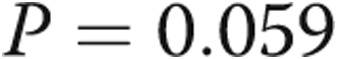 ). However, the average decrease in cardiac output after definitive revision of the AVDA (mean, 90 days) was 4.0 L/min with marked improvements in biventricular filling pressures and pulmonary artery pressure. In patients with ESRD and AVDA presenting with heart failure and PH, revision or closure of AVDA can markedly improve dyspnea as well as the clinical, echocardiographic, and hemodynamic manifestations of heart failure and PH.
). However, the average decrease in cardiac output after definitive revision of the AVDA (mean, 90 days) was 4.0 L/min with marked improvements in biventricular filling pressures and pulmonary artery pressure. In patients with ESRD and AVDA presenting with heart failure and PH, revision or closure of AVDA can markedly improve dyspnea as well as the clinical, echocardiographic, and hemodynamic manifestations of heart failure and PH.
Keywords: pulmonary hypertension, arteriovenous dialysis access, high-output heart failure, fistula, fistula closure
Pulmonary hypertension (PH) is common in end-stage renal disease (ESRD), with estimated prevalence ranging from 30% to 56% of patients.1-6 Multiple factors lead to PH in ESRD; however, the most common mechanism of PH in ESRD is passive left heart congestion due to decreased left ventricular (LV) compliance and LV diastolic dysfunction related to chronic systemic hypertension and arteriosclerosis combined with residual extracellular volume overload.1,4 An increased cardiac output (CO) can also occur, related to anemia as well as to the direct and indirect hemodynamic derangements associated with arteriovenous dialysis access (AVDA) in the form of an arteriovenous (AV) fistula or graft.7-12 The combined effects of increased left heart filling pressures and increased CO will inevitably lead to PH, whereas muscularization of the pulmonary circulation and reactive increases in the pulmonary vascular resistance will serve to further increase the pulmonary artery pressure (PAP).13-15
In patients with dyspnea and PH, the role of the AVDA must be considered, particularly given that closure of AVDA may represent a means to reverse dyspnea, heart failure, and PH in these patients. The aim of this case series is to demonstrate that closure of AVDA in patients with ESRD and dyspnea can lead to marked improvements in functional capacity as well as the clinical, echocardiographic, and hemodynamic manifestations of heart failure and PH.
Case Descriptions
Patient 1
Patient 1 was a 78-year-old man with a medical history notable for systemic hypertension and ESRD who had undergone renal transplant 3 years before presentation with a chief complaint of shortness of breath. Symptoms were consistent with New York Heart Association (NYHA) class IV functional status. He had a right brachial AV fistula that was placed 4 years earlier. Physical examination revealed a jugular venous pressure (JVP) of 14 cm of water with a prominent V-wave and 1+ lower extremity edema. Twelve-lead electrocardiogram (ECG) revealed sinus rhythm at 83 beats per minute with first-degree AV block and LV hypertrophy (LVH).
The echocardiogram (ECHO) revealed normal LV function, a moderately dilated left atrium (LA), LVH, and transmitral Doppler findings consistent with increased LA pressure. There was Doppler evidence of PH, and the right ventricle (RV) was mildly dilated with normal systolic function (Table 1).
Table 1.
Clinical and echocardiographic characteristics for each patient before and after arteriovenous dialysis access closure
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | BL | After fistula closure | BL | After fistula closure | BL | After fistula closure | BL | After fistula closure | BL | After fistula closure |
| Age, years | 78 | … | 54 | … | 70 | … | 43 | … | 75 | … |
| Sex | Male | … | Female | … | Male | … | Female | … | Female | … |
| Fistula location | Right brachial | … | Bilateral brachial | … | Left radial | … | Left femoral | … | Left brachial | … |
| NYHA | 4 | 1 | 3 | 1 | 3 | 2 | 4 | 2 | 3 | 2 |
| 6MWD, m | 384 | 438 | 228 | 375 | 347 | 396 | 91 | 329 | 128 | 292 |
| Hemoglobin, g/dL | 13 | 13.6 | 14 | 10.9 | 15.4 | 15.2 | 10 | 10.3 | 12.3 | 13.8 |
| Pulse oximetry, % | 95 | 95 | 99 | 100 | 95 | 96 | 99 | 99 | 95 | 95 |
| ECHO parameter: | ||||||||||
| RVIDed, mm | 49 | 37 | 59 | 44 | 66 | 51 | 40 | 39 | 39 | 35 |
| RV:LV ratio | 1.1 | 0.8 | 1.3 | 0.9 | 1.2 | 0.8 | 0.8 | 0.7 | 1.2 | 0.8 |
| RVOT VTI, cm | 24 | 22 | 29 | 24 | 25 | 18 | 16 | 15 | 26 | 22 |
| RVOT AccT, ms | 102 | 130 | 80 | 96 | 95 | 110 | 90 | 110 | 95 | 130 |
| PASP, mmHg | 58 | 17 | 65 | 50 | 77 | 48 | 40 | 32 | 89 | 72 |
BL: baseline; NYHA: New York Heart Association functional class; 6MWD: 6-minute walk distance; ECHO: echocardiographic; RVIDed: right ventricular internal diameter end-diastolic RV: right ventricle; LV: left ventricle; RVOT: right ventricular outflow tract; VTI: velocity time integral; AccT: acceleration time; PASP: pulmonary artery systolic pressure.
Right heart catheterization (RHC) revealed a right atrial pressure (RAP) of 13 mmHg, PAP of 78/30/46 mmHg, pulmonary arterial wedge pressure (PAWP) of 27 mmHg, CO of 10.7 L/min, cardiac index (CI) of 5.4 L/min/m2, pulmonary vascular resistance (PVR) of 1.7 Wood units, and systemic vascular resistance (SVR) of 593 dynes·s/cm5 (Table 2). During catheterization, the patient was exercised on a supine bicycle to 50 Watts, resulting in an increase in CO to 15.5 L/min and PAWP to 38 mmHg.
Table 2.
Hemodynamic characteristics for each patient before and after arteriovenous dialysis access closure
| Patient 1 | Patient 2 | Patient 3 | Patient 4a | Patient 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hemodynamic characteristic | BL | After fistula closure | BL | After fistula closure | BL | After fistula closure | BL | After fistula closure | BL | After fistula closure |
| RA, mmHg | 13 | 4 | 17 | 4 | 2 | 6 | 17 | … | 13 | 2 |
| PASP, mmHg | 78 | 37 | 101 | 40 | 71 | 65 | 45 | … | 71 | 58 |
| Mean PAP, mmHg | 46 | 20 | 62 | 25 | 34 | 33 | 31 | … | 38 | 29 |
| PAWP, mmHg | 27 | 11 | 11 | 8 | 17 | 18 | 18 | … | 22 | 11 |
| TPG, mmHg | 19 | 9 | 51 | 17 | 17 | 15 | 13 | … | 16 | 18 |
| DPG, mmHg | 3 | 1 | 29 | 7 | 5 | 2 | 0 | … | 0 | 4 |
| CO, L/min | 10.7 | 5.9 | 13.1 | 7.66 | 8.3 | 5.1 | 10.5 | … | 7.5 | 4.8 |
| CO on acute occlusion, L/min | … | … | 11.8 | … | 7.4 | … | … | … | 7 | … |
| Cardiac index, L/min/m2 | … | … | 7.7 | … | 3.7 | … | … | … | 3.8 | … |
| SVI, mL/beat/m2 | 68 | 48 | 112 | 56 | … | 40 | … | … | 68 | 40 |
| PVR, Wood units | 1.7 | 1.5 | 2.1 | 2.1 | 3.0 | 2.9 | 1.5 | … | 2.1 | 3.8 |
| SVR, dyn.s.cm−5 | 593 | 1,200 | 411 | 1,036 | 1,112 | 1,511 | 536 | … | 965 | 1,672 |
BL: baseline; RA: right atrium; PASP: pulmonary artery systolic pressure; PAP: pulmonary artery pressure; PAWP: pulmonary artery wedge pressure; TPG: transpulmonary gradient (mean  ); DPG: diastolic pulmonary gradient (diastolic
); DPG: diastolic pulmonary gradient (diastolic 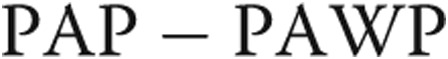 ); CO: cardiac output; SVI: stroke volume index; PVR: pulmonary vascular resistance; SVR: systemic vascular resistance.
); CO: cardiac output; SVI: stroke volume index; PVR: pulmonary vascular resistance; SVR: systemic vascular resistance.
Patient 4 declined a repeat right heart catheterization.
There was no evidence of intracardiac shunting by oximetry or echocardiographic Doppler examination. On the basis of the available data, the patient received a diagnosis of high-output congestive heart failure. The decision was made to surgically close the fistula.
Patient 2
Patient 2 was a 54-year-old woman with a medical history notable for systemic hypertension and ESRD due to glomerulonephritis; she had undergone renal transplantation 1 year before presentation. She presented with a chief complaint of exertional syncope. She also described dyspnea on exertion and NYHA functional class III limitations. She had bilateral brachial AV fistulae. The left arm fistula had been placed 15 years earlier, and the right arm fistula had been placed 2 years earlier. Physical examination revealed a JVP of 16 cm water with a prominent V-wave as well as 2+ lower extremity edema. The ECG revealed normal sinus rhythm at 60 beats per minute with first-degree AV block, incomplete right bundle branch block, and LVH.
The ECHO revealed normal LV function, LVH, and a moderately dilated LA with transmitral Doppler findings consistent with increased LA pressure. There was also Doppler evidence of PH, increased PVR, moderate RV dilatation and moderate RV systolic dysfunction (Table 1).
RHC revealed a RAP of 17 mmHg, PAP of 101/40/62 mmHg, PAWP of 11 mmHg, CO of 13.1 L/min, CI of 7.7 L/min/m2, PVR of 2.1 Wood units, and SVR of 411 dynes·s/cm5 (Table 2). After 1 minute of manual occlusion of left upper arm AV fistula, her CO decreased to 11.8 L/min and decreased further to 8.4 L/min on manual occlusion of both upper arm fistulae, with no other significant changes in her other hemodynamic characteristics.
There was no evidence of intracardiac shunting by oximetry or ECHO-Doppler examination. On the basis of the available data, she received a diagnosis of high-output congestive heart failure. The decision was made to surgically close her left upper arm AV fistula. The decision to close the left brachial fistula over the right was based on the fact that this fistula was more dilated and aneurysmal, as well as more chronic, than the right-sided fistula.
Patient 3
Patient 3 was a 70-year-old man with a medical history notable for systemic hypertension, type II diabetes, sleep apnea, and a renal transplant 2 years before presentation who presented with a chief complaint of dyspnea on exertion and NYHA functional class III limitations. He had a left radial AV fistula that was placed 7 years earlier. Physical examination revealed a JVP of 6 cm water without peripheral edema. The ECG revealed sinus bradycardia at 45 beats per minute, first-degree AV block, left bundle branch block, and LVH.
The ECHO revealed normal LV function, LVH, a dilated LA, and transmitral Doppler evidence of increased LA pressure. There was also Doppler evidence of PH, with normal RV size and function (Table 1).
RHC revealed an RAP of 2 mmHg, PAP of 71/22/34 mmHg, PAWP of 17 mmHg, CO of 8.25 L/min, CI of 3.66 L/min/m2, PVR of 3.0 Wood units, and SVR of 1,112 dynes·s/cm5 (Table 2). After 1 minute of manual fistula occlusion, the CO was 7.35 L/min with no other changes in hemodynamic characteristics. There was no evidence of intracardiac shunting by oximetry or ECHO-Doppler examination. On the basis of the available data, the patient received a diagnosis of high-output congestive heart failure. The decision was made to surgically close the fistula.
Patient 4
Patient 4 was a 43-year-old woman with a history notable for systemic hypertension, sleep apnea, and ESRD due to glomerulonephritis; she was receiving hemodialysis at the time of presentation with dyspnea on exertion and class IV functional limitations. She had a left femoral AV fistula that had been placed 10 years earlier. Physical examination revealed a JVP of 15 cm water without peripheral edema or rales. The ECG revealed normal sinus rhythm at 75 beats per minute and LVH.
With the patient lying at a 30° angle, firm pressure was held on the femoral AV fistula to temporarily occlude the fistula. With the fistula occluded, the patient was able to lie comfortably at a 30° angle for 1–2 minutes. Immediately upon releasing manual pressure on the AV fistula, the patient began to cough violently, became short of breath, and quickly sat up to catch her breath. This finding strongly suggested that flow through her AV fistula was leading to symptomatic left heart congestion.
The ECHO revealed normal LV function, a moderately dilated LA, LVH, and transmitral Doppler evidence of increased LA pressure. RV size and function were normal. Doppler methodology estimated the CO to be 10 L/min (Table 1).
An RHC had been done 6 weeks before the physical examination and showed a RAP of 17 mmHg, PAP of 45/18/31 mmHg, PAWP of 18 mmHg, CO of 10.5 L/min, CI of 5.3 L/min/m2, PVR of 1.5 Wood units, and SVR of 580 dynes·s/cm5 (Table 2).
There was no evidence of intracardiac shunting by oximetry or ECHO-Doppler examination. On the basis of the available data, the patient received a diagnosis of high-output congestive heart failure. The decision was made to partially band the fistula, thus reducing flow through the patient’s AVDA, yet allowing the patient to continue to receive hemodialysis using this access.
Patient 5
Patient 5 was a 75-year-old man with a history of systemic hypertension, diabetes, sleep apnea, aortic stenosis (status after bioprosthetic aortic valve replacement), and mitral annuloplasty 4 years before presentation as well as ESRD status post renal transplantation 2 years earlier. He presented with dyspnea on exertion and exertional lightheadedness. He reported NYHA functional class III limitations. He had a left brachial AV graft that was placed 6 years before presentation. Physical examination revealed a JVP of 15 cm of water and 2+ lower extremity edema. The ECG revealed sinus bradycardia at 52 beats per minute with bifascicular block.
The ECHO revealed normal LV function, a mildly dilated LA, LVH, and transmitral Doppler evidence of increased LA pressure. There was also Doppler evidence of PH and mild RV dysfunction (Table 1).
RHC revealed an RAP of 13 mmHg, PAP of 71/22/38 mmHg, PAWP of 22 mmHg, CO of 7.5 L/min, CI of 3.8 L/min/m2, PVR of 2.1 Wood units, and SVR of 965 dynes·s/cm5 (Table 2). Of note, the CO was 7.5 L/min at a heart rate of 56 beats per minute with a stroke volume index (SVI) of 68 mL/m2/beat. On the basis of the increase in heart rate during the 6-minute walk test, the patient’s CO would be predicted to be 9.8 L/min during mild exertion.
There was no evidence of intracardiac shunting by oximetry or ECHO-Doppler examination. On the basis of the available data, the patient received a diagnosis of high-output congestive heart failure. The decision was made to surgically close the graft.
Follow-up after fistula or graft revision
After fistula closure, all 5 patients had an improvement in heart failure symptoms (Fig. 1). The mean NYHA class improved from  to
to  (
( ). The mean JVP decreased from
). The mean JVP decreased from 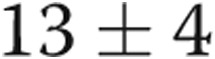 to
to 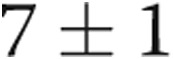 cm of water (
cm of water ( ), and lower extremity edema resolved completely. In the case of patient 1, his symptoms resolved within 1 hour of fistula closure. For patient 2, her exertional syncope completely resolved. Patients 3 and 4 had marked symptomatic improvements, and patient 4 was reactivated on the renal transplant list. Patient 5 improved sufficiently in the first 3 months after graft closure that he resumed ambulating without the use of a wheelchair. The 6-minute walk distance improved in all subjects, with the mean distance improving from
), and lower extremity edema resolved completely. In the case of patient 1, his symptoms resolved within 1 hour of fistula closure. For patient 2, her exertional syncope completely resolved. Patients 3 and 4 had marked symptomatic improvements, and patient 4 was reactivated on the renal transplant list. Patient 5 improved sufficiently in the first 3 months after graft closure that he resumed ambulating without the use of a wheelchair. The 6-minute walk distance improved in all subjects, with the mean distance improving from  to
to 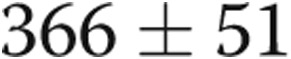 m (
m (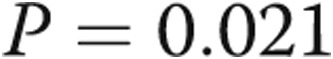 ; Table 1, Fig. 2). No patients experienced any adverse effects in terms of medical or surgical complications from the revision of the AVDA.
; Table 1, Fig. 2). No patients experienced any adverse effects in terms of medical or surgical complications from the revision of the AVDA.
Figure 1.

New York Heart Association (NYHA) functional classification at baseline and after arteriovenous dialysis access closure.
Figure 2.
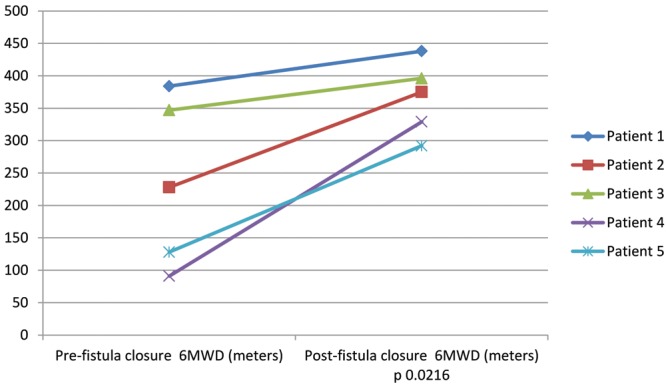
Baseline and follow-up 6-minute walk distance (6MWD) before and after arteriovenous dialysis access closure.
On ECHO, it was noteworthy that, at baseline, all patients had evidence of LVH (diastolic interventricular septal thickness, 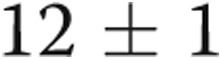 mm) and LA enlargement (systolic LA diameter,
mm) and LA enlargement (systolic LA diameter, 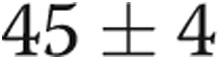 mm), whereas the LV ejection fraction was normal (LVEF,
mm), whereas the LV ejection fraction was normal (LVEF, 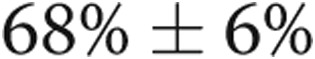 ).
).
After fistula or graft closure, repeat ECHO (mean time after closure, 135 days) revealed that the mean end-diastolic RV internal diameter decreased from  to
to 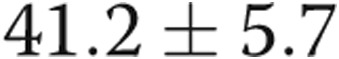 mm (
mm ( ), RV: LV ratio decreased from
), RV: LV ratio decreased from  to
to 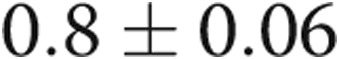 (
( ), and RV outflow track velocity time integral decreased from
), and RV outflow track velocity time integral decreased from 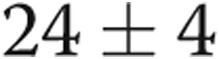 to
to  cm (
cm (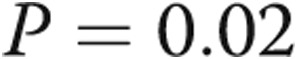 ), consistent with improved RV size, decreased right to left heart disproportion, and decreasing pulmonary blood flow, respectively.
), consistent with improved RV size, decreased right to left heart disproportion, and decreasing pulmonary blood flow, respectively.
The mean RVOT acceleration time increased from  to
to  ms (
ms (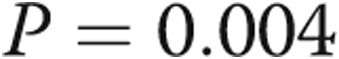 ), and pulmonary artery systolic pressure (PASP) decreased from
), and pulmonary artery systolic pressure (PASP) decreased from 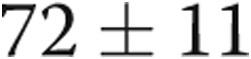 to
to 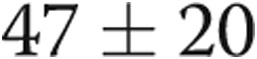 (
(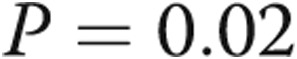 ; Table 1; Fig. 3), consistent with significant improvements in mean and systolic PAP, respectively. LV size remained unchanged in all subjects. There was a trend toward improvement in the ratio of early (E) to late (A) diastolic left heart filling, or E/A ratio, from
; Table 1; Fig. 3), consistent with significant improvements in mean and systolic PAP, respectively. LV size remained unchanged in all subjects. There was a trend toward improvement in the ratio of early (E) to late (A) diastolic left heart filling, or E/A ratio, from  to
to  (
(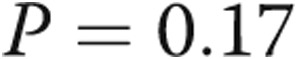 ).
).
Figure 3.
Cardiac output and echocardiographic-Doppler parameters before and after arteriovenous dialysis access closure. AccT: acceleration time; ECHO: transthoracic echocardiogram; PASP: pulmonary artery systolic pressure; RVIDed: right ventricular internal diameter end-diastolic; RVOT: right ventricular outflow tract. Data are presented as 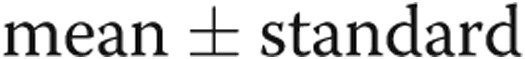 deviation. P values represent comparisons with baseline.
deviation. P values represent comparisons with baseline.
Hemodynamic characteristics
Four of the 5 subjects underwent repeat catheterization after AVDA closure. Patient 4 declined a repeat RHC. In the 3 subjects who underwent acute fistula occlusion in the catheterization laboratory by manual compression, CO acutely decreased by an average of 1.1 L/min, from 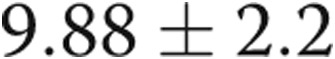 to
to 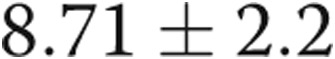 L/min (
L/min (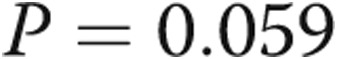 ). There were no changes in PAP, PAWP, and RAP after acute manual fistula occlusion. However, on repeat catheterization after definitive fistula or graft closure or banding (mean time after closure, 90 days), the mean decrease in CO was 4.0 L/min (Table 2). It was striking to note that the chronic decrease in CO after surgical closure was nearly 4-fold greater than the average decrease in CO seen during acute manual compression of the fistula or graft in the catheterization laboratory (Fig. 3), with marked improvements in biventricular filling pressures and PAP observed. Thus, acute fistula occlusion in the catheterization laboratory often markedly underestimated the net reduction in CO and hemodynamic improvements that were seen with chronic, definitive AVDA revision. The marked difference in the CO response to alterations in AVDA flow likely explains why hemodynamic characteristics, such as mean PAP and PAWP, did not change after acute fistula occlusion yet were markedly improved after chronic closure of the AVDA.
). There were no changes in PAP, PAWP, and RAP after acute manual fistula occlusion. However, on repeat catheterization after definitive fistula or graft closure or banding (mean time after closure, 90 days), the mean decrease in CO was 4.0 L/min (Table 2). It was striking to note that the chronic decrease in CO after surgical closure was nearly 4-fold greater than the average decrease in CO seen during acute manual compression of the fistula or graft in the catheterization laboratory (Fig. 3), with marked improvements in biventricular filling pressures and PAP observed. Thus, acute fistula occlusion in the catheterization laboratory often markedly underestimated the net reduction in CO and hemodynamic improvements that were seen with chronic, definitive AVDA revision. The marked difference in the CO response to alterations in AVDA flow likely explains why hemodynamic characteristics, such as mean PAP and PAWP, did not change after acute fistula occlusion yet were markedly improved after chronic closure of the AVDA.
PAP improved, with a decrease in PASP from 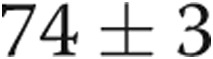 to
to 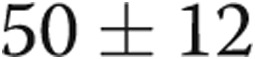 mmHg (
mmHg (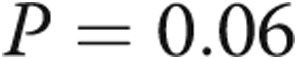 ) and a decrease in mean PAP from
) and a decrease in mean PAP from  to
to 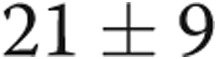 mmHg (
mmHg (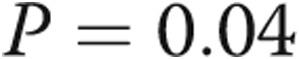 ). Biventricular filling pressures improved significantly, with the mean RA pressure decreasing from
). Biventricular filling pressures improved significantly, with the mean RA pressure decreasing from  to
to 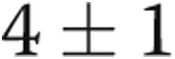 mmHg (
mmHg ( ) and PAWP decreasing from
) and PAWP decreasing from 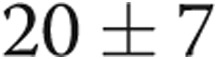 to
to 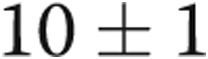 mmHg (
mmHg (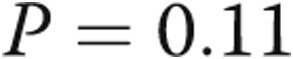 ). Transpulmonary gradient (TPG) decreased from
). Transpulmonary gradient (TPG) decreased from  to
to 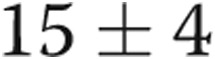 mmHg (
mmHg (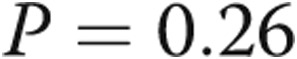 ), and diastolic PAP to PAWP gradient (DPG) decreased from
), and diastolic PAP to PAWP gradient (DPG) decreased from 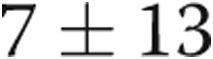 to
to  mmHg (
mmHg (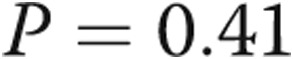 ). PVR, which was within normal range in all patients at baseline, did not change after revision of AVDA (
). PVR, which was within normal range in all patients at baseline, did not change after revision of AVDA ( to
to 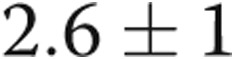 Wood units;
Wood units;  ), whereas SVR increased significantly in response to AVDA revision (
), whereas SVR increased significantly in response to AVDA revision ( to
to  dynes·s/cm5;
dynes·s/cm5;  ).
).
Discussion
In this case series, we report on the clinical, echocardiographic, and hemodynamic findings of 5 subjects with dyspnea, heart failure, and PH in association with AVDA both before and after surgical revision of AVDA. Our case series highlights that surgical closure or partial closure of AVDA in these circumstances led to marked improvements in functional capacity, hemodynamic characteristics, and overall clinical status. Several important themes emerged from our case series that may provide important insight into the significance of AVDA in the setting of dyspnea in patients with ESRD, which may inform the clinician.
Clinically, all patients had a history of systemic hypertension, and all patients manifested findings of hypertensive heart disease by echocardiography, including LVH, LA enlargement, and Doppler evidence of increased LA pressure. These findings set a critically important backdrop to these patients, because all had LV diastolic dysfunction, which would make them particularly susceptible to left heart congestion in the setting of AVDA-related increases in CO.16-20 In the absence of such findings, an increase in CO related to an AV fistula or graft is likely to be much better tolerated hemodynamically.21,22
Also of note, 4 of the 5 patients had proximal fistulae or graft access, such as brachial or femoral arteriovenous access. This is also likely relevant, because more proximal arteriovenous connection is likely more permissive to increased flow and a greater overall volume load returning to the heart.23-28 In keeping with this observation, earlier data reveal that brachial AVDA is more associated with PH than radial dialysis access. Lastly, our patients, on average, had AVDA in place for a prolonged duration of time, ranging from 6 to 15 years. Chronicity also likely plays a role in higher flow through the AVDA, given that, with time, the fistula or graft may dilate and beget higher flow.29-34
Earlier literature has emphasized the role of acute hemodynamic changes associated with fistula occlusion in the catheterization laboratory.35-37 However, our findings strongly indicate that such acute maneuvers in the catheterization laboratory can lead to a dramatic under appreciation of the overall hemodynamic sequelae of the AVDA. Of the 3 patients who underwent acute AVDA occlusion in the catheterization laboratory, none demonstrated changes in right or left heart filling pressures or PAP. Similarly, the average decrease in CO acutely was approximately 1 L/min, which suggests that this was the amount of flow through the AV fistula. However, the average reduction in CO after surgical closure of the AVDA was, in fact, 4 L/min, with dramatic reductions in RAP, PAWP, and mPAP. Why was such a large discrepancy in the hemodynamic characteristics seen after acute fistula occlusion versus chronic surgical closure? This finding speaks to the acute and direct hemodynamic effects of the AVDA as well as more chronic changes that occur in response to the arteriovenous connection.
The direct role of AVDA relates to shunting of blood flow from the high pressure/resistance of the systemic arterial system into the low pressure/resistance systemic venous system, leading to an increase in venous return and thus an increase in CO. Indirectly, the AVDA can serve as a sink to systemic arterial impedance and lesser LV afterload. The lowering of SVR may also reduce the effective circulating volume of the systemic circulation, activating arterial baroreceptor function, leading to secondary increases in cardiac sympathetic tone. This is supported by the significant increase in SVR (into a normal range) seen in our subjects after AVDA revision and may in part account for the normalization of flow seen in these subjects as LV afterload returned to a more normal range. In addition, the increased venous return permissible from the AVDA serves to increase cardiac preload, keeping the heart functioning on a higher portion of the cardiac function curve. In total, these effects can lead to marked increases in the CO in patients with AVDA, seemingly in disproportion to the cross-sectional area of the arteriovenous system or what can be appreciated through an acute assessment of flow.
Given the degree of hypertensive heart disease seen in our patients and the marked reduction in CO after surgical AVDA closure, it is logical that the overall hemodynamic profile improved to the extent observed. We observed marked reductions in biventricular filling pressures and improved PAP, whereas the PVR remained relatively similar, owing to the fact that the PVR was not an important contributor to the PH of these patients. The improved hemodynamic characteristics translated into marked improvements in functional class, 6-minute walk distance, and symptoms and signs of heart failure. In keeping with this, there were also significant improvements in several echocardiographic parameters; most notably, there were improvements in RV size and RV-to-LV proportion as well as Doppler evidence of improved LA pressure.38-40
It was interesting to note that the TPG was >12 mmHg in all patients before AVDA occlusion. However, the PVR was ≤3 Wood units in all 5 subjects, and the DPG was ≤7 mmHg in 4 of the 5 subjects, emphasizing that the PH in our subjects was largely due to the interaction of high flow and increased LA pressure as opposed to intrinsic pulmonary vascular disease. Our findings also support earlier work showing that PH reversibility is well predicted in the setting of a low DPG.41,42 In our only subject with a markedly elevated DPG, the CO and index were massively elevated, making the decision to close the fistula straightforward regardless. However, given that this patient had dramatic reversal of her PH after fistula closure, this observation also highlights a limitation of the DPG in terms of predicting intrinsic pulmonary vascular disease in the setting of very high-flow states.43
Four of 5 patients in our series had undergone renal transplantation; thus, their AVDA was not in use. One patient had not received a transplant. In this patient, not only was the AVDA in use, but the patient had many earlier failed access sites. In this patient, instead of ligating the fistula entirely, we partially banded the fistula to restrict flow but keep the AVDA viable. In this patient, symptoms and signs of heart failure and ECG-Doppler features of heart failure and PH improved dramatically and to a similar degree as in the subjects whose AVDA was ligated. The decision to ligate versus partially band a site of AVDA in the setting of high-output heart failure requires careful integration and consideration of both the hemodynamic impact of the AVDA and the potential need and alternatives for AVDA in an individual patient. In our view, if a patient has clearly demonstrated high-output heart failure that is related to the AVDA, then revision or closure of the access site should take precedence over concerns regarding the possible need for this AVDA in the future (i.e., eventual renal allograft failure) for a patient with renal transplant. In a patient currently receiving hemodialysis, AVDA revision should also take precedence, because the risks and benefits of reversing severe heart failure and PH compared with the risks and logistics of alternate means of renal replacement therapy weigh in favor of fistula banding or closure.44 In our patient who was receiving hemodialysis and under evaluation for renal transplant, heart failure and PH had led to her removal from the transplant list; after AVDA banding, her heart failure resolved, and she was reactivated for transplant. Future studies may address the issues of complete versus partial closure of AVDA as well as the optimal approach to patients both before and after receipt of a transplant more closely, which will require larger patient cohorts.
This case series highlights the clinical, echocardiographic, and hemodynamic manifestations of heart failure and PH in association with a high-flow state related to AVDA. Our findings should also serve to underscore the importance of a complete evaluation of heart failure and PH in this context. Patients should not be treated empirically for heart failure or PH in this setting, because failure to properly characterize the nature of the heart failure and PH will both delay definitive intervention and potentially increase patient morbidity. The use of pulmonary vasodilators in these patients would likely have been poorly tolerated and only served to aggravate left-sided cardiac congestion.
There are isolated reported cases of improvement of PH after closure of AVDA and acquired fistulae. Malhotra et al.45 reported a case in which closure of AVDA in a patient after receipt of transplant led to improvement of heart failure symptoms and PH based on echocardiogram and RHC. Scheir et al.46 used shunt flow as a parameter for AVDA closure and reported that patients who achieved clinical improvement after AVDA closure had a shunt flow >2,200 mL/min. In our study, we did not directly quantify flow through the AVDA before closure, given that we feel it is not the absolute flow through the AVDA itself but rather the interaction between the excess flow and the cardiovascular substrate that determines the net impact on the individual patient. A patient with severe hypertensive heart disease may prove intolerant to lesser degrees of excess flow, whereas a patient with a structurally normal heart may tolerate significantly higher flows without untoward effect. Improved outcomes have also been described in isolated reports of closure of iatrogenic arteriovenous fistulas leading to resolution of PH and heart failure symptoms.47,48
This report represents a relatively small case series with limitations that include the size of the cohort and the relatively robust phenotype of high flow, PH, and heart failure. Larger studies will be needed to gain insight into the different iterations of heart failure and PH in the setting of AVDA and whether a less severe phenotype of patient would benefit from AVDA revision in the setting of dyspnea. One of our subjects refused follow-up RHC after AVDA revision; however, the degree of improvement in heart failure and PH in this subject was commensurate with that in the other subjects and suggests that marked hemodynamic improvements would likely have been observed in this subject also.
Herein we report the baseline and follow-up clinical status of 5 patients with ESRD, dyspnea, heart failure, and PH in the setting of AVDA. After surgical revision of the AVDA, patients experienced dramatic improvements in clinical status and hemodynamic characteristics, directly implicating the AVDA as the primary cause of their condition. The potentially deleterious effects of AVDA, particularly in the setting of preexisting hypertensive heart disease, should not be underestimated. We hope that this case series sheds further insight into the importance of considering the AVDA as a potential contributor to dyspnea, heart failure, and PH in patients like this and that revision of AVDA be viewed as a potential and important therapeutic approach for such patients.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Tedford RJ, Forfia P. Hemodynamic evaluation of pulmonary hypertension in chronic kidney disease. Adv Pulmonary Hypertension 2013;12(2):82–85.
- 2.Havlucu Y, Kursat S, Ekmekci C, et al. Pulmonary hypertension in patients with chronic renal failure. Respiration 2007;74(5):503–510. [DOI] [PubMed]
- 3.Dagli CE, Sayarlioglu H, Dogan E, et al. Prevalence of and factors affecting pulmonary hypertension in hemodialysis patients. Respiration 2009;78(4):411–415. [DOI] [PubMed]
- 4.Kawar B, Ellam T, Jackson C, Kiely DG. Pulmonary hypertension in renal disease: epidemiology, potential mechanisms and implications. Am J Nephrol 2013;37(3):281–290. [DOI] [PubMed]
- 5.Yigla M, Nakhoul F, Sabag A, et al. Pulmonary hypertension in patients with end-stage renal disease. Chest 2003;123(5):1577–1582. [DOI] [PubMed]
- 6.Bozbas SS, Akcay S, Altin C, et al. Pulmonary hypertension in patients with end-stage renal disease undergoing renal transplantation. Transplant Proc 2009;41(7):2753–2756. [DOI] [PubMed]
- 7.MacRae JM, Pandeya S, Humen DP, Krivitski N, Lindsay RM. Arteriovenous fistula-associated high-output cardiac failure: a review of mechanisms. Am J Kidney Dis 2004;43(5):e17–e22. [DOI] [PubMed]
- 8.Guyton AC, Sagawa K. Compensations of cardiac output and other circulatory functions in areflex dogs with large A-V fistulas. Am J Physiol 1961;200:1157–1163. [DOI] [PubMed]
- 9.Johnson G Jr, Blythe WB. Hemodynamic effects of arteriovenous shunts used for hemodialysis. Ann Surg 1970;171(5):715–723. [DOI] [PMC free article] [PubMed]
- 10.Frank CW, Wang H, Lammerant J, Miller R, Wegria R. An experimental study of immediate hemodynamic adjustments to acute arteriovenous fistulae of various sizes. J Clin Invest 1955;34(5):722–731. [DOI] [PMC free article] [PubMed]
- 11.Ling Y, Johnson MK, Kiely DG, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension. Am J Respir Crit Care Med 2012;186(8):790–796. [DOI] [PubMed]
- 12.Fang JC, DeMarco T, Givertz MM, et al. World Health Organization Pulmonary Hypertension group 2: pulmonary hypertension due to left heart disease in the adult—a summary statement from the Pulmonary Hypertension Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2012;31(9):913–933. [DOI] [PubMed]
- 13.Sheikh AQ, Lighthouse JK, Greif DM. Recapitulation of developing artery muscularization in pulmonary hypertension. Cell Rep 6:809–817. [DOI] [PMC free article] [PubMed]
- 14.Delgado JF. Pulmonary circulation in heart failure. Rev Esp Cardiol 2010;63(3):334–345. [DOI] [PubMed]
- 15.Haddad F, Kudelko K, Mercier O, Vrtovec B, Zamanian RT, Perez VJ. Pulmonary hypertension associated with left heart disease: characteristics, emerging concepts, and treatment strategies. Prog Cardiovasc Dis 2011;54:154–167. [DOI] [PubMed]
- 16.Thenappan T, Shah SJ, Gomber-Maitland M, et al. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail 2011;4(3):257–265. [DOI] [PubMed]
- 17.Savage MT, Ferro CJ, Sassano A, Tomson CR. The impact of arteriovenous fistula formation on central hemodynamic pressures in chronic renal failure patients: a prospective study. Am J Kidney Dis 2002;40(4):753–759. [DOI] [PubMed]
- 18.Pabst S, Hammerstingl C, Hundt F, et al. Pulmonary hypertension in patients with chronic kidney disease on dialysis and without dialysis: results of the PEPPER-study. PLoS ONE 2012;7(4):e35310. [DOI] [PMC free article] [PubMed]
- 19.Basile C, Lomonte C, Vernaglione L, et al. The relationship between the flow of arteriovenous fistula and cardiac output in haemodialysis patients. Nephrol Dial Transplant 2008;23:282–287. [DOI] [PubMed]
- 20.Yigla M, Banderski R, Azzam ZS, et al. Arterio-venous access in end-stage renal disease patients and pulmonary hypertension. Ther Adv Respir Dis 2008;2:49–53. [DOI] [PubMed]
- 21.Acarturk G, Albayrak R, Melek M, et al. The relationship between arteriovenous fistula blood flow rate and pulmonary artery pressure in hemodialysis patients. Int Urol Nephrol 2008;40:509–513. [DOI] [PubMed]
- 22.Unal A, Tasdemir K, Oymak S, et al. The long-term effects of arteriovenous fistula creation on the development of pulmonary hypertension in hemodialysis patients. Hemodial Int 2010;14:398–402. [DOI] [PubMed]
- 23.Frank CW, Wang H, Lammerant J, Miller R, Wegria R. An experimental study of the immediate hemodynamic adjustments to acute arteriovenous fistulae of various sizes. J Clin Invest 1955;34(5):722–731. [DOI] [PMC free article] [PubMed]
- 24.Sapru RP, Hutchison DC, Hall JI. Pulmonary hypertension in patients with pulmonary arteriovenous fistulae. Br Heart J 1969;31(5):559–569. [DOI] [PMC free article] [PubMed]
- 25.Ahearn DJ, Maher JF. Heart failure as a complication of hemodialysis arteriovenous fistula. Ann Intern Med 1972;77:201–204. [DOI] [PubMed]
- 26.Dagli CE, Sayarlioglu H, Dogan E, et al. Prevalence of and factors affecting pulmonary hypertension in hemodialysis patients. Respiration 2009;78:411–415. [DOI] [PubMed]
- 27.Tarrass F, Benjelloun M, Medkouri G, et al. Doppler echocardiograph evaluation of pulmonary hypertension in patients undergoing hemodialysis. Hemodial Int 2006;10(4):356–359. [DOI] [PubMed]
- 28.Schinstock CA, Albright RC, Williams AW, et al. Outcomes of arteriovenous fistula creation after the fistula first initiative. Clin J Am Soc Nephrol 2011;6(8):1996–2002. [DOI] [PMC free article] [PubMed]
- 29.Patel P, Abraham G, Pratap B, et al. Clinical and biochemical parameters in chronic kidney disease with pulmonary hypertension. Indian J Nephrol 2007;17(1):4–6.
- 30.Ozdogan O, Kayikcioglu M, Asci G, et al. Left atrial volume predicts mortality in low-risk dialysis population on long-term low-salt diet. Am Heart J 2010;159(6):1089–1094. [DOI] [PubMed]
- 31.Havlucu Y, Kursat S, Ekmekci C, et al. Pulmonary hypertension in patients with chronic renal failure. Respiration 2007;74:503–510. [DOI] [PubMed]
- 32.Acarturk G, Albayrak R, Melek M, et al. The relationship between arteriovenous fistula blood flow rate and pulmonary artery pressure in hemodialysis patients. Int Urol Nephrol 2008;40(2):509–513. [DOI] [PubMed]
- 33.Yigla M, Fruchter O, Aharonson D, et al. Pulmonary hypertension is an independent predictor of mortality in hemodialysis patients. Kidney Int 2009;75:969–975. [DOI] [PubMed]
- 34.Ramasubbu K, Deswal A, Herdejurgen C, Aguilar D, Frost AE. A prospective echocardiographic evaluation of pulmonary hypertension in chronic hemodialysis patients in the United States: prevalence and clinical significance. Int J Gen Med 2010;3:279–286. [DOI] [PMC free article] [PubMed]
- 35.Epstein FH, Post RS, McDowell M. The effect of an arteriovenous fistula on renal hemodynamics and electrolyte excretion. J Clin Invest 1953;32(3):233–241. [DOI] [PMC free article] [PubMed]
- 36.Johnson GJ, Blythe WB. Hemodynamic effects of arteriovenous shunts used for hemodialysis. Ann Surg 1970;171(5):715–723. [DOI] [PMC free article] [PubMed]
- 37.Velez-Roa S, Neubauer I, Wissing M, et al. Acute arterio-venous fistula occlusion decreases sympathetic activity and improves baroreflex control in kidney transplanted patients. Nephrol Dial Transplant 2004;19(6):1606–1612. [DOI] [PubMed]
- 38.Philippe U, Martin WK, Luc DP, Jolanta N, Philippe VDB. Reduction of left ventricular diameter and mass after surgical arteriovenous fistula closure in renal transplant recipients. Transplantation 2002;74(1):73–79. [DOI] [PubMed]
- 39.Unger P, Velez-Roa S, Wissing KM, Anh Dung Hoang AD, Borne PVD. Regression of left ventricular hypertrophy after arteriovenous fistula closure in renal transplant recipients: a long-term follow-up. Am J Transplant 2004;4(12):2038–2044. [DOI] [PubMed]
- 40.van Duijnhoven EC, Cheriex EC, Tordoir JH, Kooman JP, van Hooff JP. Effect of closure of the arteriovenous fistula on left ventricular dimensions in renal transplant patients. Nephrol Dial Transplant 2001;16(2):368–372. [DOI] [PubMed]
- 41.Gerges C, Gerges M, Lang MB, et al. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest 2013;143(3):758–766. [DOI] [PubMed]
- 42.Naeije R, Vachiéry JL, Yerly P, Vanderpool R. The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. Eur Respir J 2013;41(1):217–223. [DOI] [PubMed]
- 43.Abassi Z, Nakhoul F, Khankin E, et al. Pulmonary hypertension in chronic dialysis patients with arteriovenous fistula: pathogenesis and therapeutic prospective. Curr Opin Nephrol Hypertens 2006;15(4):353–360. [DOI] [PubMed]
- 44.Quarello F, Forneris G, Borca M, Pozzato M. Do central venous catheters have advantages over arteriovenous fistulas or grafts? J Nephrol 2006;19:265–279. [PubMed]
- 45.Malhotra K, Dhawan V, Dalal P, et al. Decompensated high-output congestive heart failure in a patient with AVF and the role of right heart catheterization: a case study. Hemodial Int 2012;16:S58–S61. [DOI] [PubMed]
- 46.Schier T, Göbel G, Bösmüller C, et al. Incidence of arteriovenous fistula closure due to high output cardiac failure in kidney transplanted patients. Clin Transplant 2013;27:858–865. [DOI] [PubMed]
- 47.Gerke AK, Wilson J. Complete resolution of severe high output heart failure and pulmonary hypertension after repair of longstanding arteriovenous fistula. Chest 2007;132(4):729a.
- 48.Sopeña B, Gimena B, Pérez-Rodríguez MT, Rivera A. An unusual cause of pulmonary hypertension and refractory edema. Int J Cardiol 2011;148:e1–e2. [DOI] [PubMed]



