Abstract
Daphnanes and tiglianes are diterpenes with a shared tricyclic 5-7-6 ring system. Many members exhibit significant biological activities often associated with protein kinase C signaling. Many of these natural products (~100) have a C6-C7 α-epoxide whose influence on biological activity is little studied. Using the more readily available phorbol ester PDBu as a test substrate, we report an efficient, and potentially general, α-epoxidation method based on a vanadium-catalyzed asymmetric epoxidation with bishydroxamic acid (BHA) ligands.
Keywords: Protein kinase C, Daphnane, Tigliane, Epoxidation
1. Introduction
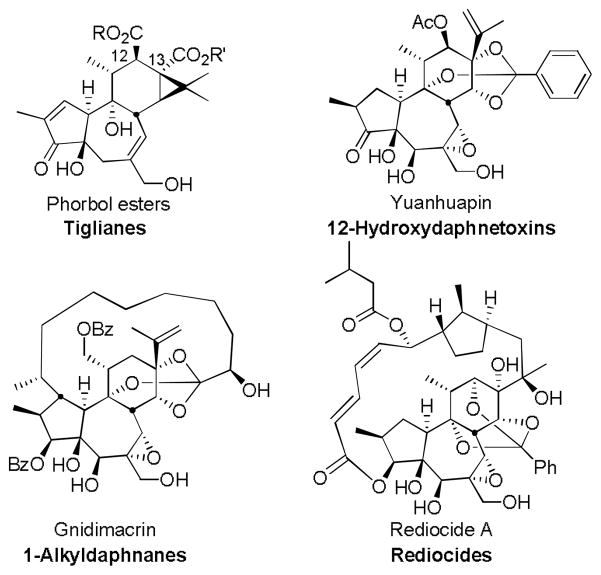
The daphnane and tigliane diterpenes are large families of structurally complex and densely functionalized natural products that share a common 5-7-6 tricyclic ring system.1 Plant extracts of the Euphorbiaceae and Thymelaeaceae families, from which these diterpenes are derived, have been used for medicinal purposes for millennia.2 Only recently, however, have scientists begun to structurally characterize the active compounds and elucidate their molecular modes of action. Representative members of these families (Figure 1) include the phorbol esters, yuanhuapin,3,4 gnidimacrin,5 and rediocide A6 which, along with many other family members, have emerged as significant therapeutic leads and research tools.1 The phorbol esters, for example, are the most studied and potent tumor promoters known7 while prostratin, 12-deoxy-phorbol 13-acetate, is a preclinical lead in studies directed at an as yet unattained but hugely important goal, the eradication of HIV/AIDS.8,9 Other members of these families have shown promising activity as antiviral, anti-cancer, neurotropic and insecticidal agents.10,11,1
Figure 1.
Representative members of the daphnane and tigliane families
The biological activities exhibited by many of these diterpenes are associated with their modulation of one or more protein kinase C (PKC) isoforms.7,12 These isoforms play important roles in several signal transduction cascades and respond to many stimulators including hormones, growth factors and other ligands related to membrane receptors. Separately or in combination, PKC isoforms are implicated in numerous clinical indications including cancer,13 Alzheimer’s disease,14 cardiovascular disease,15 immune and inflammatory diseases,16 metabolic disorders,17 stroke,18 and atherosclerosis.19 While relatively little is known about the PKC isoform selectivities of the PKC active diterpenes, some agents have shown promising PKC selective activity and therapeutic potential. Gnidimacrin (NSC 252940),20 for example, is a member of the daphnane family and shows potent antitumor activity against murine tumors in vivo and several human tumor cell lines in vitro.21 Importantly, gnidimacrin is proposed to operate by activation of PKC βII, arresting the cell cycle at the G1 phase through inhibition of cyclin-dependent kinase-2 (cdk2) activity in human K562 leukemia cells.
Studies on the modes of action and therapeutic potential of tigliane and daphnane diterpenes have been hampered by their scarce and often variable supply, high cost (generally >$50/mg), difficulty in accessing sources due to geopolitical issues, and challenges associated with their synthesis and chemical modification. To date, phorbol22 is the only tigliane and resiniferatoxin23 is the only daphnane for which total syntheses have been reported. A semi-synthesis of prostratin from phorbol has also been reported,24 enabling synthetic access to more potent analogs now being studied as latency reversing agents in strategies to eradicate HIV.25 In 2011, we also reported a study directed at producing an advanced daphnane precursor that could be used to synthetically access most members of the large daphnane diterpene family. This “gateway strategy” resulted in the synthesis of a general precursor to potentially >70 daphnanes and led to the synthesis of des-epoxy-yuanhuapin.4 This study also led to the identification of PKC as a target for yuanhuapin and the additional finding that des-epoxy-yuanhuapin is a potent PKC modulator with a Ki of 1.6 nM.
In the course of our studies on yuanhuapin,4 we found that epoxidation of des-epoxy-yuanhuapin resulted in exclusive formation of C6,C7-epi-yuanhuapin. In other words, epoxidation of the C6,C7 double bond occurred exclusively on the β-face. Notwithstanding the presence of the α-epoxide in most daphnanes, this epoxidation problem and stereochemistry have received little attention. Tyler and Howden have reported that under conditions similar to the ones used with des-epoxy-yuanhuapin to make the β-epoxide, phorbol 12,13-dibenzoate was converted to the α-epoxide.26 This stands in contrast to the yuanhuapin study4 and an earlier report by Hecker and Schmidt that epoxidation of phorbol 12,13,20-triacetate proceeds on the β-face of the C6,C7 double bond (Scheme 1).27 Given that over 90 members of the daphnane family possess an α-epoxide and that epoxidation would preferably be done as a final synthetic step when most delicate functionalities would be in place, we sought, as described herein, to determine the intrinsic facial selectivity for direct epoxidations of such complex targets and to develop mild strategies to selectively access either epoxide as needed for synthesis and structure-function studies.
Scheme 1.

Hecker’s β-face epoxidation of phorbol-triacetate 27
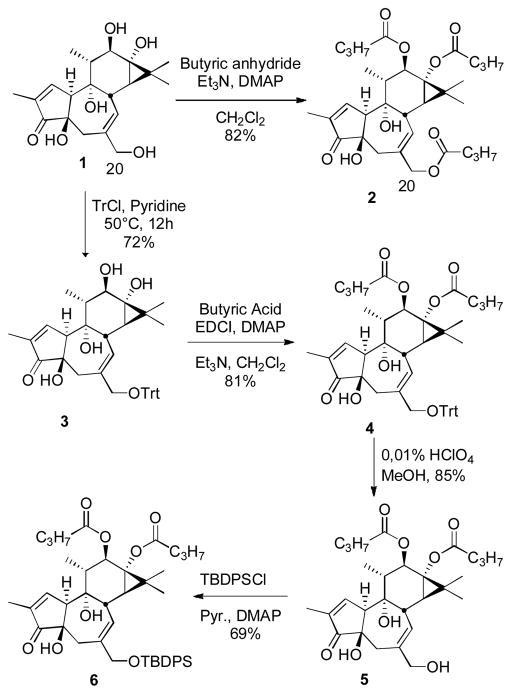
To establish a reliable protocol for stereoselective epoxidation of tiglianes and daphnanes, the readily available phorbol 12,13-dibutyrate (PDBu, 5) was selected as our test system. Phorbol (1) itself was obtained from abundantly available croton oil by first hydrolyzing various naturally occurring ester derivatives in the oil and extracting the resultant free phorbol in 1.6% yield after flash chromatography (see supporting information). Phorbol was then converted to PDBu (5) by a standard three-step procedure (Scheme 2). Direct epoxidation of PDBu or a C20 protected derivative led to β-face epoxidation, consistent with our yuanhuapin (daphnane) study and the earlier report of Hecker and Schmidt on the tigliane phorbol triacetate (Scheme 1). This is consistent with the phorbol B-ring assuming a conformation with a fold between C4 and C8 and thus a more accessible convex β-face. In cases where a direct epoxidation gives the undesired stereoisomer, one can often produce the desired epoxide isomer by using a two-step procedure in which a bromine is delivered to the more accessible substrate face to form a bromonium ion which with water would give a halohydrin whose closure in base would give the complementary epoxide stereochemistry.28 However, when PDBu 5 is treated with Br2 in a mixture of acetone and water (1:1), it undergoes preferential oxidation to the C20 aldehyde with only a small amount of the desired bromohydrin (<5%) being formed. The same results were observed using NBS instead of Br2 on the same substrate. It was expected that the C20 butyric acid ester 2 could prevent this oxidation. However, the reaction with PTBu 2 and 1.3 eq. of NBS was very slow and again when heated led to aldehyde formation presumably by hydrolysis of the primary ester and subsequent allylic alcohol oxidation.
Scheme 2.
Synthesis of PTBu 3, C20-Trityl PDBu 4 and C20-TBDPS PDBu 6
Another useful reagent for bromonium ion formation is N-bromoacetamide (NBA).29 NBA has been used in steroid syntheses to produce epoxides complementary in stereochemistry to those obtained with peroxyacids such as mCPBA.30 In our case, the same conditions (NBA in dioxane/water and a catalytic amount of HClO4) led again to the C20 aldehyde and many other unidentified products, likely resulting from acid-catalyzed opening of the cyclopropane (D) ring. In 2002, White reported the formation of bromohydrins from silyl ether protected allylic alcohols using NBS/H2O in THF.31 This procedure proceeds without deprotection and oxidation of the alcohol. To test this method, we prepared the C20-TBDPS PDBu 6 from phorbol in 4 steps (Scheme 2). Unfortunately, the reported conditions also led to oxidation of 6 to the C20 aldehyde, proceeding in 6 h at 0°C and in nearly quantitative yield. The same results were observed with a trityl protecting group (Table 1).
Table 1.
Halohydrin formation attempts from PDBu derivatives

| |||
|---|---|---|---|
| Entry | R | Conditions | Results |
| 1 | H | 1.3 eq. NBS. Acetone/H2O, rt | Mixture, C20 Aldehyde |
| 2 | COC3H7 | 1.1 eq. NBS, DME/H2O (16:1), 0°C to rt | No Rxn |
| 3 | COC3H7 | NBA, HClO4, Dioxane/H2O, rt | No Rxn |
| 4 | COC3H7 | NBA, HClO4, Dioxane/H2O, 50°C | Mixture, C20 Aldehyde |
| 5 | Trityl | NBS, DME/H2O, rt, 3h | > 75% of C20 aldehyde |
| 6 | Trityl | NBS, CaCO3, DME/H2O, 0°C to rt | No Rxn |
| 7 | Trityl | NBS, H2O, DMSO/THF (4:1), 0°C to rt | C20 aldehyde |
| 8 | Trityl | I2, Cu(OAc)2, Dioxane/H2O, 0 to 60°C, 36h | No Rxn |
| 9 | TBDPS | 1.1 eq. NBS, H2O/THF, 0°C | C20 Aldehyde |
| 10 | TBDPS | 1.1 eq. NBS, H2O/THF, NaOAc, rt | No Rxn |
| 11 | TBDPS | 4 eq. NBS, H2O/THF, NaOAc, rt | Dibrominated |
Due to competitive oxidation at C20 in procedures directed at halohydrin formation, we next decided to explore a different strategy involving reagent-controlled epoxidation. Dioxiranes from chiral alcohols are known to react with a variety of alkenes to produce epoxides in a highly enantioselective fashion.32 For our studies, the Shi D-fructose ligand 8 was synthesized in 2 steps from the commercially available D-fructose. Reaction of C20-trityl PDBu 4 with this ketone in the presence of oxone and K2CO3 under reported conditions cleanly produced an epoxide 7 in nearly quantitative yield (Scheme 3). Unfortunately, this proved to be the β-epoxide. The enantiomeric ketone (ent-8) was then prepared in 8 steps from L-sorbose.33 Unfortunately, this system did not react with C20-trityl PDBu 4 at room temperature and at higher temperature decomposed.
Scheme 3.
Reaction under Shi epoxidation conditions with catalyst 8 and ent-8
The Sharpless asymmetric epoxidation represents another procedure for controlling facial selectivity in epoxidations of allylic alcohols and thus for overcoming intrinsic substrate facial selectivity through catalyst control.34 However, with PDBu 5 this epoxidation was especially slow and variable and led to only trace amounts of the α-epoxide. In the best case, a 1.5:1 mixture in favor of the β-epoxide was obtained using 10 eq. of Ti(OiPr)4, 12 eq. of (+)-DIPT and 2 eq. of TBHP after 7 days of reaction in dichloromethane at −20°C. While not optimal, the formation of some of the α-epoxide provided encouragement that the intrinsic facial barrier presented by the substrate could be overcome through reagent control.
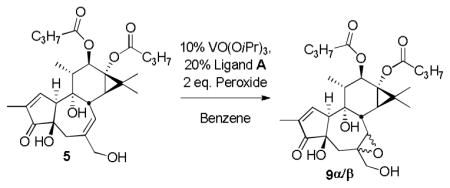
Yamamoto and co-workers reported a method for the facially selective epoxidation of a variety of allylic alcohols using VO(OiPr)3 modified with a chiral (1S,2S)-N,N′-Dihydroxy-N,N′-bis(diphenylacetyl)-1,2-cyclohexane-diamine (BHA) ligand. With 2 mol% of the BHA ligand and 1 mol% of VO(OiPr)3 and tert-butyl hydroperoxide (TBHP) in air at temperatures between 0°C and −20°C, many allylic alcohols are efficiently converted to epoxy alcohols with high enantioselectivities.35 Significantly, in our studies, using 10% of VO(acac)2 and Yamamoto ligand A for 12h at room temperature in dichloromethane, we observed the conversion of PDBu 5 to a 50:50 mixture of epoxides in 80% isolated yield (Scheme 4). The use of VO(OiPr)3 was subsequently found to be more reproducible. Using our preferred conditions (10% of VO(OiPr)3, 20% of ligand A, and 2 eq. of aqueous t-butylhydroperoxide), a screen of different solvents was then performed at different temperatures (Table 2). As previously shown, when no ligand was added, only β-epoxide was observed. No difference in selectivity was observed using dichloromethane, toluene, or chloroform. Running the reaction in benzene led to a ratio of 55 to 45 in favor of the α-epoxide in less than 12h. Progress was being made.
Scheme 4.
Vanadium epoxidation with Yamamoto ligand A
Table 2.
Solvent effect on Yamamoto epoxidation with PDBu and ligand A

| |||||
|---|---|---|---|---|---|
| Entry | Solvent | Time (h) | Conversion (%) | Yield (%) | (α:β)a |
| 1 | CH2Cl2 | 24 | 100 | 81 | 1:1 |
| 2 | PhMe | 24 | 100 | 84 | 1:1 |
| 3 | Benzene | 12 | 100 | 84 | 55:45 |
| 4 | Acetone | 24 | 48 | 44 | 22:78 |
| 5 | THF | 24 | 65 | 62 | β major |
| 6 | CHCl3 | 24 | 91 | 81 | 1:1 |
The ratios of 9α to 9β were determined by 1H NMR of the crude mixture using the C18 1H-NMR signal
The effect of the peroxide source was also investigated (Table 3). The selectivity is slightly better with tert-butyl hydroperoxide in decane and the reaction proceeds in approximately the same yield. Aqueous t-butyl hydroperoxide (TBHP) and cumene hydroperoxide gave slightly decreased selectivities and increased reaction times. As expected, the temperature of the reaction also affects the selectivity. Selectivity is slightly increased at 4°C relative to room temperature without any effect on conversion. However, the time of the reaction is much longer. At −20°, the reaction is very slow and incomplete after 3 weeks.
Table 3.
Peroxide effect on Yamamoto epoxidation with PDBu and ligand A
| |||||
|---|---|---|---|---|---|
| Entry | Peroxide | Time (h) | Conversion (%) | Yield (%) | (α:β) |
| 1 | Aq. TBHP | 24 | 100 | 84 | 55:45 |
| 2 | TBHP (5.5M in decane) | 24 | 100 | 79 | 60:40 |
| 3 | Cumene hydroperoxide | 48 | 80 | 82 | 55:45 |
Finally, Yamamoto also demonstrated that the ligand can modulate diastereoselectivity. Using the best conditions developed so far, epoxidation of PDBu 5 using ligand C led to a ratio of 80:20 in favor of the α-epoxide in very good yield in about 12 h at room temperature. Decreasing the reaction temperature to 4°C increases the selectivity to produce the α-epoxide in an 89:11 ratio. Moreover, with ligand C, the reaction time was considerably decreased compared to ligand A and B while still providing an excellent yield (90%) of product (Table 4).
Table 4.
Ligand effect on Yamamoto epoxidation with PDBu

| ||||||
|---|---|---|---|---|---|---|
| Entry | Liganda | Temp | Time (h) | Conv. (%) | Yield (%) | (α:β) |
| 1 | A | 0°C to r.t. | 12 | 100 | 84 | 60:40 |
| 2 | A | 4°C | 96 | 100 | 78 | 63:37 |
| 3 | B | 0°C to r.t | 36–48 | 100 | 85 | 75:25 |
| 4 | B | 4°C | 120 | 100 | 73 | 79:19 |
| 5 | C | 0°C to r.t | >12 | 100 | 89 | 80:20 |
| 6 | C | 4°C | 24 | 100 | 90 | 89:11 |
Ligands A-C shown in Scheme 4
In summary, we have established that epoxidation of phorbol (tigliane) and des-epoxy-yuanhuapin (daphnane) derivatives, and thus by analogy structurally related tiglianes and daphnanes, with peracids occurs preferentially from the convex β-face of the Bring and is not assisted by directed delivery of the C9-OH. Direct epoxidation leads to formation of the β-epoxide with high selectivity. This would be expected for most daphnanes and tiglianes that share a similar B-ring conformation and thus preferred convex face accessibility. While there are many methods that have been developed with simple substrates to reverse such a preference using reagent-based facial control, many of these methods encounter problems as found here with the more complex, densely functionalized and labile structures represented by phorbol derivatives. Moreover, many of these compounds undergo competitive, if not exclusive, oxidation of the allylic alcohol functionality. Many members of these families are also sensitive to acid and base, thus limiting reagent selection. Importantly, however, with respect to syntheses and structure-function studies in the tigliane and daphnane series, we have now established a procedure, based on elegant studies by the Yamamoto group, for the selective formation of the naturally occurring α-epoxide. This study produces the first α-epoxide in the phorbol series and the opportunity to begin to explore the role of this functionality in the many activities associated with phorbol derivatives and the large tigliane and daphnane families. This study also provides a more exacting synthetic reference point for testing existing or emerging epoxidation strategies and reagents and now opens the way for synthesis and structure function studies related to biologically promising tigliane and daphnane diterpenes. Studies on the application of this methodology to the less readily available alkene precursors of tigliane and daphnane epoxides will be reported in due course.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health (CA31841; CA31845). Pierre-Luc Boudreault acknowledges FQRNT for award of a Postdoctoral Research Fellowship.
Footnotes
Dedication
This manuscript is dedicated to the memory of Harry Wasserman, an inspiring scientist, teacher, artist, and friend.
Supplementary data (experimental conditions, spectroscopic data (1H and 13C NMR spectra) and HRMS) associated with this article can be found, in the online version.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.For recent reviews, see: Vasas A, Hohmann J. Chem Rev. 2014;114:8579–8612. doi: 10.1021/cr400541j.Liao SG, Chen HD, Yue JM. Chem Rev. 2009;109:1092–1140. doi: 10.1021/cr0782832.
- 2.Appendino G, Szallasi A. Life Sci. 1997;60:681–696. doi: 10.1016/s0024-3205(96)00567-x. [DOI] [PubMed] [Google Scholar]
- 3.Hu B-H, Huai S, He Z-W, Wu X-C. Acta Chim Sinica. 1986;44:843–845. [Google Scholar]
- 4.Wender PA, Buschmann N, Cardin NB, Jones LR, Kan C, Kee JM, Kowalski JA, Longcore KE. Nat Chem. 2011;3:615–619. doi: 10.1038/nchem.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kupchan SM, Shizuri Y, Murae T, Sweeny JG, Haynes HR, Shen MS, Barrick JC, Bryan RF. J Am Chem Soc. 1976;98:5719–5720. doi: 10.1021/ja00434a063. [DOI] [PubMed] [Google Scholar]
- 6.Jayasuriya H, Zink DL, Singh SB, Borris RP, Nanakorn W, Beck HT, Balick MJ, Goetz MA, Slayton L, Gregory L, Zakson-Aiken M, Shoop W, Singh SB. J Am Chem Soc. 2000;122:4998–4999. [Google Scholar]
- 7.Griner EM, Kazanietz MG. Nat Rev. 2007;7:281–284. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 8.For the current status of latency-reversing agents in HIV therapy research along with challenges of this treatment method, see: Chan CN, Dietrich I, Hosie M, Willet BJ. Gen Virol. 2013;94:917–932. doi: 10.1099/vir.0.049296-0.Bullen CK, Laird G, Durand C, Siliciano J, Siliciano R. Nat Med. 2014;20:425–430. doi: 10.1038/nm.3489.Archin N, Margolis D. Curr Opin Infect Dis. 2014;27:29–35. doi: 10.1097/QCO.0000000000000026.Gulakowski RJ, McMahon JB, Buckheit RW, Jr, Gustafson KR, Boyd MR. Antiviral Res. 1997;33:87–97. doi: 10.1016/s0166-3542(96)01004-2.
- 9.AIDS Research Alliance. [Accessed November 18, 2014];Our Progress in Developing Prostratin. Available at http://aidsresearch.org/cure-research/our-progress.
- 10.He W, Cik M, Appendino G, Puyvelde LV, Leysen JE, DeKimpe N. Mini-Rev Med Chem. 2002;2:185–200. doi: 10.2174/1389557024605492. [DOI] [PubMed] [Google Scholar]
- 11.Wang HB, Liu LP, Wang XY. Magn Reson Chem. 2013;51:580–592. doi: 10.1002/mrc.3978. [DOI] [PubMed] [Google Scholar]
- 12.For reviews of PKC, see: Steinberg S. Physiol Rev. 2008;88:1341–1378. doi: 10.1152/physrev.00034.2007.Newton A. Am J Physiol Endocrinol Metab. 2010;298:E395–E402. doi: 10.1152/ajpendo.00477.2009.Mochly-Rosen D, Das K, Grimes K. Nat Rev Drug Discovery. 2012;11:937–954. doi: 10.1038/nrd3871.Wu-Zhang A, Newton A. Biochem J. 2013;452:195–209. doi: 10.1042/BJ20130220.
- 13.Kang J-H. New J Sci. 2014:Article ID 231418. [Google Scholar]
- 14.(a) Etcheberrigaray R, Tan M, Dewachter I, Kuipéri C, Van der Auwera I, Wera S, Qiao L, Bank B, Nelson TJ, Kozikowski AP, Van Leuven F, Alkon DL. Proc Natl Acad Sci USA. 2004;101:11141–11146. doi: 10.1073/pnas.0403921101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Khan TK, Nelson TJ, Verma VA, Wender PA, Alkon DL. Neurobiol Dis. 2009;34:332–339. doi: 10.1016/j.nbd.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Sun M-K, Alkon DL. Prog In Molec Biol And Translational Sci. 2014;122:31–59. doi: 10.1016/B978-0-12-420170-5.00002-7. [DOI] [PubMed] [Google Scholar]; (d) Lucke-Wold BP, Turner RC, Zhenjun T, Rosen CL, Logsdon AF, Smith KE, Huber JD, Simpkins JW, Chen Y-W, Alkon DL. J Alz Dis. 2014 ASAP. [Google Scholar]
- 15.(a) Churchill E, Budas G, Vallentin A, Koyanagi T, Mochly-Rosen D. Ann Rev Pharmacol Tox. 2008;48:569–599. doi: 10.1146/annurev.pharmtox.48.121806.154902. [DOI] [PubMed] [Google Scholar]; (b) Palaniyandi SS, Sun L, Ferreira JCB, Mochly-Rosen D. Cardio Res. 2009;82:229–239. doi: 10.1093/cvr/cvp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Lee MR, Duan W, Tan S. Expert Op Ther Targets. 2008;5:535–552. doi: 10.1517/14728222.12.5.535. [DOI] [PubMed] [Google Scholar]; (b) Shaha SP, Tomic J, Shi Y, Pham T, Mero P, White D, He L, Baryza JL, Wender PA, Booth JW, Spaner DE. Clin Exp Immunol. 2009;158:186–198. doi: 10.1111/j.1365-2249.2009.04003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farese RV, Sajan MP. Curr Op Lipidology. 2012;23:175–181. doi: 10.1097/MOL.0b013e328352c4c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Bright R, Mochly-Rosen D. Stroke. 2005;36:2781–2790. doi: 10.1161/01.STR.0000189996.71237.f7. [DOI] [PubMed] [Google Scholar]; (b) Sun M-K, Hongpaisan J, Alkon DL. Proc Natl Acad Sci USA. 2009;106:14676–14680. doi: 10.1073/pnas.0907842106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan HC, Fernández-Hernando C, Lai JH. Biochem Pharmacol. 2014;88:139–49. doi: 10.1016/j.bcp.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida M, Heike Y, Ohno S, Ikekawa T, Wakasugi H. Int J Cancer. 2003;105:601–606. doi: 10.1002/ijc.11157. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida M, Feng W, Nishio K, Takahashi M, Heike Y, Saijo N, Wakasugi H, Ikekawa T. Int J Cancer. 2001;94:348–352. doi: 10.1002/ijc.1476. [DOI] [PubMed] [Google Scholar]
- 22.Wender PA, Kogen H, Lee HY, Munger JD, Jr, Wilhelm RS, Williams PD. J Am Chem Soc. 1989;111:8957–8958.Wender PA, McDonald FE. J Am Chem Soc. 1990;112:4956–4958.Wender PA, Rice KD, Schnute ME. J Am Chem Soc. 1997;119:7897–7898.For a formal synthesis see: Lee K, Cha JK. J Am Chem Soc. 2001;123:5590–5591. doi: 10.1021/ja010643u.
- 23.Wender PA, Jesudason CD, Nakahira H, Tamura N, Tebbe AL, Ueno Y. J Am Chem Soc. 1997;119:12976–12977. [Google Scholar]
- 24.Wender PA, Kee JM, Warrington JM. Science. 2008;320:649–652. doi: 10.1126/science.1154690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beans EJ, Fournogerakis D, Gauntlett C, Heumann LV, Kramer R, Marsden MD, Chun T-W, Zack JA, Wender PA. Proc Natl Acad Sci USA. 2013;110:11698–11703. doi: 10.1073/pnas.1302634110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tyler MI, Howden MEH. Aust J Chem. 1987;40:193–200. [Google Scholar]
- 27.Hecker E, Schmidt R. Prog Chem Org Nat Prod. 1974;31:377–467. doi: 10.1007/978-3-7091-7094-6_7. [DOI] [PubMed] [Google Scholar]
- 28.Yang YW, Huang M. J Org Chem. 2008;73:4702–4704. doi: 10.1021/jo800123m. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez J, Dulcere JP. Synthesis. 1993;12:1177–1205. [Google Scholar]
- 30.Drozdov FV, Mekhtiev AP, Morozevich GE, Timofeev VP, Misharin A. Russ J Bioorg Chem. 2007;33:326–333. doi: 10.1134/s1068162007030090. [DOI] [PubMed] [Google Scholar]
- 31.White JD, Choi YG. Helvetica Chimica Acta. 2002;85:4306–4327. [Google Scholar]
- 32.(a) Shi Y. Acc Chem Res. 2004;37:488–496. doi: 10.1021/ar030063x. [DOI] [PubMed] [Google Scholar]; (b) Wong OA, Shi Y. Chem Rev. 2008;108:3958–3987. doi: 10.1021/cr068367v. [DOI] [PubMed] [Google Scholar]
- 33.Zhao MX, Shi Y. J Org Chem. 2006;71:5377–5379. doi: 10.1021/jo060335k. [DOI] [PubMed] [Google Scholar]
- 34.Katsuki T, Sharpless KB. J Am Chem Soc. 1980;102:5974–5976. [Google Scholar]
- 35.(a) Zhang W, Basak A, Kosugi Y, Hoshino Y, Yamamoto H. Angew Chem, Int Ed. 2005;44:4389–4391. doi: 10.1002/anie.200500938. [DOI] [PubMed] [Google Scholar]; (b) Li Z, Zhang W, Yamamoto H. Angew Chem Int Ed. 2008;47:7520–7522. doi: 10.1002/anie.200802523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.