Abstract
Natural killer T (NKT) cells are known to play a role against certain microbial infections, including malaria and HIV, two major global infectious diseases. Strategies that acn harness and amplify the immunotherapeutic potential of NKT cells can serve as powerful tools in the fight against such diseases. 7DW8-5, a novel glycolipid, may be one such tool. The interaction of 7DW8-5 with CD1d molecules induces activation of NKT cells, thereby activating various immune-competent cells including dendritic cells (DCs) to provide a significant adjuvant effect for several vaccines. This review discusses the discovery and characterization of 7DW8-5 and the practical considerations of its preclinical and clinical development as a potential glycolipid adjuvant for candidate malaria and HIV vaccines.
Keywords: NKT cell, Glycolipid, CD1d, 7DW8-5, HIV, Malaria, Vaccine, Adjuvant
1. Introduction
1.1. NKT cells, CD1d molecules and glycolipids
Natural killer T cells (NKT) are distinct from conventional T cells and natural killer (NK) cells [1] as they are characterized by the expression of both a T cell antigen receptor (TCR) and NK1.1 (NKR-P1 or CD161c), a C-lectin type natural killer (NK) receptor. As the TCR is invariant for the majority of murine and human NKT cells, encoded by the Vα14 and Jα18 gene segments [2], or Vα24 and Jα18 segments [1], respectively, these NKT cells are called invariant NKT (iNKT) cells. The Vβ repertoire utilized by NKT cells is somewhat more diverse [2–4]. Murine NKT cells usually belong to either CD4−CD8− (double negative) or CD4+CD8− sub-populations [2–4]. Human NKT cells are more heterogeneous and a significant percentage of human NKT cells are CD4−CD8+, both in the blood and the liver [5].
The only characterized ligand of NKT cells to date is CD1d, an MHC class I-like molecule [2,6–8]. While the CD1d protein is not encoded by the MHC gene locus [6,7], this molecule associates non-covalently with β2-microglobulin and exhibits limited, yet significant, homology as well as overall structural similarity with MHC class I heavy chains [6,7]. CD1d is constitutively expressed by many cells, including dendritic cells (DCs), macrophages, and B and T lymphocytes [9,10]. Although all iNKT cells recognize and are restricted by CD1d molecules, some NKT cells having more diversified TCR also recognize CD1d [1]. There is a population of NKT cells that does not depend on the recognition of CD1d molecules and known as non-CD1d-restricted NKT cells [1].
A synthetic glycolipid designated α-galactosylceramide (α-GalCer), originally identified in an Okinawan marine sponge by a research group at the Kirin Brewery Co. in Japan [1,11], was shown to bind to CD1d. This was documented by surface plasmon resonance using immobilized α-GalCer and soluble murine [12,13] or human CD1d [12]. This glycolipid, when presented by the CD1d molecule, activates both murine and human NKT cells in vivo and in vitro [14–17]. X-ray crystallography studies have revealed that the lipid portion of the glycolipid fits tightly into the CD1d binding groove, wherein the sphingosine chain associates with one pocket in the groove and the longer acyl chain anchors within a separate pocket [18,19]. A more recent structural study using a tri-molecular complex consisting of CD1d-glycolipid and Vα24 TCR confirmed that the galactose ring extends above the surface of the lipid-binding groove, and thereby is exposed for recognition by the TCR of NKT cells [20].
Eliminating or exchanging this moiety with various sugars has been shown to either diminish or abrogate activity [14], indicating the importance of the galactose head group to α-GalCer function. We and others have shown that the α-anomeric conformation of the glycolipid as well as the equatorial configuration of the 2-hydroxyl group of the sugar moiety and the 3-hydroxyl group of the phytosphingosine are also crucial for α-GalCer to bind CD1d molecules and to activate NKT cells through their TCR [1,13,21,22]. One study has shown that, albeit at lower potency than α-anomeric GalCer, β-anomeric GalCer can induce CD1d-dependent biological activities in mice [23]. Earlier research has shown that α-GalCer has been amendable to modification in the acyl tails of the molecule, since varying the hydrocarbon lengths and/or introducing unsaturation in the fatty acid chains [24,25], as well as the truncation of the fatty acid chain from 24 to 2 carbons [21] did not significantly affect mouse NKT cell responses. Interestingly, a compound that has only 9 carbons at the sphingosine chain, OCH, is shown to skew the cytokine release profile towards Th-2 cytokines [26]. In contrast, we have previously found that a C-glycoside analog of α-GalCer, α-C-GalCer, preferentially stimulates a Th1-type response in mice [27,28]. This analog, in comparison to α-GalCer, consistently stimulated prolonged production of IFN-γ, increased production of IL-12, and decreased production of the Th2 cytokine IL-4.
1.2. Function of glycolipid-activated NKT cells and their adjuvant effect
A number of studies have documented the protective role of α-GalCer-activated NKT cells in anti-tumor immunity [29,30], autoimmune diseases [31-34] and infectious diseases [35-39]. α-GalCerand α-C-GalCer have recently been shown to induce full maturation of DCs, as determined by an increased expression of co-stimulatory molecules, which include CD40, CD80 and CD86, as well as MHC class II molecules on DCs [40,41]. The presence of NKT cells is required in order for the glycolipids to activate DCs. Although the precise mechanisms for this DC maturation process and for the functional consequence of the DC maturation are still unknown, it is thought to involve multi-factorial pathways in order to lead to full maturation/activation of DCs [42].
To date, several clinical trials have been conducted using α-GalCer primarily for the treatment of cancer, but also as a potential therapeutic agent against Hepatitis B and Hepatitis C [43-48]. Direct intravenous (IV) administration of α-GalCer did not result in a noticeable clinical benefit against cancer, hepatitis B, or hepatitis C infection [43,48]. In order to improve the activity in cancer patients, subsequent clinical trials conducted were based on the transfer of DCs pulsed with α-GalCer [45,46]. These trials again failed to display any noticeable anti-tumor effect. In a recent Phase I clinical trial, however, APCs were pulsed with α-GalCer in the presence of autologous Vα24 NKT cells, before transferring them back to the cancer patients. In short, the combined use of the intra-arterial infusion of activated Vα24 NKT cells and the submucosal injection of α-GalCer-pulsed APC induced significant antitumor immunity and had measurable beneficial clinical effects in the management of advanced head and neck squamous cell carcinoma [44].
Our recent work has demonstrated that α-GalCer-activated NKT cells enhance malaria-specific T cell responses and, more importantly, protective anti-malarial immunity elicited by various immunogens, including a recombinant adenovirus expressing a malarial antigen [49]. The adjuvant effects of α-GalCer in this model require CD1d molecules, Vα14 NKT cells, and IFN-γ(Fig. 1). However, two recent studies have shown that α-GalCer or its analogues can display their adjuvant effects on T cell responses elicited by a soluble protein, possibly through CD40–CD40L interaction, as well as by cytokines such as type I interferon and IFN-γ [40,50]. It was also shown that these interactions may ultimately lead to the full maturation of dendritic cells, and are therefore responsible for mediating the adjuvant activity of glycolipids [40,41,50,51]. More recently, a number of studies have reported on the adjuvant effect displayed by α-GalCer and α-C-GalCer on vaccines against viruses and cancers in a mouse model [52–56]. Interestingly, a very recent study has shown that non-glycosidic analogues of α-GalCer could activate iNKT cells resulting in dendritic cell maturation and the priming of antigen-specific T and B cells, thereby acting as a vaccine adjuvant [57].
Figure 1.

Role of NKT cells in adaptive immunity against malaria and HIV. In the context of CD1d molecules, a glycolipid can stimulate NKT cells through their invariant T cell receptor (invTCR). Upon activation, NKT cells rapidly secrete cytokines such as IFN-γ, and together with CD40–CD40L interaction, induce the activation and maturation of dendritic cells. Thus, the co-administration of a glycolipid (such as 7DW8-5) with vaccines expressing malaria or HIV antigens may be able to enhance the efficacy of the vaccines by augmenting the levels of antigen-specific T cell and humoral responses.
1.3. The discovery of a lead clinical compound, 7DW8-5, and its adjuvant activity
In an attempt to search for a more potent glycolipid, we synthesized a compound library of 25 glycolipids analogous to α-GalCer and evaluated their biological activity. The evaluation process included testing the ability of each glycolipid to stimulate human NKT cell lines and secrete cytokines including IFN-γ, as well as their ability to stimulate autologous DCs and secrete cytokines including IL-12. After selecting 5 glycolipids which displayed strong biological activity in in vitro assays, we carefully assessed the binding affinity of selected glycolipids to the human CD1d molecule, as well as to the invariant TCR (invTCR) of human NKT cells in vitro. The binding affinity of selected compounds to invTCR has been shown to have a strong correlation with its biological activity. Thus, after evaluating the biological activity of these glycolipids, we identified 7DW8-5 as a lead compound for further adjuvant testing [58].
After selecting 7DW8-5 as a lead compound, we compared its adjuvant effect with that of α-GalCer against various HIV vaccine platforms, namely Ad5–p24 and DNA–p24. For this purpose, different doses of each compound were co-administered intramuscularly with a sub-optimal dose of either Ad5–p24 or DNA–p24. Two weeks after a single immunizing dose of Ad5–p24 co-administered with each glycolipid, 7DW8-5 was shown to enhance the most the level of p24-specific CD8+, as well as CD4+ T cell responses that secrete IFN-γ, as determined by ELISpot assay [58]. Similarly, when mice were primed with a DNA–p24 vaccine plus either 7DW8-5 or α-GalCer, and then boosted with the DNA–p24 vaccine alone, 7DW8-5 displayed a stronger adjuvant effect than α-GalCer, eliciting significantly higher p24-specific CD8+ T cell and humoral responses. Next, in order to test whether 7DW8-5 displays its adjuvant effect on “protective” immunity induced by vaccines, we used Ad5 expressing a malarial antigen, Ad5-PyCS, as a vaccine platform. Two weeks after vaccinating mice with Ad5-PyCS together with or without a glycolipid, we challenged the vaccinated mice with live rodent malaria parasites. Then, we determined the amounts of parasite loads in the liver of infected mice. Non-immunized mice were used as a control. Through this challenge experiment, we confirmed that 7DW8-5 displays a more potent adjuvant effect than α-GalCer, leading to a significantly higher degree of “protection” against malaria in mice [58]. Finally, to ensure that the adjuvant effect of 7DW8-5 was mediated byCD1d molecules, we immunized mice lacking CD1d, as well as wild-type mice, with Ad5-p24 co-administered with 7DW8-5, and measured the level of p24-specific T cell responses. Not surprisingly, the adjuvant effect of 7DW8-5 was totally abolished in mice lacking CD1d molecules, confirming the CD1d mechanism-based adjuvant effect of 7DW8-5. These studies have led us to select 7DW8-5 as the lead compound for further clinical development [58].
2. Preclinical development of 7DW8-5 as an adjuvant for a candidate malaria vaccine
2.1. NMRC-M3V-Ad-PfCA candidate malaria vaccine
AdPfCA is a non-replicating adenovirus serotype 5 based malaria vaccine candidate developed by the US Military Malaria Vaccine Program (USMMVP), GenVec, Inc. and USAID, and expresses two Plasmodium falciparum antigens– circumsporozoite protein (CSP) and apical membrane antigen-1 (AMA1) [59,60]. The CSP is expressed by pre-erythrocytic stages, whereas AMA1 antigen is expressed by both pre-erythrocytic and erythrocytic stages [61,62], of the Plasmodium parasite life cycle. Thus, a combined immune response directed against these two antigens has the potential to reduce the number of malaria infections in healthy individuals by killing sporozoites and liver stage parasites and also to reduce the severity of malaria disease in those that do become infected by killing blood stage parasites. Based upon the ability of 7DW8-5 to adjuvanate Ad5- and DNA-based vaccines as described above, we hypothesized that 7DW8-5 coadministered with AdPfCA would boost the immune response against both CSP and AMA1 in comparison to AdPfCA alone, thus affording a potentially better clinical outcome against malaria.
2.2. Go/No-Go studies
To test this hypothesis, we designed a series of critical “Go/No-Go” studies in the murine model that would serve as a gatekeeper to advancing this antigen–adjuvant combination into a Phase 1 proof-of-concept clinical trial. The key study was developed to determine whether an optimal dose of 7DW8-5 could significantly augment specific immune responses elicited by varying doses of AdPfCA. The predefined criterion for adjuvant efficacy was a two-fold increase in cell-mediated immune responses or a three-fold increase in humoral immune responses induced by immunization with AdPfCA in the presence of 7DW8-5 compared to those induced by AdPfCA immunization alone.
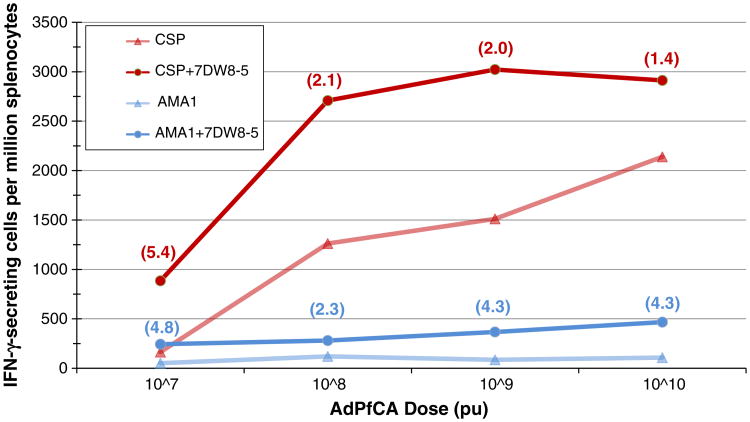
In summary, six BALB/c mice per group were immunized intramuscularly with AdPfCA doses ranging from 2×106 to 2×1010 particle units (pu) with or without an optimal dose of 7DW8-5 identified in previous murine studies [58]. Spleno-cytes and sera were collected from each group of mice two weeks post-immunization and used to determine the number of IFNγ-secreting cells (by ELISpot) and level of antibody titers (by ELISA) induced against CSP and AMA1. Results demonstrated that the presence of 7DW8-5 during immunization provided a greater than two-fold enhancement of cell-mediated immune responses against both CSP and AMA1 at AdPfCA doses of 107, 108, and 109 pu (Fig. 2). Notably, in some cases, the cell-mediated immune response levels induced by immunization with AdPfCA alone were surpassed using 100- to 1000-fold lower dosages of AdPfCA when co-administered with 7DW8-5. For example, the AMA1 specific response observed after mice were immunized with 107 pu of AdPfCA in combination with 7DW8-5 was almost two-fold higher than the AMA1 specific response observed with up to 1010pu of AdPfCA alone. These data indicate the potential for a significant dose-sparing adjuvant effect when 7DW8-5 is used in combination with AdPfCA, which would provide substantial cost reduction in widespread vaccine manufacture. A dose-sparing effect would also reduce the volume of vaccine needed per injection to achieve the same efficacy as the same vaccine administered without adjuvant. This opens the door to combining multiple vaccine strategies, usually prohibited by maximum volume limitations, into one single injection that could provide greater and broader protection. Also notably, the adjuvanted vaccine achieved ELISpot responses that were higher than any achieved by the unadjuvanted vaccine. Even more significant than the dose-sparing effect, the ability to achieve absolute increases in the magnitude of immune responses is promising for future applications. While a trend towards increased humoral immune responses against CSP and AMA1 was also noted, their level of enhancement did not meet our pre-defined criteria. However, the degree of enhancement achieved in antibody responses will remain an important consideration as future testing occurs. Refinement in non-human primates of the optimal AdPfCA and 7DW8-5 doses determined in mice and further evaluation of any potential safety and toxicity concerns will be critical before moving into a Phase 1 clinical trial in healthy human volunteers.
Figure 2.
7DW8-5 enhancement of cell-mediated immune response to AdPfCA. Groups of BALB/c mice (n=6) were immunized with varying doses of AdPfCA (adenovirus co-expressing Plasmodium falciparum CSP and AMA1) together with or without 0.1ug 7DW8-5. Splenocytes were collected two weeks after immunization to determine the relative number of IFN-γ-secreting peripheral blood mononuclear cells (PBMCs) by an ELISpot assay. Numbers in parentheses indicate the fold increase of CSP-specific (red) and AMA1-specific (blue) IFN-γ-secreting PBMCs after immunization with AdPfCA+7DW8-5 (circles) as compared to after immunization with AdPfCA alone (triangles).
3. Practical considerations in the clinical development of glycolipid adjuvants
3.1. Manufacturing
Unlike laboratory reagents for use in small animal studies, all biologic substances must be manufactured under Good Manufacturing Practice (GMP) conditions (Code of Federal Regulations Title 21, Part 211, April 2009) before use in humans. This requires that compound synthesis be reproducible and scalable at large quantities. One of the practical advantages of glycolipids is their straightforward synthesis pathway from chemical compounds that are readily available at relatively low cost. In order for glycolipids to function as effective adjuvants that provide dose-sparing of vaccines against infectious diseases with high prevalence in the developing world, low cost and ease of manufacturing are crucial.
3.2. Formulation
Glycolipids are amphipathic molecules consisting of a hydrophilic sugar head, which engages the invariant T cell receptor on NKT cells, and two highly hydrophobic lipid tails, which lie in the CD1d binding groove [63]. In small animal studies, they are often solubilized in dimethyl sulfoxide (DMSO), which is toxic to cells in large quantities and therefore unacceptable for use in clinical product formulation. The majority of clinical trials of glycolipids to date have employed pulsing autologous dendritic cells with α-GalCer ex vivo [45,64–67], thus minimizing formulation issues of the compound alone. Only three clinical trials to date have involved direct administration of a glycolipid compound to humans [43,47,48]. In all studies, the active formulation was composed of 0.2 mg lyophilized α-GalCer vialed with sucrose, L-histidine, and polysorbate 20, a surfactant used in several commercial products as a detergent and emulsifier (Kirin Pharmaceutical Co. Ltd., Gunma, Japan). Yet even with the use of polysorbate, the concentration of α-GalCer was 0.2 mg/mL when reconstituted with sterile water. Thus, the volume of administration required to achieve the target doses necessitated IV administration of each dose.
While this approach is suitable for treating patients with cancer or hepatitis, it is not practical for administering vaccines for the prevention or treatment of infectious diseases on a global scale. Vaccines are typically administered intramuscularly (IM), with a maximum tolerable injection volume of 1 mL in humans. Administration into the subcutaneous space may allow for slightly greater volumes, but with a maximum of 3 mL depending upon the injection site [68]. Because glycolipids provide their optimal adjuvant effect when co-administered at the same time and location as the target vaccine [36], the total injection volume must allow for both vaccine antigen and adjuvant together. Therefore, the application of glycolipids as vaccine adjuvants in humans necessitates improved formulation methods to achieve a high enough concentration to provide an effective adjuvant dose in a small enough volume for injection with vaccine. Several newer methods to achieve higher concentration of biologic compounds are being developed, including hydrogels, liposomes, and micro-spheres [69,70].
3.3. Safety
Giaccone et al. reported administering IV α-GalCer to patients with solid tumors in doses of 50–4800 μg/m2, on days 1, 8, and 15 of a 4-weekly cycle, for up to 6 cycles [43]. In this study, α-GalCer was well-tolerated at a wide range of doses, with no drug-related serious adverse events, and no evidence of hepatotoxity, which had been previously reported in the mice exposed to IV α-GalCer [71,72]. Similarly, Veldt et al. observed that IV administration of 0.1 μg/kg, 1 μg/kg or 10 μg/kg (versus placebo) was well tolerated in patients with chronic hepatitis C infection. Patients experienced a transient decrease in circulating NKT cell levels, but only the patient with the highest pre-treatment NKT cell level showed signs of immune activation and a subsequent 1.3 log decrease in HCV-RNA levels. Woltman et al. administered 0.1 μg/kg, 1 μg/kg or 10 μg/kg IV α-GalCer to patients with chronic hepatitis B at weeks 0, 4 and 8 [47]. In contrast to the previous studies, subjects reported a high frequency of fever and headache (78% and 63% respectively), likely due to systemic cytokine secretion resulting from generalized immune activation. One of eight patients in the mid dose group and three of eight patients in the high dose group did not complete the therapeutic regimen due to side effects. The authors concluded that the poor tolerability made α-GalCer unsuitable as an alternative monotherapy to current treatment regimens for hepatitis B. This trial underscores the need to characterize the level of immune activation and cytokine response to each glycolipid in stringent preclinical animal models. An ideal glycolipid adjuvant would provide local immune activation without a strong systemic cytokine release in order to minimize the risk-benefit ratio.
3.4. Dosing
Determining the dose of glycolipid required to provide the optimal adjuvant effect of a particular vaccine in humans is a non-trivial exercise which depends upon a number of factors. For many small molecule compounds, the effectiveness increases with increasing dose, so the maximum dose is determined by the level at which toxicity and safety considerations outweigh the benefits provided by the drug, the so-called maximum tolerated dose (MTD). The dose effect of glycolipids as vaccine adjuvants is significantly different because of their mechanism of action. While too low a dose will not provide sufficient NKT cell stimulation to recruit and activate secondary immune cells at the site of vaccination, high concentrations of glycolipid drive the NKT cells to an anergic state, also abrogating the adjuvant effect. In addition, various glycolipids may have differing dose-response curves. Fig. 3 depicts the differing dose curves of the adjuvant effect of 7DW8-5 on the IFN-γ ELISpot response to an adenovirus serotype 5 based vaccine encoding the CS antigen [58]. The maximum effective dose is 0.1 μg of 7DW8-5, which decreases at a higher dose of 1 μg. Thus, determining the optimal dose of glycolipid adjuvant for each vaccine is critical in order to optimize immunogenicity.
Figure 3.
Dose effect of 7DW8-5 on a malaria vaccine in mice. Groups of BALB/c mice (n=5) were immunized with AdPyCS (adenovirus expressing a Plasmodium yoelii CS protein) together with indicated amounts of 7DW8-5. Splenocytes were collected two weeks after immunization to determine the relative number of IFN-γ-secreting CD8+ T cells by an ELISpot assay. Adapted from Li et al., 2010.
In addition, the optimal amount of glycolipid required may vary depending upon the type of vaccine used. For example, viral vectors may require a lower level of NKT cell stimulation than DNA-based vaccines, given the inherently larger inflammatory response to viral vectors, which contain foreign antigens independent of the vaccine insert. Furthermore, the ratio of the optimal glycolipid dose to vaccine dose may play a role in the dose response, and may vary by antigen. Studies to answer these critical questions are in progress in our laboratory.
Finally, many small molecule drugs are developed in small animals and then translated to doses in humans by administering an equivalent number of milligrams per kilogram, on a linear weight-based scale. The immunologic activity of biologic compounds such as glycolipids or cytokine adjuvants is complex and often more localized than the small molecule drug compounds, and therefore not predictable using linear weight-based scaling. This critical limitation necessitates dose-finding studies in larger animal species, such as non-human primates in order to determine effective doses for clinical testing.
3.5. Species selection
The NKT cell response to glycolipids in rhesus macaques has been studied, but to a limited extent. Like humans, rhesus macaques possess Vα24 T cell receptor positive T cells. The amino acid sequence of the V–J junction of the VαTCR is 93% homologous to humans, and the CD1d alpha1–alpha2 domains of both species have 95.6% homology [73], which would imply that the rhesus macaque model is predictive of glycolipid responses in humans. However, Gansvud et al. subsequently demonstrated that rhesus NKT cells in the spleen have increased CD3+CD8+ and CD3+CD56+ coexpression, and display a more Th2 skewed phenotype in comparison to peripheral blood rhesus NKT cells [74]. As described above, the generation of an effective adjuvant response relies primarily on eliciting a strong IFN-γ response, a Th1 response, whereas Th2-skewed NKT cells are involved in the protection from autoimmune diseases such as diabetes [75]. Given that the alteration of the glycolipid structure can shift the Th1/Th2 balance [24,26,76], it is critical to test each new compound in the desired target species and the desired target cell type for replication of the appropriate response before utilizing that species for safety, dosing or other preclinical studies.
4. Clinical trials and future development
4.1. Clinical testing of the safety and immunogenicity of 7DW8-5 as a vaccine adjuvant
Although the ability of glycolipids to act as vaccine adjuvants has been well established in the animal model, neither α-GalCer nor any compound derivatives have been tested in combination with vaccines in clinical trials. In a Phase 1 clinical trial conducted by the USMMVP, one dose of the unadjuvanted vaccine elicited strong cellular immunogenicity as measured by ex vivo IFN-γ ELISpot assay [60]. In a follow-on clinical trial, in which NMRC-M3V-Ad-PfCA was given as a boost sixteen weeks after priming with three doses of DNA (also encoding PfCSP and PfAMA1), a significant proportion of the volunteers was sterilely protected against P. falciparum sporozoite challenge [59].
Glycolipid adjuvants offer the potential to augment this vaccine regimen for a number of reasons. First, glycolipids have been shown to effectively augment adenovirus based vaccines encoding a number of antigens in small animals, including NMRC-M3V-Ad-PfCA, as described earlier. Second, they preferentially boost cellular immunity, which may be particularly important for protection against malaria due to its complex intracellular lifecycle [77,78]. Third, in prior experiments in small animals, α-GalCer has been shown to assist in overcoming pre-existing anti-vector immunity to adenoviral vectored vaccines [49].
We therefore intend to evaluate the adjuvant effect of 7DW8-5 in healthy volunteers in a Phase 1 clinical trial by administering a single fixed dose of NMRC-M3V-Ad-PfCA with or without varying doses of 7DW8-5. As this will be the first administration of this adjuvant in humans, the primary objective will be the safety of the 7DW8-5/NMRC-M3V-Ad-PfCA combination. Secondary objectives will include the evaluation of adjuvant effect on the cellular and humoral immunogenicity of both CSP and AMA1, as well as the determination of the optimal dose of 7DW8-5 required for the adjuvant effect. As outlined in the previous section, given the potential of overdosing to induce NKT cell anergy, the determination of the optimal dose will be critical in pursuing further adjuvant studies with this compound. This clinical trial will be conducted after the critical animal studies to assess dosing, safety and toxicity are completed. If safety and immunogenicity results are promising, a further study is planned to measure the impact of 7DW8-5 on protection against malaria challenge. Of particular interest will be the ability of the glycolipid to counteract any negative effect of the pre-existing adenovirus 5 neutralizing antibodies on immunogenicity and protection.
4.2. Future applications
If the promising results demonstrating the strong adjuvant effect of glycolipid compounds on AdPfCA translate to humans, there are a number of potential future applications. Adenoviral vectors are being utilized for a number of candidate vaccines against malaria, HIV-1, and other pathogens [79,80]. It would be reasonable to predict that the adjuvant effect on adenoviral vaccines against differing antigens would be similar, although this hypothesis would need to be tested in separate clinical trials with each vaccine, as adjuvants must be licensed in combination with each individual antigen, rather than as standalone compounds. α-GalCer enhances the immunogenicity of a sindbis viral vector vaccine [49], and α-C-galactosylceramide, an α-GalCer analog, provides an adjuvant effect to influenza vaccine in mice [54]. It is important to determine whether glycolipid adjuvants can elicit similar effects on other viral vaccine vectors. These studies are underway in our laboratory.
In addition, α-GalCer has been shown to have an adjuvant effect on DNA-based vaccines in small animals [52]. Further clinical trials will be needed to assess whether this effect translates to humans. DNA vaccines are advantageous for a number of reasons, both immunologic and practical. DNA vaccines confer the ability to “prime” the response to viral vaccines, resulting both in the increased magnitude of the response, as well as a skewing toward a stronger CD4+ T cell response, which can act to provide help for more durable humoral and cellular immunogenicity [81-83]. On a practical level, DNA vaccines are easier to produce at lower cost than viral vaccines, and have a more favorable thermostability profile, which can be advantageous for mass production and distribution of vaccines to prevent infectious diseases in the developing world. Lastly, because DNA vaccines do not elicit anti-vector immunity, they can effectively continue boosting the immune response with multiple vaccinations (Y. Huang, personal communication), unlike the viral-vectored vaccines. Thus, boosting the immune response to DNA vaccines with glycolipid adjuvants could allow for therapeutic vaccine regimens against infectious diseases, cancer, or other diseases.
5. Conclusions
While glycolipid compounds have been studied as a potential cancer treatment in a number of trials to date, the use of glycolipid compounds as vaccine adjuvants has thus far been limited to research in small animals, with promising results to date. Clinical development of a novel glycolipid compound, 7DW8-5, is underway to determine whether it can provide an adjuvant effect on an adenoviral-based malaria vaccine in healthy human volunteers. Should this concept translate to humans, glycolipids offer a promising potential mechanism to augment the immunogenicity and efficacy of vaccines against a variety of infectious diseases at relatively low cost.
Acknowledgments
The authors wish to thank Captain Thomas Richie, Noelle Patterson and Dr. Martha Sedegah, US Military Malaria Vaccine Program at the United States Naval Medical Research Center/Walter Reed Army Institute of Research, Dr. David Ho, the Bill and Melinda Gates Foundation Collaboration for AIDS Vaccine Discovery, and the Malaria Vaccine Initiative.
References
- 1.Tsuji M. Glycolipids and phospholipids as natural CD1d-binding NKT cell ligands. Cell Mol Life Sci. 2006;63:1889–1898. doi: 10.1007/s00018-006-6073-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 3.Eberl G, Lees R, Smiley ST, Taniguchi M, Grusby MJ, MacDonald HR. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J Immunol. 1999;162:6410–6419. [PubMed] [Google Scholar]
- 4.Hammond KJ, Pelikan SB, Crowe NY, Randle-Barrett E, Nakayama T, Taniguchi M, Smyth MJ, van Driel IR, Scollay R, Baxter AG, Godfrey DI. NKT cells are phenotypically and functionally diverse. Eur J Immunol. 1999;29:3768–3781. doi: 10.1002/(SICI)1521-4141(199911)29:11<3768::AID-IMMU3768>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 5.Ishihara S, Nieda M, Kitayama J, Osada T, Yabe T, Ishikawa Y, Nagawa H, Muto T, Juji T. CD8(+)NKR-P1A (+)T cells preferentially accumulate in human liver. Eur J Immunol. 1999;29:2406–2413. doi: 10.1002/(SICI)1521-4141(199908)29:08<2406::AID-IMMU2406>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 6.Brossay L, Burdin N, Tangri S, Kronenberg M. Antigen-presenting function of mouse CD1: one molecule with two different kinds of antigenic ligands. Immunol Rev. 1998;163:139–150. doi: 10.1111/j.1600-065x.1998.tb01193.x. [DOI] [PubMed] [Google Scholar]
- 7.Porcelli SA, Modlin RL. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol. 1999;17:297–329. doi: 10.1146/annurev.immunol.17.1.297. [DOI] [PubMed] [Google Scholar]
- 8.Teitell M, Holcombe HR, Brossay L, Hagenbaugh A, Jackson MJ, Pond L, Balk SP, Terhorst C, Peterson PA, Kronenberg M. Nonclassical behavior of the mouse CD1 class I-like molecule. J Immunol. 1997;158:2143–2149. [PubMed] [Google Scholar]
- 9.Balk SP, Bleicher PA, Terhorst C. Isolation and expression of cDNA encoding the murine homologues of CD1. J Immunol. 1991;146:768–774. [PubMed] [Google Scholar]
- 10.Bleicher PA, Balk SP, Hagen SJ, Blumberg RS, Flotte TJ, Terhorst C. Expression of murine CD1 on gastrointestinal epithelium. Science. 1990;250:679–682. doi: 10.1126/science.1700477. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol Res. 1995;7:529–534. [PubMed] [Google Scholar]
- 12.Naidenko OV, Maher JK, Ernst WA, Sakai T, Modlin RL, Kronenberg M. Binding and antigen presentation of ceramide-containing glycolipids by soluble mouse and human CD1d molecules. J Exp Med. 1999;190:1069–1080. doi: 10.1084/jem.190.8.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakai T, Naidenko OV, Iijima H, Kronenberg M, Koezuka Y. Syntheses of biotinylated alpha-galactosylceramides and their effects on the immune system and CD1 molecules. J Med Chem. 1999;42:1836–1841. doi: 10.1021/jm990054n. [DOI] [PubMed] [Google Scholar]
- 14.Brossay L, Chioda M, Burdin N, Koezuka Y, Casorati G, Dellabona P, Kronenberg M. CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med. 1998;188:1521–1528. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burdin N, Brossay L, Koezuka Y, Smiley ST, Grusby MJ, Gui M, Taniguchi M, Hayakawa K, Kronenberg M. Selective ability of mouse CD1 to present glycolipids: alpha-galactosyl-ceramide specifically stimulates V alpha 14+ NK T lymphocytes. J Immunol. 1998;161:3271–3281. [PubMed] [Google Scholar]
- 16.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 17.Spada FM, Koezuka Y, Porcelli SA. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J Exp Med. 1998;188:1529–1534. doi: 10.1084/jem.188.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koch M, Stronge VS, Shepherd D, Gadola SD, Mathew B, Ritter G, Fersht AR, Besra GS, Schmidt RR, Jones EY, Cerundolo V. The crystal structure of human CD1d with and without alpha-galactosylceramide. Nat Immunol. 2005;6:819–826. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]
- 19.Zajonc DM, Cantu C, III, Mattner J, Zhou D, Savage PB, Bendelac A, Wilson IA, Teyton L. Structure and function of a potent agonist for the semi-invariant natural killer T cell receptor. Nat Immunol. 2005;6:810–818. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borg NA, Wun KS, Kjer-Nielsen L, Wilce MC, Pellicci DG, Koh R, Besra GS, Bharadwaj M, Godfrey DI, McCluskey J, Rossjohn J. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–49. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 21.Brossay L, Naidenko O, Burdin N, Matsuda J, Sakai T, Kronenberg M. Structural requirements for galactosylceramide recognition by CD1-restricted NK T cells. J Immunol. 1998;161:5124–5128. [PubMed] [Google Scholar]
- 22.Xing GW, Wu D, Poles MA, Horowitz A, Tsuji M, Ho DD, Wong CH. Synthesis and human NKT cell stimulating properties of 3-O-sulfo-alpha/beta-galactosylceramides. Bioorg Med Chem. 2005;13:2907–2916. doi: 10.1016/j.bmc.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 23.Parekh VV, Singh AK, Wilson MT, Olivares-Villagomez D, Bezbradica JS, Inazawa H, Ehara H, Sakai T, Serizawa I, Wu L, Wang CR, Joyce S, Van Kaer L. Quantitative and qualitative differences in the in vivo response of NKT cells to distinct alpha-and beta-anomeric glycolipids. J Immunol. 2004;173:3693–3706. doi: 10.4049/jimmunol.173.6.3693. [DOI] [PubMed] [Google Scholar]
- 24.Goff RD, Gao RD, Mattner J, Zhou D, Yin N, Cantu C, III, Teyton L, Bendelac A, Savage PB. Effects of lipid chain lengths in alpha-galactosylceramides on cytokine release by natural killer T cells. J Am Chem Soc. 2004;126:13602–13603. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 25.Yu KO, Im JS, Molano A, Dutronc Y, Illarionov PA, Forestier C, Fujiwara N, Arias I, Miyake S, Yamamura T, Chang YT, Besra GS, Porcelli SA. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of alpha-galacto-sylceramides. Proc Natl Acad Sci USA. 2005;102:3383–3388. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 27.Schmieg J, Yang G, Franck RW, Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand alpha-galactosylcer-amide. J Exp Med. 2003;198:1631–1641. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmieg J, Yang G, Franck RW, Van Rooijen N, Tsuji M. Glycolipid presentation to natural killer T cells differs in an organ-dependent fashion. Proc Natl Acad Sci USA. 2005;102:1127–1132. doi: 10.1073/pnas.0408288102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med. 2002;196:119–127. doi: 10.1084/jem.20020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Sato H, Kondo E, Harada M, Koseki H, Nakayama T, Tanaka Y, Taniguchi M. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc Natl Acad Sci USA. 1998;95:5690–5693. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong S, Wilson MT, Serizawa I, Wu L, Singh N, Naidenko OV, Miura T, Haba T, Scherer DC, Wei J, Kronenberg M, Koezuka Y, Van Kaer L. The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat Med. 2001;7:1052–1056. doi: 10.1038/nm0901-1052. [DOI] [PubMed] [Google Scholar]
- 32.Jahng AW, Maricic I, Pedersen B, Burdin N, Naidenko O, Kronenberg M, Koezuka Y, Kumar V. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1789–1799. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharif S, Arreaza GA, Zucker P, Mi QS, Sondhi J, Naidenko OV, Kronenberg M, Koezuka Y, Delovitch TL, Gombert JM, Leite-De-Moraes M, Gouarin C, Zhu R, Hameg A, Nakayama T, Taniguchi M, Lepault F, Lehuen A, Bach JF, Herbelin A. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune Type 1 diabetes. Nat Med. 2001;7:1057–1062. doi: 10.1038/nm0901-1057. [DOI] [PubMed] [Google Scholar]
- 34.Singh AK, Wilson MT, Hong S, Olivares-Villagomez D, Du C, Stanic AK, Joyce S, Sriram S, Koezuka Y, Van Kaer L. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1801–1811. doi: 10.1084/jem.194.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chackerian A, Alt J, Perera V, Behar SM. Activation of NKT cells protects mice from tuberculosis. Infect Immun. 2002;70:6302–6309. doi: 10.1128/IAI.70.11.6302-6309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez-Aseguinolaza G, de Oliveira C, Tomaska M, Hong S, Bruna-Romero O, Nakayama T, Taniguchi M, Bendelac A, Van Kaer L, Koezuka Y, Tsuji M. Alpha-galactosylceramide-activated Valpha 14 natural killer T cells mediate protection against murine malaria. Proc Natl Acad Sci USA. 2000;97:8461–8466. doi: 10.1073/pnas.97.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kakimi K, Guidotti LG, Koezuka Y, Chisari FV. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med. 2000;192:921–930. doi: 10.1084/jem.192.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawakami K, Kinjo Y, Yara S, Koguchi Y, Uezu K, Nakayama T, Taniguchi M, Saito A. Activation of valpha14(+) natural killer T cells by alpha-galactosylceramide results in development of Th1 response and local host resistance in mice infected with Cryptococcus neoformans. Infect Immun. 2001;69:213–220. doi: 10.1128/IAI.69.1.213-220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vasan S, Tsuji M. A double-edged sword: the role of NKT cells in malaria and HIV infection and immunity. Semin Immunol. 2010;22:87–96. doi: 10.1016/j.smim.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujii S, Shimizu K, Hemmi H, Fukui M, Bonito AJ, Chen G, Franck RW, Tsuji M, Steinman RM. Glycolipid alpha-C-galactosylceramide is a distinct inducer of dendritic cell function during innate and adaptive immune responses of mice. Proc Natl Acad Sci USA. 2006;103:11252–11257. doi: 10.1073/pnas.0604812103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009;9:28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 43.Giaccone G, Punt CJ, Ando Y, Ruijter R, Nishi N, Peters M, von Blomberg BM, Scheper RJ, van der Vliet HJ, van den Eertwegh AJ, Roelvink M, Beijnen J, Zwierzina H, Pinedo HM. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8:3702–3709. [PubMed] [Google Scholar]
- 44.Kunii N, Horiguchi S, Motohashi S, Yamamoto H, Ueno N, Yamamoto S, Sakurai D, Taniguchi M, Nakayama T, Okamoto Y. Combination therapy of in vitro-expanded natural killer T cells and alpha-galactosylceramide-pulsed antigen-presenting cells in patients with recurrent head and neck carcinoma. Cancer Sci. 2009;100:1092–1098. doi: 10.1111/j.1349-7006.2009.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nieda M, Okai M, Tazbirkova A, Lin H, Yamaura A, Ide K, Abraham R, Juji T, Macfarlane DJ, Nicol AJ. Therapeutic activation of valpha24+Vbeta11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103:383–389. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- 46.Okai M, Nieda M, Tazbirkova A, Horley D, Kikuchi A, Durrant S, Takahashi T, Boyd A, Abraham R, Yagita H, Juji T, Nicol A. Human peripheral blood valpha24+ Vbeta11+ NKT cells expand following administration of alpha-galactosylceramide-pulsed dendritic cells. Vox Sang. 2002;83:250–253. doi: 10.1046/j.1423-0410.2002.00217.x. [DOI] [PubMed] [Google Scholar]
- 47.Woltman AM, Ter Borg MJ, Binda RS, Sprengers D, von Blomberg BM, Scheper RJ, Hayashi K, Nishi N, Boonstra A, van der Molen R, Janssen HL. Alpha-galactosylceramide in chronic hepatitis B infection: results from a randomized placebo-controlled phase I/II trial. Antivir Ther. 2009;14:809–818. doi: 10.3851/IMP1295. [DOI] [PubMed] [Google Scholar]
- 48.Veldt BJ, van der Vliet HJ, von Blomberg BM, van Vlierberghe H, Gerken G, Nishi N, Hayashi K, Scheper RJ, de Knegt RJ, van den Eertwegh AJ, Janssen HL, van Nieuwkerk CM. Randomized placebo controlled phase I/II trial of alpha-galactosylceramide for the treatment of chronic hepatitis C. J Hepatol. 2007;47:356–365. doi: 10.1016/j.jhep.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez-Aseguinolaza G, Van Kaer L, Bergmann CC, Wilson JM, Schmieg J, Kronenberg M, Nakayama T, Taniguchi M, Koezuka Y, Tsuji M. Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J Exp Med. 2002;195:617–624. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silk JD, Hermans IF, Gileadi U, Chong TW, Shepherd D, Salio M, Mathew B, Schmidt RR, Lunt SJ, Williams KJ, Stratford IJ, Harris AL, Cerundolo V. Utilizing the adjuvant properties of CD1d-dependent NK T cells in T cell-mediated immunotherapy. J Clin Invest. 2004;114:1800–1811. doi: 10.1172/JCI22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taraban VY, Martin S, Attfield KE, Glennie MJ, Elliott T, Elewaut D, Van Calenbergh S, Linclau B, Al-Shamkhani A. Invariant NKT cells promote CD8+ cytotoxic T cell responses by inducing CD70 expression on dendritic cells. J Immunol. 2008;180:4615–4620. doi: 10.4049/jimmunol.180.7.4615. [DOI] [PubMed] [Google Scholar]
- 52.Huang Y, Chen A, Li X, Chen Z, Zhang W, Song Y, Gurner D, Gardiner D, Basu S, Ho DD, Tsuji M. Enhancement of HIV DNA vaccine immunogenicity by the NKT cell ligand, alpha-galactosyl-ceramide. Vaccine. 2008;26:1807–1816. doi: 10.1016/j.vaccine.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 53.Ko SY, Ko HJ, Chang WS, Park SH, Kweon MN, Kang CY. Alpha-galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J Immunol. 2005;175:3309–3317. doi: 10.4049/jimmunol.175.5.3309. [DOI] [PubMed] [Google Scholar]
- 54.Kopecky-Bromberg SA, Fraser KA, Pica N, Carnero E, Moran TM, Franck RW, Tsuji M, Palese P. Alpha-C-galactosyl-ceramide as an adjuvant for a live attenuated influenza virus vaccine. Vaccine. 2009;27:3766–3774. doi: 10.1016/j.vaccine.2009.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu K, Idoyaga J, Charalambous A, Fujii S, Bonito A, Mordoh J, Wainstok R, Bai XF, Liu Y, Steinman RM. Innate NKT lymphocytes confer superior adaptive immunity via tumor-capturing dendritic cells. J Exp Med. 2005;202:1507–1516. doi: 10.1084/jem.20050956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teng MW, Westwood JA, Darcy PK, Sharkey J, Tsuji M, Franck RW, Porcelli SA, Besra GS, Takeda K, Yagita H, Kershaw MH, Smyth MJ. Combined natural killer T-cell based immunotherapy eradicates established tumors in mice. Cancer Res. 2007;67:7495–7504. doi: 10.1158/0008-5472.CAN-07-0941. [DOI] [PubMed] [Google Scholar]
- 57.Reddy BG, Silk JD, Salio M, Balamurugan R, Shepherd D, Ritter G, Cerundolo V, Schmidt RR. Nonglycosidic agonists of invariant NKT cells for use as vaccine adjuvants. ChemMed-Chem. 2009;4:171–175. doi: 10.1002/cmdc.200800354. [DOI] [PubMed] [Google Scholar]
- 58.Li X, Fujio M, Imamura M, Wu D, Vasan S, Wong CH, Ho DD, Tsuji M. Design of a potent CD1d-binding NKT cell ligand as a vaccine adjuvant. Proc Natl Acad Sci USA. 2010;107:13010–13015. doi: 10.1073/pnas.1006662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chuang I, Sedegah M, Cicatelli S, Spring M, Tamminga C, Bennett J, Guerrero M, Polhemus M, Cummings J, Angov E, Bruder J, Patterson N, Limbach K, Murphy J, Bergmann-Leitner E, Soisson L, Diggs C, Ockenhouse C, Richie T. Keystone Symposium — Malaria: New Approaches to Understanding Host–Parasite Interactions. Copper Mountain, CO; 2010. Phase 1/2a Clinical Trial on Safety, Tolerability, Immunogenicity and Efficacy of Prime Boost Regimen of DNA- and Adenovirus-vectored Malaria Vaccines Encoding Plasmodium falciparum Circumsporozoite Protein (CSP) and Apical Membrane Antigen (AMA1) in Malaria-Naïve Adults. [Google Scholar]
- 60.Tamminga C, Sedegah M, Chuang I, Regis D, Epstein JE, Mendoza-Silveiras J, Steinbeiss V, Reyes S, Fedders C, Parekh F, Williams F, Smith K, Maiolatesi S, Sedegah M, Doolan D, Limbach K, Patterson NB, Bruder J, King CR, Soisson L, Diggs C, Ockenhouse C, Richie T. Safety and Tolerability of a Multi-Stage, Multi-Antigen Adenovirus-Vec-tored P. falciparum Malaria Vaccine, in Healthy, Malaria-Naïve Adults. 12th Annual Conference on Vaccine Research; Baltimore, MD. 2009. [Google Scholar]
- 61.Krzych U, Lyon JA, Jareed T, Schneider I, Hollingdale MR, Gordon DM, Ballou WR. T lymphocytes from volunteers immunized with irradiated Plasmodium falciparum sporozoites recognize liver and blood stage malaria antigens. J Immunol. 1995;155:4072–4077. [PubMed] [Google Scholar]
- 62.Silvie O, Franetich JF, Charrin S, Mueller MS, Siau A, Bodescot M, Rubinstein E, Hannoun L, Charoenvit Y, Kocken CH, Thomas AW, Van Gemert GJ, Sauerwein RW, Blackman MJ, Anders RF, Pluschke G, Mazier D. A role for apical membrane antigen 1 during invasion of hepatocytes by Plasmodium falciparum sporozoites. J Biol Chem. 2004;279:9490–9496. doi: 10.1074/jbc.M311331200. [DOI] [PubMed] [Google Scholar]
- 63.Zeng Z, Castano AR, Segelke BW, Stura EA, Peterson PA, Wilson PA. Crystal structure of mouse CD1: An MHC-like fold with a large hydrophobic binding groove. Science. 1997;277:339–345. doi: 10.1126/science.277.5324.339. [DOI] [PubMed] [Google Scholar]
- 64.Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M, Hutchinson A, Geller M, Liu N, Annable R, Shay J, Kirchhoff K, Nishi N, Ando Y, Hayashi K, Hassoun H, Steinman RM, Dhodapkar MV. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201:1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ishikawa A, Motohashi S, Ishikawa E, Fuchida H, Higashino K, Otsuji M, Iizasa T, Nakayama T, Taniguchi M, Fujisawa T. A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2005;11:1910–1917. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]
- 66.Motohashi S, Nagato K, Kunii N, Yamamoto H, Yamasaki K, Okita K, Hanaoka H, Shimizu N, Suzuki M, Yoshino I, Taniguchi M, Fujisawa T, Nakayama T. A phase I–II study of alpha-galactosylceramide-pulsed IL-2/GM-CSF-cultured peripheral blood mononuclear cells in patients with advanced and recurrent non-small cell lung cancer. J Immunol. 2009;182:2492–2501. doi: 10.4049/jimmunol.0800126. [DOI] [PubMed] [Google Scholar]
- 67.Uchida T, Horiguchi S, Tanaka Y, Yamamoto H, Kunii N, Motohashi S, Taniguchi M, Nakayama T, Okamoto Y. Phase I study of alpha-galactosylceramide-pulsed antigen presenting cells administration to the nasal submucosa in unresectable or recurrent head and neck cancer. Cancer Immunol Immun-other. 2008;57:337–345. doi: 10.1007/s00262-007-0373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Administer Intramuscular, Subcutaneous, And Intradermal Injections. S MD0552. U.S. Army Medical Department Center and School; Fort Sam Houston: pp. 1–64. [Google Scholar]
- 69.Larsen C, Larsen SW, Jensen H, Yaghmur A, Ostergaard J. Role of in vitro release models in formulation development and quality control of parenteral depots. Expert Opin Drug Deliv. 2009;6:1283–1295. doi: 10.1517/17425240903307431. [DOI] [PubMed] [Google Scholar]
- 70.Pisal DS, Kosloski MP, Balu-Iyer SV. Delivery of therapeutic proteins. J Pharm Sci. 2010;99:2557–2575. doi: 10.1002/jps.22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakagawa R, Nagafune I, Tazunoki Y, Ehara H, Tomura H, Iijima R, Motoki K, Kamishohara M, Seki S. Mechanisms of the antimetastatic effect in the liver and of the hepatocyte injury induced by alpha-galactosylceramide in mice. J Immunol. 2001;166:6578–6584. doi: 10.4049/jimmunol.166.11.6578. [DOI] [PubMed] [Google Scholar]
- 72.Osman Y, Kawamura T, Naito T, Takeda K, Van Kaer L, Okumura K, Abo T. Activation of hepatic NKT cells and subsequent liver injury following administration of alpha-galactosylceramide. Eur J Immunol. 2000;30:1919–1928. doi: 10.1002/1521-4141(200007)30:7<1919::AID-IMMU1919>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 73.Kashiwase K, Kikuchi A, Ando Y, Nicol A, Porcelli SA, Tokunaga K, Omine M, Satake M, Juji T, Nieda M, Koezuka Y. The CD1d natural killer T-cell antigen presentation pathway is highly conserved between humans and rhesus macaques. Immunogenetics. 2003;54:776–781. doi: 10.1007/s00251-002-0527-8. [DOI] [PubMed] [Google Scholar]
- 74.Gansuvd B, Goodwin J, Asiedu CK, Jiang XL, Jargal U, Andrades P, Exley MA, Thomas JM. Invariant natural killer T cells from rhesus macaque spleen and peripheral blood are phenotypically and functionally distinct populations. J Med Primatol. 2008;37:1–11. doi: 10.1111/j.1600-0684.2007.00222.x. [DOI] [PubMed] [Google Scholar]
- 75.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fujio M, Wu D, Garcia-Navarro R, Ho DD, Tsuji M, Wong CH. Structure-based discovery of glycolipids for CD1d-mediated NKT cell activation: tuning the adjuvant versus immunosuppression activity. J Am Chem Soc. 2006;128:9022–9023. doi: 10.1021/ja062740z. [DOI] [PubMed] [Google Scholar]
- 77.Tsuji M. A retrospective evaluation of the role of T cells in the development of malaria vaccine. Exp Parasitol. 2010;126:421–425. doi: 10.1016/j.exppara.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsuji M, Zavala F. T cells as mediators of protective immunity against liver stages of Plasmodium. Trends Parasitol. 2003;19:88–93. doi: 10.1016/s1471-4922(02)00053-3. [DOI] [PubMed] [Google Scholar]
- 79.Lasaro MO, Ertl HC. New insights on adenovirus as vaccine vectors. Mol Ther. 2009;17:1333–1339. doi: 10.1038/mt.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Limbach KJ, Richie TL. Viral vectors in malaria vaccine development. Parasite Immunol. 2009;31:501–519. doi: 10.1111/j.1365-3024.2009.01141.x. [DOI] [PubMed] [Google Scholar]
- 81.Cox KS, Clair JH, Prokop MT, Sykes KJ, Dubey SA, Shiver JW, Robertson MN, Casimiro DR. DNA gag/adenovirus type 5 (Ad5) gag and Ad5 gag/Ad5 gag vaccines induce distinct T-cell response profiles. J Virol. 2008;82:8161–8171. doi: 10.1128/JVI.00620-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koup RA, Roederer M, Lamoreaux L, Fischer J, Novik L, Nason MC, Larkin BD, Enama ME, Ledgerwood JE, Bailer RT, Mascola JR, Nabel GJ, Graham BS. Priming immunization with DNA augments immunogenicity of recombinant adenoviral vectors for both HIV-1 specific antibody and T-cell responses. PLoS ONE. 2010;5:e9015. doi: 10.1371/journal.pone.0009015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vuola JM, Keating S, Webster DP, Berthoud T, Dunachie S, Gilbert SC, Hill AV. Differential immunogenicity of various heterologous prime-boost vaccine regimens using DNA and viral vectors in healthy volunteers. J Immunol. 2005;174:449–455. doi: 10.4049/jimmunol.174.1.449. [DOI] [PubMed] [Google Scholar]