Abstract
Although ErbB receptors have been implicated in prostate cancer progression, ErbB-directed drugs have not proven effective for prostate cancer treatment. The ErbB3-binding protein EBP1 affects both ErbB2 and androgen receptor signaling, two components of the response to ErbB-targeted therapies. We therefore examined the effects of EBP1 expression on the response to the ErbB1/2 tyrosine kinase inhibitor lapatinib. We found a negative correlation between endogenous EBP1 levels and lapatinib sensitivity in prostate cancer cell lines. We then overexpressed or inhibited expression of EBP1. Silencing EBP1 expression increased lapatinib sensitivity and overexpression of EBP1 increased resistance in androgen-containing media. Androgen depletion resulted in an increased sensitivity of androgen-dependent EBP1 expressing cells to lapatinib, but did not affect the lapatinib sensitivity of hormone resistant cells. However, EBP1 silenced cells were still more sensitive to lapatinib than EBP1-expressing cells in the absence of androgens. The increase in sensitivity to lapatinib following EBP1 silencing was associated with increased ErbB2 levels. In addition, lapatinib treatment increased ErbB2 levels in sensitive cells that express low levels of EBP1, but decreased ErbB2 levels in resistant EBP1-expressing cells. In contrast, ErbB3 and phospho ErbB3 levels were not affected by either changes in EBP1 levels or lapatinib treatment. The production of the ErbB3/4 ligand heregulin was increased in EBP1-silenced cells. EBP1-induced changes in AR levels were not associated with changes in lapatinib sensitivity. These studies suggest that the ability of EBP1 to activate ErbB2 signaling pathways results in increased lapatinib sensitivity.
Keywords: EBP1, Prostate cancer, ErbB2, Lapatinib
Introduction
ErbB receptors are critical regulators of prostate cancer progression. EGFR and ErbB2 are overexpressed in castration-resistant sublines of human prostate cancer xenografts [1, 2]. High nuclear ErbB3 predicts recurrences associated with castration-resistant prostate cancer (CRPC) [3]. Preclinical studies have indicated that inhibition of ErbB2/3 signaling potently suppresses the growth of CRPC xenografts [4–6]. Although the use of tyrosine kinase inhibitors (TKIs) such as lapatinib is still being evaluated in clinical trials [7, 8], initial results have been disappointing. A greater understanding of the complexities of ErbB signaling in prostate cancer is needed to design more effective ErbB-targeted therapies.
Part of the failure of ErbB2-directed agents may be due to the fact that ErbB signaling can also inhibit prostate cancer cell growth and AR expression and activity. For example, Heparin-binding EGF (HB-EGF), an ErbB1/4 ligand, decreases AR protein expression through activation of mTOR and decreased AR mRNA translation [9–11]. ErbB stimulation of AKT in LNCaP cells results in enhanced AR phosphorylation, leading to increased ubiquitination and degradation of AR by Mdm2 [12]. Activation of EGFR or ErbB2/3 via EGF or the ErbB3/4 ligand heregulin (HRG) decreases expression of endogenous AR and PSA due to enhanced AR mRNA degradation. This downregulation can be abrogated by EGFR/ErbB2 inhibitors [13].
We have demonstrated that EBP1, a protein isolated in our laboratory by its ability to bind ErbB3, is an AR corepressor that suppresses protein levels of AR and AR target genes and inhibits growth of prostate cancer cells both in vitro and in animal models [14, 15]. EBP1 decreases transcription of AR-activated genes in part by recruiting the corepressors Sin3A and HDAC2 to AR-regulated promoters [16]. In addition to its DNA-binding properties, EBP1 has been demonstrated to be an RNA-binding protein by our group [17] and several others [18, 19]. We have found that EBP1 destabilizes AR mRNA in an HRG inducible manner [17]. In addition, EBP1 modulates ErbB2 expression at both the transcriptional and post-translational levels [20, 21]. As both AR and ErbB2 signaling play a role in determining lapatinib sensitivity, we postulated that the interplay of EBP1-driven changes in the AR and ErbB2 pathways may affect the cellular response to lapatinib. The purpose of this study was to test the effect of EBP1 on lapatinib sensitivity in prostate cancer cell lines.
Materials and methods
Cell culture and reagents
The generation and maintenance of LNCaP EBP1-silenced C13 and control A16 cells and the C81 vector and FLAG-tagged EBP1-transfected cell lines were previously described [15]. The derivation and maintenance of the GFP vector and GFP-EBP1-transfected C4-2B cells have also been described [22]. HRGβ1 was purchased from R&D Systems (Mpls, MN), lapatinib from LC Labs (Woburn, MA), and the synthetic androgen R1881 from Sigma (St. Louis, MO).
Transfection of A16 cells
Subconfluent A16 cells in 100-mm tissue culture dishes were transfected with 10 μg of EGFP or ERBB2-ECFP expression plasmids (AddGene, Cambridge, MA) using an Amaxa Nucleofector™ (Lonza, Walkersville, MD) according to the manufacturer’s protocol. Cells were selected in G418 (800 μg/ml).
Cell growth assays
Cells (2 × 103) were plated in 96-well plates in medium containing 2 % FBS and lapatinib at the indicated concentrations. Relative live cell numbers were determined at day 5 using a Promega Proliferation Reagent (Promega, Madison, WI) as per manufacturer’s instructions with absorbance being read at 490 nm in a Thermo Multiskan Ascent plate reader (Thermo-Fisher, Pittsburgh, PA). For experiments determining lapatinib sensitivity in the absence of androgens, cells were grown in RPMI 1640 phenol red-free media and 10 % charcoal-stripped serum (CSS) for 4 days prior to being used in the growth assay. Growth assays were performed in phenol red-free RPMI 1640 media and 5 % CSS.
Western blotting analysis
Western blot analysis was performed as described previously [23]. The EBP1 antibody was from Millipore (Billerica, MA), the monoclonal anti-GADPH antibody was from Sigma, the polyclonal antibody against ErbB2 and monoclonal antibodies to ErbB3 and phospho ErbB3 (Tyr 1289) were from Cell Signaling (Danvers, MA), and the monoclonal anitbody against GFP was from Clontech (Palo Alto, CA). The monoclonal antibody against AR was from Santa Cruz Biotechnology (Santa Cruz, CA).
Measurement of heregulin levels
Cells were grown to approximately 80 % confluency in 100 mm3 dishes in complete media. Cells were washed once in PBS and serum starved in RPMI 1640 media and 0.5 % serum overnight. Medium was harvested and cell debris was pelleted at 1500 rpm for 5 min. The conditioned medium was then concentrated 10-fold using Millipore Amicon Ultra (3 K) centrifugal filters at 4000×g for 40 min. HRG levels were determined using a NRG ELISA kit from R&D (Mpls, MN) as described by the manufacturer.
Statistical analysis
Western blotting assays were repeated three times. All data presented represent one individual experiment. Where appropriate, data were analyzed using a two-tailed Students t test. Differences with a p < 0.05 were deemed significant.
Results
Effect of EBP1 expression on lapatinib sensitivity
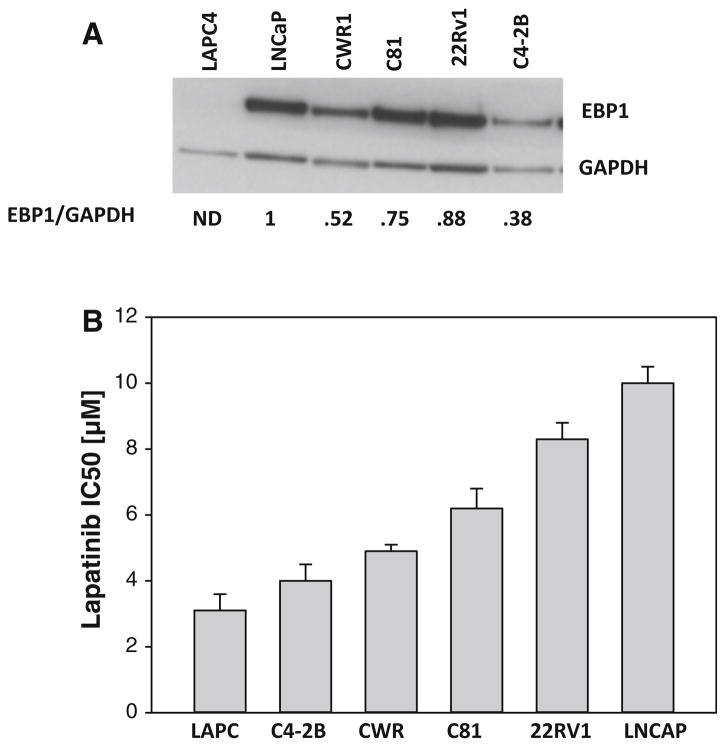
We first determined lapatinib sensitivity of a panel of AR positive prostate cancer cell lines with varying levels of expression of endogenous EBP1. Lower expression of EBP1 was associated with increased sensitivity to lapatinib (r = 0.87 p = 0.03) (Fig. 1).
Fig. 1.
Relationship between EBP1 expression and lapatinib sensitivity. a Lysates of logarithmically growing prostate cancer cell lines were collected and analyzed by Western blotting with antibodies to EBP1 or GAPDH as indicated. ND not detected. The numbers below the blots indicate the relative densities of EBP1 normalized to GAPDH. b The indicated cell lines were treated with lapatinib at concentrations varying from 0.5 to 8.0 μM in androgen-containing media. Cell number was determined 5 days later as described in the “Materials and Methods” section. IC50 values were calculated using Prism software. IC50 values from three independent experiments for each cell line were averaged. Bars represent mean ± SEM
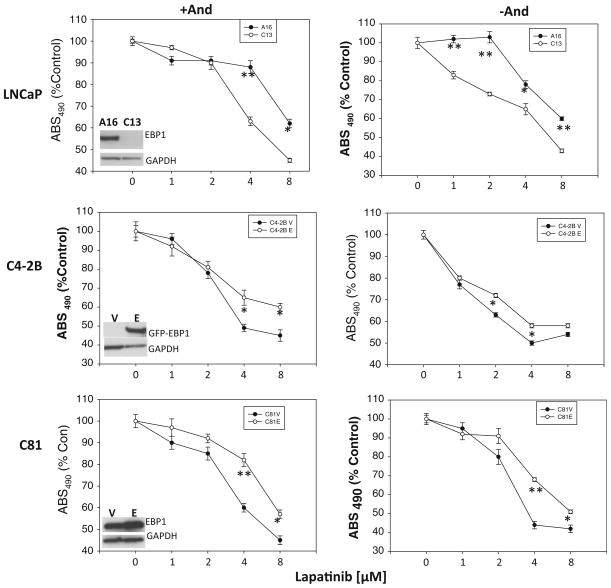
To provide more mechanistic insight into the regulation of lapatinib sensitivity by EBP1, we overexpressed or inhibited expression of EBP1. We found that EBP1-silenced LNCaP cells (C13 cells) were more sensitive to lapatinib in androgen-containing media than the shRNA controls (A16) (Fig. 2 top panel, left). The IC50 for A16 cells was 11.3 and 6.5 μM for C13 cells. Conversely, overexpression of EBP1 in the androgen-independent LNCaP derivatives C4-2B or C81 cells, which express low endogenous levels of EBP1, made cells more resistant to lapatinib (Fig. 2 middle and bottom panels, left). The IC50 for C4-2B vector control cells was 4 and 8.0 μM for C4-2B EBP1 transfectants. Similarly, the IC50 for C81 vector control cells was 6.7 and 11 μM for C81 EBP1 transfectants. Finally, overexpression of EBP1 in androgen-independent PC3 cells, which do not express AR and express low levels of EBP1 [22], resulted in an increased resistance to lapatinib (Suppl. Fig. 1).
Fig. 2.
Effect of EBP1 expression on lapatinib sensitivity in androgen-containing and androgen-depleted conditions. Cells were treated with lapatinib at the indicated concentrations in either androgen-containing (+And, left panel) or androgen-depleted (−And, right panel) media. Cell number was determined 5 days later as described in the “Materials and Methods” section. Each data point represents the Mean ± SD of 6 wells. Similar results were observed in two independent experiments. A16 and C13 cells are LNCaP derivatives in which EBP1 is expressed (A16) or silenced (C13). The EBP1 overexpressing (E) and vector control (V) C4-2B and C81 cells are indicated. Insets indicate EBP1 expression as determined by Western blotting using EBP1 (LNCaP and C81) or GFP (C4-2B) and GAPDH antibodies as indicated. *p < 0.05; **p < 0.01 EBP1-silenced or EBP1-transfected cells versus vector control cells
The complex interplay between AR and ErbB levels might factor into lapatinib sensitivity. For example, androgens increase EGFR levels and decrease ErbB2 and ErbB3 levels [24–26]. Therefore, lapatinib sensitivity in the absence of androgens was also examined. EBP1 expressing LNCaP A16 cells were slightly more sensitive to lapatinib in the absence of androgens (IC50 = 9.7 μM in the absence of androgens vs. 11.3 μM in the presence of androgens) in keeping with previous findings [26]. This increase in sensitivity has been postulated to be due to increased reliance on the ErbB pathway upon androgen depletion in these AR responsive cells. The sensitivity of C13 cells to lapatinib in the absence of androgens was approximately the same as that found in the presence of androgens (IC50 = 6.9 vs. 6.5 μM in the presence of androgens). However, C13 cells remained more sensitive to lapatinib than A16 cells in androgen-free conditions (Fig. 2 top panel, right). Androgen withdrawal did not affect the sensitivity of C4-2B control cells to lapatinib (IC50 = 4 μM in both the absence and presence of androgens), which might be expected as these cells have lost responsiveness to androgens [27]. EBP1 overexpressing C4-2B transfectants were more sensitive to lapatinib in androgen-depleted media than in androgen-containing media (IC50 = 6.1 vs. 8.0 μM). However, the EBP1 transfectants remained more resistant to lapatinib than C4-2B controls in androgen-depleted media (Fig. 2 middle panel, right). Similarly, the lapatinib sensitivity of androgen-independent C81 control cells was only slightly changed by androgen withdrawal (IC50 = 5.4 vs. 6.7 μM in androgen-containing media). The sensitivity of C81 EBP1-overexpressing cells, which are more androgen responsive than C81 controls [15], to lapatinib was increased in androgen-depleted media (IC50 = 7.8 μM in the absence of androgens vs. 11 μM in the presence of androgens (Fig. 2 bottom panel, right). C81V cells were still more sensitive to lapatinib than EBP1 transfectants in the absence of androgens. The IC50 values in androgen complete and androgen-depleted media are summarized in Table 1 and indicate that the absence of androgen renders EBP1-expressing cells more sensitive to lapatinib. EBP1 expression was not affected by androgen withdrawal in any of the cell lines tested (Suppl. Fig. 2).
Table 1.
IC50 concentrations of lapatinib in the presence and absence of androgens
| Cell line | +And | −And |
|---|---|---|
| A16 | 11.3 | 9.7 |
| C13 | 6.5 | 6.9 |
| C4-2B vector | 4.0 | 4.0 |
| C4-2B EBP1 | 8 | 6.1 |
| C81 vector | 6.7 | 5.4 |
| C81 EBP1 | 11 | 7.8 |
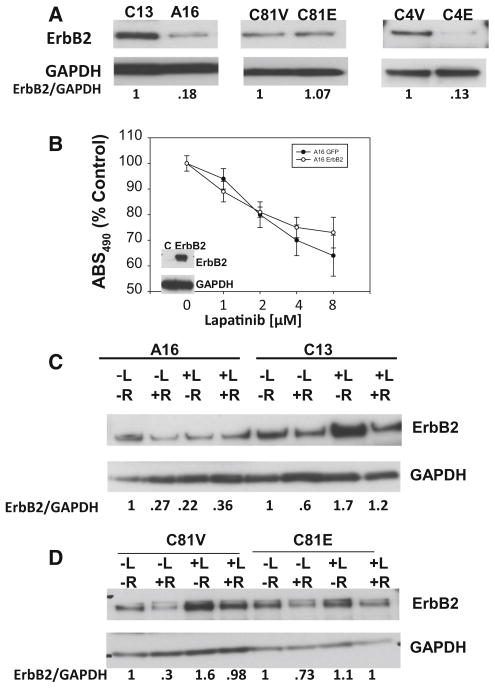
To determine a mechanism for the changes in lapatinib responsiveness in cells in which EBP1 expression has been altered, we first examined ErbB2 expression. We have previously reported that EBP1 regulates ErbB2 expression at both the transcriptional [20] and post-transcriptional levels [21]. We found that ErbB2 expression was increased in C13 EBP1-silenced cells as compared with A16 cells (Fig. 3a, left panel) in keeping with our previous findings [15]. Ectopic expression of EBP1 in C81 cells did not change ErbB2 expression (Fig. 3a, middle panel). In C4-2B cells, overexpression of EBP1 decreased ErbB2 expression (Fig. 3c, right panel). Thus, changes in lapatinib responsiveness were related to EBP1 regulation of ErbB2 levels in 2/3 cell lines.
Fig. 3.
Changes in ErbB2 levels in EBP1-silenced and overexpressing cells and relationship to response to lapatinib. a Expression of ErbB2 in EBP1-silenced or overexpressing cells. Lysates of logarithmically growing C13 or A16 cells, C81 vector control or EBP1-overexpressing cells and C4-2B vector (C4V) or EBP1-transfected (C4E) cells were immunoblotted for ErbB2 or GAPDH as indicated. Representative of 3 experiments. The numbers below the blots indicate the relative densities of ErbB2 and AR normalized to GAPDH. b Effects of ErbB2 levels on the response to lapatinib. A16 cells were transfected with ErbB2. Growth was assessed 4 days later using a Promega Cell Proliferation assay as described in the “Materials and Methods” section. Each data point represents the Mean ± SD of 6 wells. Representative of two experiments. Inset Western blot for ErbB2 expression. c, d A16 and C13 (c) and C81V and C81E (d) cells were grown in androgen-depleted media for 3 days. R1881 (10−9) M and lapatinib (1 μM) were added as indicated 24 h prior to harvesting cells. Western blotting was performed for ErbB2 and GAPDH as shown. Representative of 3 experiments. The numbers below the blots indicate the relative densities of ErbB2 normalized to GAPDH. The value of each untreated cell line was set at 1
To determine if the increased sensitivity of C13 EBP1-silenced cells was only due to higher levels of ErbB2, we transfected A16 cells with ErbB2. There was no significant change in lapatinib sensitivity in these cells as compared to controls (Fig. 3b).
Lapatinib sensitivity has also been correlated with decreases in ErbB2 levels after lapatinib treatment [28]. In addition, androgen withdrawal affects ErbB2 levels, which also can affect lapatinib sensitivity [6, 26]. Therefore, we measured changes in ErbB2 levels in the presence and absence of lapatinib and androgens in cells with different levels of EBP1 expression. ErbB2 levels were decreased by androgen treatment alone in A16 cells in agreement with previous findings [24]. Lapatinib treatment decreased ErbB2 levels in the absence of androgens and resulted in a 33 % increase in the presence of androgens. In EBP1-silenced C13 cells, androgens decreased the levels of ErbB2 in the presence and absence of lapatinib. Lapatinib increased ErbB2 levels in C13 cells in both the absence (70 %) and presence (100 %) of androgens (Fig. 3c). A somewhat similar pattern was observed for C81V and C81 EBP1-transfected cells. In C81 EBP1 transfectants, androgens decreased ErbB2 expression in the absence of lapatinib and had little effect in the presence of lapatinib. As in A16 cells, lapatinib increased ErbB2 expression 30 % in the presence of androgens, but had little effect on ErbB2 expression in the absence of androgens. In C81V cells, androgens decreased ErbB2 expression in the presence and absence of lapatinib. Lapatinib increased ErbB2 expression in both the presence (236 %) and absence (60 %) of androgens (Fig. 3d). Thus, a more robust increase in ErbB2 expression was observed in lapatinib-sensitive lines as compared with lapatinib-resistant lines following lapatinib treatment.
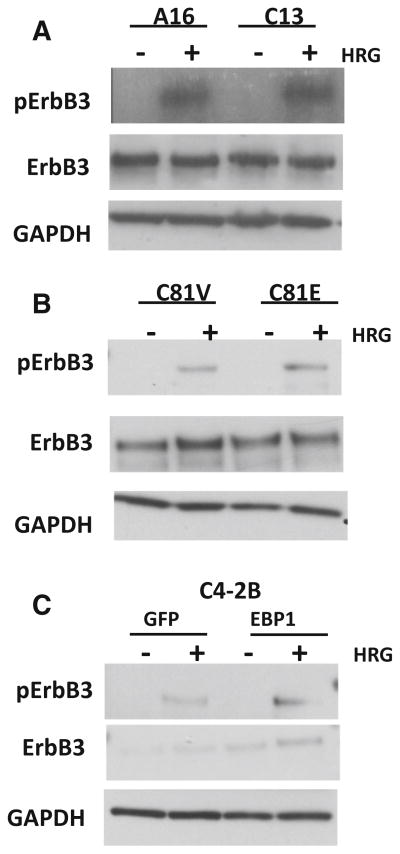
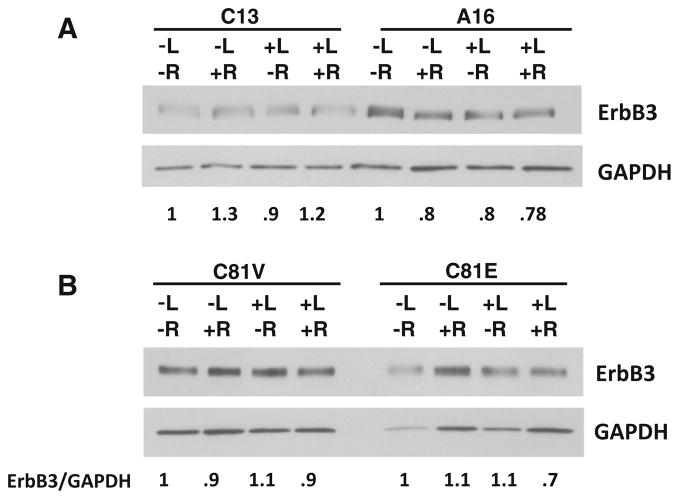
Basal phosphorylation of ErbB3 has been associated with sensitivity to lapatinib [29]. In addition, androgen withdrawal has been demonstrated to increase ErbB3 levels [26], which might also affect the response to lapatinib. We therefore first examined basal and HRG-stimulated activation of ErbB3 in cells which express different levels of EBP1. Basal activation of ErbB3 was not observed in either A16 control or C13 EBP1-silenced cells. ErbB3 was activated to an equal extent in response to HRG in A16 and C13 cells. Total ErbB3 levels were unaffected by EBP1 silencing (Fig. 4a) in keeping with our previous findings in breast cancer cell lines [30]. Similarly, ErbB3 was not basally phosphorylated in either C81V or C81 EBP1-transfected cells. ErbB3 was phosphorylated in response to HRG in both cell lines to an equal extent (Fig. 4b). Total ErbB3 expression in C81V and C81 EBP1-transfected cells was not changed by overexpression of EBP1. A similar pattern was observed in C4-2B GFP and EBP1-overexpressing cells (Fig 4c).
Fig. 4.
Effects of EBP1 expression on ErbB3 levels and phosphorylation of ErbB3 in response to HRG. a A16 and C13 cells b C81 vector control or EBP1-transfected cells and c C4-2B vector control and GFP-EBP1-transfected cells were serum starved overnight and then treated with HRG (20 ng/ml) for 1 hour. Cell lysates were immunoblotted with antibodies to phospho ErbB3, total ErbB3, and GAPDH as indicated
We also determined if lapatinib sensitivity in EBP1-silenced cells was associated with a change in ErbB3 levels after lapatinib treatment. A small (30 %) increase in ErbB3 expression was observed in the presence of androgens alone in C13 cells and a small decrease (20 %) in A16 cells. Lapatinib treatment in the absence or presence of androgens did not significantly affect ErbB3 expression. Similarly, neither androgens nor lapatinib affected ErbB3 expression in hormone-independent C81V cells. Androgen treatment resulted in a small (30 %) decrease in ErbB3 only in the presence of lapatinib (Fig. 5).
Fig. 5.
Effects of EBP1 expression on ErbB3 levels after lapatinib treatment. A16 and C13 (a) and C81V and C81E (b) cells were grown in androgen-depleted media for 3 days. R1881 (10−9) M and lapatinib (1 μM) were added as indicated 24 h prior to harvesting cells. Western blotting was performed for ErbB3 and GAPDH as indicated. Representative of 2 experiments. The numbers below the blots indicate the relative densities of ErbB3 normalized to GAPDH. The value of each untreated cell line was set at 1
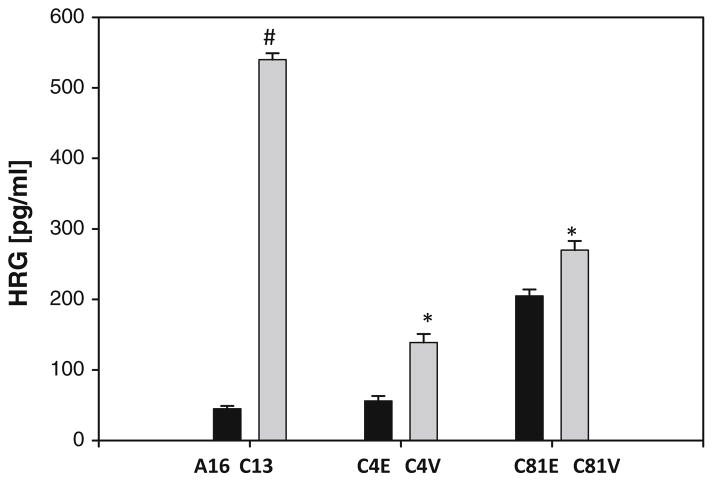
To further explore the mechanism of the increased lapatinib sensitivity in cells expressing low levels of EBP1, we examined HRG secretion in cells with different levels of EBP1 expression. Recent data show that increased production of HRG is associated with increased sensitivity of non-ErbB2 amplified cells to lapatinib [29]. Levels of HRG in conditioned media were quantified by ELISA assay. We found that silencing EBP1 in C13 cells resulted in increased levels of HRG in conditioned media as compared to A16 controls. Conversely, overexpression of EBP1 in C4-2B and C81 cells resulted in a small decrease in secreted HRG (Fig. 6).
Fig. 6.
Effect of EBP1 expression on secreted levels of HRG. The levels of HRG in conditioned media of A16 (control) and C13 (EBP1 silenced) and EBP1 or vector control-transfected C4-2B (C4E, C4V) and C81 (C81E, C81V) cells were measured by ELISA as described in the “Materials and Methods” section. The results represent the average of three independent experiments. #p < 0.01 compared to untreated control *p < 0.05 compared to untreated control
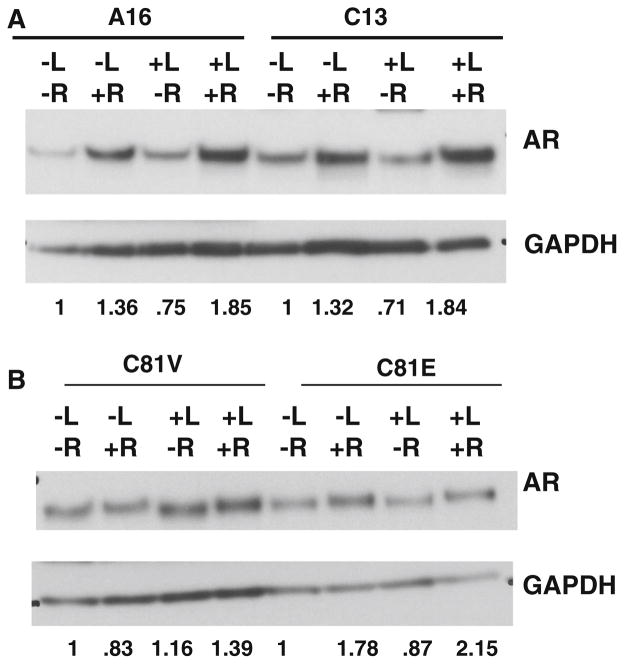
Due to the importance of the AR in prostate cancer cell proliferation, we examined the regulation of AR by lapatinib in control and EBP1-silenced or overexpressing cells. Cells were treated with lapatinib in the presence or absence of androgens. In A16 cells, androgens increased AR protein expression as has been reported for parental LNCaP cells [31]. Lapatinib treatment resulted in a small decrease in AR expression in the absence of androgens and an increase in AR expression in the presence of androgens as has been previously published [13]. This increase in AR levels in the presence of lapatinib and androgens has been postulated to be due to the fact that ErbB2 kinase activity can destabilize AR mRNA. A similar pattern was observed in EBP1-silenced C13 cells. Androgen treatment increased AR levels in the presence or absence of lapatinib (Fig. 7a). Lapatinib treatment decreased AR levels in androgen-free media, but increased AR levels in androgen-containing media. In C81-EBP1-overexpressing cells, R1881 stabilized AR levels as expected as these cells have become more androgen responsive [15]. Lapatinib resulted in a small decrease of AR in androgen-free media as observed for A16 cells and a moderate increase in AR levels in androgen-containing media. In C81V cells, lapatinib treatment resulted in a small increase in AR levels in the absence of androgens and a larger increase in AR levels in the presence of androgens as observed for C13 EBP1-silenced cells (Fig. 7b). Thus, changes in AR levels after lapatinib treatment were the same in both lapatinib-sensitive (EBP1 silenced) and resistant (EBP1 expressing) cell lines.
Fig. 7.
Effect of EBP1 and lapatinib on AR expression. A16 and C13 cells (a) or C81V and C81 EBP1 cells (b) were grown in androgen-depleted media for 3 days. R1881(10−9 M) and lapatinib (1 μM) were added 24 h prior to harvesting cells. Western blotting was performed for AR and GAPDH as indicated. Representative of 3 experiments. The numbers below the blots indicate the relative densities of AR normalized to GAPDH. The value of each untreated cell line was set at 1
Discussion
The ErbB family of tyrosine kinases has been implicated in the progression of prostate cancer. However, the clinical response to ErbB-directed therapies such as pertuzumab [32] and lapatinib [7] has been disappointing. Thus, there is a need for a better understanding of the factors that contribute to the response to ErbB-directed therapies in prostate cancer. We have previously demonstrated that the ErbB3-binding protein EBP1 represses AR activity [33], decreases transcription of AR [14], and lowers steady-state levels of AR mRNA [17]. EBP1 also regulates ErbB2 levels via transcriptional and post-transcriptional mechanisms [20]. The activity of EBP1 is regulated by the ErbB3 ligand HRG [17, 34] placing it in a downstream ErbB2/3 pathway. Thus, we postulated that EBP1 may be involved in the cellular response to lapatinib via its ability to mediate signals from the ErbB2/3 heterodimer to AR, ultimately resulting in changes in cell growth. We demonstrate in this paper that the response to lapatinib is associated with EBP1 expression. The effects of EBP1 on lapatinib response appear to be mediated in part by changes in expression of ErbB2 and HRG.
We first demonstrated that the sensitivity to lapatinib in a series of androgen receptor-expressing prostate cancer cell lines inversely correlated with endogenous EBP1 expression. To provide a more mechanistic basis for this effect, we silenced EBP1 expression and found that this resulted in increased sensitivity to lapatinib. Conversely, transfection of EBP1 into cells that express low endogenous EBP1 levels resulted in decreased responsiveness to lapatinib. We postulated that the increased sensitivity of C13 EBP1-silenced cells or cells which express low endogenous levels of EBP1 (C4-2B, C81) may be due to the increased reliance on a HRG-driven pathway for cell proliferation. We [15] and others [35] have previously shown that HRG inhibits growth of LNCaP parental cells. Suppression of EBP1 expression in these cells results in HRG enhancing cell growth [15]. Similarly, overexpression of EBP1 in C81 and C4-2B cells which express low endogenous levels of EBP1 results in HRG decreasing, rather than increasing, cell growth [15] (Suppl. Fig. 3). Thus, ablation of EBP1 results in HRG promoting growth, making cells vulnerable to lapatinib.
As there are complex interactions between AR and ErbB signaling, we further examined the effects of androgens on the differential response to lapatinib. We first found that androgen withdrawal resulted in increased sensitivity of androgen-dependent LNCaP cells (A16) to lapatinib. Withdrawal of androgens also resulted in increased lapatinib sensitivity in the EBP1-transfected cell lines, which have become more androgen dependent [15]. Similarly, Chen et al. [26] showed that androgen-dependent LNCaP cells do not respond to ErbB inhibitors in the presence of androgens. Androgen withdrawal results in enhanced sensitivity to a combination of ErbB1 and 2 inhibitors, similar to what we observed for more hormone-dependent A16, C4-2B EBP1, and C81 EBP1 cells. Interestingly, in our study, androgen withdrawal resulted in increased ErbB2 levels in hormone-dependent cells as has been previously noted [24, 25]. Chen et al. [26] postulated cells become vulnerable to ErbB inhibition after androgen withdrawal due to increased expression of ErbB2. Androgen withdrawal did not affect lapatinib sensitivity of vector control hormone-independent C81 and C4-2B cells in our study. Similarly, withdrawal of androgens had no effect on the responsiveness of androgen-insensitive cells to dual ErbB inhibitors in earlier studies [4, 26].
One obvious cause for the greater sensitivity to lapatinib would be the increased expression of ErbB2 observed when EBP1 is silenced. In the present study, silencing EBP1 resulted in increased expression of ErbB2. We have found that in breast cancer cells, EBP1 downregulates ErbB2 levels via both transcriptional [20] and post-translational [21] mechanisms. However, transfection of ERBB2 alone did not enhance the sensitivity of A16 cells, which express low levels of ErbB2, to lapatinib. In addition, we found that endogenous ErbB2 expression did not correlate with lapatinib sensitivity in a series of AR-expressing prostate cancer cell lines (Suppl. Fig. 4). Thus, the increased expression of ErbB2 might be necessary but not sufficient to cause the increase in lapatinib sensitivity in the absence of EBP1. Lapatinib sensitivity has also been correlated with decreases in ErbB2 levels in response to lapatinib treatment [28]. However, lapatinib treatment in our study generally resulted in a more robust increase in ErbB2 levels in lapatinib-sensitive cell lines expressing low levels of EBP1 as compared with lapatinib-resistant cells. Thus, EBP1 appears to prevent the increase in ErbB2 observed when cells were treated with lapatinib. Lapatinib also targets the EGFR tyrosine kinase, but EBP1 expression has no effect on EGFR levels or kinase activation [30, 34]. In addition, EBP1 does not physically interact with EGFR [34].
We also examined the expression and phosphorylation status of ErbB3 as basal phosphorylation of ErbB3 is associated with increased sensitivity to ErbB2-directed inhibitors [29]. We did not find any differences in either total ErbB3 levels, basal ErbB3 phosphorylation, or the tyrosine phosphorylation of ErbB3 in response to HRG in cells with varying levels of EBP1 expression. However, EBP1 decreases ErbB3 function [35]. If active ErbB3 is needed for lapatinib sensitivity, EBP1-induced decreases in ErbB3 activity might result in increased resistance. In keeping with the possibility that EBP1 prevents downstream signaling, we have previously shown that silencing EBP1 results in activation of AKT in LNCaP cells, while overexpressing EBP1 in C81 cells decreases AKT activity [15]. Thus, in our study, lapatinib sensitivity was increased under circumstances where AKT was activated. This is in contrast to findings indicating sensitivity of prostate cancer cells to lapatinib was associated with decreased activity of the AKT pathway [36]. However, inhibition of AKT has been shown to result in increased AR activity leading to cell survival [37]. It is possible that increased reliance of EBP1-silenced cells on AKT signaling makes them more vulnerable to ErbB2-directed drugs.
We also interrogated whether lapatinib sensitivity was associated with increased production of the ErbB3/4 ligand HRG. Wilson et al. [29] demonstrated that sensitivity of a large panel of human cancer cell lines derived from multiple tissue types was related to neuregulin (HRG) secretion which drove an ErbB3-regulated pathway. They suggested that a subset of cancer patients which do not have amplification of the ERBB2 gene may still benefit from ErbB-directed therapies. In contrast, they found in other cases that addition of ligands such as HRG resulted in increased resistance to ErbB2-directed therapies [38]. We found that low levels of EBP1 were associated with increased expression of secreted HRG and lapatinib sensitivity. As EBP1 has been shown to be a transcriptional repressor for a number of genes [39–41], future studies should determine EBP1’s effect on HRG transcription. One caveat was that basal ErbB3 phosphorylation was not observed in EBP1-silenced cells, suggesting that the levels of secreted HRG were too low to increase phosphorylation of the receptor or that the secreted HRG may have been inactivated by post-translational modification such as nitration [42].
Due to the importance of AR in prostate cancer cell growth and the ability of EBP1 to affect AR expression [14], we questioned whether lapatinib differentially affected AR expression in cells with varying expression of EBP1. Increased expression of AR has been associated with a decreased sensitivity to ErbB1/2 inhibitors [26]. We found that lapatinib increased AR expression in both A16 and C13 and C81V and C81 EBP1-transfected cells in the presence of androgens. This was somewhat surprising as ErbB2 can stimulate AR transcription in LNCaP cells [43] and inhibition of ErbB2 kinase activity by lapatinib has been found to increase AR levels. However, ErbB2 kinase activity has been demonstrated to destabilize AR mRNA [13] and lapatinib enhances levels of endogenous AR expression. Therefore, in our studies, ErbB2-mediated destabilization of AR mRNA in androgen-containing media may have been more important for maintenance of AR protein levels than ErbB2 activation of AR transcription. In contrast, lapatinib decreased AR levels in both C13, A16, and C81V cells in the absence of androgens. Thus, cells may be more dependent on the ErbB2 transcriptional activation of AR in the absence of androgen. However, overall changes in AR levels after lapatinib treatment exhibited a similar pattern in lapatinib-sensitive cells with low levels of EBP1 expression and lapatinib-resistant cells with higher levels of EBP1 expression.
In conclusion, EBP1 expression affects the sensitivity of prostate cancer cells to lapatinib. In the presence of EBP1, AKT activity and ErbB2 expression are decreased and HRG decreases cellular proliferation. This is associated with lapatinib resistance. In the absence of EBP1, AKT activity and ErbB2 expression are increased and HRG promotes growth. This is associated with lapatinib sensitivity. Thus, our results suggest that this increased sensitivity to lapatinib may be due to EBP1-modulated changes that result in a greater reliance on ErbB signal transduction pathways. We suggest that prostate cancer cells with low expression of EBP1 may be more vulnerable to ErbB-directed therapies.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant 1R01CA138583 (AWH). We thank Dr. M. Lin (University of Nebraska Medical Center) for permission to use the C81 cells and Dr. Yun Qiu (University of Maryland School of Medicine) for providing these cells.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11010-015-2409-z) contains supplementary material, which is available to authorized users.
References
- 1.Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5:280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 2.Yeh S, Lin HK, Kang HY, Thin TH, Lin MF, Chang C. From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc Natl Acad Sci USA. 1999;96:5458–5463. doi: 10.1073/pnas.96.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koumakpayi IH, Diallo JS, Le PC, Lessard L, Gleave M, Begin LR, Mes-Masson AM, Saad F. Expression and nuclear localization of ErbB3 in prostate cancer. Clin Cancer Res. 2006;12:2730–2737. doi: 10.1158/1078-0432.CCR-05-2242. [DOI] [PubMed] [Google Scholar]
- 4.Mellinghoff IK, Tran C, Sawyers CL. Growth inhibitory effects of the dual ErbB1/ErbB2 tyrosine kinase inhibitor PKI-166 on human prostate cancer xenografts. Cancer Res. 2002;62:5254–5259. [PubMed] [Google Scholar]
- 5.Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K, Scher HI, Sliwkowski MX. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 6.Mendoza N, Phillips GL, Silva J, Schwall R, Wickramasinghe D. Inhibition of ligand-mediated HER2 activation in androgen-independent prostate cancer. Cancer Res. 2002;62:5485–5488. [PubMed] [Google Scholar]
- 7.Liu G, Chen YH, Kolesar J, Huang W, Dipaola R, Pins M, Carducci M, Stein M, Bubley GJ, Wilding G. Eastern Cooperative Oncology Group phase II trial of lapatinib in men with biochemically relapsed, androgen dependent prostate cancer. Urol Oncol. 2013;31:211–218. doi: 10.1016/j.urolonc.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whang YE, Armstrong AJ, Rathmell WK, Godley PA, Kim WY, Pruthi RS, Wallen EM, Crane JM, Moore DT, Grigson G, Morris K, Watkins CP, George DJ. A phase II study of lapatinib, a dual EGFR and HER-2 tyrosine kinase inhibitor, in patients with castration-resistant prostate cancer. Urol Oncol. 2013;31:82–86. doi: 10.1016/j.urolonc.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Adam RM, Kim J, Lin J, Orsola A, Zhuang L, Rice DC, Freeman MR. Heparin-binding epidermal growth factor-like growth factor stimulates androgen-independent prostate tumor growth and antagonizes androgen receptor function. Endocrinology. 2002;143:4599–4608. doi: 10.1210/en.2002-220561. [DOI] [PubMed] [Google Scholar]
- 10.Cinar B, De BA, Freeman MR. Post-transcriptional regulation of the androgen receptor by Mammalian target of rapamycin. Cancer Res. 2005;65:2547–2553. doi: 10.1158/0008-5472.CAN-04-3411. [DOI] [PubMed] [Google Scholar]
- 11.Mukhopadhyay NK, Kim J, Cinar B, Ramachandran A, Hager MH, Di VD, Adam RM, Rubin MA, Raychaudhuri P, De BA, Freeman MR. Heterogeneous nuclear ribonucleoprotein K is a novel regulator of androgen receptor translation. Cancer Res. 2009;69:2210–2218. doi: 10.1158/0008-5472.CAN-08-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin HK, Wang L, Hu YC, Altuwaijri S, Chang C. Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. EMBO J. 2002;21:4037–4048. doi: 10.1093/emboj/cdf406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai C, Portnoy DC, Wang H, Jiang X, Chen S, Balk SP. Androgen receptor expression in prostate cancer cells is suppressed by activation of epidermal growth factor receptor and ErbB2. Cancer Res. 2009;69:5202–5209. doi: 10.1158/0008-5472.CAN-09-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Wang XW, Jelovac D, Nakanishi T, Yu MH, Akinmade D, Goloubeva O, Ross DD, Brodie A, Hamburger AW. The ErbB3-binding protein Ebp1 suppresses androgen receptor-mediated gene transcription and tumorigenesis of prostate cancer cells. Proc Natl Acad Sci USA. 2005;102:9890–9895. doi: 10.1073/pnas.0503829102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Linn D, Liu Z, Melamed J, Tavora F, Young CY, Burger AM, Hamburger AW. EBP1, an ErbB3-binding protein, is decreased in prostate cancer and implicated in hormone resistance. Mol Cancer Ther. 2008;7:3176–3186. doi: 10.1158/1535-7163.MCT-08-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Akinmade D, Hamburger AW. The ErbB3 binding protein Ebp1 interacts with Sin3A to repress E2F1 and AR-mediated transcription. Nucl Acids Res. 2005;33:6024–6033. doi: 10.1093/nar/gki903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou H, Mazan-Mamczarz K, Martindale JL, Barker A, Liu Z, Gorospe M, Leedman PJ, Gartenhaus RB, Hamburger AW, Zhang Y. Post-transcriptional regulation of androgen receptor mRNA by an ErbB3 binding protein 1 in prostate cancer. Nucl Acids Res. 2010;38:3619–3631. doi: 10.1093/nar/gkq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Squatrito M, Mancino M, Sala L, Draetta GF. Ebp1 is a dsRNA-binding protein associated with ribosomes that modulates eIF2alpha phosphorylation. Biochem Biophys Res Commun. 2006;344:859–868. doi: 10.1016/j.bbrc.2006.03.205. [DOI] [PubMed] [Google Scholar]
- 19.Bose SK, Sengupta TK, Bandyopadhyay S, Spicer EK. Identification of Ebp1 as a component of cytoplasmic bcl-2 mRNP (messenger ribonucleoprotein particle) complexes. Biochem J. 2006;396:99–107. doi: 10.1042/BJ20051548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh A, Awasthi S, Hamburger AW. ErbB3-binding protein EBP1 decreases ErbB2 levels via a transcriptional mechanism. Oncol Rep. 2013;29:1161–1166. doi: 10.3892/or.2012.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Y, Zhou H, Chen W, Zhang Y, Hamburger AW. The ErbB3 binding protein EBP1 regulates ErbB2 protein levels and tamoxifen sensitivity in breast cancer cells. Breast Cancer Res Treat. 2011;126:27–36. doi: 10.1007/s10549-010-0873-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Ali TZ, Zhou H, D’Souza DR, Lu Y, Jaffe J, Liu Z, Passaniti A, Hamburger AW. ErbB3 binding protein 1 represses metastasis-promoting gene anterior gradient protein 2 in prostate cancer. Cancer Res. 2010;70:240–248. doi: 10.1158/0008-5472.CAN-09-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia X, Lessor TJ, Zhang Y, Woodford N, Hamburger AW. Analysis of the expression pattern of Ebp1, an ErbB-3-binding protein. Biochem Biophys Res Commun. 2001;289:240–244. doi: 10.1006/bbrc.2001.5942. [DOI] [PubMed] [Google Scholar]
- 24.Pignon JC, Koopmansch B, Nolens G, Delacroix L, Waltregny D, Winkler R. Androgen receptor controls EGFR and ERBB2 gene expression at different levels in prostate cancer cell lines. Cancer Res. 2009;69:2941–2949. doi: 10.1158/0008-5472.CAN-08-3760. [DOI] [PubMed] [Google Scholar]
- 25.Berger R, Lin DI, Nieto M, Sicinska E, Garraway LA, Adams H, Signoretti S, Hahn WC, Loda M. Androgen-dependent regulation of Her-2/neu in prostate cancer cells. Cancer Res. 2006;66:5723–5728. doi: 10.1158/0008-5472.CAN-05-3928. [DOI] [PubMed] [Google Scholar]
- 26.Chen L, Mooso BA, Jathal MK, Madhav A, Johnson SD, van Spyk E, Mikhailova M, Zierenberg-Ripoll A, Xue L, Vinall RL, deVere White RW, Ghosh PM. Dual EGFR/HER2 inhibition sensitizes prostate cancer cells to androgen withdrawal by suppressing ErbB3. Clin Cancer Res. 2011;17:6218–6228. doi: 10.1158/1078-0432.CCR-11-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu HC, Hsieh JT, Gleave ME, Brown NM, Pathak S, Chung LW. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int J Cancer. 1994;57:406–412. doi: 10.1002/ijc.2910570319. [DOI] [PubMed] [Google Scholar]
- 28.Konecny GE, Pegram MD, Venkatesan N, Finn R, Yang G, Rahmeh M, Untch M, Rusnak DW, Spehar G, Mullin RJ, Keith BR, Gilmer TM, Berger M, Podratz KC, Slamon DJ. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–1639. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 29.Wilson TR, Lee DY, Berry L, Shames DS, Settleman J. Neuregulin-1-mediated autocrine signaling underlies sensitivity to HER2 kinase inhibitors in a subset of human cancers. Cancer Cell. 2011;20:158–172. doi: 10.1016/j.ccr.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Akinmade D, Hamburger AW. Inhibition of heregulin mediated MCF-7 breast cancer cell growth by the ErbB3 binding protein EBP1. Cancer Lett. 2008;265:298–306. doi: 10.1016/j.canlet.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dehm SM, Tindall DJ. Molecular regulation of androgen action in prostate cancer. J Cell Biochem. 2006;99:333–344. doi: 10.1002/jcb.20794. [DOI] [PubMed] [Google Scholar]
- 32.Agus DB, Sweeney CJ, Morris MJ, Mendelson DS, McNeel DG, Ahmann FR, Wang J, Derynck MK, Ng K, Lyons B, Allison DE, Kattan MW, Scher HI. Efficacy and safety of single-agent pertuzumab (rhuMAb 2C4), a human epidermal growth factor receptor dimerization inhibitor, in castration-resistant prostate cancer after progression from taxane-based therapy. J Clin Oncol. 2007;25:675–681. doi: 10.1200/JCO.2006.07.0649. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Fondell JD, Wang Q, Xia X, Cheng A, Lu ML, Hamburger AW. Repression of androgen receptor mediated transcription by the ErbB-3 binding protein, Ebp1. Oncogene. 2002;21:5609–5618. doi: 10.1038/sj.onc.1205638. [DOI] [PubMed] [Google Scholar]
- 34.Yoo JY, Wang XW, Rishi AK, Lessor T, Xia XM, Gustafson TA, Hamburger AW. Interaction of the PA2G4 (EBP1) protein with ErbB-3 and regulation of this binding by heregulin. Br J Cancer. 2000;82:683–690. doi: 10.1054/bjoc.1999.0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tal-Or P, Di Segni A, Lupowitz Z, Pinkas-Kramarski R. Neuregulin promotes autophagic cell death of prostate cancer cells. Prostate. 2003;55:147–157. doi: 10.1002/pros.10200. [DOI] [PubMed] [Google Scholar]
- 36.Wu Z, Gioeli D, Conaway M, Weber MJ, Theodorescu D. Restoration of PTEN expression alters the sensitivity of prostate cancer cells to EGFR inhibitors. Prostate. 2008;68:935–944. doi: 10.1002/pros.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, Arora VK, Le C, Koutcher J, Scher H, Scardino PT, Rosen N, Sawyers CL. Reciprocal feedback regulation of PI3 K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–586. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, Peng J, Lin E, Wang Y, Sosman J, Ribas A, Li J, Moffat J, Sutherlin DP, Koeppen H, Merchant M, Neve R, Settleman J. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012;487:505–509. doi: 10.1038/nature11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xia X, Cheng A, Lessor T, Zhang Y, Hamburger AW. Ebp1, an ErbB-3 binding protein, interacts with Rb and affects Rb transcriptional regulation. J Cell Physiol. 2001;187:209–217. doi: 10.1002/jcp.1075. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Y, Hamburger AW. Heregulin regulates the ability of the ErbB3-binding protein Ebp1 to bind E2F promoter elements and repress E2F-mediated transcription. J Biol Chem. 2004;279:26126–26133. doi: 10.1074/jbc.M314305200. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Hamburger AW. Specificity and heregulin regulation of Ebp1 (ErbB3 binding protein 1) mediated repression of androgen receptor signalling. Br J Cancer. 2005;92:140–146. doi: 10.1038/sj.bjc.6602257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nethery DE, Ghosh S, Erzurum SC, Kern JA. Inactivation of neuregulin-1 by nitration. Am J Physiol Lung Cell Mol Physiol. 2007;292:L287–L293. doi: 10.1152/ajplung.00058.2006. [DOI] [PubMed] [Google Scholar]
- 43.Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongvipat J, Sawyers CL. HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell. 2004;6:517–527. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.