Summary
The family of p90 ribosomal S6 kinases (RSK) are pleiotropic effectors for extracellular signal-regulated kinase (ERK) signaling pathways. Recently, RSK3 was shown to be important for pathological remodeling of the heart. While cardiac myocyte hypertrophy can be compensatory for increased wall stress, in chronic heart diseases this non-mitotic cell growth is usually associated with interstitial fibrosis, increased cell death, and decreased cardiac function. Although RSK3 is less abundant in the cardiac myocyte than other RSK family members, RSK3 appears to serve a unique role in cardiac myocyte stress responses. A potential mechanism conferring RSK3’s unique function in the heart is anchoring by the scaffold protein muscle A-kinase Anchoring Protein β (mAKAPβ). Recent findings suggest that RSK3 should be considered as a therapeutic target for the prevention of heart failure, a clinical syndrome of major public health significance.
Keywords: Ribosomal S6 Kinase, Heart, Hypertrophy, Remodeling, Signal Transduction, Scaffold, mAKAP
Introduction
In the United States, heart failure affects 5.1 million people, and each year 825,000 new cases are diagnosed (1). Heart failure occurs when the heart is unable to fill with (diastolic dysfunction) or pump out (systolic dysfunction) sufficient blood to meet the needs of the body, and is associated with a high health expenditure which has been estimated to amount to more than $35 billion annually in the United States (1). The prevalence and incidence of heart failure are increasing, mostly because of increasing life span, but also because of the increased prevalence of risk factors (hypertension, diabetes, dyslipidemia, and obesity) and improved survival rates from other types of cardiovascular disease such as myocardial infarction and arrhythmias (2). First-line therapy for patients with heart failure includes angiotensin-converting enzyme (ACE) inhibitors and β-adrenergic receptor blockers (β-blockers) that can improve survival and quality of life in heart failure patients and that have been shown to reduce mortality in patients with left ventricular dysfunction in randomized trials (2). Additional therapies include oral loop diuretics, angiotensin receptor blockers, vasodilators, and aldosterone receptor antagonists. However, despite these therapies the 5-year mortality for heart failure remains at 50% (1). In addition, heart failure patients can be divided almost evenly into those with heart failure with reduced ejection fraction (HFrEF) and those with heart failure with preserved ejection fraction (HFpEF) (2). There are currently no effective therapies for HFpEF, which is generally thought to be attributable mainly to diastolic dysfunction (3, 4). Therefore, there is a clear need to investigate the molecular mechanisms of this syndrome to identify new molecular targets and to develop effective targeted therapies to treat patients with heart failure.
Myocytes are the contracting cells of the heart and are terminally differentiated. The endogenous potential for new cardiac myocyte generation is normally negligible, such that myocyte hypertrophy is the main compensatory mechanism for the heart in response to chronic stress. Hypertrophy is characterized by an increase in myocyte size, enhanced protein synthesis, reorganization of sarcomeres, and stereotypical changes in gene expression (5, 6). Concentric cardiac hypertrophy can decrease wall stress (LaPlace’s Law). However, in disease hypertrophy is also associated with decreased contractility, increased myocyte apoptosis and the development of myocardial interstitial fibrosis, ultimately resulting in heart failure (7). Research over the last decade in mice has revealed that the extent of hypertrophy present in disease is not required for compensation during pressure overload and that blocking the common pathways for remodeling can result in beneficial long-term outcomes (8, 9). Therefore, there is great interest in the elucidation of the mechanisms controlling this response, with the overall goal of identifying new molecular targets for therapeutic intervention in heart failure. Within the individual myocyte, the response to stress is controlled by a network of mitogen-activated protein kinases (MAPK), cyclic nucleotide, Ca2+ and phosphoinositide-dependent intracellular signaling pathways (10). In this review, we focus on the p90 ribosomal S6 kinase RSK3, an effector for extracellular signal-regulated kinase (ERK) pathways that we recently discovered to regulate pathological remodeling of cardiac tissue. Therefore, we have proposed RSK3 as a candidate molecular target for heart failure therapy (11, 12).
p90 Ribosomal S6 Kinases as ERK Effectors
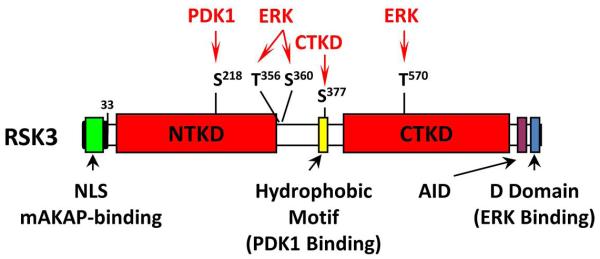
The p90 ribosomal S6 kinases (RSKs) constitute a family of serine/threonine kinases that are involved in the regulation of gene expression and protein translation and cell growth, proliferation, and survival (13, 14). RSK activation occurs in response to growth factors, hormones, chemokines, neurotransmitters, and other stimuli (15). All four RSK isoforms (RSK1-4) contain two kinase domains, an N-terminal kinase domain (NTKD) and a C-terminal kinase domain (CTKD). The NTKD is homologous to AGC kinases, while the CTKD is homologous to Ca2+/calmodulin-dependent protein kinases. The NTKD is responsible for the phosphorylation of RSK substrates and, like other AGC kinases, can phosphorylate sites containing the consensus R/KxRxxS (16). The RSK NTKD is activated by sequential RSK phosphorylation by ERK (1, 2, or 5), the CTKD (autophosphorylation), and 3-phosphoinositide-dependent protein kinase 1 (PDK1) (Fig. 1) (13, 14) (14). ERK is poised to activate RSK by binding inactive RSK on a C-terminal docking domain, while PDK1 is recruited to RSK following CTKD autophosphorylation of the RSK hydrophobic motif (17, 18). Following cessation of stimulation, RSK can be inactivated by protein phosphatase 2A and 2Cδ-catalyzed dephosphorylation (19, 20).
Fig. 1. RSK3 Structure.
Like the related mitogen- and stress-activated kinases (MSK), all four RSKs contain two catalytic domains, N-terminal (NTKD) and C-terminal (CTKD) kinase domains (13). In inactive RSK, the CTKD binds the autoinhibitory domain (AID) α-helix. When activated, ERK pre-bound to the D-domain phosphorylates RSK residues, including the CTKD activation loop (T570). The CTKD then autophosphorylates S377, permitting PDK1 binding and PDK1 phosphorylation of the NTKD activation loop (S218). The NTKD then phosphorylates RSK substrates.
Cloning and initial functional characterization of RSK3 was completed in the mid-1990s (19). RSK3 shares significant homology with other RSK isoforms throughout both kinase catalytic domains; however, RSK3 is structurally distinct from the other RSKs in that it has a different 33-amino-acid N-terminal domain that contains a nuclear localization sequence (19). Studies using antibodies raised against the unique RSK3 N-terminus established that RSK3 translocates to the nucleus of HeLa cells arrested in G0/G1 transition and that it phosphorylates nuclear proteins such as c-Fos and histones (19). Less abundant, alternatively spliced-forms of RSK3 have been identified that contain N-terminal domains similar to RSK1 and RSK2 and that are not localized to the nucleus (21). Another characteristic of RSK3 is that ERK 1/2 does not dissociate from RSK3 as readily as it does from RSK1/2 following mitogen stimulation and that RSK3 has prolonged activity following mitogen termination (18). RSKs 1-3 are expressed in all adult tissues tested thus far (19, 22). Due to the differences in RSK isoform regulation, it is perhaps not surprising that RSKs are not functionally redundant (23, 24). Notably, RSK2 mutations cause the X-linked human disease Coffin-Lowry Syndrome characterized by mental and growth retardation and skeletal and facial anomalies (25, 26).
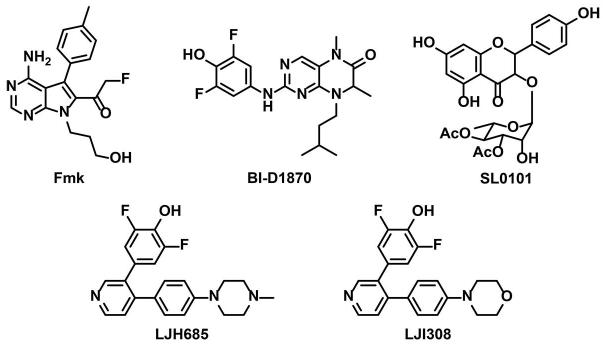
In the cardiac myocyte, it is established that total RSK activity is elevated following stimulation with most hypertrophic agents (27-34), as well as in explanted hearts from patients with end-stage dilated cardiomyopathy (35). RSK has been shown to phosphorylate and activate the sarcolemmal Na+/H+ exchanger NHE1, and α-adrenergic-induced NHE1 phosphorylation in myocytes is blocked by the small chemical inhibitor fluoromethylketone (FMK, Fig. 2) which inhibits all RSKs except RSK3 through binding to the CTKD (36-38). Increased NHE1 activity can cause intracellular sodium and calcium overload. Thus, the improved myocyte survival following ischemia-reperfusion of a mouse expressing a dominant negative RSK1 (dnRSK1) transgene has been attributed to attenuated RSK-activation of NHE1 (39). While unstressed dnRSK1 transgenic mice were overtly normal, transgenic mice over-expressing wild-type RSK1 gradually developed cardiac hypertrophy, with evidence of interstitial fibrosis, myocyte apoptosis and impaired contractility (40). Interestingly, these mice also exhibited QT prolongation associated with RSK1 inhibition of outward K+ channel activity (Kv4.3) (41). There are many potential other targets for RSKs in the myocyte (13), of which myocyte-specific data exist for the phosphorylation of the transcription factor GATA4 (42) and the sarcomeric proteins troponin I and myosin-binding protein-C (43, 44).
Fig 2. Structures of the Known RSK Inhibitors.
As discussed below, RSK3 appears to selectively regulate cardiac remodeling (11, 12). It is worth noting that very little else has been published regarding the physiological function of RSK3 in any organ system, except for a few recent studies regarding the role of RSK3 in cancer. For example, RSK3 has been proposed to be a tumor suppressor in ovarian cancer (45, 46). Conversely, a role has been reported for RSK3 and RSK4 as mediators of resistance to PI3K inhibitors in breast cancer cells both in vitro and in vivo (47). As expected, this resistance could be overcome by the addition of MEK and RSK inhibitors to RSK-overexpressing cells. Similarly, RSK3 depletion was synergistic with epidermal growth factor receptor (EGFR) inhibition in inducing the apoptosis of pancreatic cancer cell lines (48). Since the clinical use of MEK inhibitors may lead to undesirable side effects (49), RSK inhibitors have been suggested to be safer and equally as effective as MEK inhibitors for anti-proliferative therapy in cancer (47).
Role of RSK3 in Cardiac Hypertrophy
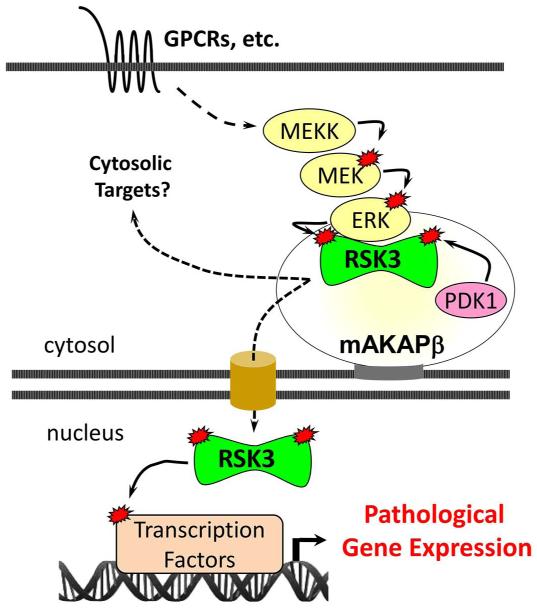
Our group initially became interested in RSK3 due to its association with mAKAP, a scaffold for PDK1 and ERK5 located at the nuclear envelope of striated myocytes and neurons (Fig. 3) (50, 51). The formation of multimolecular enzyme complexes by scaffold proteins is an important mechanism responsible for specificity in intracellular signal transduction (52). By binding scaffold proteins, signaling enzymes may be selectively localized within the cell together with its upstream activators and/or target substrates, constituting a mechanism for efficient and specific isoform signaling. “Signalosome” formation is likely to be especially important for enzymes like RSK3 that are low in abundance or that have broad intrinsic substrate specificity, thereby necessitating targeting of their activity to select locations within the cell. As the organizers of “nodes” in the intracellular signaling network, these scaffold proteins may be of interest as potential therapeutic targets (53). In myocytes, mAKAPβ (the alternatively-spliced form expressed in myocytes) organizes multimolecular complexes that transduce cAMP, MAPK, Ca2+, phosphoinositide, and hypoxic signals regulating the transcription factors NFATc, MEF2 and HIF-1α, as well as the histone deacetylase HDAC4 (54-62). Like RSK3, mAKAPβ is required for the pathological remodeling and development of heart failure in response to pressure overload (61). Remarkably, a discrete site within mAKAP binds the unique RSK3 N-terminal domain, conferring the preferential binding of this RSK isoform to the scaffold and defining a novel protein-protein interaction (11). RSK3-mAKAPβ binding is direct and high affinity (KD = 1.6 nM) as assayed by surface plasmon resonance (11). The high energy of binding is likely due to ionic interactions due to the large number of basic and acidic residues within the docking sites on RSK3 and mAKAPβ, respectively. Interestingly, RSK3 phosphorylation by either ERK or both ERK and PDK1 decreased RSK3-mAKAPβ binding affinity 5- and 8-fold, respectively (11), consistent with a model in which RSK3 would be activated at the scaffold by resident ERK and PDK1 and then translocate to the nucleus following unmasking of the RSK3 nuclear localization signal.
Fig. 3. Model for mAKAPβ-RSK3 Signaling.
ERK pathways are activated by stress signaling, including α1-adrenergic receptors (α1-AR). Anchored by the perinuclear scaffold muscle A-kinase anchoring protein (mAKAPβ), RSK3 is an ERK effector that phosphorylates cytosolic and nuclear substrates, contributing to gene expression that promotes pathological remodeling.
α-Adrenergic stimulation induces the hypertrophy of cultured neonatal rat ventricular myocytes. Consistent with the activation of RSKs by sequential ERK and PDK1 phosphorylation (Fig. 1), acute stimulation of α-adrenergic receptors induced the phosphorylation of RSK3 ERK (S360) and PDK1 (S218) sites through both MEK1/2- and MEK5-dependent signaling (11). SL0101 and BI-D1870 (Fig. 2) are small molecule pan-RSK inhibitors that differ from Fmk in that they bind to the NTKD and inhibit all the RSK family members including RSK3 (46, 63). These compounds have been found to prevent the induction of myocyte hypertrophy by different stimuli including α-adrenergic agonists (42, 64, 11, 65). The specific role for RSK3 in hypertrophy was shown by RNA interference (RNAi) (11). Myocytes transfected with small interfering RNA oligonucleotides (siRNA) for RSK3 showed an attenuated hypertrophic response to α-adrenergic stimulation even though RSK3 is expressed in these cells at much lower levels than other RSK family members (11). Remarkably, the inhibition of myocyte hypertrophy by RSK3 siRNA and active site inhibitors was mimicked by expression of an anchoring disruptor peptide based upon the RSK3-binding site in mAKAPβ. Overexpression of a green fluorescent protein – RSK3 binding domain (mAKAP 1694- 1833) fusion protein blocked RSK3-mAKAPβ association in myocytes, as well as inhibited α-adrenergic induced hypertrophy (11). These results established RSK3 as a key intermediate in the hypertrophic signaling network whose function is conferred by scaffold anchoring.
Results obtained using cultured myocytes have been corroborated in vivo using a global RSK3 knock-out mouse that when unstressed has preserved heart function (11). RSK3 knock-out attenuated the hypertrophy induced by pressure overload or chronic catecholamine infusion without negatively impacting cardiac function or inducing myocyte apoptosis. In particular, RSK3 was important for the “concentric” growth of cardiac myocytes in width, as opposed to the “eccentric” growth of myocytes in length. Besides hypertrophy, RSK3 was also required for the induction of myocardial fibrosis in a non-hypertrophic, mouse heart failure model - the α-tropomyosin Glu180Gly transgenic (TM180) mouse in a mixed C57BL/6:FVB/N background (12). RSK3 knock-out improved both systolic and diastolic dysfunction in the TM180 mouse. These results suggest that targeting of RSK3 may be efficacious in diverse types of heart disease. Interestingly, atrial fibrosis was also attenuated in the TM180 mouse (12), suggesting that RSK3 should be studied further with regards to atrial fibrillation, another cardiac disease with little satisfactory therapy.
RSK3 and Cardiac Remodeling – Many Open Questions
While RSK3 has been established as important in both cardiac myocyte hypertrophy and interstitial fibrosis, many questions remain regarding its mechanism of action. No relevant substrates have yet been shown to be phosphorylated by RSK3 in cardiac remodeling. In addition, while mAKAPβ is a scaffold that binds both upstream activators of RSK3 and despite the overwhelming evidence that mAKAPβ itself is required for cardiac remodeling (61), it is possible that RSK3 signaling in hypertrophy is not limited to mAKAPβ signalosomes. mAKAPβ has only been shown to bind ERK5 and not ERK1/2 (56). However, ERK5 signaling has traditionally been associated with eccentric hypertrophy, while, like RSK3, ERK1/2 signaling is generally considered to promote concentric myocyte hypertrophy (66, 67), albeit the ERK5 knock-out mouse does have an attenuated response to pressure overload-induced hypertrophy (68). It is possible that other scaffolds anchoring RSK3 through its N-terminal domain also facilitate hypertrophic signaling, and the identification of the additional RSK3 scaffolds both in the cardiac myocyte and other cell types will be important for a complete understanding of RSK3 signaling.
Therapeutic Perspectives-RSK3 Inhibitors
Although much remains to be learned regarding the mechanisms of RSK3 action, the ability of RSK3 knock-out to inhibit remodeling in multiple mouse models for heart disease makes RSK3 a candidate drug target for the prevention and/or treatment of heart failure (11, 12). Several kinases have been proposed as possible targets in heart failure, but because of their pleiotropism have been difficult to target therapeutically. For example, inhibitors of MEKs (that activate ERKs and RSKs) are associated with significant ophthalmologic and neurologic side effects (69). In addition, conditional ERK2 knock-out in the adult mouse heart was associated with increased myocyte apoptosis and heart failure following prolonged pressure overload (70). As a specific downstream effector for ERKs, therapeutic intervention by RSK3 inhibition is less likely to produce the severe side effects associated with the inhibition of the upstream master regulators Raf, MEKs and ERKs.
There are currently no specific RSK3 inhibitors. A number of pan-RSK inhibitors have been identified and include BI-D1870, SL0101, LJH685 and LJI308 (Fig. 2) that are all potent NTKD inhibitors (71, 63, 72). Interestingly, the irreversible inhibitor FMK, that was designed to covalently react with the conserved cysteine residue in the CTKD of the RSK family, does not inhibit RSK3 due to the differing ATP-binding domain gatekeeper residue in this isoform (37, 73). Other RSK inhibitors such as staurosporine and quercitrin have been reported, but these compounds have broad spectrum activities (74, 75). Two approaches may be considered to develop a RSK3-selective inhibitor. First, it may be possible to exploit subtle differences in the active site of the RSK3 NTKD and CTKD, such as the gatekeeper residue, to develop a conventional RSK3-selective small molecule inhibitor. Second, given the efficacy of the RSK3 anchoring disruptor peptide in vitro (11), blockade of RSK3-scaffold protein binding in vivo may be considered as a novel therapeutic strategy. While not traditionally considered feasible, recent work has shown that protein-protein interactions may be specifically inhibited in vivo with directed physiologic affects, and several drugs that target protein-protein interactions are currently the subject of clinical trials (76-79).
Conclusion
Elucidating the mechanisms which govern specificity in cellular signaling during cardiac disease has become a major goal of current research. Recent work has identified RSK3 as an important mediator of cardiac remodeling in disease. Research aiming at providing novel mechanistic insights into the regulation and function of RSK3 in the cardiac myocyte is necessary, as this may provide a better understanding of the events involved in RSK3-mediated pathological cardiac remodeling and a conceptual framework necessary for the further development of RSK3 as a therapeutic target for heart failure.
Acknowledgments
None
Funding Sources
This work was supported by NIH grant HL075398 and State of Florida James & Esther King Biomedical Research Program Grant 4KB08 to MSK.
Footnotes
Disclosures
Drs. Kapiloff, Li, Passariello, and Dodge-Kafka are co-inventors of intellectual property concerning the use of RSK3 inhibitors for the treatment of heart failure, for which a patent is pending and which may yield future royalties. This patent is currently assigned to Anchored RSK3 Inhibitors, LLC., in which Dr. Kapiloff holds equity. He and the University of Miami may gain royalties from future commercialization.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics C. Stroke Statistics S Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braunwald E. Heart failure. JACC Heart failure. 2013;1:1–20. doi: 10.1016/j.jchf.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Loffredo FS, Nikolova AP, Pancoast JR, Lee RT. Heart failure with preserved ejection fraction: molecular pathways of the aging myocardium. Circ Res. 2014;115:97–107. doi: 10.1161/CIRCRESAHA.115.302929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma K, Kass DA. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res. 2014;115:79–96. doi: 10.1161/CIRCRESAHA.115.302922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finn SG, Dickens M, Fuller SJ. c-Jun N-terminal kinase-interacting protein 1 inhibits gene expression in response to hypertrophic agonists in neonatal rat ventricular myocytes. Biochem J. 2001;358:489–495. doi: 10.1042/0264-6021:3580489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 8.The CONSENSUS Trial Study Group Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 9.Esposito G, Rapacciuolo A, Naga Prasad SV, Takaoka H, Thomas SA, Koch WJ, Rockman HA. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation. 2002;105:85–92. doi: 10.1161/hc0102.101365. [DOI] [PubMed] [Google Scholar]
- 10.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Kritzer MD, Michel JJ, Le A, Thakur H, Gayanilo M, Passariello CL, Negro A, Danial JB, Oskouei B, Sanders M, Hare JM, Hanauer A, Dodge-Kafka K, Kapiloff MS. Anchored p90 ribosomal S6 kinase 3 is required for cardiac myocyte hypertrophy. Circ Res. 2013;112:128–139. doi: 10.1161/CIRCRESAHA.112.276162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Passariello CL, Gayanilo M, Kritzer MD, Thakur H, Cozacov Z, Rusconi F, Wieczorek D, Sanders M, Li J, Kapiloff MS. p90 ribosomal S6 kinase 3 contributes to cardiac insufficiency in alpha-tropomyosin Glu180Gly transgenic mice. Am J Physiol Heart Circ Physiol. 2013;305:H1010–1019. doi: 10.1152/ajpheart.00237.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9:747–758. doi: 10.1038/nrm2509. [DOI] [PubMed] [Google Scholar]
- 14.Romeo Y, Zhang X, Roux PP. Regulation and function of the RSK family of protein kinases. Biochem J. 2012;441:553–569. doi: 10.1042/BJ20110289. [DOI] [PubMed] [Google Scholar]
- 15.Hauge C, Frodin M. RSK and MSK in MAP kinase signalling. J Cell Sci. 2006;119:3021–3023. doi: 10.1242/jcs.02950. [DOI] [PubMed] [Google Scholar]
- 16.Leighton IA, Dalby KN, Caudwell FB, Cohen PT, Cohen P. Comparison of the specificities of p70 S6 kinase and MAPKAP kinase-1 identifies a relatively specific substrate for p70 S6 kinase: the N-terminal kinase domain of MAPKAP kinase-1 is essential for peptide phosphorylation. FEBS Lett. 1995;375:289–293. doi: 10.1016/0014-5793(95)01170-j. [DOI] [PubMed] [Google Scholar]
- 17.Frodin M, Jensen CJ, Merienne K, Gammeltoft S. A phosphoserine-regulated docking site in the protein kinase RSK2 that recruits and activates PDK1. EMBO J. 2000;19:2924–2934. doi: 10.1093/emboj/19.12.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roux PP, Richards SA, Blenis J. Phosphorylation of p90 ribosomal S6 kinase (RSK) regulates extracellular signal-regulated kinase docking and RSK activity. Mol Cell Biol. 2003;23:4796–4804. doi: 10.1128/MCB.23.14.4796-4804.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y, Bjorbaek C, Weremowicz S, Morton CC, Moller DE. RSK3 encodes a novel pp90rsk isoform with a unique N-terminal sequence: growth factor-stimulated kinase function and nuclear translocation. Mol Cell Biol. 1995;15:4353–4363. doi: 10.1128/mcb.15.8.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doehn U, Gammeltoft S, Shen SH, Jensen CJ. p90 ribosomal S6 kinase 2 is associated with and dephosphorylated by protein phosphatase 2Cdelta. Biochem J. 2004;382:425–431. doi: 10.1042/BJ20040948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bignone PA, Lee KY, Liu Y, Emilion G, Finch J, Soosay AE, Charnock FM, Beck S, Dunham I, Mungall AJ, Ganesan TS. RPS6KA2, a putative tumour suppressor gene at 6q27 in sporadic epithelial ovarian cancer. Oncogene. 2007;26:683–700. doi: 10.1038/sj.onc.1209827. [DOI] [PubMed] [Google Scholar]
- 22.Zeniou M, Ding T, Trivier E, Hanauer A. Expression analysis of RSK gene family members: the RSK2 gene, mutated in Coffin-Lowry syndrome, is prominently expressed in brain structures essential for cognitive function and learning. Hum Mol Genet. 2002;11:2929–2940. doi: 10.1093/hmg/11.23.2929. [DOI] [PubMed] [Google Scholar]
- 23.Lin JX, Spolski R, Leonard WJ. Critical role for Rsk2 in T-lymphocyte activation. Blood. 2008;111:525–533. doi: 10.1182/blood-2007-02-072207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lara R, Mauri FA, Taylor H, Derua R, Shia A, Gray C, Nicols A, Shiner RJ, Schofield E, Bates PA, Waelkens E, Dallman M, Lamb J, Zicha D, Downward J, Seckl MJ, Pardo OE. An siRNA screen identifies RSK1 as a key modulator of lung cancer metastasis. Oncogene. 2011;30:3513–3521. doi: 10.1038/onc.2011.61. [DOI] [PubMed] [Google Scholar]
- 25.Dufresne SD, Bjorbaek C, El-Haschimi K, Zhao Y, Aschenbach WG, Moller DE, Goodyear LJ. Altered extracellular signal-regulated kinase signaling and glycogen metabolism in skeletal muscle from p90 ribosomal S6 kinase 2 knockout mice. Mol Cell Biol. 2001;21:81–87. doi: 10.1128/MCB.21.1.81-87.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Facher JJ, Regier EJ, Jacobs GH, Siwik E, Delaunoy JP, Robin NH. Cardiomyopathy in Coffin-Lowry syndrome. Am J Med Genet A. 2004;128A:176–178. doi: 10.1002/ajmg.a.30056. [DOI] [PubMed] [Google Scholar]
- 27.Sadoshima J, Izumo S. Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine/paracrine mechanism. EMBO J. 1993;12:1681–1692. doi: 10.1002/j.1460-2075.1993.tb05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamazaki T, Tobe K, Hoh E, Maemura K, Kaida T, Komuro I, Tamemoto H, Kadowaki T, Nagai R, Yazaki Y. Mechanical loading activates mitogen-activated protein kinase and S6 peptide kinase in cultured rat cardiac myocytes. J Biol Chem. 1993;268:12069–12076. [PubMed] [Google Scholar]
- 29.Sadoshima J, Qiu Z, Morgan JP, Izumo S. Angiotensin II and other hypertrophic stimuli mediated by G protein-coupled receptors activate tyrosine kinase, mitogen-activated protein kinase, and 90-kD S6 kinase in cardiac myocytes. The critical role of Ca(2+)-dependent signaling. Circ Res. 1995;76:1–15. doi: 10.1161/01.res.76.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Baliga RR, Pimental DR, Zhao YY, Simmons WW, Marchionni MA, Sawyer DB, Kelly RA. NRG-1-induced cardiomyocyte hypertrophy. Role of PI-3-kinase, p70(S6K), and MEK-MAPK-RSK. Am J Physiol. 1999;277:H2026–2037. doi: 10.1152/ajpheart.1999.277.5.H2026. [DOI] [PubMed] [Google Scholar]
- 31.Takeishi Y, Abe J, Lee JD, Kawakatsu H, Walsh RA, Berk BC. Differential regulation of p90 ribosomal S6 kinase and big mitogen-activated protein kinase 1 by ischemia/reperfusion and oxidative stress in perfused guinea pig hearts. Circ Res. 1999;85:1164–1172. doi: 10.1161/01.res.85.12.1164. [DOI] [PubMed] [Google Scholar]
- 32.Kodama H, Fukuda K, Pan J, Sano M, Takahashi T, Kato T, Makino S, Manabe T, Murata M, Ogawa S. Significance of ERK cascade compared with JAK/STAT and PI3-K pathway in gp130-mediated cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2000;279:H1635–1644. doi: 10.1152/ajpheart.2000.279.4.H1635. [DOI] [PubMed] [Google Scholar]
- 33.Snabaitis AK, Muntendorf A, Wieland T, Avkiran M. Regulation of the extracellular signal-regulated kinase pathway in adult myocardium: differential roles of G(q/11), Gi and G(12/13) proteins in signalling by alpha1-adrenergic, endothelin-1 and thrombin-sensitive protease-activated receptors. Cell Signal. 2005;17:655–664. doi: 10.1016/j.cellsig.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension. 2007;49:241–248. doi: 10.1161/01.HYP.0000254415.31362.a7. [DOI] [PubMed] [Google Scholar]
- 35.Takeishi Y, Huang Q, Abe J, Che W, Lee JD, Kawakatsu H, Hoit BD, Berk BC, Walsh RA. Activation of mitogen-activated protein kinases and p90 ribosomal S6 kinase in failing human hearts with dilated cardiomyopathy. Cardiovasc Res. 2002;53:131–137. doi: 10.1016/s0008-6363(01)00438-2. [DOI] [PubMed] [Google Scholar]
- 36.Moor AN, Gan XT, Karmazyn M, Fliegel L. Activation of Na+/H+ exchanger-directed protein kinases in the ischemic and ischemic-reperfused rat myocardium. J Biol Chem. 2001;276:16113–16122. doi: 10.1074/jbc.M100519200. [DOI] [PubMed] [Google Scholar]
- 37.Cohen MS, Zhang C, Shokat KM, Taunton J. Structural bioinformatics-based design of selective, irreversible kinase inhibitors. Science. 2005;308:1318–1321. doi: 10.1126/science1108367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cuello F, Snabaitis AK, Cohen MS, Taunton J, Avkiran M. Evidence for direct regulation of myocardial Na+/H+ exchanger isoform 1 phosphorylation and activity by 90-kDa ribosomal S6 kinase (RSK): effects of the novel and specific RSK inhibitor fmk on responses to alpha1-adrenergic stimulation. Mol Pharmacol. 2007;71:799–806. doi: 10.1124/mol.106.029900. [DOI] [PubMed] [Google Scholar]
- 39.Maekawa N, Abe J, Shishido T, Itoh S, Ding B, Sharma VK, Sheu SS, Blaxall BC, Berk BC. Inhibiting p90 ribosomal S6 kinase prevents (Na+)-H+ exchanger-mediated cardiac ischemia-reperfusion injury. Circulation. 2006;113:2516–2523. doi: 10.1161/CIRCULATIONAHA.105.563486. [DOI] [PubMed] [Google Scholar]
- 40.Itoh S, Ding B, Shishido T, Lerner-Marmarosh N, Wang N, Maekawa N, Berk BC, Takeishi Y, Yan C, Blaxall BC, Abe J. Role of p90 ribosomal S6 kinase-mediated prorenin-converting enzyme in ischemic and diabetic myocardium. Circulation. 2006;113:1787–1798. doi: 10.1161/CIRCULATIONAHA.105.578278. [DOI] [PubMed] [Google Scholar]
- 41.Lu Z, Abe J, Taunton J, Lu Y, Shishido T, McClain C, Yan C, Xu SP, Spangenberg TM, Xu H. Reactive oxygen species-induced activation of p90 ribosomal S6 kinase prolongs cardiac repolarization through inhibiting outward K+ channel activity. Circ Res. 2008;103:269–278. doi: 10.1161/CIRCRESAHA.107.166678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li T, Liu Z, Hu X, Ma K, Zhou C. Involvement of ERK-RSK cascade in phenylephrine-induced phosphorylation of GATA4. Biochim Biophys Acta. 2012;1823:582–592. doi: 10.1016/j.bbamcr.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 43.Itoh S, Ding B, Bains CP, Wang N, Takeishi Y, Jalili T, King GL, Walsh RA, Yan C, Abe J. Role of p90 ribosomal S6 kinase (p90RSK) in reactive oxygen species and protein kinase C beta (PKC-beta)-mediated cardiac troponin I phosphorylation. J Biol Chem. 2005;280:24135–24142. doi: 10.1074/jbc.M413015200. [DOI] [PubMed] [Google Scholar]
- 44.Cuello F, Bardswell SC, Haworth RS, Ehler E, Sadayappan S, Kentish JC, Avkiran M. Novel role for p90 ribosomal S6 kinase in the regulation of cardiac myofilament phosphorylation. J Biol Chem. 2011;286:5300–5310. doi: 10.1074/jbc.M110.202713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clark DE, Errington TM, Smith JA, Frierson HF, Jr., Weber MJ, Lannigan DA. The serine/threonine protein kinase, p90 ribosomal S6 kinase, is an important regulator of prostate cancer cell proliferation. Cancer Res. 2005;65:3108–3116. doi: 10.1158/0008-5472.CAN-04-3151. [DOI] [PubMed] [Google Scholar]
- 46.Smith JA, Poteet-Smith CE, Xu Y, Errington TM, Hecht SM, Lannigan DA. Identification of the first specific inhibitor of p90 ribosomal S6 kinase (RSK) reveals an unexpected role for RSK in cancer cell proliferation. Cancer Res. 2005;65:1027–1034. [PubMed] [Google Scholar]
- 47.Serra V, Eichhorn PJ, Garcia-Garcia C, Ibrahim YH, Prudkin L, Sanchez G, Rodriguez O, Anton P, Parra JL, Marlow S, Scaltriti M, Perez-Garcia J, Prat A, Arribas J, Hahn WC, Kim SY, Baselga J. RSK3/4 mediate resistance to PI3K pathway inhibitors in breast cancer. J Clin Invest. 2013;123:2551–2563. doi: 10.1172/JCI66343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Milosevic N, Kuhnemuth B, Muhlberg L, Ripka S, Griesmann H, Lolkes C, Buchholz M, Aust D, Pilarsky C, Krug S, Gress T, Michl P. Synthetic lethality screen identifies RPS6KA2 as modifier of epidermal growth factor receptor activity in pancreatic cancer. Neoplasia. 2013;15:1354–1362. doi: 10.1593/neo.131660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimizu T, Tolcher AW, Papadopoulos KP, Beeram M, Rasco DW, Smith LS, Gunn S, Smetzer L, Mays TA, Kaiser B, Wick MJ, Alvarez C, Cavazos A, Mangold GL, Patnaik A. The clinical effect of the dual-targeting strategy involving PI3K/AKT/mTOR and RAS/MEK/ERK pathways in patients with advanced cancer. Clin Cancer Res. 2012;18:2316–2325. doi: 10.1158/1078-0432.CCR-11-2381. [DOI] [PubMed] [Google Scholar]
- 50.Michel JJ, Townley IK, Dodge-Kafka KL, Zhang F, Kapiloff MS, Scott JD. Spatial restriction of PDK1 activation cascades by anchoring to mAKAPalpha. Mol Cell. 2005;20:661–672. doi: 10.1016/j.molcel.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 51.Pare GC, Easlick JL, Mislow JM, McNally EM, Kapiloff MS. Nesprin-1alpha contributes to the targeting of mAKAP to the cardiac myocyte nuclear envelope. Exp Cell Res. 2005;303:388–399. doi: 10.1016/j.yexcr.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Scott JD, Pawson T. Cell signaling in space and time: where proteins come together and when they’re apart. Science. 2009;326:1220–1224. doi: 10.1126/science.1175668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Negro A, Dodge-Kafka K, Kapiloff MS. Signalosomes as Therapeutic Targets. Prog Pediatr Cardiol. 2008;25:51–56. doi: 10.1016/j.ppedcard.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 55.Kapiloff MS, Jackson N, Airhart N. mAKAP and the ryanodine receptor are part of a multi-component signaling complex on the cardiomyocyte nuclear envelope. J Cell Sci. 2001;114:3167–3176. doi: 10.1242/jcs.114.17.3167. [DOI] [PubMed] [Google Scholar]
- 56.Dodge-Kafka KL, Soughayer J, Pare GC, Carlisle Michel JJ, Langeberg LK, Kapiloff MS, Scott JD. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005;437:574–578. doi: 10.1038/nature03966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong W, Goehring AS, Kapiloff MS, Langeberg LK, Scott JD. mAKAP compartmentalizes oxygen-dependent control of HIF-1alpha. Sci Signal. 2008;1:ra18. doi: 10.1126/scisignal.2000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kapiloff MS, Piggott LA, Sadana R, Li J, Heredia LA, Henson E, Efendiev R, Dessauer CW. An adenylyl cyclase-mAKAPbeta signaling complex regulates cAMP levels in cardiac myocytes. J Biol Chem. 2009;284:23540–23546. doi: 10.1074/jbc.M109.030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li J, Negro A, Lopez J, Bauman AL, Henson E, Dodge-Kafka K, Kapiloff MS. The mAKAPbeta scaffold regulates cardiac myocyte hypertrophy via recruitment of activated calcineurin. J Mol Cell Cardiol. 2010;48:387–394. doi: 10.1016/j.yjmcc.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vargas MA, Tirnauer JS, Glidden N, Kapiloff MS, Dodge-Kafka KL. Myocyte enhancer factor 2 (MEF2) tethering to muscle selective A-kinase anchoring protein (mAKAP) is necessary for myogenic differentiation. Cell Signal. 2012;24:1496–1503. doi: 10.1016/j.cellsig.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kritzer MD, Li J, Passariello CL, Gayanilo M, Thakur H, Dayan J, Dodge-Kafka K, Kapiloff MS. The scaffold protein muscle A-kinase anchoring protein beta orchestrates cardiac myocyte hypertrophic signaling required for the development of heart failure. Circulation Heart failure. 2014;7:663–672. doi: 10.1161/CIRCHEARTFAILURE.114.001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Passariello CL, Li J, Dodge-Kafka K, Kapiloff MS. mAKAP - A Master Scaffold for Cardiac Remodeling. J Cardiovasc Pharmacol. 2014 doi: 10.1097/FJC.0000000000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sapkota GP, Cummings L, Newell FS, Armstrong C, Bain J, Frodin M, Grauert M, Hoffmann M, Schnapp G, Steegmaier M, Cohen P, Alessi DR. BI-D1870 is a specific inhibitor of the p90 RSK (ribosomal S6 kinase) isoforms in vitro and in vivo. Biochem J. 2007;401:29–38. doi: 10.1042/BJ20061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amirak E, Fuller SJ, Sugden PH, Clerk A. p90 ribosomal S6 kinases play a significant role in early gene regulation in the cardiomyocyte response to G(q)-protein-coupled receptor stimuli, endothelin-1 and alpha(1)-adrenergic receptor agonists. Biochem J. 2013;450:351–363. doi: 10.1042/BJ20121371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Z, Liu R, Townsend PA, Proud CG. p90(RSK)s mediate the activation of ribosomal RNA synthesis by the hypertrophic agonist phenylephrine in adult cardiomyocytes. J Mol Cell Cardiol. 2013;59:139–147. doi: 10.1016/j.yjmcc.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 66.Nicol RL, Frey N, Pearson G, Cobb M, Richardson J, Olson EN. Activated MEK5 induces serial assembly of sarcomeres and eccentric cardiac hypertrophy. EMBO J. 2001;20:2757–2767. doi: 10.1093/emboj/20.11.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kehat I, Davis J, Tiburcy M, Accornero F, Saba-El-Leil MK, Maillet M, York AJ, Lorenz JN, Zimmermann WH, Meloche S, Molkentin JD. Extracellular signal-regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth. Circ Res. 2011;108:176–183. doi: 10.1161/CIRCRESAHA.110.231514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kimura TE, Jin J, Zi M, Prehar S, Liu W, Oceandy D, Abe J, Neyses L, Weston AH, Cartwright EJ, Wang X. Targeted deletion of the extracellular signal-regulated protein kinase 5 attenuates hypertrophic response and promotes pressure overload-induced apoptosis in the heart. Circ Res. 2010;106:961–970. doi: 10.1161/CIRCRESAHA.109.209320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gelb BD, Tartaglia M. RAS signaling pathway mutations and hypertrophic cardiomyopathy: getting into and out of the thick of it. J Clin Invest. 2011;121:844–847. doi: 10.1172/JCI46399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ulm S, Liu W, Zi M, Tsui H, Chowdhury SK, Endo S, Satoh Y, Prehar S, Wang R, Cartwright EJ, Wang X. Targeted deletion of ERK2 in cardiomyocytes attenuates hypertrophic response but provokes pathological stress induced cardiac dysfunction. J Mol Cell Cardiol. 2014;72:104–116. doi: 10.1016/j.yjmcc.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maloney DJ, Hecht SM. Synthesis of a potent and selective inhibitor of p90 Rsk. Organic letters. 2005;7:1097–1099. doi: 10.1021/ol0500463. [DOI] [PubMed] [Google Scholar]
- 72.Aronchik I, Appleton BA, Basham SE, Crawford K, Del Rosario M, Doyle LV, Estacio WF, Lan J, Lindvall MK, Luu CA, Ornelas E, Venetsanakos E, Shafer CM, Jefferson AB. Novel potent and selective inhibitors of p90 ribosomal S6 kinase reveal the heterogeneity of RSK function in MAPK-driven cancers. Molecular cancer research: MCR. 2014;12:803–812. doi: 10.1158/1541-7786.MCR-13-0595. [DOI] [PubMed] [Google Scholar]
- 73.Cohen MS, Hadjivassiliou H, Taunton J. A clickable inhibitor reveals context-dependent autoactivation of p90 RSK. Nat Chem Biol. 2007;3:156–160. doi: 10.1038/nchembio859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ikuta M, Kornienko M, Byrne N, Reid JC, Mizuarai S, Kotani H, Munshi SK. Crystal structures of the N-terminal kinase domain of human RSK1 bound to three different ligands: Implications for the design of RSK1 specific inhibitors. Protein Sci. 2007;16:2626–2635. doi: 10.1110/ps.073123707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Derewenda U, Artamonov M, Szukalska G, Utepbergenov D, Olekhnovich N, Parikh HI, Kellogg GE, Somlyo AV, Derewenda ZS. Identification of quercitrin as an inhibitor of the p90 S6 ribosomal kinase (RSK): structure of its complex with the N-terminal domain of RSK2 at 1.8 A resolution. Acta crystallographica Section D, Biological crystallography. 2013;69:266–275. doi: 10.1107/S0907444912045520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moellering RE, Cornejo M, Davis TN, Del Bianco C, Aster JC, Blacklow SC, Kung AL, Gilliland DG, Verdine GL, Bradner JE. Direct inhibition of the NOTCH transcription factor complex. Nature. 2009;462:182–188. doi: 10.1038/nature08543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Picaud S, Wells C, Felletar I, Brotherton D, Martin S, Savitsky P, Diez-Dacal B, Philpott M, Bountra C, Lingard H, Fedorov O, Muller S, Brennan PE, Knapp S, Filippakopoulos P. RVX-208, an inhibitor of BET transcriptional regulators with selectivity for the second bromodomain. Proc Natl Acad Sci U S A. 2013;110:19754–19759. doi: 10.1073/pnas.1310658110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DC, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park CM, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH, Elmore SW. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 79.Wang S, Sun W, Zhao Y, McEachern D, Meaux I, Barriere C, Stuckey JA, Meagher JL, Bai L, Liu L, Hoffman-Luca CG, Lu J, Shangary S, Yu S, Bernard D, Aguilar A, Dos-Santos O, Besret L, Guerif S, Pannier P, Gorge-Bernat D, Debussche L. SAR405838: an optimized inhibitor of MDM2-p53 interaction that induces complete and durable tumor regression. Cancer Res. 2014;74:5855–5865. doi: 10.1158/0008-5472.CAN-14-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]