Abstract
Objectives
To determine the acute effects of cigarette smoking on hypothalamic-pituitary-adrenal axis (HPA) hormones and subjective states as a function of the menstrual cycle in nicotine-dependent women.
Methods
Seventeen healthy nicotine-dependent women were studied during the follicular and/or luteal phase of the menstrual cycle. Due to observation of a possible bimodal distribution of progesterone levels within the luteal phase group, we performed a set of a posteriori analyses. Therefore, we divided the luteal group into a low progesterone and a high progesterone groups.
Results
Smoked nicotine activated HPA, measured by ACTH, cortisol, and DHEA response and affected subjective states in both follicular and luteal phases, with increased “High”, “Rush”, and decreased “Craving”. The HPA stimulation revealed a blunting of ACTH response. There was only modest evidence for a blunting of subjective state responses in the luteal phase. However upon post hoc analyses, the high progesterone luteal group showed a marked blunting of measures of subjective states and a blunted ACTH response. Examining the association between hormone and measures of subjective states revealed tentative associations of ACTH stimulation with increased “Rush” and “Craving”, and DHEA stimulation with increased “Craving”.
Conclusions
This pilot study suggests that menstrual cycle phase differences in progesterone levels may attenuate nicotine’s addictive effects via diminution of its reinforcing properties, and augmentation of its aversive effects interfering with the pleasure associated with cigarette smoking.
INTRODUCTION
Cigarette smoking is a preventable cause of death and disease, and results in about 1 of every 5 deaths annually (CDC, 2005, 2008), with direct medical costs of at least $50 billion per year (CDC, 1994). A few FDA approved medications for nicotine dependence have shown a low success and high relapse rate. Sixteen percent of women age 18 or older in the US smoke cigarettes (Centers for Disease Control, 2014), and women are less successful in quitting smoking compared to men (Bjornson et al., 1995; Epperson et al., 2010). Therefore, neuroactive gonadal steroids, such as estradiol (E2) and progesterone, which dominate follicular and luteal phase, respectively, may differentially modulate women’s response to nicotine and its treatment outcomes. Previous studies indicated that the abuse-related effects of smoking in women are influenced by menstrual cycle phase (Carpenter et al, 2006; Gray et al 2010, Marks et al 1999). Progesterone may augment nicotine’s aversive effects (including withdrawal effects) and decrease its pleasurable effects (Sofuoglu and Mooney, 2009, Sofuoglu et al., 2009, Allen et al., 2008, Allen et al., 1999) and therefore may have a therapeutic use in smoking cessation (Lynch and Sofuoglu, 2010). An underlying mechanism for nicotine’s effects on the brain may be a striking decline in cortical GABA levels predominantly in the follicular phase (Epperson et al 2005) and an inverse correlation between progesterone levels and the beta 2*-nAChR availability in the cortex and cerebellum of female smokers (Cosgrove et al., 2012) suggesting that progesterone may be a negative allosteric modulator of the beta 2*-nAChR known to be a primary mediator of nicotine’s reinforcing effects in the follicular phase. However, to date, no study has examined the potential role of progesterone as an element of the neuroendocrine response to smoking by comparing responses in women during the luteal phase of the menstrual cycle when progesterone levels are high compared with the follicular phase when they are low.
PARTCIPANTS AND METHODS
Participants
Through online and newspaper advertisements, we recruited women who fulfilled the DSM-IV criteria for current nicotine dependence, and who had normal and regular menstrual cycles. We excluded treatment seeking and nicotine patch wearing women with severe premenstrual syndrome or premenstrual dysphoric disorder who had any lifetime major Axis I or Axis II disorder; a body-mass index below 18 or above 27; any clinically significant current medical problems and clinically significant abnormalities on electrocardiogram, blood chemistry screen, hematology screen, or physical examination. We also excluded women undergoing any hormonal treatments and/or taking birth control medications, including contraceptives administered by orally or via other routes. This study was approved by the McLean Hospital Institutional Review Board, and written informed consent was obtained before any study procedures were performed.
Study Procedures
Screening evaluation
The following were obtained or conducted at the initial screening: written informed consent; Structured Clinical Interview for DSM-IV (APA, 1994; First et al 2002); psychiatric and medical history; Fagerström Test for Nicotine Dependence (Heatherton et al., 1991); physical examination; vital signs; routine blood chemistry and hematology; serum pregnancy test; and urine drug screen.
Overview of study design
We carried out studies in mid-follicular phase (4–8 days after the onset of menses) and the mid-luteal phase (18–23 days after the onset of menses) per 28 day cycle.
Within 24 hours prior to each study day, we verified menstrual cycle status by measuring serum levels of progesterone, luteinizing hormone, and estradiol and performing a serum pregnancy test. We asked participants to abstain from cigarette smoking, caffeine intake after midnight on the night before the study, and alcohol consumption 3 days prior to the study. We conducted studies at the same time of day, beginning at approximately 10:00 am. Prior to the smoking session, we obtained a urine sample, tested subjects for breath alcohol (Alco-Sensor, IV Intoximeters, Inc. St. Louis, MO) and breath CO (>10 ppm was exclusionary). The details of the assays described elsewhere (Mendelson 2008). Participants then underwent a smoking session and a follow-up period, as described below. After study completion, participants remained on the clinical research ward for 2 or more hours to ensure stability of vital signs, and were discharged when medically stable.
Nicotine dose
We used commercially available, high-nicotine content cigarettes (Marlboro Red, Philip Morris, Richmond, VA, USA, which contained 1.2 mg of nicotine and 16 mg of tar (Federal Trade Commision), which were identical to the cigarettes used in our studies in men (Mendelson et al., 2005; Mendelson et al., 2008).
Nicotine administration
Participants were studied in a semi-supine position. We collected physiological and subjective state data at baseline (10 min before smoking session), throughout a 12 min smoking session, and after smoking for a total of 120 min after the beginning of the smoking session. We used a paced smoking procedure to standardize inter-puff intervals and duration of inhalation (Griffiths et al, 1982; Mendelson et al., 2008). Participants took one puff from a cigarette every 30 sec, held the smoke for 5 sec, and exhaled. Using this paced smoking paradigm, individuals absorbed approximately the amount of nicotine contained in one cigarette.
Safety precautions
We monitored pulse, blood pressure, and electrocardiogram continuously with a non-invasive patient monitor for 20 min before smoking the first cigarette and for 2 hours after smoking session initiation as well as completion of the study. We measured systolic and diastolic blood pressure at baseline and 5, 10, 20, 30, 40, 50, 60, 80, 100, 120 min after smoking session started.
Sample collection
We collected plasma or serum samples for analysis of ACTH, cortisol, DHEA, progesterone, and nicotine from an intravenous catheter placed in the antecubital vein 10 min before the smoking began, and then at 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 25, 30, 40, 50, 60, 80, and 120 min following the onset of smoking. This frequency of sampling was based upon our previous observations that nicotine levels increase rapidly after smoking begins and reach peak levels within 14 min (Mendelson et al., 2005; Mendelson et al., 2008). Details of the specimen collection and processing are described elsewhere (Mendelson 2008).
Assay Procedures
We measured serum ACTH using an immunoradiometric assay kit from Nichols Institute (San Juan Capistrano, CA); serum cortisol using the GammaCoat RIA kit from DiaSorin (Stillwater, MN); plasma DHEA by Coated Tubes RIA method using kits purchased from Diagnostic Systems Laboratories Inc. (Webster, TX); and serum nicotine using gas chromatography mass spectrometry (Jacob et al., 2000) (for assay sensitivity and interassay coefficients of variation, see Mendelson et al., 2008). We measured serum progesterone using a direct, double-antibody RIA (ICN Biomedicals Inc.); the serum samples were extracted and reconstituted in zero standard before the assay. The assay sensitivity was 0.12 ng/ml, and the intra- and interassay coefficients of variance were 5.9 and 10.1%, respectively.
Measures of subjective states
Participants rated how “High” they felt, how much of a “Rush” they felt, and how much were they “Craving” cigarettes and rated their reactions to smoking on a Visual Analog Scale (0–100 mm), using the identical method used in two previous studies (Mendelson et al., 2005; 2008), with verbal anchors that 0 was “not at all” and 100 was “as strongly as possible.” Participants rated subjective states at the same time points as samples were collected for hormones.
Data Analysis
For inferential analyses, we used linear regression with adjustment of standard errors for the correlation of observations within individuals using generalized estimating equations. This approach accommodates well observations within and between subjects and does not require complete case data (Fitzmaurice et al., 2011). Specifically, for the model for the mean in comparisons of the levels of hormones or rating of subjective states between groups, the outcome variables were hormone or subjective state measures and the predictor was group status (modeled as indicator variables). For the model for the mean for the association between measures of subjective states and hormones, the outcome variables were ratings of subjective state and the predictor variables were hormone levels.
The study was designed to assess women at both phases of the menstrual cycle. However, it was recognized that, due to the challenges in retaining women to be studied in both phases (because of the extended experimental period), not all participants would have data at both time points. Therefore, we planned to attempt to study women at both phases, but to perform a statistical analysis that could validly utilize all available information from all participants, regardless of whether they were studied at one or both phases of the menstrual cycle.
Because we observed a bimodal distribution of progesterone levels within the luteal phase group (with 7 values below 7.0 ng/ml and 4 values above 11.0 ng/ml), we also performed post hoc analyses in which we divided the luteal group into a low progesterone group (baseline progesterone levels below 10 ng/ml; N=4) and a high progesterone group (baseline progesterone at or above 10 ng/ml; N=7). Note that we had used a cut-off 5 ng/ml on the basis of data supporting it to be valid marker of the luteal phase; e.g., it has been established that progesterone levels of 5 ng/ml are indicative of competent secretory endometrium (Barbieri, 2013). However, since peak levels of progesterone of 10 ng/ml or greater are necessary to achieve ultrasonographic confirmation ovulation (Hughes, 2001), it is possible that the effects of progesterone on hormones and measures of subjective states at this higher level would be different from those at lower levels. These observations thus provided a theoretical rationale for examining women in the luteal phase at two different levels of progesterone, with the hypothesis that there may be dose-dependent effects of progesterone.
We set alpha at 0.05, 2-tailed and performed all analyses using Stata, Version 9.2 (College Station, TX).
Note that the study generated multiple comparisons, increasing the likelihood of type I errors. However, it is likely that Bonferroni and similar correction procedures for these comparisons would be too conservative and would potentially inflate type II error rates, which would obscure potential signals arising from this small study that has very limited power. Thus, we present the results without correction. However, when evaluating the tests of significance reported below, one must consider the greater possibility of chance associations
RESULTS
Participant Characteristics
Seventeen women were studied during at least one phase of their menstrual cycle; 6 participants were studied in the follicular and luteal phases. Their characteristics (mean (SD) at baseline were: age 26.7 (4.6) years; age of menarche 13.1 (1.7) years; years of smoking 8.7 (3.3); body-mass index 23.6 (2.6); breath CO 4.6 (2.7) ppm; serum nicotine 3.3 (1.7 ng/ml; and Fagerström score 5.7 (1.8). The mean (SD) serum progesterone level was 0.6 (0.3) ng/ml for participants in the follicular phase and 8.4 (3.5) ng/ml for participants in the luteal phase; for high- and low-progesterone subgroups, the mean level was 12.7 (1.3) and 5.9 (0.5), respectively.
Nicotine Levels
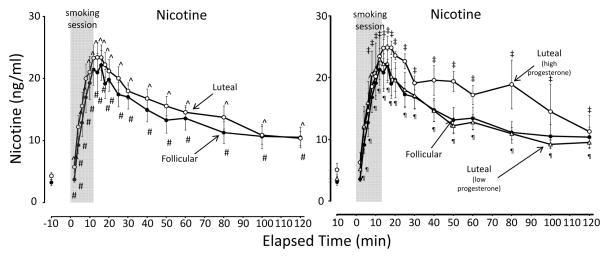
For all groups, mean nicotine levels for all groups peaked within 12–16 minutes after smoking onset (profile depicted in Figure 1). Mean peak levels (Table 1) were similar for all groups
Figure 1.
Mean (± SE) nicotine levels after smoking high-nicotine cigarettes during the follicular and luteal phases of the menstrual cycle. The graph on left depicts the follicular phase in comparison with the luteal phase; the graph on the right depicts the follicular phase in comparison with the two luteal phase subgroups (high progesterone and low progesterone). The 12-min cigarette smoking period is indicated by a gray rectangle. Significant changes (p < 0.05) from the presmoking baseline in follicular phase group are indicated by #; in luteal phase group by ^; in the high progesterone luteal phase subgroup by ‡; and in the low progesterone luteal phase subgroup by ¶.
Table 1.
Effects on Smoked Nicotine on Nicotine Levels, Hypothalamic-Pituitary-Adrenal Axis Hormone Levels, and Mood in Womena in the Follicular and Luteal Phases of the Menstrual Cycle
| Measure | Median Values [Interquartile Range]
|
Comparison Between Groups
|
||||||
|---|---|---|---|---|---|---|---|---|
| Follicular (N=12) | Luteal
|
Follicular vs. All Luteal Coefficientb (SE); P |
Follicular vs. Luteal High Progesterone Coefficientb (SE); P |
Follicular vs. Luteal Low Progesterone Coefficientb (SE); P |
Luteal High vs. Luteal Low Progesterone Coefficienta (SE); P |
|||
| All (N=11) | High Progesterone (N=4) | Low Progesterone (N=7) | ||||||
| Nicotine (ng/ml) | ||||||||
| Peak levelc | 27.4 [20.5, 29.0] | 26. [18.8, 30.1] | 26.1 [25.2, 28.2] | 22.0 [16.0, 34.6] | 0.4 (3.0); .90 | −1.1 (2.0); .60 | 1.2 (4.0); .30 | 2.3 (3.6); .53 |
| Hormones | ||||||||
| Peak levelc | ||||||||
| ACTH, pmol/L | 9.9 [4.9, 30.7] | 8.6 [5.2, 9.4] | 6.9 [4.4, 9.0] | 8.6 [5.6, 12.8] | 8.7 (4.8); .070 | 10.7 (5.2); .038 | 8.1 (5.3); .13 | −2.6 (2.2);. 23 |
| Cortisol, nmol/L | 400 [242, 602] | 343 [288, 539] | 379 [163, 580] | 343 [296, 396] | 48 (76); .53 | 58 (111); .60 | 48 (90); .59 | −10 (121); .93 |
| DHEA, pmol/L | 13.5 [9.6, 24.4] | 12.0 [8.4, 23.5] | 9.5 [7.6, 17.1] | 15.4 [8.7, 23.7] | 1.0 (3.2); .76 | 3.0 (4.2); .73 | −0.7 (3.3); .82 | −3.8 (4.1); .36 |
| Area under the curvee | ||||||||
| ACTH, pmol/L | 6.6 [4.2, 16.6] | 5.0 [3.7, 6.8] | 3.9 [3.3, 5.4] | 5.2 [4.2, 10.4] | 4.3 (2.3); .055 | 5.7 (2.3); .012 | 3.5 (2.5); .15 | −2.3 (1.4); .12 |
| Cortisol, nmol/L | 350 [181, 504] | 281 [217, 419] | 323 [111, 552] | 281 [253, 324] | 65 (63); .30 | 39 (117); .74 | 80 (61); .19 | 40 (119); .74 |
| DHEA, pmol/L | 11.5 [8.6, 20.4] | 9.6 [7.9, 17.3] | 8.8 [6.4, 17.0] | 11.9 [7.9, 17.3] | 1.9 (2.5); .46 | 2.4 (4.5); .58 | 1.6 (2.3); .50 | −0.9 (4.3); .83 |
| Subjective statesf | ||||||||
| Peak leveld | ||||||||
| High | 55 [27, 90] | 40 [20, 100] | 35 [15, 45] | 60 [20, 100] | 5.5 (12.3); .66 | 24.6 (11.6); .033 | −5.4 (17.8); .76 | −30.0 (17.8); .093 |
| Rush | 55 [40, 75] | 60 [10, 80] | 10 [5, 20] | 80 [60, 100] | 0.5 (11.3);. 96 | 47.7 (11.4); <.001 | −23.0 (12.2); .059 | −64.6 (9.5); <.001 |
| Craving (nadirg) | 0 [0, 7] | 0 [0, 0] | 0 [0, 0] | 0 [0, 10] | −0.1 (3.2); .97 | −3.8 (1.8); .041 | 2.0 (4.5); .66 | 5.7 (4.1); .16 |
| Area under the curvee | ||||||||
| High | 10.9 [3.0, 30.4] | 3.6 [0.8 17.8] | 1.1 [0.4, 2.5] | 1.6 [1.5, 1.9] | 12.5 (8.7); .13 | 20.9 (8.4); .013 | 7.7 (9.3); .41 | −13.2 (5.4); .014 |
| Rush | 10.9 [2.0, 26.4] | 3.3 [7.8, 11.8] | 0.6 [0.2, 1.3] | 5.7 [3.3, 23.0] | 7.7 (6.7); .25 | 16.2 (5.4); .002 | 2.8 (8.6); .75 | −13.4 (6.1); .028 |
| Craving (inverseg) | 98.6 [76.0, 108.9] | 102.1 [55.7, 112.7] | 113.8 [98.2, 115.4] | 93.2 [32.3, 104.1] | 6.1 (10.1); .55 | 17.8 (13.8); .20 | −14.4 (9.2); .12 | 32.2 (15.0)* .032- |
Abbreviations: ACTH, adrenocorticotropin hormone; DHEA, dehydroepiandrosterone; min, minute; SE, standard error.
Statistically significant coefficients are bolded and italicized.
17 women were studied: 6 in both follicular and luteal phase, 6 in follicular phase only, 5 in luteal phase only.
Coefficient from regression analysis, using generalized estimating equations, represents estimated mean difference between groups.
For nicotine and hormones, peak level within 120 min after start of smoking session.
Peak level within 120 min after start of smoking session (except nadir, for the case of Craving).
Area under the curve for 120 min after start of smoking session, expressed in units used for peak values divided by 100.
Visual Analog Scales (range 0–100 for each scale).
Analyses reported for the inverse of craving (100-craving), so that the peak represents the lowest point (nadir) of craving, and area under the curve represents the area above the curve for craving.
HPA Axis Hormones: ACTH, Cortisol and DHEA
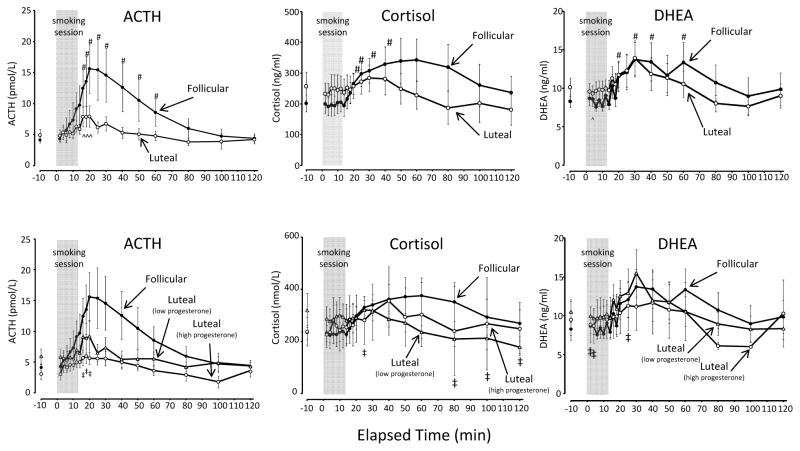
Smoking significantly increased levels of ACTH, cortisol, and DHEA (Figure 2) from baseline. For all groups, the mean peak level for each hormone was significantly greater than baseline (Figure 2; Table 1).
Figure 2.
Mean (± SE) ACTH, cortisol, and DHEA levels after smoking high-nicotine cigarettes during the follicular and luteal phases of the menstrual cycle. The three graphs above depict the follicular phase in comparison with the luteal phase; the three graphs below depict the follicular phase in comparison with the two luteal phase subgroups (high progesterone and low progesterone). The 12-min cigarette smoking period is indicated by a gray rectangle. Significant changes (p < 0.05) from the presmoking baseline in follicular phase group are indicated by #; in luteal phase group by ^; in the high progesterone luteal phase subgroup by ‡; and in the low progesterone luteal phase subgroup by ¶.
ACTH
Comparing the follicular with the luteal phase, neither the mean peak level nor the AUC were significantly different (Table 1). Examining luteal subgroups compared with the follicular group, the high progesterone luteal group showed a blunted response compared with the follicular group, with a significantly lower peak level, and a significantly lower area under the curve (Table 1). For the low progesterone group, the mean peak level and mean area under the curve were not significantly different (Table 1). The low and high progesterone luteal groups did not differ significantly on any comparison.
Cortisol
There were no significant differences between groups (including analysis of luteal subgroups) on peak level or area under the curve (Table 1).
DHEA
There were no significant differences between groups (including analysis of luteal subgroups) on peak level or area under the curve (Table 1).
Subjective States: Ratings of High, Rush and Craving
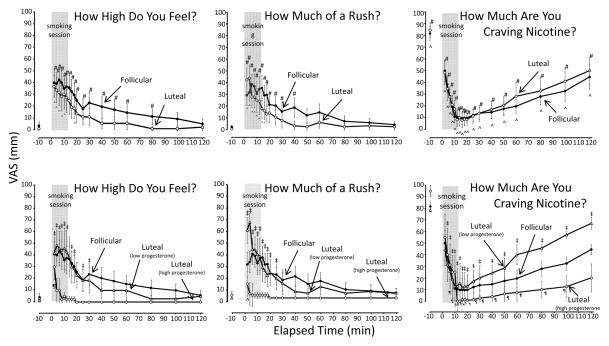
Smoking increased subjective reports of High and Rush, and a decrease reports of Craving (Figure 3).
Figure 3.
Mean (± SE) ratings of High, Rush, and Craving for Nicotine after smoking high-nicotine cigarettes during the follicular and luteal phases of the menstrual cycle. The three graphs above depict the follicular phase in comparison with the luteal phase; the three graphs below depict the follicular phase in comparison with the two luteal phase subgroups (high progesterone and low progesterone). The 12-min cigarette smoking period is indicated by a gray rectangle. Significant changes (p < 0.05) from the presmoking baseline in follicular phase group are indicated by #; in luteal phase group by ^; in the high progesterone luteal phase subgroup by ‡; and in the low progesterone luteal phase subgroup by ¶.
High
Comparing the follicular with the luteal phase, neither the mean peak level nor the mean area under the curve was significantly different (Table 1). Examining luteal subgroups compared with the follicular group, the high progesterone luteal group showed a markedly blunted response, with a significantly lower peak level, and a significantly lower area under the curve (Table 1). The low progesterone group showed a pattern of ratings similar to that of the follicular group. The high progesterone group showed a blunted response relative to the low progesterone group, with a significantly lower mean area under the curve, although the mean peak level was not significantly different (Table 1).
Rush
Comparing the follicular with the luteal phase, neither the mean peak rating nor the mean area under the curve was significantly different (Table 1). Examining luteal subgroups compared with the follicular group, the high progesterone luteal group showed a markedly blunted response, with a significantly lower mean peak level, and a significantly lower mean area under the curve (Table 1). The low progesterone group did not differ significantly from the follicular group on either peak levels or area under the curve (Table 1). The high progesterone group experienced a blunted response relative to the low progesterone group, with a significantly greater lower area under the curve, although the mean peak level was not significantly different (Table 1).
Craving
Comparing the follicular with the luteal phase, the mean ratings of Craving were very similar (Table 1). Examining luteal subgroups compared with the follicular group, the high progesterone luteal group displayed a blunted response compared the follicular phase, with a significantly higher mean nadir, although the mean area under the inverse curve was not significantly different (Table 1). The high progesterone group also had a blunted response relative to the low progesterone group, with a significantly lower mean area under the inverse curve, although the mean nadir was not significantly different (Table 1).
Association of Measures of Hormones with Measures of Subjective States
There was a significant positive association between peak ACTH levels and peak “Rush” ratings (Table 2). There were no significant associations of cortisol measures with ratings of subjective states (Table 2), The only significant association was the positive association between area under the curve for DHEA and area under the inverse curve of Craving (that is, greater DHEA was associated with more Craving) (Table 2).
Table 2.
Association of Measures of Subjective States with Hypothalamic-Pituitary-Adrenal Axis Hormone Levels in Women in the Follicular and Luteal Phases of the Menstrual Cycle
| Measure | ACTH | Cortisol | DHEA |
|---|---|---|---|
| Coefficienta (SE); P | Coefficienta (SE); P | Coefficienta (SE); P | |
| Peak levelsb | |||
| High | 6.0 (5.8); .31 | 0.30 (0.45); .51 | 3.6 (9.2); .70 |
| Rush | 8.5 (3.9); .031 | −0.10 (.45); .83 | 4.9 (8.4); .56 |
| Craving (nadir) | 1.4 (0.8); .061 | 1.1 (0.08); .14 | 1.4 (2.0); .50 |
| AUCc | |||
| High | 12.3 (9.4); .19 | −0.21 (0.36); .56 | 3.1 (7.0); .66 |
| Rush | 7.5 (5.9); .20 | −0.02 (0.22); .90 | −1.5 (4.9); .76 |
| Inverse of Craving | 11.1 (8.0); .17 | 0.37 (0.35); .29 | 15.6 (6.4); .015 |
Statistically significant coefficients are bolded and italicized.
Coefficient from regression analysis, using generalized estimating equations, scaled to represent estimated change in subjective state for each 10-unit increase in hormone level.
Peak level within 120 min after start of smoking session.
Area under the curve for 120 min after start of smoking session, expressed in units used for peak values divided by 100.
DISCUSSION
This pilot study investigated nicotine-induced activation of the HPA axis in association with changes in measures of subjective states as a function of the menstrual cycle phase. Smoked tobacco activated the HPA axis (ACTH, cortisol, and DHEA) in both the follicular and luteal phases of the menstrual cycle, and these endocrine responses paralleled serum nicotine levels and ratings of subjective states. Upon further scrutiny, we noted that the luteal phase women could be separated on the basis of their progesterone levels and therefore we conducted a post hoc analysis of high and low progesterone groups, which revealed that the high progesterone group produced an even more blunted response on ratings of subjective state and a blunted response to ACTH. ACTH stimulation was associated with increased reports of Rush and DHEA levels were associated with increased Craving.
The attenuation of ACTH and subjective measures related to reward in the high progesterone group after nicotine administration may be indicative of an effect of progesterone generally on reward circuits activated by drugs of abuse, particularly stimulants such as cocaine (Mello, 2010). For example, in a clinical study with similarities to the present study, Fox and associates (2013) found that ACTH and behavioral response to cocaine cues in cocaine-dependent women was attenuated with administration of progesterone as compared with placebo.
Despite emerging evidence of a differential response to drugs of abuse, women remain an understudied, yet potentially more vulnerable, population when it comes to addictive disorders. For example, while women often become addicted to drugs later in life, the magnitude of their dependence seems to be greater and the speed with which the process develops is faster than with men (Ziegler, 2008). Relapse appears to be more challenging and as a result, women have a more difficult time quitting smoking (McVay and Copeland 2011). It is possible that the fluctuating hormonal milieu contributes to the difficulties in adhering to various treatment strategies.
The similar time effect curves of plasma ACTH with serum nicotine levels and self-reports of rush suggests that these elements are related. The inverse relationship to craving is expected because the abstinence-induced increase in tobacco craving was quickly satisfied after smoking onset. ACTH release may well be mediated by E2, which acts synergistically with nicotine on several neurotransmitter systems (Centeno M.L et al., 2006, Watkins et al., 2000).
In contrast to E2, progesterone appears to have an inverse effect on the reinforcing effects of nicotine. HPA activation was diminished in the luteal phase with a blunted mood state response in the high progesterone group. Although there is no established mechanism linking progesterone with ACTH released induced by nicotine, serotonin (5-HT), dopamine (DA) and γ-Aminobutyric acid (GABA) are candidate neurotransmitters modulating the ACTH/progesterone response to nicotine (Stone 1996). The possibility that progesterone is responsible for attenuating nicotine-induced activation of the HPA axis is supported, potentially having important clinical implications as advocated by poorer smoking cessation outcomes during the follicular phase (Allen et al., 2008).
There are two naturally higher progesterone states physiologically unique to a woman: luteal phase (post-ovulatory) characterized by higher progesterone levels and pregnancy, characterized by the highest concentrations of progesterone. Less tobacco consumption was reported during the first trimester of pregnancy in 209 pregnant smokers, potentially supporting the hypothesis that progesterone exerts a negative modulatory effect on cigarette smoking (Pletsch PK et al 2008). Alternatively, it is equally plausible that a good deal of the observed reduction in smoking was associated with the psychosocial pressure placed on pregnant women to cease smoking in an interest of the health of the fetus. Nicotine abstinence was achieved by approximately 80–90% of women after initiation of pregnancy (CDC, 2005; Mathews & Rivera, 2004), however, as many as 70% of these women (DiClemente et al 2000; Kahn et al., 2002; McBride et al., 1999) re-established the usual patterns between three (Kahn et al 2002; McBride et al., 1999) and twelve months post parturition (DiClemente et al, 2000; Kahn et al., 2002; McBride et al., 1999). Given this evidence, we propose that increased progesterone may facilitate smoking cessation and temporarily protect against relapse, just as it occurs naturally during pregnancy.
Several limitations of the study should be considered.
First, the major limitation was that the results were derived from a post hoc analysis on a relatively small sample size.
Second, the study design lacked a placebo or sham-smoking (e.g., denicotinized cigarettes) control. Thus, expectation effects might have influenced the results, especially in those participants who underwent two smoking challenge sessions. Further, it is possible that women studied at the second time point would develop other aspects of habituation to the study procedures and that this habituation would influence the results of the study.
Third, because participants had undergone an overnight period of abstinence, they could be expected to be in an early phase of withdrawal from nicotine, which may in turn have influenced the hormonal responses and measures of subjective states. Furthermore, since we did not have any measures of withdrawal, it is not possible to examine the potential association of levels of withdrawal within the group with the outcomes measures under study.
Fourth, a number of participants were studied at only one phase and consequently the lack of data from their second phase resulted in missing data, thus raising a potential concern that the results may be biased due to failure to observe these missing outcomes. However, for missing data to result in a biased estimate, the mechanism for the missingness must be related to the outcomes of interest. We believe that the reason that a participant did not undergo a second study session would likely be unrelated to their hormonal or subjective responses to nicotine administration. Therefore, bias due to missing data is likely to be minimal.
Looking at future directions, further studies are warranted to strategically administer progesterone in postovulatory doses to simulate and prolong the luteal phase of the menstrual cycle to potentially alter the acute rewarding effects of tobacco smoking, which therefore may result in a reduction in smoking, prevention of rapid relapse, and facilitation of abstinence. Such an approach would also address individual needs that are currently not being met by conventional therapies as it would aid in the development of more specific, possibly more successful and cost-effective sex-specific treatment for nicotine dependence in women.
In conclusion, the results of this study suggest that menstrual cycle phase differences in progesterone levels may modulate nicotine-induced behavioral/mood state changes that may have an impact on its addictive potential in women.
Acknowledgments
The authors acknowledge Ms. Haley Duncanson for their assistance; and the late Drs. Nancy K. Mello and Jack H. Mendelson for the grant support for this study.
Financial Support : Supported in part by grants R01-DA15067 AND T32-DA07252
References
- Allen S, Bade T, Center B, Finstad D, et al. Menstrual phase effects on smoking relapse. Addiction. 2008;103:809–821. doi: 10.1111/j.1360-0443.2008.02146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen S, Hatsukami D, Christianson D, et al. Withdrawal and pre-menstrual symptomatology during the menstrual cycle in short-term smoking abstinence: effects of menstrual cycle on smoking abstinence. Nicotine Tob Res. 1999;1:129–142. doi: 10.1080/14622299050011241. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. APA Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, D.C: 1994. [Google Scholar]
- Barbieri RL. Female infertility. In: Strauss JF, Barbieri RL, editors. Yen & Jaffe’s reproductive endocrinology (seventh edition): physiology, pathophysiology, and clinical management. Philadelphia: Elsevier Saunders; 2013. pp. 512–537. [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. Nicotine dependencemajor depression. New evidence from a prospective investigation. Arch Gen Psychiatry. 1993;50:31–35. doi: 10.1001/archpsyc.1993.01820130033006. [DOI] [PubMed] [Google Scholar]
- Bjornson W, Rand C, Connett JE, et al. Gender differences in smoking cessation after 3 years in the lung health study. Am J Public Health. 1995;85:223–230. doi: 10.2105/ajph.85.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter M, Upadhyaya H, LaRowe S, et al. Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: a review. Nicotine Tob Res. 2006;8:627–638. doi: 10.1080/14622200600910793. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Medical-care expenditures attributable to cigarette smoking—United States, 1993. Morb Mortal Weekly Report. 1994;43:469–472. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Annual smoking-attributable mortality, years of potential life cost and productivity losses - United States, 1997–2001. Morb Mortal Weekly Report. 2005;54:625–628. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Current cigarette smoking among adults—United States, 2005–2012. Morb Mortal Weekly Report. 2014;63:29–34. [PMC free article] [PubMed] [Google Scholar]
- Centeno M, Henderson J, Pau K, et al. Estradiol increases alpha7 nicotinic receptor in serotonergic dorsal raphe and noradrenergic locus coeruleus neurons of macaques. J Comp Neurol. 2006;497(3):489–501. doi: 10.1002/cne.21026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove K, Esterlis I, McKee S, et al. Sex differences in availability of β2*-nicotinic acetylcholine receptors in recently abstinent tobacco smokers. Arch Gen Psychiatry. 2012;69(4):418–27. doi: 10.1001/archgenpsychiatry.2011.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiClemente C, Dolan-Mullen P, Windsor RA. The process of pregnancy smoking cessation: Implications for interventions. Tobacco Control. 2000;9:16–21. doi: 10.1136/tc.9.suppl_3.iii16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson C, O’Malley S, Czarkowski K, et al. Sex, GABA, and nicotine: the impact of smoking on cortical GABA levels across the menstrual cycle as measured with proton magnetic resonance spectroscopy. Biol Psychiatry. 2005;57(1):44–8. doi: 10.1016/j.biopsych.2004.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Toll B, Wu R, et al. Exploring the impact of gender and reproductive status on outcomes in a randomized clinical trial of naltrexone augmentation of nicotine patch. Drug Alcohol Dep. 2010;112:1–8. doi: 10.1016/j.drugalcdep.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal Trade Commission. (FTC) FTC Technical Report. Washington DC: 2000. Report of “tar”, nicotine, and carbon monoxide of the smoke of 1294 varieties of domestic cigarettes for the year 1998. [Google Scholar]
- First M, Spitzer R, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Non–patient Edition (SCID–I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fitzmaurice GM, Laird NM, Ware JH, editors. Applied longitudinal analysis. 2. New York: Wiley; 2011. [Google Scholar]
- Fox HC, Sofuoglu M, Morgan PT, Tuit KL, Sinha R. The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: Impact of gender and cue type. Psychopharmacology. 2013;38:1532–1544. doi: 10.1016/j.psyneuen.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman A, Helzer J, Covey L, et al. Smoking, smoking cessation, and major depression. JAMA. 1990;264:1546–1549. [PubMed] [Google Scholar]
- Gray K, DeSantis S, Carpenter M, et al. Menstrual cycle and cue reactivity in participants smokers. Nicotine Tob Res. 2010;12:174–178. doi: 10.1093/ntr/ntp179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R, Henningfield J, Bigelow G. Human cigarette smoking: manipulation of number of puffs per bout, interbout interval and nicotine dose. J Pharmacol Exp Ther Feb. 1982;220(2):256–65. [PubMed] [Google Scholar]
- Heatherton T, Kozlowski L, Frecker R, et al. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hugues J-N. Induction of ovulation in World Health Organization group II anovulatory participants undergoing follicular stimulation with recombinant human follicle-stimulating hormone: A comparison of recombinant human chorionic gonadotropin (rhCG) and urinary hCG. Fertility and Sterility. 2001;75:753–760. doi: 10.1016/s0015-0282(01)01803-9. [DOI] [PubMed] [Google Scholar]
- Jacob P, Wu S, Yu L, Benowitz N. Simultaneous determination of mecamylamine, nicotine, and cotinine in plasma by gas chromatography-mass spectrometry. J Pharm Biomed Anal. 2000;23:653–661. doi: 10.1016/s0731-7085(00)00343-5. [DOI] [PubMed] [Google Scholar]
- Kahn R, Certain L, Whitaker R. A re-examination of smoking before, during, and after pregnancy. American Journal of Public Health. 2002;92:1801–1808. doi: 10.2105/ajph.92.11.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch W, Sofuoglu M. Role of progesterone in nicotine addiction: evidence from initiation to relapse. Exp Clin Psychopharmacol. 2010;18(6):451–61. doi: 10.1037/a0021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani S, O’Malley B. Mechanisms of progesterone receptor action in the brain. Hormones, Brain and Behavior. 2002;3:643–682. [Google Scholar]
- Marks J, Pomerleau C, Pomerleau O. Effects of menstrual phase on reactivity to nicotine. Addict Behav. 1999;24:127–134. doi: 10.1016/s0306-4603(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Marx C, Trost W, Shampine L, et al. Neuroactive steroids, negative affect, and nicotine dependence severity in male smokers. Psychopharmacology. 2006;186:463–472. doi: 10.1007/s00213-005-0226-x. [DOI] [PubMed] [Google Scholar]
- Mathews T, Rivera C. Smoking during pregnancy—United States, 1990–2002. Morbidity Mortality. 2004;53:911–915. [PubMed] [Google Scholar]
- McBride C, Pirie P. Postpartum smoking relapse. Addictive Behaviors. 1990;15:165–168. doi: 10.1016/0306-4603(90)90020-x. [DOI] [PubMed] [Google Scholar]
- McVay M, Copeland A. Smoking Cessation in Peri- and Postmenopausal Women: A Review. Experimental and Clinical Psychopharmacology. 2011;19(3):192–202. doi: 10.1037/a0023119. [DOI] [PubMed] [Google Scholar]
- Mello N. Hormones, nicotine, and cocaine: Clinical studies. Hormones and Behavior. 2010;58:57–71. doi: 10.1016/j.yhbeh.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson J, Goletiani N, Sholar M, et al. Effects of smoking successive low- and high-nicotine cigarettes on hypothalamic-pituitary-adrenal axis hormones and mood in men. Neuropsychopharmacology. 2008;33:749–760. doi: 10.1038/sj.npp.1301455. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Sholar MB, Goletiani NV, Siegel AJ, Mello NK. Effects of low and high nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacology. 2005;30:1751–1763. doi: 10.1038/sj.npp.1300753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Vender J, Grobe J, Picciotto M, Brunzell D, Caldarone B. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13:1097–1106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- Pletsch P, Pollak K, Peterson B, et al. Olfactory and gustatory sensory changes to tobacco smoke in pregnant smokers. Res Nurs Health. 2008;31(1):31–41. doi: 10.1002/nur.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Babb D, Hatsukami D. Progesterone treatment during the early follicular phase of the menstrual cycle: effects on smoking behavior in participants. Pharmacol Biochem Behav. 2001;69:299–304. doi: 10.1016/s0091-3057(01)00527-5. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Mooney M. Progesterone effects on subjective and physiological responses to intravenous nicotine in male and female smokers. Hum Psychopharmacol. 2009;24(7):559–64. doi: 10.1002/hup.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Mooney M. Subjective responses to intravenous nicotine: greater sensitivity in women than in men. Exp Clin Psychopharmacol. 2009;17(2):63–9. doi: 10.1037/a0015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone TW. CNS Neurotransmitters and Neuromodulators. CRC Press: Taylor & Francis Group, LLC; 1996. [Google Scholar]
- Watkins S, Koob G, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine Tob Res. 2000;2:19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- Ziegler P. Addiction in older women: American health care’s best-kept secret. J Calif Dent Assoc. 2008;36(2):115–8. [PubMed] [Google Scholar]