Abstract
Background
The neural projections from the infralimbic region of the prefrontal cortex (IL) to the amygdala are important for the maintenance of conditioned fear extinction. Neurons in this pathway exhibit a unique pattern of structural plasticity that is sex-dependent, but the relationship between the morphological characteristics of these neurons and successful extinction in males and females is unknown.
Methods
Using classic cued fear conditioning and extinction paradigm in large cohorts of male and female rats, we identified subpopulations of both sexes that exhibited high (HF) or low (LF) levels of freezing on an extinction retrieval test, representing failed or successful extinction maintenance, respectively. We then combined retrograde tracing with fluorescent intracellular microinjections to perform 3D reconstructions of IL neurons that project to the basolateral amygdala (BLA) in these groups.
Results
HF/LF males exhibited neuroanatomical distinctions that were not observed in HF vs. LF females. A retrospective analysis of behavior during fear conditioning and extinction revealed that despite no overall sex differences in freezing behavior, HF/LF phenotypes emerged in males during extinction, but in females during fear conditioning, which does not involve IL-BLA neurons.
Conclusion
Our results suggest that the neural processes underlying successful or failed extinction maintenance may be sex-specific. These findings are not only relevant to future basic research on sex differences in fear conditioning and extinction, but also to exposure-based clinical therapies, which are similar in premise to fear extinction, and which are primarily used to treat disorders that are more common in women than in men.
Keywords: sex differences, fear extinction, dendritic morphology, infralimbic cortex, basolateral amygdala, individual differences
Introduction
In both humans and animals, extinction of a conditioned fear response depends upon communication between the medial prefrontal cortex (mPFC) and the amygdala (1; 2). Disrupted connectivity of this circuit is thought to underlie some symptoms of mental illnesses like post-traumatic stress disorder (PTSD); in particular, unwanted or inappropriate fear responses may result from a failure of the mPFC to regulate amygdala activity (3; 4). PTSD is twice as common in women as in men (5), but whether sex differences in mPFC-amygdala circuit function underlie this discrepancy is unknown.
In rodents, efferents from the infralimbic (IL) region of the mPFC activate a microcircuit within the basolateral area of the amygdala (BLA) that results in fear response suppression after extinction (6). We have previously shown that neurons in this pathway can undergo unique patterns of dendritic remodeling in response to stress in males and females (7; 8), suggesting that structural plasticity in IL-BLA neurons is sex-specific. However, a direct relationship between IL morphology and extinction success has not been demonstrated in a circuit-based manner, let alone in both males and females. In fact, less than 2% of all fear conditioning and extinction research has been conducted in female animals (9), and thus our understanding of the mechanisms and neural processes that mediate extinction in the female brain is comparatively limited. However, multiple reports of sexually dimorphic plasticity in the mPFC (10; 11) lead us to hypothesize that the neuroanatomical features associated with successful vs. failed extinction retrieval may be distinct in males and females.
Our primary objective for the current work was to define sex-specific structure-function relationships in IL-BLA neurons. We took advantage of naturally occurring behavioral variability in male and female rats, identifying subpopulations of animals that exhibited high or low levels of freezing during an extinction retrieval test. We then used a combination of retrograde tracing and 3D neuronal reconstructions to create detailed structural profiles of IL-BLA projection neurons in these groups. We found that high- and low-freezing males, but not females, exhibited distinct morphological features. Our data suggest that this discrepancy may be due to sex differences in the behavioral trajectories that lead to successful extinction maintenance.
Methods and Materials
Subjects
Young adult (8–10 weeks) male (n=58) and female (n=57) Sprague Dawley rats were individually housed in the Nightingale Animal Facility at Northeastern University on a 12:12 light:dark cycle with access to food and water ad libitum. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Northeastern University Institutional Animal Care And Use Committee.
Estrous cycle monitoring
Females were vaginally swabbed daily to ensure normal estrous cycling. Collected cells were smeared on a microscope slide, stained with Nissl, and examined with a light microscope for cytology.
Surgery
All animals underwent aseptic stereotaxic surgery as described in (7). Animals were anaesthetized with an i.p. injection of a ketamine (90 mg/kg) and xylazine (4 mg/kg) cocktail. Animals’ heads were shaved and secured into a stereotaxic apparatus (Stoelting Co. Wood Dale, IL), and an incision was made to reveal the skull. Once bregma was clearly visible, a burr hole was drilled above the BLA (−3.0mm AP, +/− 5.0mm ML; Paxinos and Watson 2005) and a 1μl syringe attached to a stereotaxic arm was lowered to 8.0mm DV. After allowing tissue to settle for 2 min, an infusion pump (Harvard Apparatus, Holliston, MA) delivered 0.2 μl 5% Fluorogold (FG; Fluorochrome, LLC; Denver CO) at 0.02 microliters per minute. The syringe was left in place for 10 minutes, and then slowly removed from the brain and the incision was sealed with VetBond surgical glue (3M, St Paul, MN). Animals were kept in cages atop heated recovery pads until righting reflexes returned. For three days after surgery, animals received s.c. injections of 0.3 ml Buprenorphine, and were monitored for healthy eating and drinking habits. Behavior testing began one week after surgery.
Behavioral Testing
Apparatus and stimuli
Rats underwent habituation, fear conditioning and fear extinction as in (12) in one of four identical chambers constructed of aluminum and Plexiglas walls (Rat Test Cage, Coulbourn Instruments, Allentown, PA), with metal stainless steel rod flooring that was attached to a shock generator (Model H13–15; Coulbourn Instruments). The chambers were lit with a single house light, and each chamber was enclosed within a sound-isolation cubicle (Model H10–24A; Coulbourn Instruments). An infrared digital camera allowed videotaping during behavioral procedures. Chamber grid floors, trays, and walls were thoroughly cleaned with water and dried between sessions. Rats were allowed to freely explore the chamber for 4 min before tone presentation on each day began.
Fear conditioning procedure
All rats were exposed to five tone (CS) presentations (habituation), followed by seven conditioning trials (CS–US pairings) on day 1. The CS was a 30-s, 5 kHz, 80 dB SPL sine wave tone, which co-terminated with a 0.5-s, 0.7 mA footshock US during fear conditioning. Mean intertrial interval was 4 min (2–6 min range) throughout habituation and fear conditioning. Fear behavior was measured using freezing, defined as the cessation of all movement with the exception of respiration-related movement and non-awake or rest body posture. Freezing was continuously recorded during the conditioning session and analyzed using FreezeFrame Software. Minimum bout was set at 2sec. After conditioning, rats were returned to their home cages.
Extinction procedures
Freezing was recorded continuously during the extinction training (20 tone alone trials, day 2) and retrieval sessions (3 tone alone trials, day 3). Both extinction training and testing took place in the same chamber as fear conditioning, but with different contextual cues (floor, light, and odor). Mean inter-trial interval was 4min (2–6 min range). To ensure that low freezing (LF) animals were not those that simply failed to learn the tone-shock association, animals that did not reach criterion for fear conditioning (>40% average freezing on 1st 2 extinction tone presentations, as in (13)) were removed from overall analysis.
Experimental groups
For evaluation of estrous cycle effects, female animals were analyzed according to estrous phase during extinction learning. We and others have previously demonstrated that animals in proestrus (high estrogen levels) during extinction exhibit facilitated extinction retrieval the following day (14; 15). Notably, this effect is specific to extinction learning, as freezing in animals in proestrus during extinction retrieval is indistinguishable from that of animals in other estrous phases (14). To identify high-and low-freezing populations of males and females, animals were sorted by freezing during extinction retrieval. The eight highest- (HF) and lowest-freezing (LF) animals in each sex were selected for morphological and retrospective behavioral analyses (Figure 2). Estrous distribution in selected animals was: HF – 3 diestrus, 2 estrus, 3 metaestrus. LF – 1 diestrus, 1 estrus, 2 metaestrus, 4 proestrus.
Euthanasia and tissue preparation
Twenty-four hours after the completion of Extinction Retrieval testing, animals were anesthetized and sacrificed by transcardial perfusion of 4% paraformaldehyde (PFA) in 0.1M phosphate buffer (PBS, pH 7.4). Brains were extracted and post-fixed in PFA for six hours, and then placed in 0.1% sodium azide in PBS at 4°C for storage.
Morphology analysis
After proper BLA injection targets were confirmed, 250 μm, IL-containing sections were collected using a vibrating microtome (Leica). FG-positive IL neurons were visualized through a DAPI filter on a Zeiss Axio Examiner A.1 microscope. Iontophoretic microinjections of fluorescent dye Lucifer Yellow were done into FG-positive, IL layer II/III pyramidal neurons using a DC current of 1–6 nA for 5–10 min, until distal processes were filled with dye and no further loading could be observed. Sections were mounted and coverslipped in Vectashield (Vector Laboratories, Burlingame CA), and filled neurons were selected for imaging and analysis based on completeness criteria described previously (7; 8). 3–6 neurons per animal were included in the analysis. For off-line tracing of apical dendrites, multiple z-stacks of whole neurons were acquired using an Olympus FV1000 confocal microscope with a 60x lens and a step-size of 1μm. Montages of all images covering the apical dendrite of one neuron were created and traced using Neurolucida (MBF Bioscience). Apical length, branch number and sholl analyses were performed with Neurolucida Explorer. For spine analysis, spine segments from apical dendrites were selected in 50μm increments from the cell body, and approximately 8 segments per neuron were sampled. Z-stacks were acquired using a 100x lens with a zoom of 3.3, NA 1.4, and step size of 0.08μm. Raw z-stacks were deconvolved with AutoQuant (Media Cybernetics) and analyzed for spine number and shape (thin, stubby or mushroom) using Neuron Studio software (classification criteria described in detail in (17)), after which they were manually verified. We focused on apical dendrites because basal dendrites have been repeatedly shown to exhibit little or no structural plasticity (18; 19).
Statistical analysis
All statistical analyses were conducted using Graphpad Prism software. Behavioral data (percent freezing during tone presentation or in 30-sec period after tone) were analyzed using 2-way ANOVAs corrected for multiple comparisons, with factors of sex or phenotype and trial number for each day of testing. Bonferroni post-hoc tests were conducted when appropriate. Dendritic length and spine density (spines/μm) were averaged for each animal, and then group means calculated. For all analyses, significance was set at p<0.05. It should be noted that with the exception of the data presented in Fig 1a, we intentionally conducted within-sex comparisons only. We took this approach because the overarching goal of this work was not to demonstrate whole-group sex differences (of which there seem to be very few), but to identify sex-specific patterns of variability.
Figure 1.
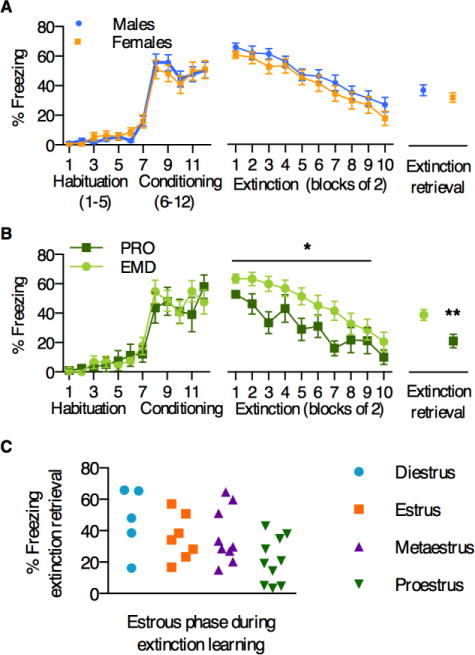
A) Large cohorts of males and females did not differ in average freezing levels across fear conditioning, extinction, and extinction retrieval. B) Female animals that extinguished in proestrus (high estrogen, PRO) froze significantly less during extinction and extinction retrieval than those that extinguished in estrus, metaestrus, or diestrus (low estrogen, EMD). C) Variability in freezing during extinction retrieval, based on estrous phase during extinction learning. *p<0.03, **p<0.01 PRO vs. EMD
Results
Overall sex differences and estrous effects on fear conditioning, extinction, and extinction retrieval
Gonadally intact, young adult male and female rats were subjected to auditory cued fear conditioning, extinction, and extinction retrieval procedures, as in (15). On average, we did not observe sex differences in percent freezing during the tone across fear conditioning, extinction, and extinction retrieval (Figure 1A; test for main effect of sex: 2-way ANOVAs for fear conditioning [F1,63=0.01, p=0.92] and extinction [F1,63=1.46, p=0.23]; unpaired t-test for extinction retrieval [p=0.33]), suggesting that as general populations, males and females do not differ in these measures. To examine the influence of the estrous cycle, we also analyzed female animals alone, grouped by estrous phase during extinction learning, as in (14; 15)—either proestrus (PRO), or estrus, metaestrus, diestrus (EMD). We found that PRO animals exhibited significantly less overall freezing during extinction learning (main effect of estrous cycle [F1,29=5.46, p<0.03];), as well as less freezing during extinction retrieval (p<0.01), consistent with previous reports (Fig 1B). Figure 1C shows freezing during extinction retrieval for animals in each estrous phase (again, grouped by phase during extinction learning), demonstrating variability in behavior in all phases of the estrous cycle. A 1-way ANOVA with planned multiple comparisons (F3,27=3.1, p<0.05) revealed a significant difference between animals in proestrus and diestrus only (adjusted post-hoc p<0.03).
Identification of high- and low-freezing subpopulations
We next assessed the range of freezing responses during extinction retrieval in all animals (Figure 2), and observed broad but comparable variability in both males and females. The eight highest (HF) and lowest freezing (LF) males and females were selected for morphological analyses (data points in shaded regions).
Figure 2.
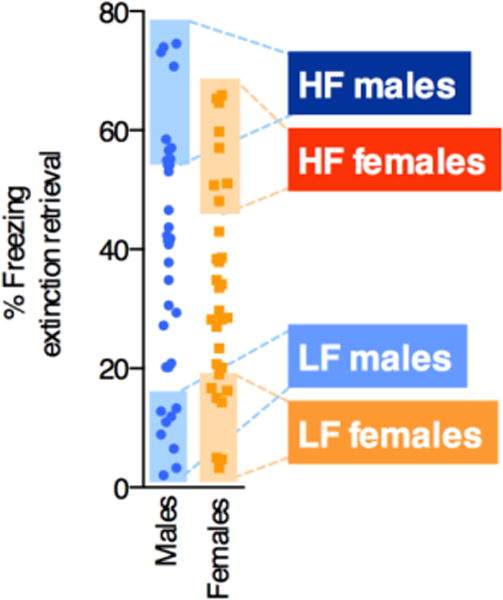
Eight high- (HF) and eight low-freezing (LF) phenotypes were selected from male and female cohorts based on freezing during extinction retrieval (data points within shaded regions). Animals in these groups were used for subsequent analysis.
Morphological analysis of IL-BLA neurons
Figure 3A illustrates the retrograde tracing strategy to label BLA-projecting IL neurons, and 3B shows representative localization of FG in the BLA. FG-positive layer II/III neurons in the IL of HF and LF males and females were iontophoretically filled with Lucifer Yellow (7; 8), imaged (Figure 3C), and reconstructed in 3D for analysis of apical dendrite morphology (Figure 3D). Overall, we found HF/LF differences in males, but not females. Although there were no group differences in branch number in either sex (2-way ANOVA, no main effect of sex (p=0.29) or phenotype (p=0.61); Figure 3E), HF males had significantly less dendritic length compared to LF males (2-way ANOVA, main effect of phenotype [F1,21=16.43, p<0.001]; adjusted Bonferroni post-hoc p<0.001; Figure 3F). In contrast, dendritic length was similar between HF and LF females. To determine whether any structural differences were localized along the apical dendrite, we performed a Sholl analysis, with 50-μm bins radiating outwards from the cell soma. This analysis revealed that HF males specifically lack dendritic material at the most distal points of the apical dendrite (2-way ANOVA: main effect of phenotype [F1,10=11.2, p<0.01]; Figure 2G), which corresponds to cortical layer I. Interestingly, this layer is the synaptic target for the vast majority of IL afferents from the bed nucleus of the stria terminalis (BNST; Oh et al., 2014), which plays a well-documented role in fear and anxiety expression (21; 22). Thus, the HF phenotype in male animals may be due in part to disrupted communication in BNST-IL-BLA circuitry.
Figure 3. IL-BLA dendritic arborization in HF/LF males and females.
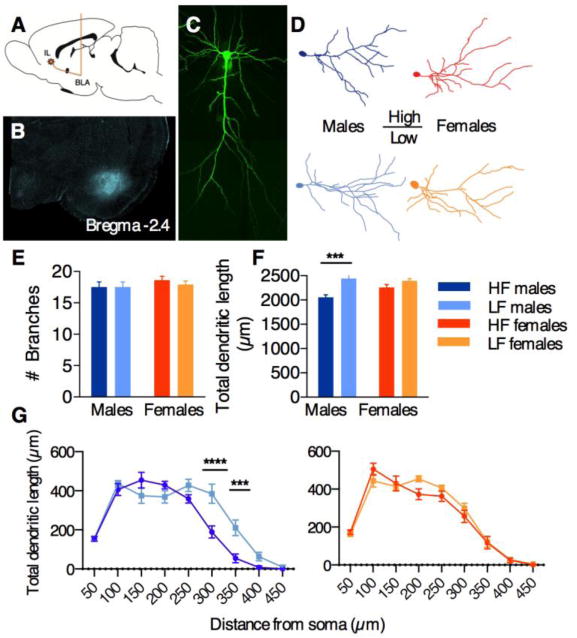
A) Illustration of stereotaxic surgery to inject retrograde tracer Fluorogold into the BLA, for future identification of IL-BLA neurons. B) representative image of FG spread in the BLA C) FG-positive layer II/III IL neurons were iontophoretically filled with Lucifer Yellow and imaged in their entirety. D) representative Neurolucida tracings of neurons from HF and LF males and females. E) no group differences in branch number were observed. F) HF males had significantly shorter apical dendrite length compared to LF males. G) HF vs. LF differences in males were observed at distal points along the apical dendrite. All data points represent mean +/− SEM. ***p<0.001; ****p<0.0001, within-sex HF vs. LF adjusted post-hoc comparisons.
We next examined the morphological features of dendritic spines in IL-BLA neurons of HF vs. LF males and females. We collected high-magnification z-stack images of dendrite segments (Figure 4A & 4C) and analyzed them for spine density, type, and head diameter using NeuronStudio software (Dumitriu et al., 2011; Figure 4B & 4D). Intriguingly, we again observed HF vs. LF distinctions in males, but not females. Spine density was greater in HF males (2-way ANOVA, main effects of spine type [F2,20=1185, p<0.0001] and phenotype [F1,10=14.2, p<0.01]; Figure 4A–B vs. 4C–D), an effect that was fully accounted for by thin spines, and not stubby or mushroom spines (Bonferroni adjusted post-hoc test, p<0.0001; Figure 4E). To assess whether these differences were localized to one area of the apical dendrite, we separately analyzed spine density at either proximal or distal branches (< or >150μm from cell soma, respectively; Figure 4F). We found that the difference in spines in HF males occurred exclusively on proximal branches (2-way ANOVA, main effect of phenotype [F1,19=9.3, p<0.01]; Figure 4F), which corresponds to cortical layer 2. Interestingly, this layer receives reciprocal inputs from BLA neurons (24), suggesting that alterations in feedback between the IL and BLA may contribute to HF/LF behavioral distinctions in males. Finally, we examined head diameter in mushroom spine populations, and once again observed significant differences in HF vs. LF males, but not females. Frequency distribution analysis revealed a leftward shift in HF males only (Kolmolgorov-Smirnoff D=0.059, p=0.001; Figure 4G), suggesting that these animals have proportionately more small-headed mushroom spines.
Figure 4. IL-BLA spine density in HF/LF males and females.
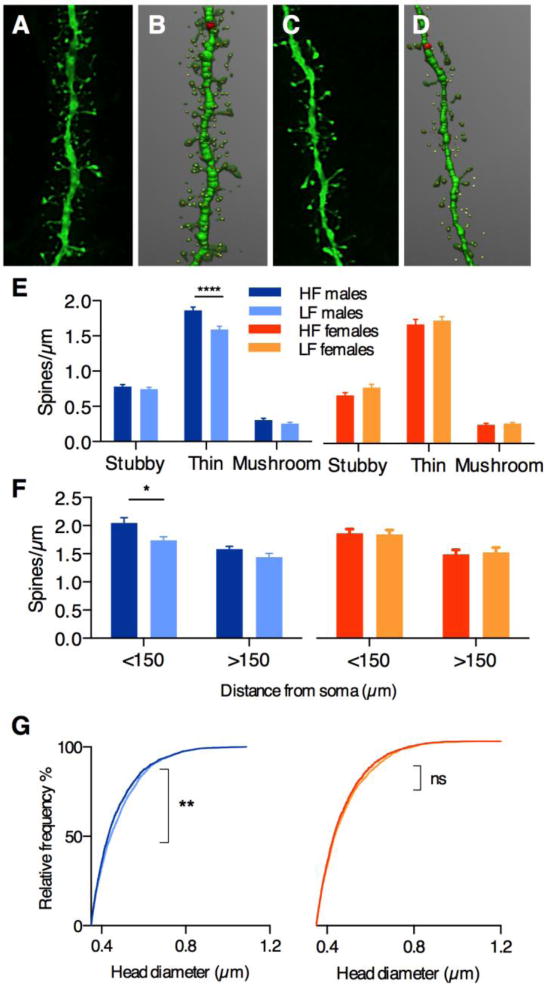
Confocal images (A,C) and NeuronStudio renderings (B,D) of dendritic segments in IL-BLA neurons of HF and LF males, respectively. E) greater density of thin spines was observed in HF males compared to LF males, but no HF/LF distinctions were observed in females. F) thin spine density differences in HF vs. LF males were restricted to proximal dendrites <150 μm from the cell soma. (G) Frequency distribution of mushroom spine head diameter distribution revealed a significant leftward shift in HF males compared to LF males, but no differences in HF vs. LF females. All data points represent mean +/− SEM except (G). *p<0.02; **p=0.001; ****p<0.0001, within-sex HF vs. LF adjusted post-hoc comparisons.
Retrospective behavioral analyses in HF vs. LF males and females
Our morphological analyses suggest that the relationship between IL-BLA structure and freezing behavior during extinction retrieval is different for males and females. To investigate whether this was due to pre-existing sex differences in HF/LF behavioral patterns, we re-plotted their freezing levels across all three days of testing. Remarkably, we found that the HF/LF phenotypes emerged during distinct learning phases in males and females. HF and LF males exhibited comparable levels of freezing during fear conditioning, but diverged at the beginning of extinction (2-way ANOVA F1,14=39.1; p<0.0001), and remained separated throughout the course of the session (Figure 5A). In contrast, HF and LF females’ freezing levels were significantly different during fear conditioning as well as extinction (test for main effect of phenotype: 2-way ANOVAs for fear conditioning [F1,14=8.6], p=0.01 and extinction [F1,14=35.3], p<0.0001; Figure 5B).
Figure 5. High- and low-fear phenotypes emerge during distinct phases of learning in males and females.
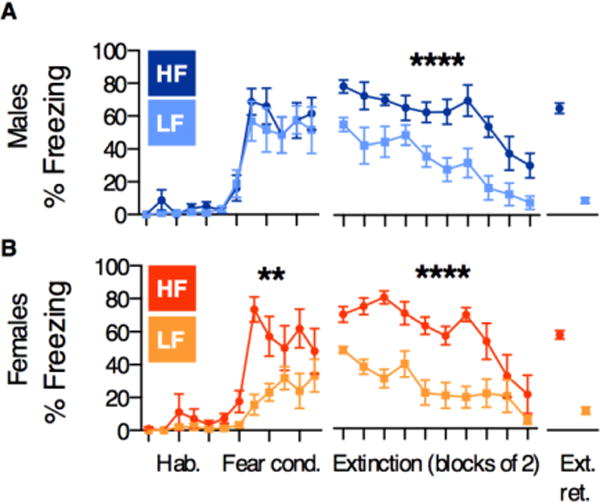
A) HF and LF males differed only during extinction B) HF and LF females differed during both fear conditioning and extinction. All data points represent mean +/− SEM. All significant effects are within-sex HF vs. LF main effects of phenotype. **p=0.01, **** p<0.0001.
Discussion
To our knowledge, this study represents the first large-scale evaluation of fear conditioning and extinction processes that includes both male and female animals, addressing a fundamental problem in basic neuroscience research—specifically, a glaring lack of knowledge about even basic sex differences in this classic model. Collectively, our data demonstrate that the behavioral and neuroanatomical correlates of freezing during extinction retrieval are sex-specific. It is important to note, however, that the freezing levels that defined the HF and LF subpopulations during extinction retrieval did not differ between the sexes; thus, the finding that these phenotypes emerged during distinct phases of learning in males and females suggests that freezing during extinction retrieval—even at identical levels—may in fact reflect separate neural processes in males and females. Consistent with this hypothesis, we observed structural differences in IL-BLA projections in HF vs. LF males only.
We recognize that because our neuroanatomical analyses were necessarily conducted after animals had been through fear conditioning, extinction, and extinction retrieval, it is impossible to know for certain whether HF/LF distinctions reflect pre-existing differences in morphology, or different patterns of structural plasticity in response to the learning and memory experiences. We hypothesize that the answer may be a combination of both. Experience-based changes in dendritic arborization are usually observed only after an extended period of time – for example, after at least one week of stress exposure (25). We would therefore speculate that the shorter dendritic length in HF males to be indicative of a pre-existing condition that may have predisposed this group to high freezing during extinction. In contrast, dendritic spines can undergo rapid structural changes over the course of fear conditioning and extinction. In particular, dendrites in the frontal association cortex lose thin spines after fear conditioning, but then regrow new spines in essentially the same spot after extinction (26). Although this intriguing phenomenon has not yet been demonstrated in the IL, the localized, increased spine density observed in HF males may reflect a disruption of this pruning-regrowth pattern. Specifically, if these neurons fail to undergo fear conditioning-related spine pruning, extinction-related new spines may compete for “synaptic control” over the neuron, which may then result in failed extinction retrieval. Finally, the observed shift in mushroom spine head diameter size in HF males may be related to consolidation of the extinction memory. In contrast to thin spines, which are more dynamic, mushroom spines are thought to play a role in the stabilization and long-term maintenance of memories (27). Learning elicits AMPA receptor insertion into the cell membrane of mushroom spines exclusively (28), and thus the smaller mushroom spines in in HF males may indicate decreased AMPA receptor insertion in response to extinction learning, thus impeding stronger memory consolidation.
In our female animals, the lack of observed morphological differences between HF vs. LF groups is consistent with the emergence of these phenotypes during fear conditioning, which does not engage the IL (29). This surprising finding suggests that freezing during extinction retrieval in females may in fact be related to the initial processing of the tone-shock association, rather than consolidation of extinction learning. Indeed, unlike in male HF/LF phenotypes, both HF and LF females exhibited comparably low levels of freezing by the completion of extinction. The lack of HF/LF differences in any morphological measure in females suggests that an alternate circuit may drive freezing during extinction retrieval. One intriguing possibility is that the IL modulates extinction in females not through direct projections to the BLA, but via connections with the prelimbic cortex (PL). The PL also projects to the BLA, but unlike the IL, PL activity is related to increased fear expression after fear conditioning (30). In physiological studies, PL activity has been linked to elevated freezing during extinction in females but not males (31), suggesting that the PL may play a distinctly important role in females. We recently found that impaired extinction retrieval in female rats was associated with a switch in the balance of IL and PL c-fos expression, such that PL neurons were more active than those in the IL, while the reverse was the case in animals exhibiting good extinction retrieval (12). Therefore, freezing behavior in females may rely more closely on IL-PL and/or PL-BLA circuits, while IL-BLA projections are less involved. This hypothesis would be consistent with findings that enhanced extinction in females is associated with increased c-fos mRNA in whole-IL (as opposed to circuit-specific) assays (32), and we look forward to exploring the idea further in future experiments.
It is also important to consider the role that circulating ovarian hormones play in modulating the learning and memory processes involved in fear conditioning and extinction. Milad and colleagues have convincingly demonstrated in both humans and animals that estradiol—either administered or circulating at high levels at the time of extinction learning—leads to reduced fear responses during an extinction retrieval test (14; 16; 32). Administration of an estrogen-receptor agonist can mimic these effects (33), suggesting that estradiol signaling mechanisms may enhance consolidation of the extinction memory. Our findings here support this idea; females in proestrus during extinction learning froze significantly less during extinction retrieval than animals that had been in estrus, metaestrus, or diestrus. We have also observed this effect in a recent study (15). However, it is notable that in the current study, animals in each phase exhibited a range of freezing responses during extinction retrieval (Fig 1C), suggesting that success or failure was not solely determined by the estrous cycle. A critical step in better understanding fear conditioning and extinction processes in females will be the identification of other hormone and neurotransmitter systems that interact with estrogen to modulate learning and memory.
Extinction retrieval is widely considered to be a test of fear suppression—the ability of an animal to use the information it learned during extinction to actively inhibit a conditioned response (34). Studies in male rodents show that on a physiological level, IL activation results in decreased amygdala output and decreased freezing (29; 30), and thus high freezing is commonly interpreted as a failure of the IL to regulate amygdala-driven behavior (35). Our findings in male animals support this narrative: HF males did not differ from LF males in acquisition of the tone-shock association, but exhibited impaired extinction and extinction retrieval, suggesting that the mechanisms that underlie the HF/LF phenotype are primarily related to extinction processes. Accordingly, HF males exhibited morphological alterations in IL-BLA projection neurons that are consistent with compromised extinction maintenance: shorter dendrites extending into cortical layer I, localized increases in thin spines, and a population of mushroom spines characterized by smaller head diameters.
In contrast, our results in female animals suggest a more complex story. If women are more prone to disorders like PTSD, one might intuitively predict that a greater proportion of our female animals would exhibit failed or impaired fear extinction compared to males. We were therefore somewhat surprised to find a lack of overall sex differences in these measures. This may mean that the parameters of our behavioral paradigm do not appropriately mimic the conditions that lead to sex differences in trauma effects in humans, or that a more severe stressor is necessary in addition to the footshock. However, it should be noted that the lack of sex differences in fear conditioning and extinction is consistent with previous reports from our lab and others (15; 36–38). This seeming paradox is discussed in greater detail in our recent review (39).
Taken together, our data provide evidence for fundamental sex differences in fear circuitry. That extinction in males correlated with structural differences in IL-BLA projections supports the current literature – which has been conducted almost exclusively in males – that this pathway plays a critical role in suppressing freezing during extinction retrieval. The lack of a discernable relationship between IL-BLA structure and extinction retrieval in females suggests that variability in freezing during extinction retrieval in females may instead reflect activity in mechanisms that drive fear expression, rather than suppression. Alternately, freezing may be an incomplete indicator of fear in females. This distinction could hold implications for identifying sex-specific risk factors for PTSD, which is characterized by poor extinction retrieval and altered prefrontal-amygdala connectivity (4). Clearly, much work needs to be done in order to fully define sex differences in the mechanisms that drive fear conditioning and extinction, and we look forward to continuing our investigations. Our findings not only highlight the need for increased inclusion of female animals in basic neuroscience, but also demonstrate the value of taking a within-sex approach to sex differences research.
Acknowledgments
This work was supported by a grant from the National Institute of Mental Health (R21-MH098006-01) to R.M.S. We thank Jennifer Lipps for technical assistance, and Natalie Tronson and Mark Baxter for manuscript comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: The authors declare no biomedical financial interests or potential conflicts of interest.
Author Contributions T.M.G., E.R., V.T., and A.R. performed the experiments and analyzed the data. T.M.G. and R.M.S. designed the experiments and wrote the manuscript.
References
- 1.Quirk GJ, Likhtik E, Pelletier JG, Paré D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–7. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–4. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 3.Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B, Ressler KJ. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res. 2013;47:1469–78. doi: 10.1016/j.jpsychires.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 66: Elsevier Inc. 2009:1075–82. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breslau N. The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma Violence Abuse. 2009;10:198–210. doi: 10.1177/1524838009334448. [DOI] [PubMed] [Google Scholar]
- 6.Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Nature. Vol. 454. Macmillan Publishers Limited; 2008. Switching on and off fear by distinct neuronal circuits; pp. 600–6. All rights reserved. [DOI] [PubMed] [Google Scholar]
- 7.Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex. 2009;19:2479–84. doi: 10.1093/cercor/bhp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb Cortex. 2010;20:2560–7. doi: 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lebron-Milad K, Milad MR. Sex differences, gonadal hormones and the fear extinction network: implications for anxiety disorders. Biol Mood Anxiety Disord. 2012;2:3. doi: 10.1186/2045-5380-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrell MR, Sengelaub DR, Wellman CL. Sex differences and chronic stress effects on the neural circuitry underlying fear conditioning and extinction. Physiol Behav. 2013;122:208–15. doi: 10.1016/j.physbeh.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shansky RM. Estrogen, stress and the brain: progress toward unraveling gender discrepancies in major depressive disorder. Expert Rev Neurother. 2009;9:967–73. doi: 10.1586/ern.09.46. [DOI] [PubMed] [Google Scholar]
- 12.Gruene TM, Lipps J, Rey CD, Bouck A, Shansky RM. Heat exposure in female rats elicits abnormal fear expression and cellular changes in prefrontal cortex and hippocampus. Neurobiol Learn Mem. 2014 doi: 10.1016/j.nlm.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Bush DEA, Sotres-bayon F, Ledoux JE. Individual Differences in Fear: Isolating Fear Reactivity and Fear Recovery Phenotypes. J Trauma Stress. 2007;20:413–422. doi: 10.1002/jts.20261. [DOI] [PubMed] [Google Scholar]
- 14.Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164:887–95. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rey CD, Lipps J, Shansky RM. Dopamine D1 receptor activation rescues extinction impairments in low-estrogen female rats and induces cortical layer-specific activation changes in prefrontal-amygdala circuits. Neuropsychopharmacology. 2014;39:1282–9. doi: 10.1038/npp.2013.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, Goldstein JM. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–8. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez A, Ehlenberger DB, Dickstein DL, Hof PR, Wearne SL. Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS One. 2008;3:e1997. doi: 10.1371/journal.pone.0001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–4. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, et al. A mesoscale connectome of the mouse brain. Nature. 2014;508:207–14. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haufler D, Nagy FZ, Pare D. Neuronal correlates of fear conditioning in the bed nucleus of the stria terminalis. Learn Mem. 2013;20:633–41. doi: 10.1101/lm.031799.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elharrar E, Warhaftig G, Issler O, Sztainberg Y, Dikshtein Y, Zahut R, et al. Overexpression of corticotropin-releasing factor receptor type 2 in the bed nucleus of stria terminalis improves posttraumatic stress disorder-like symptoms in a model of incubation of fear. Biol Psychiatry. 2013;74:827–36. doi: 10.1016/j.biopsych.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 23.Dumitriu D, Rodriguez A, Morrison JH. Nat Protoc. Vol. 6. Nature Publishing Group; 2011. High-throughput, detailed, cell-specific neuroanatomy of dendritic spines using microinjection and confocal microscopy; pp. 1391–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Little JP, Carter AG. Synaptic mechanisms underlying strong reciprocal connectivity between the medial prefrontal cortex and basolateral amygdala. J Neurosci. 2013;33:15333–42. doi: 10.1523/JNEUROSCI.2385-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai CSW, Franke TF, Gan W-B. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature. 2012;483:87–91. doi: 10.1038/nature10792. [DOI] [PubMed] [Google Scholar]
- 27.Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol. 2007;17:381–6. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Matsuo N, Reijmers L, Mayford M. Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. Science. 2008;319:1104–7. doi: 10.1126/science.1149967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–38. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–33. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fenton GE, Pollard AK, Halliday DM, Mason R, Bredy TW, Stevenson CW. Persistent prelimbic cortex activity contributes to enhanced learned fear expression in females. Learn Mem. 2014;21:55–60. doi: 10.1101/lm.033514.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeidan Ma, Igoe Sa, Linnman C, Vitalo A, Levine JB, Klibanski A, et al. Biol Psychiatry. Society of Biological Psychiatry; 2011. Estradiol Modulates Medial Prefrontal Cortex and Amygdala Activity During Fear Extinction in Women and Female Rats; pp. 0–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graham BM, Milad MR. Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biol Psychiatry. 2013;73:371–8. doi: 10.1016/j.biopsych.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sepulveda-Orengo MT, Lopez AV, Soler-Cedeño O, Porter JT. Fear extinction induces mGluR5-mediated synaptic and intrinsic plasticity in infralimbic neurons. J Neurosci. 2013;33:7184–93. doi: 10.1523/JNEUROSCI.5198-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maren S, De Oca B, Fanselow MS. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res. 1994;661:25–34. doi: 10.1016/0006-8993(94)91176-2. [DOI] [PubMed] [Google Scholar]
- 37.Baran SE, Armstrong CE, Niren DC, Hanna JJ, Conrad CD. Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiol Learn Mem. 2009;91:323–32. doi: 10.1016/j.nlm.2008.11.005. 2008/12/17 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lebrón-Milad K, Tsareva A, Ahmed N, Milad MR. Sex differences and estrous cycle in female rats interact with the effects of fluoxetine treatment on fear extinction. Behav Brain Res. 2013;253:217–22. doi: 10.1016/j.bbr.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shansky RM. Sex differences in PTSD resilience and susceptibility: Challenges for animal models of fear learning. Neurobiol Stress. 2014 doi: 10.1016/j.ynstr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


