Abstract
Background
Cilia are important for Hedgehog signaling in vertebrates and many genes that encode proteins involved in ciliogenesis have been studied for their roles in embryonic development. Null mutations in many of these genes cause early embryonic lethality, hence an understanding of their roles in postnatal development is limited.
Results
The Inturned (Intu) gene is required for ciliogenesis and here we report a recessive hypomorphic mutation, resulting in substitution of a conserved hydrophobic residue (I813N) near the C-terminus, that sheds light on later functions of Intu. Mice homozygous for this Double-thumb (IntuDtm) allele exhibit polydactyly, retarded growth, and reduced survival. There is a moderate loss of cilia in IntuDtm/Dtm mutants, and IntuI813N exhibits compromised ability to increase ciliogenesis in cultured Intu null mutant cells. IntuDtm mutants show rib defects and delay of endochondral ossification in long bones, digits, vertebrae and the sternum. These skeletal defects correlate with a decrease in Hh signaling. However, patterning of the neural tube and planar cell polarity appear to be normal.
Conclusion
This hypomorphic Intu allele highlights an important role of Intu in mouse skeletal development.
Keywords: Hedgehog signaling, polydactyly, primary cilia, ciliogenesis, skeletal development, endochondral ossification
INTRODUCTION
Vertebrate embryonic development is a complex process that requires the integration of numerous molecular and cellular components under genetic control. This complexity is reflected in a diverse array of birth defects resulting from disruptions of core developmental signaling pathways, such as Wnt, Notch, FGF, TGFβ, and Hedgehog (Hh) pathways. Ciliopathies comprise a plethora of human birth defects such as Polycystic Kidney Disease (OMIM 173 900), Bardet-Biedl (OMIM 209 900), Joubert (OMIM 213 300), Meckel (OMIM 24900), and Kartagener (OMIM 244 400) Syndromes. The common cellular bases of all ciliopathies are defects in the biogenesis or function of the cilium, a microtubule-based cell surface extension present in most post-mitotic cells in vertebrates (Baker and Beales, 2009). Understanding the genetic and molecular basis of ciliogenesis and the role of the cilium in embryonic and postnatal development is critical for proper diagnosis and treatment of ciliopathies.
Many developmental defects in ciliopathies are attributed to abnormal Hh signaling. The Hh signaling pathway is essential for embryonic development, patterning, and growth (Briscoe and Therond, 2013). There are three Hh family members in the mouse, Sonic Hedgehog (Shh), Desert Hedgehog (Dhh), and Indian Hedgehog (Ihh). Shh affects cell proliferation, axis formation, and patterning in many tissues, including the neural tube and limb buds. In the developing neural tube, a decreasing ventral-to-dorsal Shh gradient regulates the expression of various genes encoding homeobox and bHLH transcription factors (Briscoe and Therond, 2013). The subsequent antagonistic interactions among these transcription factors sharpen gene expression boundaries in the neural tube and promote neuronal differentiation (Briscoe and Therond, 2013). Shh is also transiently expressed in posterior mesenchyme of the limb buds known as zone of polarizing activity (ZPA). Both gain and loss of function analyses show that Shh is critical for the establishment of the anterior/posterior polarity of the limb buds and proper formation of digits (Zeller et al., 2009).
Ihh signaling is predominant in endochondral ossification, a process in which developing cartilage expands, calcifies and is then replaced by bone tissue (Lai and Mitchell, 2005). The development of the long bones begins with the condensation of mesenchymal cells and differentiation into chondrocytes, which secrete cartilaginous extracellular matrix (ECM), proliferate and undergo hypertrophy. Terminally differentiated hypertrophic chondrocytes deposit calcified ECM and undergo apoptosis. This is followed by the invasion of osteoblasts and osteoclasts, which completes the transition from cartilage to bone. The ossification of the expanding cartilage framework causes bone lengthening. Perturbations in this process, for example due to loss of Ihh function in mice, leads to shorter bones and retarded growth.
A fast growing number of proteins have been linked to ciliopathies, including proteins originally identified based on their roles in establishing planar cell polarity (PCP). PCP is the organization and alignment of tissues and repetitive structures along an axis in a single plane (Goodrich and Strutt, 2011). Based on their functions in Drosophila, PCP proteins belong to two categories, the core proteins and effector proteins. The core PCP proteins (e.g. Frizzled and Van Gogh) interact physically and genetically to form a network regulating PCP in multiple contexts. In contrast, PCP effector proteins, such as Inturned, Fuzzy, Fritz and Multiple Wing Hairs, act downstream of core PCP proteins and the molecular mechanisms of their functions remain to be elucidated.
Studies in frogs suggested that Inturned (Intu) and Fuzzy (Fuz) were involved in both ciliogenesis and convergent extension (Park et al., 2006). We and others previously showed that null mutants of mouse Intu and Fuz were embryonic lethal with severe defects in ciliogenesis, Hh signaling and abnormal patterning in neural tube and limbs (Gray et al., 2009; Heydeck et al., 2009; Zeng et al., 2010). As Intu and Fuz null mutants rarely survive past embryonic day 15.5 (E15.5), we have not been able to investigate the roles of these proteins in later fetal and postnatal development.
Here we report the characterization of the Double-thumb (IntuDtm), a chemically-induced mutation in mouse that leads to multiple developmental defects. A single amino acid substitution near the C terminus of the Intu protein underlies these defects. Consistent with an essential role of Intu in ciliogenesis, we found reduced ciliation in multiple tissues of IntuDtm mutants, and compromised activity of an Intu protein carrying the Dtm mutation in rescuing the ciliation defects of Intu null mutant cells. Homozygous IntuDtm mutants display mild polydactyly and infrequent neural tube defects, reduced post-natal survival and stunted growth. Delayed ossification was found in vertebrae, sternum, the long bones and digits of the limbs. In addition, ribs are misaligned in IntuDtm mutants. These defects correlate with compromised Hh signaling indicated by reduced Ptch1 expression in IntuDtm mutants.
RESULTS
Dtm mutants exhibit limb abnormalities, infrequent neural tube defects, and reduced survival
In an ENU-based genetic screen aimed at identifying essential genes in mammalian embryonic development, we isolated Double-thumb (Dtm). All Dtm homozygous mutants exhibit the duplication of digit 1 in all four limbs on the C3H background (Fig. 1A; n>300). Very rarely we observed mutants with exencephaly, a cranial neural tube defect (Fig. 1B; n=2/202). In crosses between heterozygous carriers, ~22% (15 out of 68) of newborns are Dtm homozygous mutants, close to what was predicted for a single recessive mutation (Fig. 1C). By the end of the first postnatal week (PW1), Dtm mutants constitute less than 20% (13 out of 67) of the litter. By PW2, only ~17% (11 out of 66) of the pups are Dtm mutants. By PW3, only ~6% (5 out of 79) are Dtm mutants, suggesting that ~80% of Dtm mutants die between birth and weaning. This indicates that the gene affected in Dtm mutants is important for the postnatal survival of the mice.
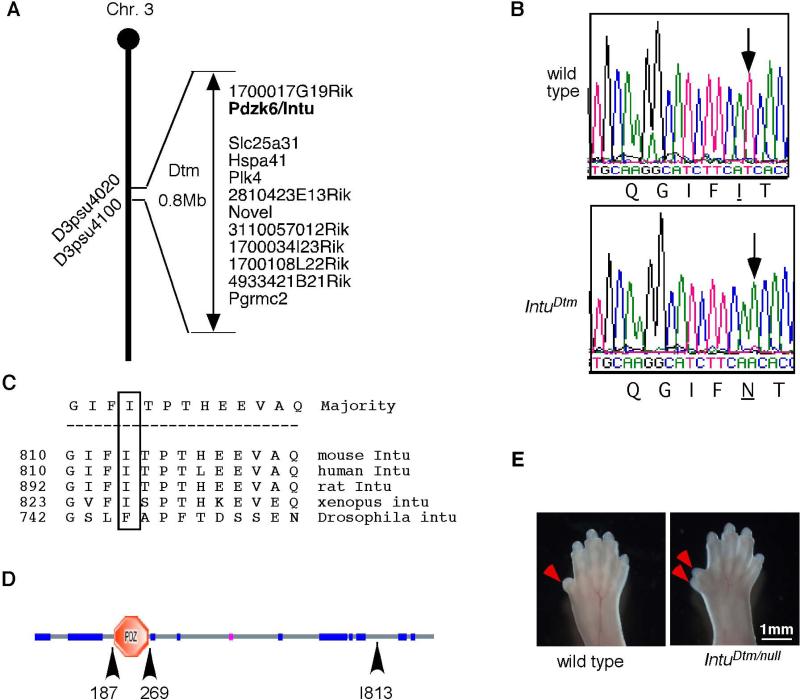
Figure 1. Dtm mice exhibit polydactyly, neural tube defects and reduced survival.
(A) Forelimbs of E18.5 wild type and Dtm mutants with thumbs indicated by red arrows. (B) Lateral views of E15.5 wild type and Dtm mutant embryos showing the cranial neural tube defect, exencephaly. (C) Quantification showing Dtm mutants as the percentage of total pups resulting from crosses between heterozygous Dtm mutant carriers.
Dtm mutants carry a point mutation in the PCP effector gene Inturned
Through positional cloning, we mapped the Dtm mutation to a 0.8 Mb region on chromosome 3 (Fig. 2A). We sequenced the exons and splice junctions of 12 candidate genes in this region and found a non-synonymous T to A base substitution in the Pdzk6 gene (Fig. 2B), which was later renamed Inturned (Intu) based on its sequence homology with the Drosophila PCP effector gene Inturned. This substitution results in the replacement of a hydrophobic amino acid Isoleucine with a hydrophilic amino acid Asparagine (I813N) near the C-terminal end of the 942-amino acid Intu protein (Fig. 2C and D). This Isoleucine residue is conserved among all vertebrate species examined, and is replaced with another hydrophobic residue phenylalanine in Drosophila (Fig. 2D). We have previously shown that a null mutation in mouse Intu results in severe polydactyly in all four limbs (Zeng et al., 2010). To confirm that a mutation in Intu underlies the polydactyly phenotype of Dtm mutants, we performed a complementation test by crossing Dtm heterozygotes to Intu null heterozygotes. Ten out of 50 embryos (E13.5-E18.5) recovered from such crossings were Dtm/Intunull transheterozygotes and exhibited polydactyly in all four limbs, suggesting that Dtm is indeed a hypomorphic allele of Intu (Fig. 2E).
Figure 2. Dtm is a new hypomorphic allele of Intu.
(A) Dtm was mapped to a 0.8Mb region on chromosome 3. (B) Dtm mutants contain a T to A mutation that changes an Isoleucine (I) to an Asparagine (N). (C) The Isoleucine mutated in Dtm is conserved among vertebrates. (D) A diagram showing the structure of mouse Intu. A predicted PDZ domain is between amino acid 187 and 269, whereas the Dtm mutation occurs at amino acid 813. (E) The forelimbs of an E18.5 wild type and an Intunull/Dtm transheterozygous embryo. Arrowheads point to the thumbs.
Dtm mutants exhibit reduced ciliation
Our previous study indicated that Intu was essential for the biogenesis of cilia and normal digit formation in the limbs (Zeng et al., 2010). Therefore, we tested whether the Dtm mutation affected the biogenesis of cilia. We evaluated ciliation in three contexts. First, we examined ciliation in kidney sections through immunofluorescence. We observed a 40% reduction in the number of multi-ciliated cells in the PW6 Dtm mutant kidneys (n=2) compared to wild type kidneys (n=3) of the same age (Fig. 3A and B, p=0.000002). Second, motile cilia that are present transiently on the ventral surface of the embryonic node were reduced in number and many of the cilia that did form were shorter than those in the wild type (Fig. 3C; n= 3 mutant and 3 control embryos). Third, cilia in the central canal of the neural tube were also reduced in length in Dtm mutants (Fig. 3D; n=4 mutant and 4 control embryos). These data indicate that the I813N mutation in Intu results in widespread ciliation defects in Dtm mutant embryos.
Figure 3. The mutant IntuI813N protein exhibits compromised ciliogenic activity in vivo and in vitro.
(A) Cilia visualized by acetylated-tubulin staining (red) are reduced in number in the IntuDtm mutant kidney compared to wild type at PW6. DAPI-stained nuclei are blue. (B) The number of cilia in the kidney is significantly less in IntuDtm than wild type mice at PW6. *: p=5x 10−6. Unpaired student t-test. N= 2 mutant and 3 wild type kidneys. (C) Fewer and shorter cilia are present in the ventral node of E8.0 IntuDtm mutant embryos as shown by scanning electron microscopy. N=3 mutant and 3 wild type embryos. (D) Transverse sections of E10.5 spinal neural tube show shorter primary cilia (marked with Arl13b, red) in the central canal of IntuDtm mutants. The basal bodies (green) are marked with γ-tubulin. (E) Both GFP-Intu and GFPIntuI813N significantly increase ciliogenesis in Intu null mutant cells, but the ciliogenic activity of GFP-IntuI813N is not as robust as that of GFP-Intu. *: p<0.05; **: p<0.01; ***: p<0.001. n= 3 independent experiments.
Intu protein containing the Dtm mutation exhibits compromised ability to increase ciliation in Intu null mutant cells
To evaluate further the impact of the Dtm mutation on Intu function, we conducted a cilia-based assay in cultured embryonic Intu null mutant limb cells. As previously reported, ciliation was almost completely disrupted in Intu null mutant cells (Zeng et al., 2010; Fig. 3E). Overexpressed GFP-Intu greatly increased ciliation in Intu null mutant cells (Fig. 3E, p=0.0005, n=3 independent experiments). GFP-IntuI813N, which carried the point mutation found in Dtm mutants, could also significantly increase ciliation in Intu null mutant cells (Fig. 3E, p=0.0056, n=3 independent experiments). However, the extent of ciliation in GFP-IntuI813N-expressing Intu mutant cells was significantly lower than GFP-Intu-expressing Intu mutant cells (p=0.038), suggesting that the Dtm mutation compromises Intu function. Taken together, evidence from genetic mapping and sequencing, the failure to complement the Intu null allele, reduced ciliogenesis in multiple tissues, and reduced function in a cilia-based assay, lead to the conclusion that Dtm represents a hypomorphic allele of Intu, which we will refer to as IntuDtm.
IntuDtm mutant embryos exhibit normal neural tube patterning
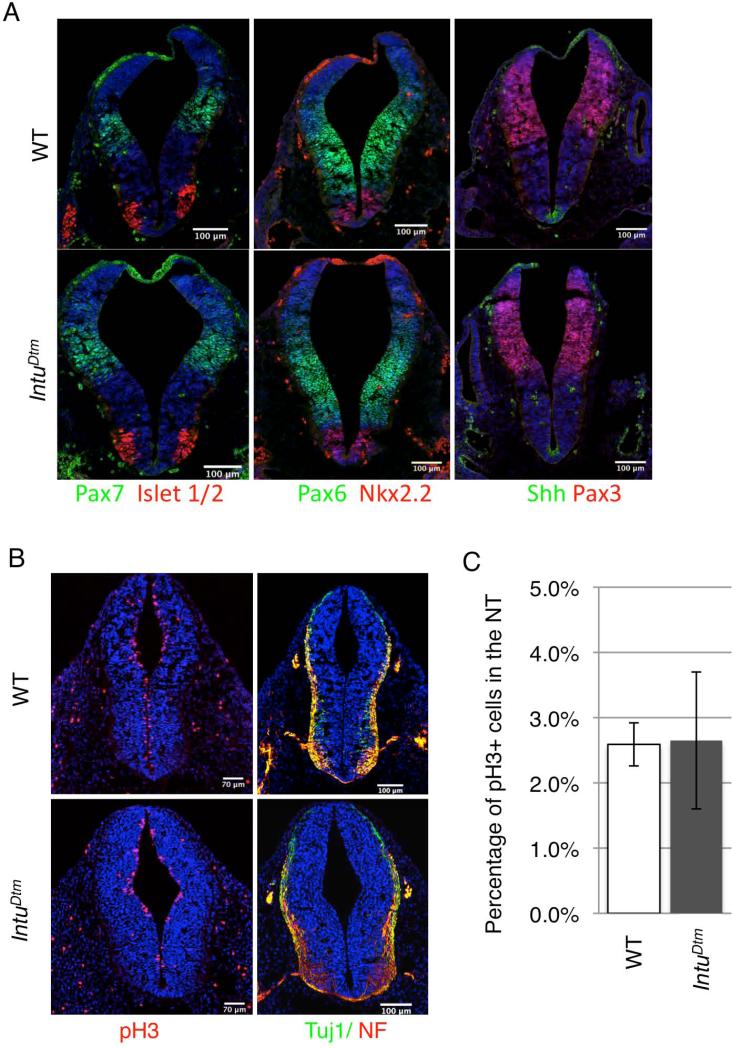
The primary cilia play critical roles in dorsal/ventral patterning of the neural tube by regulating cell responses to Shh (Huangfu and Anderson, 2005; Liu et al., 2005). To evaluate the impact of the IntuDtm mutation on Shh signaling and neural tube patterning, we examined the expression patterns of genes expressed in the neural tube and known to be regulated by Hh signaling. At E10.5, we observed no obvious difference in the expression of Pax3, Pax6, Pax7, Nkx2.2, Foxa2, and Islet1/2 in both the anterior and posterior neural tube (Fig. 4A; and data not shown; n=4 mutant and 4 wild type embryos), despite a moderate reduction in cilia length (Fig. 3D). We also observed no change in proliferation or differentiation in the neural tube (Fig. 4B and C; n=2 mutant and 2 wild type embryos). Consistent with these observations, real time quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) on E9.5 and E10.5 heads also failed to show significant decrease in the expression of direct Hh signaling targets Gli1 and Ptch1 in IntuDtm mutants (data not shown), despite the occasional occurrence of cranial neural tube defects. Therefore, it appears that the hypomorphic IntuDtm allele does not lead to a significant change in Shh pathway activity or dorsal/ventral patterning of the neural tube.
Figure 4. Neural patterning, proliferation, and differentiation are not obviously affected in IntuDtm mutant embryos.
(A) Dorsal/ventral patterning in the anterior neural tube of E10.5 IntuDtm mutant embryos is not apparently affected as shown by staining for Shh, Nkx2.2, Isl1/2, Pax6 and Pax7 as labeled. DAPI-stained nuclei are blue. N= 4 mutant and 4 control embryos. (B) Proliferation (assessed by phospho-histone H3/pH3) and differentiation (assessed by TuJ1 and Neurofilament/NF staining) appear normal in E10.5 IntuDtm mutant neural tube. N= 2 mutant and 2 control embryos. (C) The percentage of cells in the neural tube that are pH3 positive.
IntuDtm mutant mice exhibit growth retardation
In addition to reduced survival, IntuDtm mutants are significantly smaller than their wild type and heterozygote littermates at PW6 (Fig. 5A). To examine the progression of the retarded postnatal growth in IntuDtm mutants, we monitored body weight of the pups from birth to PW6. At birth, IntuDtm mice (1.29g, n=12) weighed an average of 18.4% less than their littermates (1.58g, n=15), a highly significant difference (p=0.0007), suggesting a prenatal growth defect in IntuDtm mutants. The body weights continued to diverge through all time points for which data was collected (Fig. 5B; 22% less, p=0.0023 at PW2, n=6 mutants and 11 control; 36% less, p=0.0341 at PW3, n=4 mutants and 11 control; and 48% less, p=0.0079 at PW4, n=3 mutants and 8 control), suggesting progressive growth retardation in IntuDtm mutants.
Figure 5. IntuDtm mice exhibit retarded postnatal growth.
(A) At PW6, an IntuDtm mutant pup is obviously smaller than its littermate. (B) Body weights of IntuDtm mice and their littermates diverge from birth to PW6. Note the difference between wild type and mutant mice does not reach statistical significance due to very low survival of IntuDtm mutant mice. *p<0.05; **p<0.005. unpaired student's t-test.
Delayed endochondral ossification in digits and vertebrae of IntuDtm mutants
To investigate whether the retarded growth correlates with defective skeletal development in IntuDtm mutants, we stained the cartilages and bones with Alcian Blue and Alizarin Red, respectively. Interestingly, we observed a delay in endochondral ossification in IntuDtm mutant embryos. As shown in Fig. 6A, the ossification in forelimb digits starts around E16.5 and more phalanges are ossified at E17.5 and E18.5 in wild type embryos (n=3 at each stage). The ossification of IntuDtm mutant digits appears to be delayed by one day compared to wild type littermates (n=3 at each stage).
Figure 6. IntuDtm embryos exhibit reduced ossification and misaligned ribs.
(A) Ossification within the phalanges (red arrows) is delayed by ~one day in IntuDtm mutant embryos compared to wild type at E16.5, E17.5, and E18.5. (B) Ossification of vertebrae of IntuDtm embryos is reduced and does not extend as caudally as in wild type mice at E16.5, but this difference is resolved by E17.5 and E18.5. Red arrowheads indicate the most caudal vertebra with ossification. (C) The extent of endochondral ossification in long bones (humerus, radius, and ulna) is decreased in IntuDtm mutants as quantified by measuring the size of the ossified region (red) as compared to the full length of the long bone (between joints). *p<0.05, **p<0.005. (D) Longitudinal sections of E14.5 wild type and IntuDtm mutant ulna. Red arrows mark the hypertrophic zones (hyp) and yellow arrows mark the columnar/proliferative zones (col). (E) IntuDtm mice exhibit delayed sternum ossification and rib misalignment. Ventral views of sterna with attached ribs isolated from wild type and IntuDtm mutant embryos at E17.5 and E18.5. Asterisks indicate the extra attached rib on one side of the sternum in IntuDtm mutants.
IntuDtm mutant embryos exhibit a similar delay in the ossification of the vertebrae. In E16.5 wild type embryos, we observed strong Alizarin Red staining in the centers of thoracic vertebrae, and the ossification extended caudally along the vertebral column to the pelvic level (Fig. 6B, n=3). In IntuDtm mutants, Alizarin Red staining is faint in the thoracic region, and extended caudally only a few vertebrae beyond the rib cage (Fig. 6B, n=3). At E17.5 and E18.5, the ossification patterns in the vertebrae were similar between wild type and mutant individuals (Fig. 6B, n=3 wild type and 3 mutants at each stage). These results suggest that the IntuDtm mutation results in a delay in vertebrae ossification.
Delayed ossification in long bones of IntuDtm mutant forelimbs
The long bones of the limb present a useful system to quantitate differences in the extent of ossification by measurement of the ossified region as a fraction of bone length (Fig. 6C). As shown in Fig. 6A, at E16.5, the stylopod (humerus) and zeugopod (ulna and radius) bones were ossified in both wild type and IntuDtm mutants. At E16.5, the ossified fraction of the humerus, radius, and ulna was significantly less in the mutants than in the wild type (32% less in humerus, p=0.01; 11% less in radius, p=0.03, 8.3% less in ulna, p=0.01; Fig. 6C, n=5 mutants and 5 control). At E17.5, a significant difference was observed in the humerus (11% difference, p=0.04), but not in the radius or ulna (9.7% difference, p=0.36 and 3.3% difference, p=0.27, respectively; Fig. 6C, n=6 mutants and 5 control). At E18.5, significantly less ossification was found in the radius of IntuDtm embryos than in wild type (9.9% difference, p=0.002), but not in the humerus or ulna (1.6% difference, p=0.41 and 0% difference, p=0.80, respectively; Fig. 6C, n=5 mutants and 5 control). Thus, the IntuDtm mutation causes a delay in ossification in both the long bones and vertebrae but this difference becomes less apparent at later embryonic stages.
During endochondral ossification, chondrocytes undergo hypertrophic differentiation in which cells increase in size and deposit calcium in the ECM. Our histological analysis showed drastically narrower hypertrophic zones in the E14.5 IntuDtm mutant long bones than those of the wild type littermates (Fig. 6D), suggesting that defects in hypertrophic differentiation may contribute to the delay in ossification.
Delayed ossification of the sternum and rib misalignment in IntuDtm mutants
Similar to the limbs and vertebrae, ossification of the sternum was also delayed in IntuDtm mutants (Fig. 6E, n=8 mutants and 5 controls). In addition, we observed misalignment of the ribs. In normal embryos, ribs are connected to the sternum in pairs and ossification of the sternum initiates in the inter-rib region (Fig. 6E, n=8). Interestingly, IntuDtm mice exhibited severe rib misalignment (Fig. 6E, n=8). In conjunction with the misalignment, some IntuDtm mutant mice developed an unequal number of ribs on each side of the sternum (Fig. 6E, note there are 8 attached ribs on the left and 7 attached ribs on the right side, 3 out of 8 mutants).
Planar cell polarity of the growth plate and Organ of Corti are not affected by the IntuDtm mutation
The PCP signaling pathway plays an important role in skeletal development by regulating the morphological change and columnar arrangement of proliferating chondrocytes (Gao et al., 2011; Randall et al., 2012; Kuss et al., 2014). To investigate whether defects in the PCP pathway contribute to the skeletal defects in IntuDtm mutants, we performed histological analysis of P0 growth plates. In control proximal tibia (Fig. 7A and A’, n=3 pups) and metatarsal (Fig. 7C and C’; n=3 pups) growth plates, the proliferating chondrocytes formed columns that align with the long axis of the bones. In IntuDtm mutant tibia (Fig. 7B and B’; n=3 pups) and metatarsals (Fig. 7D and D’, n=3 pups), the proliferating chondrocytes exhibited a similar flattened morphology and columnar arrangement as wild type.
Figure 7. Planar cell polarity is not altered in IntuDtm mutants.
(A, B) Longitudinal sections of the proximal tibia from control and IntuDtm mutant newborn pups stained with Hematoxylin and Eosin. A’ and B’ show high magnification view of the area highlighted in the rectangles in A and B. (C, D) Longitudinal sections of the metatarsals from control and IntuDtm mutant newborn pups stained with Hematoxylin and Eosin. C’ and D’ show high magnification view of the area highlighted in the rectangles in C and D. (E, F) Scanning EM images of the Organs of Corti from E18.5 control and IntuDtm mutant embryos. The abneural side is up.
To investigate further the roles of Intu in PCP signaling, we examined the Organ of Corti, the functional component of the inner ear. Wild type stereocilia form chevron-shaped bundles of actin-based apical protrusions that all point to the abneural side, as revealed by scanning electron microscopy (Fig. 7E). In IntuDtm mutant Organ of Corti, the polarity of the hair cells was not obviously altered (Fig. 7F). Together these data, along with our earlier study (Heydeck and Liu, 2011), suggest that the IntuDtm phenotypes do not result from abnormal PCP signaling.
Reduced Ptch1 expression in the long bones of IntuDtm mutant embryos
Previous studies indicated that Ihh promotes endochondral bone development (Lai and Mitchell, 2005). As cilia are also required for Ihh signaling, we first examined cilia number in the growth plate chondrocytes. This showed a significant decrease in ciliogenesis in IntuDtm mutant chondrocytes in both the proximal tibia and metatarsals (Fig. 8A). Next we investigated whether Ihh signaling was defective in the skeletal elements by examining the expression of a direct target of Hh, Ptch1, via X-gal staining of mouse embryos expressing a Ptch1-lacZ reporter gene (Fig. 8B-D). As previously reported, Ptch1 is highly expressed in tissues known to exhibit high levels of Hh signaling activities, including the whisker follicles, ventral neural tube and somites, as well as developing long bones (Fig. 8B, E13.5 n=3; Goodrich et al., 1997). Closer examination showed a modest reduction in Ptch1-lacZ expression in E13.5 IntuDtm mutant forelimbs (Fig. 8C n=3). The reduction of Ptch1-lacZ expression in the IntuDtm mutants was also observed in sections of the E14.5 forelimbs, where Ptch1-lacZ is expressed in the developing cartilage and perichondrium, a layer of cells surrounding the cartilage (Fig. 8D, n=3). Altogether this data shows both compromised ciliogenesis and decreased expression of a direct target of Ihh signaling in IntuDtm mutants suggesting that the ossification and growth defects of the IntuDtm mutants result from compromised Hh signaling.
Figure 8. IntuDtm embryos exhibit reduced Ptch1 expression.
(A) Cilia are reduced in number in P0 growth plate chondrocytes of IntuDtm mutant proximal tibia and metatarsals compared to wild type. (B) Lateral views of E13.5 Ptch1-lacZ embryos stained with X-gal. The wild type and mutant embryos shown were processed together. (C) Dorsal views of the dissected forelimbs of the embryos shown in (B). Note the staining in the IntuDtm mutant long bones and digits is weaker compared to wild type. Thin red arrows: humerus; thick red arrows: radius; black arrows: digits 2-4. (D) Sections along the proximal-distal axis of the forelimbs showing Ptch1-lacZ expression in the perichondrium and the developing cartilage. H: Humerus, R: radius, U: ulna.
DISCUSSION
Here we report the characterization of IntuDtm, a recessive mouse mutation that alters a conserved amino acid (I813N) in the mouse Intu protein. Consistent with the reported roles of Intu in ciliogenesis, IntuDtm mutant cells have fewer and shorter cilia. In addition, the ability of IntuI813N mutant protein to increase ciliation in Intu null mutant cells is compromised. Significantly, unlike Intu null embryos, which die during embryogenesis, IntuDtm mutants can survive to adulthood, allowing us to investigate the role of Intu in late embryonic and postnatal development. In this manuscript we describe a role for Intu in skeletal development using the new IntuDtm hypomorphic allele.
We observed significant differences between IntuDtm mutants and their normal littermates in both postnatal survival and growth rates, indicating that Intu plays an important role in postnatal development. Although we cannot rule out the contribution of nutrient or heat deprivation to the reduced survival and growth (Ding et al., 2010), our data strongly suggest that defects in skeletal development are likely the main contributing factor to the size difference.
Ihh mutants exhibit defects in endochondral ossification and smaller size at birth (St-Jacques et al., 1999; Razzaque et al., 2005). Cartilage-specific ablation of cilia in conditional Ift88 or Kif3a mutants result in reduced Ihh signaling, defects in endochondral ossification and postnatal growth (Song et al., 2007; Chang and Serra, 2013). We found delayed ossification in the vertebrae, sternum, digits and long bones of the limbs in IntuDtm mutants, consistent with an important role for cilia in skeletal development. Indeed IntuDtm mutant cartilage had reduced number of cilia and decreased Ptch1 expression, suggesting that Hh signaling is compromised inIntuDtm mutants. Therefore, it is plausible that reduced ciliation in IntuDtm mutants results in dampened Ihh signaling activity in the cartilage, which in turn leads to defects in skeletal development and animal growth.
The formation of extra digits in the IntuDtm mutants is consistent with a requirement for the primary cilia in the processing of Gli3 into a transcriptional repressor, which negatively regulates Hh signaling and the proper formation of digits (Hui and Joyner, 1993; Liu et al., 2005). Interestingly, the IntuDtm mutants only form one extra digit per limb, similar to that of Gli3 heterozygous mutants (Hui and Joyner, 1993), suggesting that Gli3 processing is not completely disrupted in IntuDtm mutants.
In the neural tube, even though ciliogenesis is compromised in IntuDtm mutants, Hh pathway activities appear sufficient, as IntuDtm mutants exhibit normal gene expression patterns along the D/V axis, and our qRT-PCR data did not show significant decrease in Hh target gene expression in the neural tube. We suggest that endochondral ossification and digit formation are more sensitive to subtle changes in the level of Hh pathway activities. It is also possible that Intu regulates skeletal development through a combination of Hh-dependent and Hh-independent mechanisms.
Intu was originally reported as a regulator of PCP in fruit flies and frogs (Adler et al., 1994; Park et al., 2006), yet our characterization of the Intu null mutants suggested that Intu does not play a major role in convergent extension in early mouse development (Heydeck and Liu, 2011). Recent studies suggested that the PCP pathway regulates the flattening and columnar organization of proliferating chondrocytes in growth plates (Gao et al., 2011; Randall et al., 2012; Kuss et al., 2014). Our histological analysis of IntuDtm mutant growth plates did not reveal any defects in the flattening or columnar formation of the proliferating chondrocytes, suggesting that the skeletal defects in IntuDtm mutants do not result from abnormal PCP signaling. Interestingly, another well-characterized tissue for PCP studies, the Organ of Corti, also did not exhibit an obvious PCP defect in IntuDtm mutants. The cranial neural tube defect in IntuDtm mutants could result from a defect in Hh or PCP signaling. However, our previous studies of the null allele of Intu failed to detect defects in convergent extension, even on the sensitized Vangl2 heterozygous mutant background (Zeng et al., 2010; Heydeck and Liu, 2011). Therefore, it is more likely the infrequent NTD in IntuDtm mutants results from a subtle decrease in Gli3 repressor activity, as also indicated by the mild polydactyly in the limbs. In conjunction with our previous studies of Intu null mutants, we conclude that Intu does not play a major role in PCP signaling in mammals.
Through the study of IntuDtm mutants, a hypomorphic allele for the gene Intu, we demonstrate that Intu is critical for mouse skeletal development, postnatal survival and growth. We show that both ciliation and Hh signaling are compromised in IntuDtm mutants, which we suggest account for the skeletal and growth defects. This new mutant mouse strain will serve as an important model for the study of other postnatal functions of Intu and the pathology of ciliopathies.
EXPERIMENTAL PROCEDURES
Mice
The ENU mutagenesis and positional cloning procedure for Dtm mutants have been previously described (Zohn et al., 2005; Liu and Eggenschwiler, 2014). Phenotypic analyses were carried out in congenic C3H/HeN background. Genomic DNA was prepared by incubating tail snips overnight in 100μl lysis buffer (50mM Tris·HCl, pH8.8, 1mM EDTA and 0.5% Tween 20) with proteinase K (100μg/ml) at 55°C. The Ptch1-lacZ transgenic mice were genotyped for lacZ by PCR on genomic DNA using primers CCG AAC CAT CCG CTG TGG TAC and CAT CCA CGC GCG CGT ACA TC.
Bioinformatics
Information on the cDNA, exon-intron structure and open reading frames was obtained from the Ensembl database (http://www.ensembl.org). Intu protein structure was predicted using the SMART program (http://smart.embl-heidelberg.de).
Scanning electron microscopy (SEM)
E8.0 mouse embryos and E18.5 cochlea were fixed overnight in 2.5% glutaraldehyde, washed in PBS and dehydrated through an ethanol series. A small portion of the embryo was removed for genotyping before dehydration. The dehydrated samples were critical-point dried, mounted to metal holders with the embryonic node and stereocilia facing up, sputter-coated with silver and visualized with a JEOL JSM 5400 SEM at the Penn State microscopy facility.
X-gal staining of Ptch1-lacZ transgenic embryos
Embryos were dissected and fixed in 4% paraformaldehyde (PFA) for 1-3 hours at 4°C. Subsequently, embryos were washed three times for 15 minutes per wash using wash buffer (0.1 M phosphate buffer, 2 mM MgCl2 and 0.02% Nonidet P-40) and stained overnight at 37°C in X-gal solution (in wash buffer: 5 mM potassium ferrocyanide [K3Fe(CN)6], 5 mM potassium ferricyanide [K4Fe(CN)6.3H2O], 1 mg/ml X-Gal [5-Bromo-4-chloro-3-indolyl β-d-galactopyranoside, Sigma] and 0.01% sodium deoxycholate for improved penetration). After staining, the embryos were washed with wash buffer three times, five minutes per wash and stored in 4% PFA at 4°C until further processing and imaging.
For sectioning, forelimbs of fixed X-gal-stained embryos were washed three times in cold phosphate buffered saline (PBS) for 15 minutes per wash. They were then submerged in 30% sucrose in PBS and incubated in Tissue-Tek OCT compound before freezing. The tissue was sectioned at 10μm and allowed to air dry for one hour. Sections were stored at −80°C until mounted and imaged on the Nikon E600 microscope.
Bone and cartilage staining
Embryos were soaked in water for 24 hours prior to skin removal and evisceration. Embryos were then fixed in 95% ethanol for three days followed by staining in cartilage-specific Alcian Blue solution for 24 hours (for 50ml solution: 10ml glacial acetic acid, 40ml 95% ethanol, 7.5mg Alcian Blue 8GX, Fluka). Subsequently, embryos were rinsed with 95% ethanol and stained in fresh Alcian Blue for an additional 24 hours. Embryos were then stained in bone-specific Alizarin Red (Harleco) solution (50mg Alizarin Red per liter of 2% potassium hydroxide [KOH]) for three hours, and stained in fresh Alizarin Red overnight. Samples were cleared in 1% KOH for a few days and then transferred from 20 to 40, 60, and 80% glycerol solutions in 1% KOH over a few weeks to remove excess dye.
All wholemount embryo images were taken on the Zeiss KD2500 microscope. Whole embryo images were taken with 16x magnification (E11.5) and 8x magnification (E16.5-18.5). Limb images were taken with 10x magnification (E16.5-18.5) and sternum images with 15x magnification.
Immunohistochemistry
Sections stored at −80°C were dried for one hour at room temperature and then incubated in blocking buffer (for patterning markers: 3% normal goat serum in PBS; for all other antibodies, 3% normal goat serum, 0.3% Triton X-100 in PBS) for one hour. Sections were incubated overnight in primary antibody at 4°C. The primary antibody used for the kidney sections was rabbit α-mouse Arl13b (1:1000 dilution, from Dr. Tamara Caspary). The primary mouse antibodies used for spinal cord sections included Nkx2.2, Pax3, Pax6, Pax7, Islet1/2 and Shh (all used at 1:10), all from the Developmental Studies Hybridoma Bank, as well as Arl13b (1:500, Antibodies, Inc, clone N295B66), γ-tubulin (1:1000, AbCam, clone GTU-88), neurofilament (1:1000), Tuj1 (1:1000), and phospho-histone H3 (1:500, Cell Signaling #9701L). Slides were washed three times for 10 minutes per wash in blocking buffer and then incubated in secondary antibody for one hour at room temperature. The secondary antibody used for the kidney sections was Cy3-conjugated goat α-rabbit (1:250, Jackson ImmunoResearch). Secondary antibodies used for the spinal cord sections were conjugated with Alexa Fluor dyes (1:500, Life Technologies). All antibodies were diluted in blocking buffer. Slides were washed three times for 10 minutes per wash and mounted with Vectashield or DABCO mounting medium. Slides were stored at 4°C until imaged.
The tissue sections were visualized with either a Nikon E600 microscope or a Zeiss 780 confocal microscope. Kidney sections were imaged at 40x magnification. Spinal cord sections were imaged at 10x magnification.
Generation of the GFP-IntuI813N construct
The GFP-IntuI813N fusion construct was generated by introducing the Dtm point mutation into the previously described GFP-Intu construct (Zeng et al., 2010). The primers used were AC ACC CCC ACC CAT GAA GAG and T GAA GAT GCC TTG CAC TGT TTC C. The PCR product was purified with the QIAquick PCR Purification Kit (Qiagen), self-ligated, and was used to transform competent cells. The Dtm point mutation was verified by digestion with HindIII (2.3kb and 5kb products) and sequencing (Primers used for sequencing are GFP primers: ATG GTC CTG CTG GAG TTC GT and GGG AGG TGT GGG AGG TTT T; Intu primers: GCT ATG AAG AGC GGT CAG GT and ATG TTG GAG AGG CTG CTG TT).
Cell culture and ciliogenesis assay
Wild type and Intu−/− mouse limb fibroblasts (E12.5, immortalized with SV40 large T antigen) (Zeng et al., 2010) were maintained at 37°C and 5% CO2 in Dulbecco's modified eagle's medium [DMEM], 10% fetal bovine serum [FBS], 2 mM GlutaMAX, penicillin [100U/ml]-streptomycin [100μg/ml]. Cells were allowed to grow to 90-95% confluence on plates coated with 0.1% gelatin before passaging.
For transfection, cells were grown on 0.1% gelatin-coated coverslips in regular culture media at a dilution that allowed cells to reach ~50% confluence by 24 hours. On the following day, the cells were transfected using JetPrime reagent (Polyplus Transfection) according to the manufacturers protocol. 16-20 hours post transfection, the cells were washed twice with, and then cultured in starvation medium (DMEM, 0.25% FBS, 2mM GlutaMAX, penicillin [100U/ml]-streptomycin [100μg/ml]) for ~48 hours to allow cilia growth.
Cells were harvested and rinsed with warm PBS prior to fixation in 4% PFA for 10 minutes at room temperature. Cells were then rinsed twice with PBST (PBS, 0.1% Triton X-100) and blocked with a solution of PBST+10% NGS for 10 minutes. Subsequently, cells were incubated in primary antibody overnight at 4°C (α-acetylated tubulin, 1:1000 dilution, Sigma; GFP, 1:1000, Life Technologies). Cells were then rinsed three times with PBST and incubated in secondary antibody for two hours at room temperature (Cy3-conjugated goat α-mouse, 1:500, Jackson ImmunoResearch; AlexaFluor 488-conjugated goat α-rabbit, 1:500, Life Technologies). All antibodies were diluted in a solution of PBS+10% NGS. Following three washes with PBST and one wash with water, the coverslips were mounted onto glass slides with Vectashield mounting medium with DAPI. The slides were stored at 4°C and visualized under a Nikon E600 microscope and imaged at 60x magnification.
Histological Analyses
E14.5 forelimbs were fixed in 4% PFA overnight and processed in a Leica TP1020 Paraffin Processor. 5μm sections were cut using Shandon Finesse microtome. Hematoxylin and eosin staining was performed using a Shandon Gemini Varistainer. Photos were taken using a Nikon E600 microscope and a QImaging digital camera.
P0 hindlimbs were fixed in Bouin's or 4% PFA overnight at 4°C. Bones were decalcified for 11 days in 14% EDTA in PBS (pH 7.0-7.4) with the solution changed daily. Proximal tibia and metatarsals were embedded in paraffin following standard protocol and 10 μm sections were cut on a Microm HM355 Microtome followed by hematoxylin and eosin staining. Photos were taken using a Nikon Eclipse 80i microscope and a Nikon DS-F12 digital color camera.
Key findings.
Dtm, a hypomorphic allele of mouse Intu carrying an I813N mutation, exhibits polydactyly, reduced survival and retarded growth.
IntuI813N exhibits compromised ciliogenic activity in vivo and in vitro.
Dtm mutants exhibit delayed endochondral ossification and defective Hh signaling.
ACKNOWLEDGMENT
We thank Huiqing Zeng and Hongchen Cai for critically reading the manuscript. We thank Dr. Matt Scott for the Ptch1-lacZ mouse and Dr. Tamara Caspary for the Arl13b antibody. We thank Hongchen Cai for performing histological analysis presented in Fig. 6D. We thank the Genomics and Microscopy Core Facilities for their technical support. The monoclonal antibodies against Foxa2, Nkx2.2, Islet1/2, Pax6 were developed by Dr. Jessell, against Pax3 developed by Dr. Ordahl and against Pax7 developed by Dr. Kawakami, and obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. AL was supported by a Penn State University new lab start-up fund and a Young Investigator Career Development Award from the PKD Foundation. RC was partially supported with a PSU fund for undergraduate research.
Grant Sponsor and Number: The PKD Foundation Young Investigator Career Development Award, #:02YI08a
Penn State University new lab start-up fund and a PSU fund for undergraduate research
REFERENCES
- Adler PN, Charlton J, Park WJ. The Drosophila tissue polarity gene inturned functions prior to wing hair morphogenesis in the regulation of hair polarity and number. Genetics. 1994;137:829–836. doi: 10.1093/genetics/137.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker K, Beales PL. Making sense of cilia in disease: the human ciliopathies. Am J Med Genet C Semin Med Genet. 2009;151C:281–295. doi: 10.1002/ajmg.c.30231. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- Chang CF, Serra R. Ift88 regulates Hedgehog signaling, Sfrp5 expression, and beta-catenin activity in post-natal growth plate. J Orthop Res. 2013;31:350–356. doi: 10.1002/jor.22237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding F, Li HH, Li J, Myers RM, Francke U. Neonatal maternal deprivation response and developmental changes in gene expression revealed by hypothalamic gene expression profiling in mice. PLoS One. 2010;5:e9402. doi: 10.1371/journal.pone.0009402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, Andre P, Robinson J, Sood R, Minami Y, Economides AN, Yang Y. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell. 2011;20:163–176. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RS, Abitua PB, Wlodarczyk BJ, Szabo-Rogers HL, Blanchard O, Lee I, Weiss GS, Liu KJ, Marcotte EM, Wallingford JB, Finnell RH. The planar cell polarity effector Fuz is essential for targeted membrane trafficking, ciliogenesis and mouse embryonic development. Nat Cell Biol. 2009;11:1225–1232. doi: 10.1038/ncb1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydeck W, Liu A. PCP effector proteins inturned and fuzzy play nonredundant roles in the patterning but not convergent extension of mammalian neural tube. Dev Dyn. 2011;240:1938–1948. doi: 10.1002/dvdy.22696. [DOI] [PubMed] [Google Scholar]
- Heydeck W, Zeng H, Liu A. Planar cell polarity effector gene Fuzzy regulates cilia formation and Hedgehog signal transduction in mouse. Dev Dyn. 2009;238:3035–3042. doi: 10.1002/dvdy.22130. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui CC, Joyner AL. A mouse model of greig cephalopolysyndactyly syndrome: the extra-toesJ mutation contains an intragenic deletion of the Gli3 gene.PG - 241-6. Nat Genet. 1993:3. doi: 10.1038/ng0393-241. [DOI] [PubMed] [Google Scholar]
- Kuss P, Kraft K, Stumm J, Ibrahim D, Vallecillo-Garcia P, Mundlos S, Stricker S. Regulation of cell polarity in the cartilage growth plate and perichondrium of metacarpal elements by HOXD13 and WNT5A. Dev Biol. 2014;385:83–93. doi: 10.1016/j.ydbio.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Lai LP, Mitchell J. Indian hedgehog: its roles and regulation in endochondral bone development. J Cell Biochem. 2005;96:1163–1173. doi: 10.1002/jcb.20635. [DOI] [PubMed] [Google Scholar]
- Liu A, Eggenschwiler J. Identifying essential genes in mouse development via an ENU-based forward genetic approach. Methods Mol Biol. 2014;1092:95–118. doi: 10.1007/978-1-60327-292-6_7. [DOI] [PubMed] [Google Scholar]
- Liu A, Wang B, Niswander LA. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132:3103–3111. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet. 2006;38:303–311. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- Randall RM, Shao YY, Wang L, Ballock RT. Activation of Wnt Planar Cell Polarity (PCP) signaling promotes growth plate column formation in vitro. J Orthop Res. 2012;30:1906–1914. doi: 10.1002/jor.22152. [DOI] [PubMed] [Google Scholar]
- Razzaque MS, Soegiarto DW, Chang D, Long F, Lanske B. Conditional deletion of Indian hedgehog from collagen type 2alpha1-expressing cells results in abnormal endochondral bone formation. J Pathol. 2005;207:453–461. doi: 10.1002/path.1870. [DOI] [PubMed] [Google Scholar]
- Song B, Haycraft CJ, Seo HS, Yoder BK, Serra R. Development of the post-natal growth plate requires intraflagellar transport proteins. Dev Biol. 2007;305:202–216. doi: 10.1016/j.ydbio.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller R, Lopez-Rios J, Zuniga A. Vertebrate limb bud development: moving towards integrative analysis of organogenesis. Nat Rev Genet. 2009;10:845–858. doi: 10.1038/nrg2681. [DOI] [PubMed] [Google Scholar]
- Zeng H, Hoover AN, Liu A. PCP effector gene Inturned is an important regulator of cilia formation and embryonic development in mammals. Dev Biol. 2010;339:418–428. doi: 10.1016/j.ydbio.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Zohn IE, Anderson KV, Niswander L. Using genomewide mutagenesis screens to identify the genes required for neural tube closure in the mouse. Birth Defects Res A Clin Mol Teratol. 2005;73:583–590. doi: 10.1002/bdra.20164. [DOI] [PubMed] [Google Scholar]