Abstract
Emotional memory consolidation has been associated with rapid eye movement (REM) sleep, and recent evidence suggests that increased electroencephalogram spectral power in the theta (4–8 Hz) frequency range indexes this activity. REM sleep has been implicated in posttraumatic stress disorder (PTSD) as well as in emotional adaption. In this cross-sectional study, thirty young healthy African American adults with trauma exposure were assessed for PTSD status using the Clinician Administered PTSD Scale. Two consecutive night polysomnographic (PSG) recordings were performed and data scored for sleep stages. Quantitative electroencephalographic spectral analysis was used to measure theta frequency components sampled from REM sleep periods of the second-night PSG recordings. Our objective was to compare relative theta power between trauma-exposed participants who were either resilient or had developed PTSD. Results indicated higher right prefrontal theta power during the first and last REM periods in resilient participants compared with participants with PTSD. Right hemisphere prefrontal theta power during REM sleep may serve as a biomarker of the capacity for adaptive emotional memory processing among trauma-exposed individuals.
Keywords: Posttraumatic stress disorder (PTSD), Resilience, Trauma, Polysomnography (PSG), Quantitative EEG (qEEG), Spectral analysis
Introduction
Posttraumatic stress disorder (PTSD) is a potentially debilitating and chronic disorder that develops in some but not all individuals exposed to severe trauma (Agaibi and Wilson 2005; Breslau 2009). Resilience refers to an ability to maintain psychological health and stability in the aftermath of exposure to profoundly life-altering or life-threatening traumatic events and is often designated based on the absence of PTSD following such experiences. While there are a number of psychosocial factors that have been linked to outcomes of PTSD versus resilience (Alim et al. 2008; Kessler et al. 1999; Yehuda and Flory 2007), there are significant gaps in our understanding of why certain individuals are resilient while others are vulnerable to the development of PTSD following trauma, and there is emerging evidence that neurobiology contributes to these outcomes.
Functional brain imaging studies (fMRI) have identified abnormalities in the circuitry involved with emotional regulation, memory formation, and fear extinction in the pathophysiology of PTSD (Bryant et al. 2008; Cohen et al. 2013; Etkin and Wager 2007). In PTSD subjects, hypoactivation in the ventral medial prefrontal cortex (vmPFC) and dorsal and mid-anterior cingulate cortex (ACC), and hyperactivation in the insula and bilateral amygdala are often reported, suggesting reduced top-down regulation of subcortical structures (Etkin and Wager 2007). Deficits in these areas are implicated in the impaired extinction of trauma-related memories and deficits in the ability to regulate emotions explained in neurocircuitry models of PTSD (Germain et al. 2008; Hayes et al. 2012). In an fMRI study of sexual assault victims, resilient individuals exhibited patterns of brain activation that were distinct from both the PTSD group and the non-traumatized controls with increased activation of the ACC during a task requiring an enhanced emotional response to negative stimuli (New et al. 2009). The authors hypothesized that this reflected a capacity by resilient individuals to engage cognitive/linguistic areas of the brain while coping with negative emotions.
While the above described studies focus on waking brain activity, investigations of neural activity during sleep could contribute critically to a comprehensive understanding of the neurobiology of resilience to trauma. Multiple lines of evidence support a role for sleep in the consolidation of memories and links REM sleep in particular with the processing of affective stimuli (Baran et al. 2012; Diekelmann and Born 2010; Groch et al. 2013; Gujar et al. 2011; Wagner et al. 2001). For example, neuroimaging studies show increased neural activation in affective brain networks during REM sleep. Experiments implicate REM sleep in enhancing consolidation of memories with a negative valence (Nishida et al. 2009; Wagner et al. 2001). Baran et al. (2012) suggested that REM sleep consolidation processes serve to preserve the emotional salience of and reactivity to memories. It has been posited that consolidation processes during REM sleep may lead to a gradual integration of these memories into existing cortical networks, resulting in an attenuation of negative emotional components (Walker and van der Helm 2009). REM-related consolidation may reduce the emotional charge of the content while preserving salient components, thus serving an adaptive or “therapeutic” function (Gujar et al. 2011; Hu et al. 2006; Walker 2009; Walker and van der Helm 2009).
Rapid eye movement sleep abnormalities have been implicated in several neuropsychiatric disorders including PTSD (Benca et al. 1997; Wulff et al. 2010). A meta-analysis of PTSD studies using polysomnographic (PSG) prior to 2007 found increased density of eye movements during rapid eye movement (REM) sleep (Kobayshi et al. 2007). In a prospective study in which hospitalized trauma victims were given PSG recordings within a month of trauma exposure, Mellman et al. (2002) found patterns of fragmented REM sleep in patients who developed PTSD compared with resilient patients. The centrality of nightmares in PTSD has also suggested the involvement of REM sleep (Ross et al. 1994).
Quantitative spectral analysis of EEG (qEEG) provides a method for identifying microstructural alterations to frequency-specific activity during sleep that is promising for yielding markers of vulnerability and resilience to trauma. REM sleep is characterized by frequencies in the theta (4–8 Hz), beta (16–32 Hz), and gamma (>32 Hz) ranges (Merica and Blois 1997). The potential significance of activity at theta frequencies is suggested by its role in encoding information during wake and in limbic and cortical structures during sleep-dependent memory consolidation processes (Mitchell et al. 2008). In a recent study of waking emotional regulation, using both fMRI and EEG measures, response to viewing negatively valenced emotional images produced early synchronized activity in prefrontal areas at theta frequencies (3–6 Hz) which modulated subsequent frontal and temperooccipital high beta (Hβ) (25–30 Hz) in healthy subjects. In contrast, PTSD subjects exhibited early and widespread theta activity that failed to modulate Hβ (Cohen et al. 2013).
Neural activity at theta frequencies has been implicated in learning and memory with recent evidence of changes to REM theta power following fear conditioning. In a rodent model, enhanced activity and synchronization between the hippocampus (HC) and amygdala occurred at theta frequencies during REM sleep (Karashima et al. 2010). Evidence from Popa et al. (2010) using an animal model of fear conditioning showed increased retention of the fear response strongly correlated with synchronized firing between the basal lateral amygdala (BLA) and the medial prefrontal cortex (mPFC) and the BLA and HC at theta frequencies during REM sleep. Synchronized activity between the HC and amygdala at theta frequencies has been previously associated with fear memory retrieval (Popa et al. 2010). Both acute and chronic stress have been shown to cause changes to REM theta activity in the HC and amygdala with stress-induced reductions in theta power and coherence after the stress was terminated (Hegde et al. 2011). In a human study of the role of sleep in processing emotional memory, Nishida et al. (2009) found that the benefit of a nap toward consolidating memory of recent emotional stimuli correlated positively with right-dominant prefrontal theta power during the REM sleep portion of the nap.
While REM sleep is associated with consolidation and possibly further processing of affective components of memory, its role in PTSD remains not well understood. Given the evidence that REM sleep can have an adaptive role in emotional memory processing and that this role is impaired among those with PTSD and the critical role for activity linked to theta frequencies we sought to determine whether there is a difference in relative theta power during REM sleep in trauma-exposed individuals with PTSD versus resilience. In this study, we use the term “resilience” to refer to individuals who experienced high impact traumas but did not develop PTSD symptoms. Based on neuroimaging findings and on the findings of Nishida et al. (2009) suggesting that higher REM theta power measured in prefrontal leads index emotional memory consolidation processes, we hypothesized that effects would manifest in frontal areas and focused our analysis on frontal derivations. Further, we hypothesized that resilient subjects would exhibit higher REM theta power than individuals with PTSD.
Methods
Participants
Participants were a subsample of African Americans (age 18–35 years) who completed laboratory PSG recording as part of a larger study examining associations between trauma, PTSD, nocturnal blood pressure, and sleep. Participants were recruited from the Washington, DC metropolitan area through flyers and referrals from prior participants. For the purpose of this report, analyses focused on the participants who had experiences meeting DSM-IV PTSD criterion A1 for trauma exposure with “high impact” but had never developed PTSD. We considered as traumas previously associated in population studies with high risk for engendering PTSD such as sexual and physical assault, abuse during childhood, and domestic violence (Kessler 2000). This group was designated “resilient” (n = 22). The resilient participants were compared with participants who had met criteria for a diagnosis of PTSD and had current active symptoms (n = 28).
During the initial screening, potential participants were excluded if they were found to have a body mass index ≥40, chronic medical conditions (such as severe asthma, cancer, diabetes mellitus, and emphysema), or severe mental disorders (psychotic disorders, bipolar disorder, severe recurrent depression) that required consistent use of medications. Additional exclusion criteria were excessive use of caffeine (>5 cups of coffee per day or its equivalent), heavy smoking (>20 cigarettes per day), regular night shift work or unusual sleep–wake schedules, sleep breathing and movement disorders (screened through the first night PSG), hazardous levels of drinking (>14 drinks/week in men, >7 drinks/week in women), current alcohol or drug abuse or dependence (screened through a structured clinical interview), and positive urine toxicology for illicit drugs. Five resilient and three PTSD participants were not included in this analysis as they did not have frontal (F−) leads which were added to the montage later in the study. Other exclusions included participants with positive toxicology screening results (three Resilient and seven PTSD); apnea and hypopnea scores >10 (one resilient); poor signal quality for staging and conducting quantitative EEG analyses (4 PTSD). Therefore, 13 resilient and 17 PTSD participants were included in our final sample. Characteristics of these participants are presented in Table 1.
Table 1.
Clinical and demographic variables
| Variable | Resilient (n = 13) Mean (SD) |
PTSD (n = 17) Mean (SD) |
t (28) |
|---|---|---|---|
| Age | 20.77 (2.68) | 22.35 (4.62) | 0.172 |
| CAPS current | 7.38 (16.1) | 47.4 (17.1) | −8.6 |
| CAPS lifetime | 21.3 (13.4) | 77.5 (16.0) | −10.1 |
| N | N | p | |
|---|---|---|---|
| Gender (male) | 5 (17 %) | 6 (20 %) | 0.86 |
| Index trauma | (Total %) | ||
| Sexual assault or rape | 1 | 5 | 20.0 |
| Non-sexual assault | 4 | 7 | 33.3 |
| Childhood physical/sexual abuse | 2 | 2 | 13.3 |
| Life-threatening accident | 2a | 1 | 13.3 |
| Witnessed violent death | 1 | 0 | 3.0 |
| Domestic violence | 3 | 2 | 16.6 |
Resulted in serious injury and hospitalization
Clinical interviews
The Clinician Administered PTSD Scale (CAPS) (Blake et al. 1995) is a structured clinical interview designed to produce dichotomous lifetime and current PTSD diagnostic status and continuous symptom severity. In the current study, the most distressing potentially traumatic event (the index event) reported by a participant was assessed at the beginning of the interview to determine whether it met the diagnostic criteria for a traumatic event (Criterion A) (DSM-IV-TR 2000). If the index event met the criteria, the frequency and intensity of each of 17 PTSD symptoms associated with the index event were rated on 5-point scales for frequency and intensity as follows: [Frequency] 0 = never, 1 = once or twice, 2 = once or twice a week, 3 = several times a week, 4 = daily or almost every day; [Intensity] 0 = none, 1 = mild or minimal distress or disruption of activities, 2 = moderate distress with some disruption of activities, 3 = severe, considerable distress, marked interruption of activities, 4 = extreme, incapacitating distress, unable to continue activities. A symptom was considered present when rated at least one for frequency and at least two for intensity (Blake et al. 1995).
Current and lifetime diagnoses of mood disorders, psychotic disorders, anxiety disorders other than PTSD, substance abuse and dependence, and eating disorders were assessed using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (SCID) (First et al. 2002). All CAPS and SCID interviews were conducted by trained staff members (psychology graduate students, medical students, and clinical psychology postdoctoral fellows), and a licensed psychiatrist reviewed all cases. Practice interviews were completed prior to data collection until the trainee and the trainer reached the 90 % agreement rate on practice cases.
Procedures
The following procedures were approved by the institutional review board at Howard University. Potential participants were first given a brief description of the study and screened for eligibility through a brief interview over the phone or in person. Eligible individuals were then invited to the initial study session at Howard University with a researcher who further described the study and obtained a written informed consent. During the session, participants completed a self-report survey packet, which included a demographic questionnaire.
A subset of participants who completed the self-report survey was invited to the laboratory phase of the study. In the laboratory phase, participants completed the clinical interview, physical examination, and urine screening, and then participants went through two consecutive overnight PSG recordings at the Howard University Hospital Clinical Research Unit (CRU). Participants received $25 for completing the self-report survey, another $25 for the clinical interview, $125 for each PSG recording.
Polysomnography (PSG) and quantitative EEG (qEEG)
Participants underwent two consecutive overnight PSG recordings in the Howard University Clinical Research Unit (CRU). Recordings were conducted using an Embla (Denver, CO) titanium portable unit. PSG collection included a standard electroencephalogram montage with bilateral frontal, central, and occipital leads, two electrooculograms, and chin electromyogram and limb electromyogram and respiratory monitors on the first night only. Study staff, who were blinded to participants’ PTSD diagnostic status, visually scored sleep records on a computer monitor applying AASM scoring rules (Iber et al. 2007). All scorers had demonstrated >90 % concordance for scoring epochs with reference records. Participants were instructed to go to bed and arise close to their habitual bedtime and rise time. We analyzed data from the second-night recordings.
Spectral analysis of the EEG recordings using fast Fourier transform (FFT) was performed using onboard power spectral software within the REMlogic data acquisition system (sampling rate 512, low-pass filter 0.3 Hz, high-pass filter 100 Hz). Prior to FFT, a low-pass filter of 0.3 Hz (smoothing function) and a Hamming window (tapering) was applied to the raw signal. Spectral power density was estimated for each 10 s segment using Welch’s averaged modified periodogram (FFT sampling rate: 512 Hz, linear detrending, 50 % overlap and Hamming windowing) providing a 0.1 Hz frequency resolution and allowing time alignment with the sleep staging of the EEG record (30 s epochs). Spectral power density for six frequency bands was obtained: delta (0.5–4.0 Hz), theta (4.0–8.0 Hz), alpha (9.0–12.0 Hz), sigma (12–14 Hz), beta (16.0–32.0 Hz), and gamma (>32 Hz). Although we restricted our current analysis to the theta frequency band in frontal leads only, all frequencies were obtained to determine relative theta power. Relative power in the theta band for right and left frontal leads for each 10 s epoch was calculated by dividing the absolute power in the theta band by the total power in the whole spectrum [(theta power/total power) × 100]. In order to standardize for the variable number of REM sleep periods among participants, the segments obtained from the first and late night REM sleep periods were used for the analysis. After determining the maximum number of continuous, artifact-free 10 s segments available per REM period per subject, we sampled, on average, 20, 10 s epochs per subject for the first REM period and 45, 10 s epochs for the last REM period.
Data analysis
Data analysis was conducted using SPSS 20 statistical software (IBM, Armonk, NY). Before running statistical tests, data were inspected for normality. For all measures in the analysis, an alpha level of 0.05 (two-tailed) was used. Continuous demographic and clinical variables and standard sleep statistics (e.g., sleep onset, sleep efficiency) were compared between the PTSD and resilient groups using independent t tests. Fisher’s exact test was used to examine the two groups for potential differences in gender distribution. To examine effects of group, hemisphere, and group by hemisphere interaction on theta power, we conducted a 2 (hemisphere) × 2 (group) mixed ANOVA separately for early and late REM periods. When we found a significant hemisphere × group interaction, we performed simple effects tests to examine group differences separately for left and right hemispheres.
Results
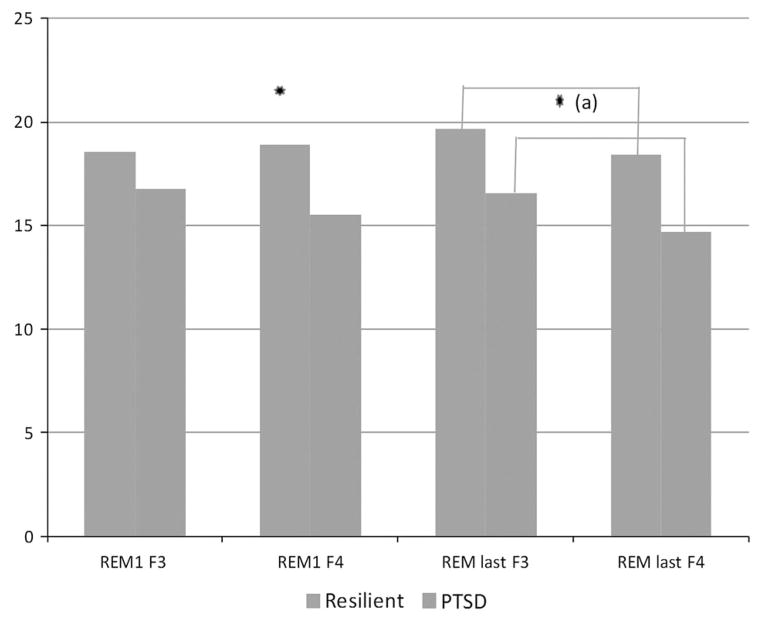
Table 1 shows demographic and clinical characteristics of participants. Age and gender representation were not significantly different between the groups. As expected, the PTSD group had significantly higher current and lifetime CAPS scores than the resilient group (current: (t = −8.664); (p < 0.001), lifetime: (t = −10.15); (p < 0.001). Trauma categorization is based on index trauma used in CAPS evaluation of PTSD status. Table 2 presents group comparisons of standard sleep measures evaluated by night two PSG recordings. Significant differences in sleep parameters between groups were not found, with the exception of REM latency which almost reached significance (p < 0.051). Included in Table 2 are the mean relative theta power for right frontal (F4) and left frontal (F3) electrodes during the first and last REM periods. Results from the mixed ANOVA for early REM indicated no main effects of hemisphere (F (1,28) = 1.60; p = 0.216) or group (F (1,28) = 3.41; p = 0.076), but a significant hemisphere by group interaction (F (1,28) = 4.90; p = 0.035). Simple effect tests showed a significant group difference in right hemisphere activity (p = 0.26) indicating greater relative theta power in the resilient group. No significant group difference was found in left hemisphere activity (p = 0.244). The ANOVA results for the last REM period showed a significant main effect of group, with higher theta power in the resilient group across hemispheres. Hemisphere by group interaction was not significant (F (1,28) = 0.118; p = 0.734). Main effect for hemisphere was significant (F (1,28) = 5.075; p = 0.032) indicating greater theta power in left hemisphere. Figure 1 shows average relative theta power for early and late REM periods.
Table 2.
Polysomnographic parameters and significant spectral findings by group
| Variable | Resilient n = 13 Mean (SD) |
PTSD n = 17 Mean (SD) |
t | p |
|---|---|---|---|---|
| Total sleep time | 391.9 (76.5) | 367.1 (70.2) | 0.92 | N.S. |
| Wake after sleep onset | 41.5 (93.6) | 39.4 (51.7) | 0.07 | N.S. |
| Sleep efficiency | 79.1 (27.9) | 88.4 (14.0) | 0.68 | N.S. |
| %Stage 1 | 1.3 (1.19) | 1.7 (0.85) | −0.86 | N.S. |
| %Stage 2 | 5.2 (5.9) | 5.8 (8.9) | −0.42 | N.S. |
| %Stage 3 | 22.6 (7.3) | 22.6 (8.2) | 0.01 | N.S. |
| %REM | 31.6 (30.1) | 22.5 (3.9) | 1.00 | N.S. |
| REM latency (min) | 59.8 (22.2) | 79.1 (27.9) | −2.10 | 0.051 |
| Simple effecta | ||||
| REM 1 relative theta (F3) | 18.6 (4.7) | 16.8 (3.4) | 0.244 | |
| REM 1 relative theta (F4) | 18.9 (4.2) | 15.5 (4.0) | 0.026* | |
| Group main effect | ||||
| REM last relative theta (F3) | 19.7 (4.4) | 16.6 (4.1) | F = 6.70 | p = 0.015* |
| REM last relative theta (F4) | 18.3 (3.4) | 14.7 (4.1) | ||
p < 0.05, REM = rapid eye movement, F3 = left frontal, F4 = right frontal
Simple effect test following a significant group × hemisphere interaction in 2 × 2 mixed ANOVA
Fig. 1.
Average relative theta power in frontal leads during REM sleep. F3 = right frontal; F4 = left frontal. (a) Significant main effect of group. Significance level: *p < 0.05
Discussion
The findings of our study support our hypothesis that resilient participants would have higher REM theta power than those with PTSD. This hypothesis and the finding are consistent with the idea that the emotional memory processing that takes place during REM sleep can be adaptive (Gujar et al. 2011; Walker and van der Helm 2009) and that such processing is indexed by right frontal theta activity (Nishida et al. 2009). That differences were found in right prefrontal areas are consistent with studies associating the processing of affective stimuli with right hemispheric frontal activation (Davidson 2002; Rauch et al. 1997).
During REM sleep in normal subjects, there is increased activity in areas which include the thalamic nuclei and limbic regions including the amygdala, areas shown to have altered activity during wake in PTSD (Shin et al. 2006; van der Helm et al. 2011). Therefore, it might be possible that reduced REM theta activity represents weaker communication between these structures. Our findings in frontal theta are also consistent with neuroimaging findings in PTSD that indicate altered activity in mPFC and limbic structures (Shin et al. 2006; Sterpenich et al. 2007).
Neural activity at theta frequencies has a well-documented role in models of learning and memory (Mitchell et al. 2008). Altered activity has recently been implicated in other disorders involving memory and affect. Within a group of patients with early Alzheimer’s disease, greater episodic memory retrieval was associated with increased theta power associated during slow wave sleep (SWS). As compared to controls, AD patients had significantly greater mean theta frequencies during REM, suggesting that sleep-specific changes to theta power may provide an important index of disease progression and severity (Hot et al. 2010). Prehn-Kristensen et al. (2013) found REM theta power positively correlated with performance on an emotional memory bias task in healthy subjects but negatively correlated with performance in children with attention-deficit hyperactivity disorder.
There is evidence for a role of REM sleep in the processing of emotional components of memory (Diekelmann et al. 2009; Hu et al. 2006; Nishida et al. 2009; Wagner et al. 2001), and therapeutic effects of such processing have been proposed (Walker and van der Helm 2009). Sterpenich et al. (2007) suggest that memories with negative emotional bias undergo stronger encoding via HC-amygdala circuitry and become potentiated in neocortical long-term memory networks in time-dependent manner. Effective emotional memory consolidation during REM sleep may serve an adaptive function by attenuating emotional reactivity. REM sleep has a unique biochemical environment, and it is speculated that amygdala activity in the absence of adrenergic tone and increased glucocorticoid levels may reduce emotional tone during REM sleep consolidation of the emotional aspects of memory (Rasch and Born 2013). In support of this hypothesis, results of a study by van der Helm et al. (2011) showed that REM sleep was associated with reduced amygdala reactivity to previously encountered emotional stimuli.
While some might argue that consolidation of negative emotional memories would result in persistence rather than amelioration of PTSD symptoms, our findings and those discussed above would support a role for REM sleep in adaptive habituation, extinction, or integration of traumatic memories. Indeed, in previous research of the senior author of this paper, fragmented patterns of REM sleep during the aftermath of trauma were associated with the development of PTSD (Mellman et al. 2002, 2007). Effective emotional processing during REM sleep may serve to integrate and ultimately modulate the affective components of memories into existing neural networks resulting in a reduction in reactivity. REM sleep disruption in PTSD may reduce the “therapeutic effect” of REM sleep (Walker and van der Helm 2009) and be a contributing factor in the development and persistence of trauma-related memories in PTSD.
Although the study provides new information to studies of sleep in PTSD, it has limitations. Our findings are limited by a relatively small sample size (30 participants) of young urban African Americans with varying trauma histories. Because the parent study was not originally designed to investigate the specific focus of this report, F-leads were not added to the PSG montage until later in the study resulting in the exclusion of 5 resilient and 3 PTSD participants. This study featured a non-clinical sample with symptom severity in the PTSD group in a mild to moderate range (Weathers et al. 2001). These participant characteristics may limit the generalizability of the findings. While results from our analysis show higher REM sleep theta power in resilient individuals, we have yet to assess whether resilient individuals have similar values to non-trauma-exposed controls. In this study, we limited our analysis exclusively to theta spectral power during REM sleep. Future studies and analyses should include central and occipital derivations in order to evaluate potential contributions and interactions of other REM and NREM frequencies in comparing neural activity during sleep in resilience and PTSD.
In summary, our findings support the possibility that right frontal theta activity during REM sleep distinguishes resilient individuals from those with PTSD, supporting the possibility suggested by the work of Nishida et al. (2009) that it is a marker of affective memory processing capacities. It will be of interest to evaluate this possibility prospectively and across the course of treatment in future studies.
Acknowledgments
This study was supported by Grant R01HL087995 to Dr. Mellman and NCATS Grant UL1RR031975. The authors thank the staff of the Georgetown-Howard Universities Center for Clinical and Translational Science and Joseph Lavela and Bryonna Wilson for their excellent technical assistance.
Contributor Information
Nancy Cowdin, Interdisciplinary Program in Neuroscience, Georgetown University, Washington, DC, USA.
Ihori Kobayashi, Department of Psychiatry, Howard University College of Medicine, 520 W Street, NW, Washington, DC 20059, USA.
Thomas A. Mellman, Email: tmellman@howard.edu, Department of Psychiatry, Howard University College of Medicine, 520 W Street, NW, Washington, DC 20059, USA
References
- Agaibi CE, Wilson JP. Trauma, PTSD and resilience. Trauma Violence Abuse. 2005;6:195–216. doi: 10.1177/1524838005277438. [DOI] [PubMed] [Google Scholar]
- Alim TN, Feder A, Graves RE, Wang Y, Weaver J, Westphal M, Alonso A, Aigbogun NU, Smith B, Doucette J, Mellman T, Lawson W, Charney DS. Trauma, resilience, and recovery in a high-risk African-American population. Am J Psychiatry. 2008;165:1566–1575. doi: 10.1176/appi.ajp.2008.07121939. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders DSM-IV-TR. 4. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Baran B, Pace-Schott EF, Ericson C, Spencer RM. Processing of emotional reactivity and emotional memory over sleep. J Neurosci. 2012;32:1035–1042. doi: 10.1523/JNEUROSCI.2532-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benca RM, Okawa M, Uchiyama M, Ozaki S, Nakajima T, Shibui K, et al. Sleep and mood disorders. Sleep Med Rev. 1997;1:45–56. doi: 10.1016/s1087-0792(97)90005-8. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Breslau N. The epidemiology of trauma, PTSD and other post-trauma disorders. Trauma Violence Abuse. 2009;10:198–210. doi: 10.1177/1524838009334448. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Kemp AH, Felmingham KL, Liddell B, Olivieri G, Peduto A, Gordon E, Williams LM. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Hum Brain Mapp. 2008;29:517–523. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JE, Shalev H, Admon R, Hefetz S, Gasho CJ, Shachar LF, Shelef I, Hendler T, Friedman A. Emotional brain rhythms and their impairment in post-traumatic patients. Hum Brain Mapp. 2013;34:1344–1356. doi: 10.1002/hbm.21516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: the role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51:61–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Wilhelm I, Born J. The whats and whens of sleep-dependent memory consolidation. Sleep Med Rev. 2009;13:309–321. doi: 10.1016/j.smrv.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edn. Biometrics Research; New York: 2002. [Google Scholar]
- Germain A, Buysse DJ, Nofzinger E. Sleep-specific mechanisms underlying posttraumatic stress disorder: integrative review and neurobiological hypothesis. Sleep Med Rev. 2008;12:185–195. doi: 10.1016/j.smrv.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groch S, Wilhelm I, Diekelmann S, Born J. The role of REM sleep in the processing of emotional memories: evidence from behavior and event-related potentials. Neurobiol Learn Mem. 2013;99:1–9. doi: 10.1016/j.nlm.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Gujar N, McDonald SA, Nishida M, Walker MP. A role for REM sleep in recalibrating the sensitivity of the human brain to specific emotions. Cereb Cortex. 2011;21:115–123. doi: 10.1093/cercor/bhq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord. 2012;2:9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde P, Jayakrishnan HR, Chattarji S, Kutty BM, Laxmi TR. Chronic stress-induced changes in REM sleep on theta oscillations in the rat hippocampus and amygdala. Brain Res. 2011;1382:155–164. doi: 10.1016/j.brainres.2011.01.055. [DOI] [PubMed] [Google Scholar]
- Hot P, Rauchs G, Bertran F, Denise P, Desgranges B, Clochon P, Eustache F. Changes in sleep theta rhythm are related to episodic memory impairment in early Alzheimer’s disease. Biol Psychol. 2010;87:334–339. doi: 10.1016/j.biopsycho.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Hu P, Stylos-Allen M, Walker MP. Sleep facilitates consolidation of emotional declarative memory. Psychol Sci. 2006;17:891–898. doi: 10.1111/j.1467-9280.2006.01799.x. [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, Quan ST. Rules, terminology and technical specifications. Am Acad Sleep Med; Westchester: 2007. The AASM manual for the scoring of sleep and associated events. [Google Scholar]
- Karashima A, Katayama N, Nakao M. Enhancement of synchronization between hippocampal and amygdala theta waves associated with pontine wave density. J Neurophysiol. 2010;103:2318–2325. doi: 10.1152/jn.00551.2009. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry. 2000;6l:4–14. [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB, Breslau N. Epidemiological risk factors for trauma and PTSD: risk factors for posttraumatic stress disorder. Am Psychiatr Assoc. 1999;21:23–59. [Google Scholar]
- Kobayshi I, Boarts J, Delahanty D. Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology. 2007;44:660–669. doi: 10.1111/j.1469-8986.2007.537.x. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159:1696–1701. doi: 10.1176/appi.ajp.159.10.1696. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Pigeon WR, Nowell PD, Nolan B. Relationships between REM sleep findings and PTSD symptoms during the early aftermath of trauma. J Trauma Stress. 2007;20:893–901. doi: 10.1002/jts.20246. [DOI] [PubMed] [Google Scholar]
- Merica H, Blois R. Relationship between the time courses of power in the frequency bands of human sleep EEG. Neurophysiol Clin. 1997;27:116–128. doi: 10.1016/S0987-7053(97)85664-X. [DOI] [PubMed] [Google Scholar]
- Mitchell DJ, McNaughton N, Flanagan D, Kirk IJ. Frontal med-line theta from the perspective of hippocampal theta. Prog Neurobiol. 2008;86:156–185. doi: 10.1016/j.pneurobio.2008.09.005. [DOI] [PubMed] [Google Scholar]
- New A, Fan J, Murrough J, Liu X, Liebman R, Guise K, Tang C, Charney D. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biol Psychiatry. 2009;66:656–664. doi: 10.1016/j.biopsych.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb Cortex. 2009;19:1158–1166. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa D, Duvarci S, Popescu AT, Lena CM, Pare D. Coherent amygdalocortical theta promotes fear memory consolidation during paradoxical sleep. Proc Natl Acad Sci. 2010;107:6516–6519. doi: 10.1073/pnas.0913016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehn-Kristensen A, Munz M, Molzow I, Wilhelm I, Wiesner CD, Baving L. Sleep promotes consolidation of emotional memory in healthy children but not in children with attention-deficit hyperactivity disorder. Proc Natl Acad Sci. 2013;8:1–10. doi: 10.1371/journal.pone.0065098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B, Born J. About sleep’s role in memory. Physiol Rev. 2013;93:681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Savage CR, Alpert MN, Fischman AJ, Jenike MA. The functional neuroanatomy of anxiety: a study of three disorders using positron emission tomography and symptom provocation. Biol Psychiatry. 1997;42:444–452. doi: 10.1016/S0006-3223(97)00145-5. [DOI] [PubMed] [Google Scholar]
- Ross RJ, Ball WA, Dinges DF, Kribbs NB, Morrison AR, Silver SM, Mulvaney FD. Rapid eye movement sleep in posttraumatic stress disorder. Biol Psychiatry. 1994;35:195–202. doi: 10.1016/0006-3223(94)91152-5. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Sterpenich V, Albouy G, Boly M, Vandewalle G, Darsaud A, Balteau E, Dang-Vu TT, Desseilles M, D’Argenbeau A, Gais S, Rauchs G, Schabus M, Degueldre C, Luxen A, Collette F, Maquet P. Sleep related hippocampocortical interplay during emotional memory recollection. PLoS Biol. 2007;5:e282. doi: 10.1371/journal.pbio.0050282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Helm E, Yao J, Dutt S, Rao V, Saletin J, Walker M. REM sleep depotentiates amygdala to previous emotional experiences. Curr Biol. 2011;21:2029–2032. doi: 10.1016/j.cub.2011.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem. 2001;8:112–119. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M. The role of sleep in cognition and emotion. Ann N Y Acad Sci. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- Walker MP, van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009;135:731–748. doi: 10.1037/a0016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13:132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 2010;11:589–599. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Flory JD. Differentiating biological correlates of risk, PTSD, and resilience following trauma exposure. J Trauma Stress. 2007;20:435–447. doi: 10.1002/jts.20260. [DOI] [PubMed] [Google Scholar]