Abstract
Mesenchymal stromal cells (MSC) exert either tumor-stimulatory or tumor-inhibitory effect. The outcome of the tumor-MSC interaction is dictated by the tumor-specific activating signals. We analyzed the alterations in MSC phenotype in response to stimulation by tumor-secreted paracrine factors. Paracrine factors from human melanoma A375 and glioblastoma 8MGBA cells were used for prolonged culture of MSC to produce derived cells designated DIFF(A)-MSC or DIFF(G)-MSC, respectively. Derived cells were analyzed for the specific surface markers, the expression pattern of MSC markers and fibroblast-specific proteins. Changes in the cell phenotype were evaluated using scratch wound assay and tube formation in vitro; and xenotransplant growth in vivo. Our data show induced expression of vascular endothelial growth factor 2, CD146, fibroblast-specific protein, vimentin and endosialin in DIFF(A)-MSC cells. This indicates their differentiation towards the cells with features of tumor-associated fibroblasts upon stimulation with melanoma-secreted cytokines. Paracrine stimulation in DIFF(G)-MSC led to up-regulation of the genes involved in the MSC differentiation. MSC-specific surface marker characteristics were preserved in derived DIFF(A)-MSC and DIFF(G)-MSC cells. However, we observed increased proportion of CD146 and GD2 (neural ganglioside) positive cells and decreased expression of marker NG2 in the MSC exposed to tumor-conditioned medium. Melanoma-CM increased MSC migration, glioblastoma-CM compromised angiogenic capacity of MSC in vitro and the protumorigenic effect in vivo. Our data directly compare the pleiotropic effects mediated by the malignant cells on the MSC. Secreted paracrine factors from melanoma or glioblastoma differently changed molecular traits in MSC, which explains the dual role of MSC in tumor growth.
Electronic supplementary material
The online version of this article (doi:10.1007/s12307-014-0151-9) contains supplementary material, which is available to authorized users.
Keywords: Human mesenchymal stromal cells, Differentiation, Melanoma, Glioblastoma, Tumor-associated fibroblasts
Introduction
Stromal compartment of a solid tumor is composed of immune cells, cells contributing to tumor vasculature and cells of mesenchymal origin. They preexist in the tissue before the tumor development; however, they are also recruited to the tumor microenvironment by tumor cell-originating signals [1, 2]. These cells are considered to be important drivers of the tumor development. It has been recognized that bone-marrow derived progenitor cells and/or mesenchymal stromal cells (MSC) of various origin can home and engraft into the developing tumor [3, 4]. The MSC represent a heterogeneous population of precursor cells with considerable plasticity [5]. They can be found in situ within all mammalian stromal tissue compartments. Bone marrow, adipose-tissue and the umbilical cord remain most commonly used tissue sources of the MSC for therapeutic purposes in regenerative medicine. Based on the surface marker expression, MSC are defined as CD73+, CD90+, CD105+, CD11b−, CD14−, CD19−, CD45− and HLA-DR− cells. MSC retain their multipotency upon culture expansion and proliferate as adherent cells with typical fibroblast-like morphology [6].
MSC and bone-marrow derived progenitor cells may acquire a phenotype of tumor-associated fibroblasts (TAF) [7, 8]. TAF (synonymously also termed cancer-associated fibroblasts, myofibroblasts or activated fibroblasts) represent cells with increased proliferation activity, enhanced secretion of extracellular-matrix proteins and specific expression of several markers: vimentin, α-SMA (α-smooth muscle actin), FSP1 (fibroblast specific protein 1), FAP (fibroblast-activation protein) and others. Several studies have demonstrated that MSC exposed to tumor-cell secreted factors expressed high levels of TAF markers [9, 10]. Activated fibroblasts and tumor-incorporated MSC communicate with tumor cells, resident epithelial cells, endothelial cells, pericytes and inflammatory cells through complex cytokine and chemokine network [11]. Accumulating experimental evidence suggested that paracrine factors secreted from the tumor cells shift MSC into activated state, thereby promoting further tumor development or tumor inhibition [12, 13]. The mechanisms responsible for the dual role of MSC in tumor development still remain a subject of investigation. Our experimental data suggested the role of VEGF- signaling and SDF-1α/CXCR4 axis in the MSC-mediated human melanoma tumor growth support [14]. MSC were also shown to become a part of the tumor microenvironment and serve as precursors for tumor-associated pericytes, endothelial cells, tumor-associated fibroblasts or even a cancer stem cell supporting cells in their niche [15]. Accumulating evidence for the antitumor effect of MSC was recently comprehensively reviewed and discussed [13, 3, 16]. Our experiments have also demonstrated the antitumor effect of MSC on human glioblastoma tumor growth [14]. Considerable variation among the studies and their experimental setup limits their direct comparison; however some common features of the tumor-inhibiting MSC type 1 and tumor-promoting MSC type 2 were suggested [13].
In the present study we focused on the molecular changes induced in the MSC by tumor-secreted soluble factors. The MSC exposed to tumor-cell conditioned medium (TCM) from the human melanoma A375 were designated (DIFF(A)-MSC). Similarly, MSC exposed to glioblastoma 8MGBA-CM were designated (DIFF(G)-MSC). Tumor cell lines were selected based on their opposing response to MSC both in vitro and in vivo [14]. We identified a specific response pattern dictated by tumor paracrine factors in the MSC. Melanoma-secreted factors induced expression of markers specific for tumor-associated fibroblasts in derived DIFF(A)-MSC. On the contrary, glioblastoma-secreted paracrine factors induced upregulation of differentiation markers in derived DIFF(G)-MSC. Taken together, we describe here that the TAF-like differentiation was present along with the tumor-promoting effect of MSC and a failure to drive TAF-like MSC differentiation was accompanied by the antitumor effect of MSC.
Material and Methods
Cells
Human glioblastoma multiforme cell line 8MGBA (kindly provided by Dr. Perzelova, Faculty of Medicine, Comenius University, Bratislava) and human melanoma cell line A375 (ECACC No. 88113005) were cultured in Dulbecco’s modified Eagle medium (PAA Laboratories, GmbH, Pasching, Austria) supplemented with 10 % fetal bovine serum (GIBCO® Invitrogen, Carlsbad, CA). Human adipose-tissue-derived mesenchymal stem cells were isolated by collagenase digestion as established previously [17]. MSC were cultured in low-glucose Dulbecco’s modified Eagle medium (GIBCO® Invitrogen) supplemented with HyClone Advance Mesenchymal Stem Cell Growth Supplement (Thermo Scientific, Waltham, MA) and Antibiotic-Antimycotic mix (GIBCO® Invitrogen). HUVEC cells were purchased and maintained in Low Serum Growth Supplement (LSGS) diluted in Medium 200PRF as recommended by the supplier (GIBCO® Invitrogen). Cells were grown at 37 °C in 5 % CO2 humidified atmosphere.
Tumor conditioned medium (TCM) was collected from 80 to 90 % confluent A375 or 8MGBA cell cultures maintained for 24 h in MSC medium, centrifuged and supernatant was passed through 0.45 μm membrane filter. Fresh TCM was changed every third day for the entire 30-day MSC culture. MSC were passaged when cultures reached 85–90 % confluence every 5 to 7 days. The MSC exposed to A375-TCM were labeled DIFF(A)-MSC. The MSC exposed to 8MGBA-TCM were labeled DIFF(G)-MSC. Untreated MSC culture-expanded in MSC medium were used as a control and were labeled PAR-MSC. Each experiment was repeated with three different MSC isolates.
Multiplex Cytokine Secretion Analysis
Tumor cells (5 × 104) PAR-MSC, DIFF(A)-MSC and DIFF(G)-MSC (2.5 × 104) were plated on 24-well plates and cultured in 2 ml of complete culture medium for 2 days. Cell-free supernatants were collected and subjected to human Bio-PlexTM 27-plex Cytokine Assay (Bio-Rad Laboratories Inc, Hercules, CA). Measurements were performed on Luminex 100 System (Luminex Corporation, Austin, TX) in duplicates. Results were expressed as mean pg/ml of culture medium ± SD.
ELISA
SDF-1α level in cell-free supernatants was determined by Quantikine Immunoassay (R&D Systems Inc., Minneapolis, MN) on xMark™ Microplate Spectrophotometer (BIO-RAD, Hercules, CA). TGFβ levels were detected by human TGFβ1 ELISA Ready-SET-Go 2nd generation (eBioscience Inc., San Diego, CA) and evaluated as above.
Analysis of the Gene Expression
Total RNA was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany) and DNase treated following the manufacturer’s protocol. cDNA was obtained using RevertAid™ H Minus First Strand cDNA Synthesis Kit (Fermentas GmbH, St. Leon-Rot, Germany). Primers (see Table 1 for the primer sequences, amplicon size and annealing temperature) were designed using NCBI Primer blast. At least one primer of a primer set was designed to span exon-exon junction to limit the amplification to mRNA only (with the exception of intron-less endosialin). The PCR reaction mixture (25 μl) contained 1 μl of cDNA, 80 nM of forward and reverse primers and 12.5 μl of ABsolute™ QPCR SYBR® Green Mix (Thermo Scientific). Quantitative PCR was performed on CFX96™ Real-Time PCR Detection System (BIO-RAD) using the following protocol: activation step at 95 °C for 15 min, 40 cycles of denaturation at 95 °C for 15 s, 15 s annealing, polymerization for 15 s at 72 °C and plate read for 5 s at 77 °C followed by final extension for 5 min at 72 °C and melt curve analysis. The size of PCR products was verified by agarose gel electrophoresis. Relative gene expression change was calculated according to the formula ΔΔCt method. Analysis was performed twice in triplicates and data are expressed as means ± SD.
Table 1.
Sequences of primers used for quantitative PCR
| Gene | Amplicon size [bp] | Primer sequence | TA [°C] |
|---|---|---|---|
| Vimentin | 163 bp | FOR: 5- CAGGGCGCGTCCTCTGCC-3 REV: 5- TGGACATGGCTGCGGAGGGT-3 |
51 |
| FAP | 169 bp | FOR: 5- GGTGGATGGTCGAGGAACAGC-3 REV: 5- TCCTCCATAGGACCAGCCCCA-3 |
60 |
| Endosialin | 170 bp | FOR: 5- CGCAGTTGCGAGGACCCCTG-3 REV: 5- ATCTGCTGGCACACACCGGC-3 |
57 |
| α-SMA | 136 bp | FOR: 5- GCACCCCTGAACCCCAAGGC-3 REV: 5- GCACGATGCCAGTTGTGCGT-3 |
57 |
For the expression array, total RNA from the MSC, DIFF(A)-MSC and DIFF(G)-MSC cells was isolated by the Agilent Total RNA isolation Mini kit (Agilent Technologies, Santa Clara CA), reverse transcribed with RT2 First strand kit (SABiosciences-QIAGEN, Frederik, MD) and mixed with RT2 SYBR® Green qPCR Mastermix (SABiosciences). MSC specific expression was analyzed by RT2 Profiler Mesenchymal Stem Cell PCR Array PAHS-082 (SABiosciences) and evaluated by an Excel- based PCR Array Data Analysis Software (SABiosciences).
MSC Differentiation Assay
Differentiation capabilities of the AT-MSCs were examined by the previously established protocols. Briefly, adipogenic differentiation was performed in the differentiation media composed of αMEM medium supplemented with 15 % FBS, 0.5 μM hydrocortisone, 0.5 mM isobutylmethylxanthine, and 60 μM indomethacin for 21 days. Cells were washed with PBS, fixed in 4 % formalin for 1 h, and stained for 15 min with fresh Oil Red O solution. Osteogenic differentiation was performed in osteogenic medium using Osteogenic stem cell kit (StemCell Technologies, Vancouver, BC, Canada) as recommended by the manufacturer. Medium αMEM was supplemented with 15 % human osteogenic stimulatory supplements, 10−8 M dexamethasone, 0.2 mM ascorbic acid, 10 mM β-glycerolphosphate. Medium was replaced every 3–4 days for 14 days. Cultures were washed with PBS, fixed in 4 % formalin for 1 h, and stained for 10 min with 1 ml of 40 mM Alizarin red (pH 4.3). Osteogenic differentiation was confirmed by detection of red stained calcium deposits. Chemicals were purchased from Sigma-Aldrich Corporation, St. Louis, MO, if not stated otherwise.
Cell Morphology
Morphological appearance was determined in PAR-MSC, DIFF(A)-MSC and DIFF(G)-MSC by the IncuCyte ZOOM™ Kinetic Imaging System (Essen BioScience, Welwyn Garden City, UK). Cells (2 × 104/well) were seeded on 24-well plates, let to adhere and proliferate. Morphological features were compared 72 h later on the cultures with 55–60 % cell confluence as determined by IncuCyte ZOOM™ 2013A software (Essen BioScience) based on the MSC specific confluence mask.
Wound Healing Assay
Thirty-five thousand MSC per well were plated in triplicates on ImageLock 96-well plates (Essen BioScience) and let to adhere for 16–24 h. Confluent monolayers were wounded with wound making tool (Essen BioScience), washed twice and supplemented with fresh TCM or culture medium. As indicated, the MSC culture medium was supplemented with 10 ng/ml IL-6, 50 ng/ml IL-8 and/or 10 ng/ml IP-10 (eBioscience, San Diego, CA). Images were taken every 2 h for the next 48 h in the IncuCyte ZOOM™ Kinetic Imaging System. Cell migration was evaluated by IncuCyte ZOOM™ 2013A software based on the relative wound density measurements and expressed as means of three independent experiments run in triplicates ± SD.
Flow Cytometry
Following antibodies were used for the detection of surface markers: human MSC Phenotyping Kit (Miltenyi Biotec, Bergisch Gladbach, Germany), CD31-PE, CD34-FITC, CD44-PE, BCRPI-PE, HLA-DR-FITC (Chemicon, Temecula, CA), CD146-APC, CD166-PE (Beckmann Coulter, Prague, Czech Republic), CD133 (Miltenyi Biotec), GD2-PE (BD PharmingenTM, San Diego, CA), CD144-PE and NG2-PE (eBioscience, San Diego, CA). Dead cells were excluded based on DAPI staining. Cells were analyzed using BD Canto II cytometer (Beckton Dickinson, Franklin Lakes, NJ) equipped with FACSDiva program. FCS Express software was used for the evaluation.
Immunofluorescence Analysis
Cells were plated on sterilized coverslips in 6-well plates and let to adhere for 24 h before fixation. Samples were fixed in ice-cold methanol for 10 min at −20 °C, washed with PBS, blocked with 10 % goat serum or 5 % milk in TBS-T for 1 h and incubated with primary monoclonal mouse anti-human antibodies diluted in blocking buffer overnight at 4 °C. Cells were immunostained for fibroblast specific protein (FSP, 1:100 in 10 % serum, Abcam, Cambridge, MA), vimentin (1:50 in 10 % serum) and α-smooth muscle actin (α-SMA, 1:50 in 5 % milk, both Santa Cruz Biotechnology, Santa Cruz, CA). Secondary polyclonal goat anti-mouse antibodies used were TexasRed (Santa Cruz) for α-SMA (diluted 1:400) and Cy5 (Millipore, Billerica, MA) for FSP and vimentin (diluted 1:200). Following further washes, the cells were embedded in Vecta Shield mounting medium with DAPI (Vector Laboratories, Burlingame, CA). Images were acquired using Zeiss LSM 510 Meta confocal microscope (Carl Zeiss Microscopy GmbH, Göttingen, Germany) and analysed with ImageJ software package using same settings for contrast and brightness adjustment for all images.
In Vitro Angiogenesis Assay
ECMatrixTM gel solution (Millipore) was thawed on ice at 4 °C overnight and diluted with 110 μl of Diluent Buffer. Fifty μl per well was dispensed in 96-well plates using ice-cooled tips and incubated for 1 h at 37 °C. Two ×104 HUVEC cells, 1 × 104 PAR-MSC, DIFF(A)-MSC or DIFF(G)-MSC per well were applied onto matrix layer in 150 μl of low-glucose DMEM supplemented with 1× Low Serum Growth Supplement (GIBCO® Invitrogen) and 50 ng/ml human VEGF (Stem Cell Technologies, Vancouver, BC, Canada). Cells were incubated at 37 °C in a 5 % CO2 humidified atmosphere. HUVEC cells served as a positive control and angiogenesis progression was monitored using phase-contrast images taken every 3 h by the IncuCyte ZOOM™ Kinetic Imaging System.
Xenotransplant Growth In Vivo
Six to eight weeks old athymic nude mice (Balb/c nu/nu) were used in accordance with the institutional guidelines under an approved protocol. Tumor xenotransplants were induced with 5 × 105 A375 cells alone or coinjected with 1 × 105 PAR-MSC, DIFF(A)-MSC or DIFF(G)-MSC s.c. Animals (n = 6 per group) were monitored for the tumor growth. Tumor volume was calculated from caliper measurements of two perpendicular diameters and expressed as mean volume ± SD.
Statistical Analysis
Data involving comparison between the two groups were analyzed by an unpaired Student’s t-test in GraphPad Prism® software (LA Jolla, CA). The value of p < 0.05 was considered statistically significant.
Results
Cytokine Production and Expression Profile of Tumor Cells
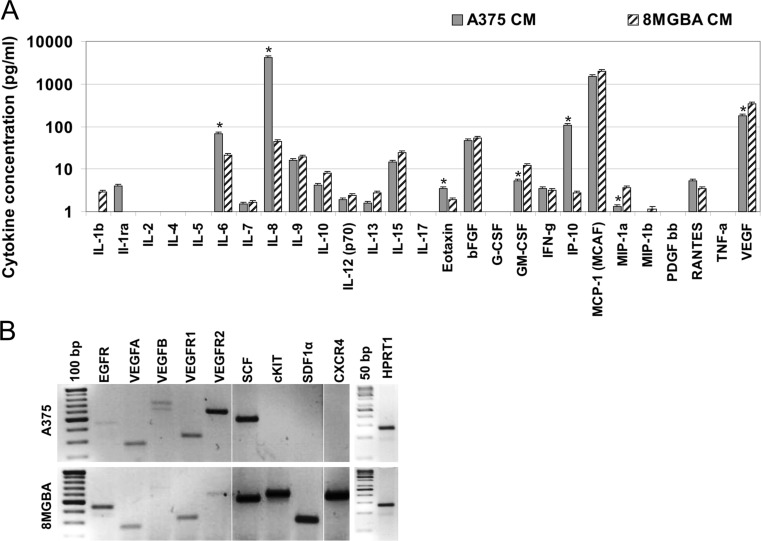
In our previous work we identified multiple effects of the MSC on tumor cell biology [18, 14, 2, 19]. However, the communication between the tumor cells and MSC is bidirectional. Thus, we decided to analyze the alterations induced in the MSC in response to tumor cell stimulation. Tumor cell lines were chosen based on their opposite response to the MSC stimulation in vitro and in vivo. We hypothesized that it was a consequence of tumor paracrine stimulation leading to the molecular changes in MSC. Both melanoma A375 and glioblastoma 8MGBA secrete multiple cytokines and chemokines into the conditioned medium (Fig. 1a). We detected significantly higher concentration of IL-6, IL-8, eotaxin-3 (CCL26) and IP-10 (CXCL10) produced by melanoma cells, as determined by multiplex cytokine assay. Glioblastoma cells released higher concentrations of GM-CSF, MIP-1a and VEGF (Fig. 1a). These tumor cell lines also express different subset of ligands and their cognate receptors as demonstrated by the reverse transcription-coupled PCR (Fig. 1b). Melanoma A375 express high levels of SCF and VEGFR2 and lack the expression of cKIT, SDF1α and CXCR4. Glioblastoma 8MGBA cells express both the ligand and cognate receptor of the SCF/cKit and SDF1α/CXCR4 signaling axes.
Fig. 1.
Differences in the secretome and the expression pattern of the tumor cells. a Cell-free melanoma A375 and glioblastoma 8MGBA cell conditioned media (CM) were collected after 48 h and subjected to multiplex analysis with Bio-PlexTM 27-plex Cytokine Assay. Significantly higher levels of IL-6, IL-8, eotaxin-3 and IP-10 were detected in the melanoma CM. Significantly lower concentrations of GM-CSF, MIP-1a and VEGF were also detected, *p < 0.05. b RNA was isolated from the tumor cells, reverse transcribed and PCR amplified to detect the gene expression. Glioblastoma 8MGBA cells express SDF1α, cKit and CXCR4 in contrast to melanoma A375 cells. HPRT1 was used as a loading control
MSC Specific Surface Markers and Their Expression Pattern in Response to TCM
In order to analyze the tumor-induced alterations, MSC were cultivated in the tumor-conditioned cell-free medium for 30 days. Melanoma A375-CM-exposed MSC were designated DIFF(A)-MSC and glioblastoma 8MGBA-CM-exposed MSC were labeled DIFF(G)-MSC. Untreated MSC from the same isolate (PAR-MSC) were cultured and passaged along with the TCM-exposed cells in order to compare the MSC at the same passage. Prolonged MSC culture in TCM did not significantly alter proliferation capacity or morphology in the MSC (Fig. 2a). Population doubling time was determined to be 56–58 h for PAR-MSC, DIFF(A)-MSC and DIFF(G)-MSC. TCM-exposed MSC retained their bipolar fibroblastoid morphology in both types of the tumor-conditioned media. Expression analysis confirmed the persistence of MSC specific markers in the TCM-exposed cells with significantly increased expression of CD146 and the adhesion molecule VCAM1 (Fig. 2b). Neither parental PAR-MSC, nor TCM-exposed MSC expressed FZD9, CD271 or CD133. PAR-MSC and TCM-exposed MSC were positive for CD44, CD73, CD90, CD105, CD146 and CD166 surface markers (Table 2). PAR-MSC, DIFF(A)-MSC and DIFF(G)-MSC were negative (<1 %) for CD14, CD20, CD34 (passages >3), CD45, CD19, CD31, CD144 (VE-cadherin), CD133, BCRP1 and HLA-DR (data not shown). Qualitative expression analysis unraveled that melanoma-TCM induced VEGFR2 expression in DIFF(A)-MSC (Fig. 2c). Moreover, higher proportion of CD146 positive cells was detected in TCM-exposed cells (Fig. 2d and Table 2). Our data further demonstrated that 46.7 % of the parental MSC were positive for neural ganglioside GD2, recently suggested to be another MSC-characteristic surface marker [20]. TCM exposure decreased the proportion of the GD2+ positive cells to 23.0 % in DIFF(A)-MSC and 31.4 % in DIFF(G)-MSC. TCM-exposed MSC did not retain the expression of proteoglycan NG2, a pericyte marker (Fig. 2d). Taken together, our data demonstrate multiple alterations induced in the MSC upon exposure to the paracrine factors secreted from the tumor cells. In addition, based on the absence of CD31, VE-cadherin (CD144) and the loss of NG2, we excluded transdifferentiation into endothelial or pericytic cells induced by TCM.
Fig. 2.
The TCM increases VEGFR2 expression in DIFF(A)-MSC, and VCAM1 and CD146 expression in the TCM-exposed MSC. a MSC morphology was not affected in the TCM-exposed MSC. Phase contrast images were taken from cultures 72 h after plating by the Incucyte ™ live-cell imaging system. Scale bar 300 μm. b Total RNA was isolated from the PAR-MSC, DIFF(A)-MSC and DIFF(G)-MSC to analyze the expression of MSC specific markers. RT2 Profiler Mesenchymal Stem Cell PCR Array has shown a significant increase of the CD146 and VCAM1 expression in the TCM-exposed MSC,*p ≤ 0.05, #p < 0.001. c Reverse transcription coupled-PCR analysis of the same samples unraveled the induction of the VEGFR2 expression in the melanoma-CM exposed DIFF(A)-MSC cells. (ACTB - β-actin was used as a loading control). d The PAR-MSC, DIFF(A)-MSC and DIFF(G)-MSC were stained with specific anti-human GD2-PE, CD146-APC or NG2-PE antibodies and analyzed by flow cytometry. The MSC were found to be positive for the neural ganglioside GD2 with median of 46.7 % GD2-positive cells in the PAR-MSC (min.36.7 %–max.49.9 %), 23.0 % positive cells in the DIFF(A)-MSC (min.21.8 %–max.40.7 %) and 31.4 % positive cells in the DIFF(G)-MSC (min.25.2 %–max.40.6 %). Human fibroblasts were negative for the GD2 neural ganglioside as expected (data not shown). MSC were also positive for the CD146 – median value for the PAR-MSC was 51.0 % positive cells and TCM increased CD146 positivity to 66.7 % CD146+ cells in the DIFF(A)-MSC and 63.5 % CD146+ cells in the DIFF(G)-MSC. Parental MSC exhibited 90.2 % positivity for NG2, but TCM exposure decreased the positivity to 4.1 % NG2+ cells in the DIFF(A)-MSC and 6.8 % NG2+ cells in the DIFF(G)-MSC. (Red line in the histogram shows the specific staining, black line stands for the isotype control)
Table 2.
Immunophenotype of TCM exposed MSC
| PAR-MSC | DIFF(A)-MSC | DIFF(G)-MSC | ||||
|---|---|---|---|---|---|---|
| CD44 | 92.7 % | (90.6–94.6) | 89.9 % | (87.4–92.3) | 87.9 % | (78.6–97.1) |
| CD73 | 99.4 % | (96.5–99.9) | 99.0 % | (98.2–99.6) | 98.6 % | (97.9–99.2) |
| CD90 | 94.7 % | (86.0–98.7) | 85.9 % | (67.9–88.3) | 92.5 % | (88.1–97.7) |
| CD105 | 98.9 % | (93.1–99.5) | 91.2 % | (83.8–98.4) | 95.9 % | (95.0–96.7) |
| CD146 | 51.0 % | (43.2–85.5) | 66.7 % | (47.5–82.4) | 63.5 % | (60.5–79.0) |
| CD166 | 99.3 % | (99.0–99.5) | 98.9 % | (98.7–99.4) | 99.5 % | (97.1–99.8) |
The proportions of positive cells are expressed as median (minimum–maximum)
Alterations in MSC Differentiation Markers and Cytokine Secretion Pattern in Response to TCM
The expression of several differentiation markers such as Runx2, PparG, RhoA, Jag1 and Smad4 was significantly higher in glioblastoma CM-exposed DIFF(G)-MSC (Fig. 3a). The upregulation of these factors did not substantially alter the capability of the MSC to differentiate into adipogenic or osteogenic lineage upon specific stimulation (Fig. 3b). However, the DIFF(G)-MSC exhibited decreased secretion of several cytokines including IL-6, IL-8 and G-CSF in comparison to the parental PAR-MSC (Fig. 3c). DIFF(A)-MSC retained secretion of proinflammatory IL-6 and IL-8 and exhibited significantly higher levels of IL-9, IL-12, IFNγ and VEGF. TCM exposure increased MCP-1 and SDF1α secretion in both MSC types and did not alter TGFβ level secreted from the TCM-exposed MSC (Fig. 3c–d). To conclude, melanoma TCM induced VCAM1 expression, upregulated VEGFR2, CD146 and supported a secretion of proinflammatory stimuli from the DIFF(A)-MSC. Glioblastoma-TCM exposed DIFF(G)-MSC increased the expression of several differentiation markers along with the decrease in secreted proinflammatory factors IL-6, IL-8, IL-12 and G-CSF. These results indicate that TCM-exposed MSC have altered multiple molecular traits in response to tumor paracrine factor stimulation.
Fig. 3.
TCM alters the expression and secretome profile in the MSC. a Mesenchymal Stem Cell PCR Array for the MSC differentiation markers identified upregulation of Runx2, PPARγ, RhoA and Jag1 in glioblastoma DIFF(G)-MSC, *p < 0.05. b The PAR-MSC, DIFF(A)-MSC and DIFF(G)-MSC were cultured in specific differentiation media and stained for calcium deposits as an indicator for osteogenesis and oil droplets for the adipogenic differentiation. There was no significant difference in the differentiation capabilities of the untreated and TCM-exposed MSC. Scale bar 300 μm. c Cell-free medium from the PAR-MSC, DIFF(A)-MSC and DIFF(G)-MSC was collected after 48 h and subjected to a multiplex analysis with Bio-PlexTM 27-plex Cytokine Assay. Significantly higher levels of IL-9, IL-12, IFNγ, MCP-1 and VEGF were detected in the DIFF(A)-MSC in comparison to the parental cells PAR-MSC. Significantly higher levels of IL-12 and MCP-1 and lower concentrations of IL-6, IL-8, G-CSF and TNFα were detected in the DIFF(G)-MSC in comparison to the parental cells PAR-MSC, *p < 0.05. d Secretion of SDF1α and TGFβ was determined in the same media collected as above by the standard ELISA assays. The MSC produce both SDF1α and TGFβ and TCM-exposure increased the level of SDF1α significantly, *p < 0.05
Markers of the Tumor Associated Fibroblasts on TCM-exposed MSC
Previously published data indicated the capability of TCM to induce a phenotype of tumor-associated fibroblasts (TAF) in the MSC [7, 8]. To confirm this, we analyzed the TCM-exposed MSC for TAF markers using quantitative PCR and immunofluorescent microscopy. The DIFF(A)-MSC had significantly higher expression of FAP, vimentin (fibroblast-specific markers) and endosialin (tumor-vasculature specific marker, Fig. 4a). The DIFF(G)-MSC also expressed increased levels of FAP in comparison to the PAR-MSC, but they downregulated endosialin and vimentin. Baseline expression of myofibroblast marker αSMA was decreased in the DIFF(A)-MSC. Immunofluorescence analysis confirmed the up-regulated expression of the FSP and vimentin in the DIFF(A)-MSC and no substantial increase in the α-SMA (Fig. 4b). Taken together, the melanoma secreted factors up-regulated markers of tumor-associated fibroblasts in the TCM-exposed MSC such as FAP, FSP and vimentin.
Fig. 4.
Melanoma TCM up-regulates the markers of tumor-associated fibroblasts in the DIFF(A)-MSC and stimulates migration of the MSC. a Total RNA was isolated from the PAR-MSC, DIFF(A)-MSC and DIFF(G)-MSC, reverse transcribed and subjected to quantitative PCR. The data show a significant up-regulation of FAP, vimentin and endosialin in the melanoma-CM exposed DIFF(A)-MSC in comparison to the DIFF(G)-MSC and PAR-MSC, *p < 0.05. b The PAR-MSC, DIFF(A)-MSC and DIFF(G)-MSC were immunostained for FSP, vimentin and αSMA with specific primary antibodies. High levels of FSP and substantial increase in the vimentin was confirmed in the DIFF(A)-MSC. c To examine the migratory properties in a wound healing assay, the MSC were plated on the ImageLock ™ 96-well plates, confluent monolayers were wounded 18 h later and cell migration was monitored by Incucyte ZOOM ™ platform for 40 h. A375-CM significantly increased the migration of the MSC as determined by higher relative wound density, *p < 0.05. d The MSC were plated as in c. Cell monolayers were wounded 24 h later and MSC culture medium was supplemented with 10 ng/ml IL-6, 50 ng/ml IL-8 or 10 ng/ml IP-10 alone or the three of them in combination. Comparison of the average relative wound density indicated significantly increased MSC migration in the combined presence of IL-6, IL-8 and IP-10, *p < 0.05. e Representative images taken 18 h after monolayer wounding exhibit higher cell confluence in the wounded area when the cells were stimulated with cytokines in combination. Black lines indicate the initial scratch wound border, scale bar 300 μm
Next, we investigated functional alterations in the TCM-exposed MSC. Conditioned medium from melanoma cells significantly increased the migration of the MSC as demonstrated in the wound healing assay in vitro (Fig. 4c). Melanoma TCM contained significantly higher levels of IL-6, IL-8 and IP-10 that could have stimulated the MSC migration. To examine this possibility, we tested the MSC migration in the MSC culture medium supplemented with each of these molecules alone or in combination. We were able to show that the combination of IL-6, IL-8 and IP-10 was able to significantly increase MSC migration by 10 % (Fig. 4d–e). Even when these cytokines was added to the A375-CM or 8MGBA-CM, the migration of the MSC increased by 12–34 % in comparison to the TCM without cytokine supplementation confirming their contribution to the increased MSC migration (data not shown).
The angiogenic potential in the MSC was examined by the tube formation assay in vitro. The DIFF(A)-MSC exhibited high branching activity and formation of many polygonal structures on matrigel layer similar to the PAR-MSC. Contrastingly, tube formation capacity of the DIFF(G)-MSC was lower with absence of polygonal alignment (Fig. 5a). Our analysis excluded any increase in the MSC senescence during the 30 day TCM exposure (data not shown), consistent with the preservation of the proliferation potential and morphology in the TCM-exposed MSC. Finally, the tumor growth support in vivo was examined in the TCM-exposed MSC. The Fig. 5b shows that the co-injection of 100,000 melanoma-CM exposed DIFF(A)-MSC with 500,000 melanoma A375 cells supported the tumor growth to the same extent as the parental PAR-MSC. The exposure to the melanoma conditioned medium did not significantly compromise tumor promoting activity of the MSC. Contrastingly, the glioblastoma-CM exposed DIFF(G)-MSC could not support the tumor growth to the same extent as the PAR-MSC, although there still was a significant support of the tumor growth. The same number of tumor cells A375 (105) injected alone did not produce tumors in 60 % of the animals and the median tumor volume of growing tumors is 50 mm3 by day 30 compared to 320 mm3 in DIFF(G)-coinjected group.
Fig. 5.
Glioblastoma TCM compromised angiogenic potential and protumorigenic capacity of the DIFF(G)-MSC. a Cells were plated onto matrigel layer and the tube formation indicative of angiogenic capacity in vitro was observed 8 h later. Both PAR-MSC and DIFF(A)-MSC formed closed polygons, although they were not able to develop complete mesh structure as control HUVEC cells. There was some cell alignment and cell sprouting in the DIFF(G)-MSC, but closed polygons did not form, so the glioblastoma-CM decreased angiogenic capacity of MSC. Experiment was performed in triplicates, representative images are shown, scale bar 300 μm. b The PAR-MSC, DIFF(A)-MSC and DIFF(G)-MSC were co-injected with the melanoma cells A375 s.c. on immunodeficient animals to evaluate the tumor-promoting activity of the MSC. There was no significant difference in the tumor growth support between the PAR-MSC and DIFF(A)-MSC demonstrating a retention of the tumor-promoting capabilities. The DIFF(G)-MSC could not support tumor growth to the same extent as the PAR-MSC, although there still was a significant tumor growth promotion. Same number of the tumor cells (5 × 105) injected alone does not give rise to tumors in each animal (the penetrance is 40 % and the median tumor volume of growing tumors is 50 mm3 by day 30),*p < 0.05
In summary, we have demonstrated that the composition of the tumor secretome determines molecular and phenotypic features of the adjacent stromal cells. Our data extend the understanding of the tumor-stromal interaction and show that tumor cells dictated the molecular fate of MSC. Melanoma cells supported immunogenic profile along with the increased expression of the markers typical for TAF. This correlates with preserved angiogenic capacity in vitro and tumor growth support. On the contrary, glioblastoma-secreted factors induced up-regulation of differentiation markers, decreased expression of TAF markers along with decreased tube formation in vitro resulting in reduced protumorigenic effect of DIFF(G)-MSC.
Discussion
The MSC are actively recruited to the sites of tumor development and capable of engraftment within the tumor, forming 20 % of its stroma [4]. Tumor cells secrete growth factors and chemokines that both attract stromal precursors and promote MSC differentiation [12]. In our previous studies we have described multiple effects of naïve MSC on tumor cells. The MSC had stimulatory effect on tumor cell proliferation in human breast carcinoma cells MCF7, MDA-231 and T47D, colon cancer cells HT-29, melanoma A375 and M4Beu cells [14, 18, 17]. The inhibitory effect of the MSC on cell proliferation and tumor growth was observed in medullary thyroid carcinoma cells TT [21], human breast carcinoma cells SKBR3 [19] and glioblastoma cells 8MGBA [14]. Importantly, the antiproliferative effect of MSC on SKBR3 cells was accompanied by the induction of EMT, increased migration and mammosphere formation, leading to potentially more tumorigenic and aggressive traits in tumor cells [19]. Based on these data, we hypothesized that the tumor cells dictated molecular changes in MSC. This resulted in their capability either to sustain or inhibit the tumor growth. Model melanoma and glioblastoma cell lines were chosen for our study based on their differential response to the MSC in vitro and in vivo. The MSC were maintained in the tumor-conditioned medium for 30 days prior to analysis with no changes in their differentiation potential, proliferation capacity, senescence susceptibility or substantial alteration in morphology. Differences in the composition of the tumor-secreted paracrine factors were demonstrated by the cytokine and expression analysis. TCM contained several paracrine factors (such as IL-7, IL-13, IL-15, bFGF and GM-CSF) that could not be detected in the secretome of the MSC. The levels of multiple cytokines and chemokines also significantly differed between the two tumor cell lines. We confirmed the capability of melanoma secreted factors to support the MSC migration. Migration of the adipose tissue-derived MSC is stimulated by many cytokines due to the high expression of chemokine and growth factor receptors on the surface of these MSC [22, 23]. We suppose that higher levels of IL-6, IL-8 and IP-10 (CXCL10) in melanoma-CM contribute to increased MSC migration as these were previously reported to affect cell migration [24–26]. Recently, it has been demonstrated that IL-8 in breast cancer conditioned medium contributed to MSC migration and invasion [27]. However, in our experiment, none of these cytokines was able to increase MSC migration substantially alone, but the combination of the three was necessary to increase the MSC migration significantly (Fig. 4d–e).
Major focus of the present study was to compare the alterations in the MSC dictated by the tumor-cell conditioned media. Previous studies suggested that the tumor secreted factors can exert pleiotropic effects due to the inherent MSC plasticity, heterogeneity in culture and a presence of the cells at different stages of their terminal differentiation. Tumor-stimulated MSC were shown to create a carcinoma stem cell niche [15] and also promote metastasis via CXCL10/CXCR3 or CCL5/CCR5 signaling [28, 29]. MSC incorporation into tumor vessels and differentiation into endothelial cells was demonstrated on the breast cancer model [30]. Although Bexell et al. also demonstrated the MSC integration within the rat malignant glioblastoma and the expression of pericyte markers, they did not confirm any endothelial differentiation of the MSC [31]. Several studies demonstrated the capability of MSC to undergo differentiation into the cells with a phenotype of cancer-associated fibroblasts [8, 7]. Similarly we were able to detect significantly increased FAP and FSP in the DIFF(A)-MSC (Fig. 4b). This demonstrates higher efficiency of the melanoma-CM to promote shift towards the phenotype of TAF in MSC in vitro. Moreover, we detected consistently high levels of HGF and SDF-1α secreted from the MSC regardless of the TCM stimulation ([18, 14] and Fig. 3d). Study published by Desai et al. showed that downregulation of αSMA and upregulation of vimentin was a consequence of bFGF exposure [32]. We observed similar phenomenon in the TCM-exposed DIFF(A)-MSC accompanied by the increased migratory properties of the MSC upon αSMA downregulation [33]. Enormous heterogeneity in the phenotype of tumor-associated fibroblasts exists in the populations, where the expression of FSP-1 vs. αSMA markers are mutually exclusive [34]. It has been shown that the αSMA and NG2 populations of the cells are overlapping which correlates with our observation that both these markers were downregulated in the TCM-exposed DIFF(A)-MSC.
Endosialin as another marker highly abundant in carcinoma-associated fibroblasts was also described to be expressed in naïve MSC [35]. Suzuki et al. reported that MSC were able to support neovascularization in a mouse model of melanoma by secretion of proangiogenic factors [36]. They observed the MSC differentiation towards endothelial cells in the absence of αSMA marker of activated myofibroblasts in the tumors. Our study did not show a capability of the melanoma-secreted molecules to promote an endothelial or pericytic transdifferentiation of the MSC. We were not able to detect a long-term incorporation of the MSC in the melanoma xenografts (unpublished data). MSC-exerted support of the tumor xenotransplant growth is critical at the early stages of the tumor onset and growth establishment. Previously published data demonstrated the potential of MSC-conditioned medium to support tumor initiation and growth [14], which illustrates their importance in the tumor initiation. PAR-MSC and DIFF(A)-MSC supported the tumor growth to the same extent (Fig. 5b) and secreted very similar level of IL-6 and IL-8 (Fig. 3c.) Significant decrease of IL-6 and IL-8 levels in the DIFF(G)-MSC correlated with the decrease of the tumor growth support (Figs. 3c and 5b). Moreover, we think that decreased IL-6 secretion in the DIFF(G)-MSC contributed to their differentiation, as is was shown that IL-6 maintains the proliferative and undifferentiated state of bone marrow-derived MSCs [37]. Study by Korkaya et al. has shown that both IL-6 and IL-8 signaling can regulate self-renewal in breast cancer stem cells [38]. Our working hypothesis is that similar mechanism operates in the melanoma initiation. Presence of IL-6 and IL-8 in both tumor-produced and MSC-produced medium promotes tumor xenograft growth. In agreement with this observation, we detected higher secretion of these two interleukins in highly aggressive melanoma cell line (Fig. 1a). Since our in vivo experiments are based on the xenograft growth in immunocompromised host, they do not provide direct link between a tumor behavior and MSC proinflammatory secretion pattern. However, they enable to dissect the direct action of these molecules on the tumor cells. We have observed substantial alterations in IL-9, IL-12, IFN-γ and VEGF levels in the melanoma-exposed MSC, which may also contribute to the dual effect of MSC. It was reported that IL-9 can exert direct effect on melanoma cells in addition to its immunomodulatory function [39].
Tumor cell-conditioned media induced multiple changes in the MSC (summarized in Table 3). Certain markers increased in both types of the TCM-cultured MSC, such as VCAM1 (CD106) and CD146. CD106 was already associated with the MSC phenotype and reported to be expressed on the adipose tissue-derived MSC [40]. Together with the ALCAM (CD166) and mCAM (CD146) they are important for an intercellular adhesion and interaction with integrins on other cell types [12]. Increased levels of MCP-1 chemokine in combination with IL-8 promoted proinflammatory action of the MSC and also may contribute to the increased invasion and adhesion of the tumor cells [41]. Neural ganglioside GD2 was suggested to be a key determinant for the bone marrow-derived MSC and a marker absent on the fibroblasts [42]. In our work we showed that the adipose tissue-derived MSC express this marker, although there is only ~50 % positivity. TCM treatment did not substantially alter the GD2+ MSC subpopulation indicative that these cells were not shifted from the mesenchymal phenotype. Taken together, the combination of multiple paracrine factors present in melanoma-CM dictated pleiotropic effects on the DIFF(A)-MSC and shifted MSC towards the cells with altered intercellular and adhesion properties, immunomodulatory potential and features of the tumor-associated fibroblasts. Barcellos-de-Souza et al. [13] proposed a model of the MSC polarization towards MSC1 and MSC2 phenotypes and linked the immunomodulatory properties to the capability of MSC to support or suppress the tumor growth. Our data were derived in the immunodeficient mouse models and therefore cannot be directly linked to their observations.
Table 3.
Major alterations in the TCM-exposed MSC
| DIFF(A)-MSC vs. PAR-MSC | DIFF(G)-MSC vs. PAR-MSC | |
|---|---|---|
| Adhesion and intercellular connections | ||
| VCAM1 | ↑↑↑ | ↑↑↑ |
| CD146 (mCAM) | ↑ | ↑ |
| CD166 (ALCAM) | >99 % | >99 % |
| Fibroblast markers | ||
| FAP | ↑↑↑ | ↑ |
| FSP | ↑ | ↑ |
| Vimentin | ↑ | ↓ |
| CD90 | ↓ | * |
| Activated myofibroblast marker | ||
| αSMA | ↓ | * |
| Tumor vasculature | ||
| Endosialin | ↑ | ↓ |
| VEGFR2 | ↑↑↑ | ± |
| VEGF | ↑ | * |
| Differentiation markers | ||
| Osteo | * | ↑Runx2 |
| Adipo | * | ↑↑↑ |
| Myogen | * | ↑Jag1 |
Table legend: ↑ - increased, ↑↑↑ - highly increased, ↓ - decreased, ± - very low- undetectable, * - no significant change
Our experimental data unraveled altered expression of several fibroblast markers and adhesion proteins accompanied by substantial change in the MSC-differentiation markers [43] upon treatment with glioblastoma-CM. Increased expression of key regulators of adipocyte differentiation PPAR-γ and RhoA indicated the shift of the DIFF(G)-MSC to more differentiated state. Glioblastoma-CM exposure also induced the expression of essential signaling molecules for the osteogenic differentiation, Runx2 and Jag1. Furthermore, Jag1/Notch1 signaling was also shown to contribute to the chondrogenic and myogenic differentiation in the MSC [44, 45]. The MSC represent a very heterogeneous cell population, therefore TCM stimulation of particular MSC progenitor subsets could induce an upregulation of specific differentiation programs. We think that these regulators are increased in the cells prone to the differentiation into particular cellular lineage, however, the paracrine factor composition does not suffice to mediate terminal differentiation of the DIFF(G)-MSC. The alterations dictated by the glioblastoma-CM are sufficient to decrease the tumor supporting capabilities of the glioblastoma-exposed MSC.
Conclusion
We have shown here that secretion of paracrine factors by tumor cells acts in concerted fashion and exerts pleiotropic effects on the MSC. In addition to certain features of the tumor-associated fibroblasts, the MSC were also subjected to the molecular changes covering wide range of alterations. These changes resulted in altered capacity to stimulate tumor growth. Tumor-stroma interaction is a very complex phenomenon, and our data stress that the composition of the tumor secretome is translated into the altered biological properties of the stromal cells.
Electronic supplementary material
(DOCX 14 kb)
Acknowledgments
We acknowledge the excellent technical help and assistance from M. Dubrovcakova and V. Frivalska. We acknowledge K. Zmajkovicova, PhD, for the kind help with the confocal microscopy. This work was supported by the Slovak Research and Development Agency under the contract No. APVV-0230-11, APVV-0052-12, VEGA grants 2/0088/11 and 2/0171/13. The experiments on the CFX96™ Real-Time PCR Detection System and the IncuCyte ZOOM™ were enabled with the kind help and the financial support from the Cancer Research Foundation RFL2009 and RFL2012.
Conflict of Interest
The authors declare no conflict of interest.
Authors Contributions
Conception, design and development of methodology: LK, JZ; data acquisition LK, JZ, SS, LD, LB, MM; analysis and data interpretation LK, JZ, MM; writing of the manuscript and review: LK, JZ. All authors have read and approved the final version of the manuscript.
Abbreviations
- ALCAM
Activated leukocyte cell adhesion molecule
- αSMA
α-smooth muscle actin
- BCRP1
ATP-binding cassette, sub-family G, ABCG2
- CCL5
RANTES chemokine
- CCR5
Receptor for CCL5 (RANTES)
- cKit
SCF receptor
- CXCR3
CXCL10 receptor
- CXCR4
SDF1α (CXCL12) receptor
- EGFR
Epidermal growth factor receptor
- ELISA
Enzyme-linked immunosorbent assay
- FAP
Fibroblast-activating protein
- FBS
Foetal bovine serum
- FSP
Fibroblast specific protein
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- G-CSF
Granulocyte-colony stimulating factor
- GD2
Neural ganglioside
- GM-CSF
Granulocyte monocyte-colony stimulating factor
- HGF
Hepatocyte growth factor
- HPRT1
Hypoxantine-guanine phosphoribosyl transferase
- IFNγ
Interferon γ
- IL
Interleukin
- IP-10
Chemokine (C-X-C motif) ligand 10, CXCL10
- mCAM
Melanoma cell adhesion molecule, CD146
- MCP-1 (CCL2)
Monocyte chemoattractant protein-1, chemokine CCL2
- MIP-1a (CCL3)
Macrophage inflammatory protein-1alpha
- MIP-1b (CCL4)
Macrophage inflammatory protein-1beta
- MSC
mesenchymal stromal cells
- PDGF-bb
Platelet-derived growth factor
- RANTES (CCL5)
Regulated on activation, normal T-cell expressed and secreted
- SCF
Stem cell factor
- SDF1α
Stroma-derived factor 1α, chemokine CXCL12
- TNFα
Tumor necrosis factor α
- TAF
Tumor associated fibroblasts
- TCM
Tumor cell-conditioned medium
- VCAM1
Vascular cell adhesion molecule 1
- VEGF
Vascular endothelial growth factor
- VEGFR
VEGF receptor
Footnotes
Lucia Kucerova and Jakub Zmajkovic contributed equally to this work
References
- 1.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Kucerova L, Skolekova S. Tumor microenvironment and the role of mesenchymal stromal cells. Neoplasma. 2013;60(1):1–10. doi: 10.4149/neo_2013_001. [DOI] [PubMed] [Google Scholar]
- 3.Kidd S, Spaeth E, Dembinski JL, Dietrich M, Watson K, Klopp A, Battula VL, Weil M, Andreeff M, Marini FC. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 2009;27(10):2614–2623. doi: 10.1002/stem.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kidd S, Spaeth E, Watson K, Burks J, Lu H, Klopp A, Andreeff M, Marini FC. Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS One. 2012;7(2):e30563. doi: 10.1371/journal.pone.0030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10(6):709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12(5):383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 7.Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, Andreeff M, Marini F. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One. 2009;4(4):e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW, Banerjee D. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68(11):4331–4339. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xouri G, Christian S. Origin and function of tumor stroma fibroblasts. Semin Cell Dev Biol. 2010;21(1):40–46. doi: 10.1016/j.semcdb.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth–bystanders turning into key players. Curr Opin Genet Dev. 2009;19(1):67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 12.Hass R, Otte A. Mesenchymal stem cells as all-round supporters in a normal and neoplastic microenvironment. Cell Commun Signal. 2012;10(1):26. doi: 10.1186/1478-811X-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barcellos-de-Souza P, Gori V, Bambi F, Chiarugi P. Tumor microenvironment: bone marrow-mesenchymal stem cells as key players. Biochim Biophys Acta. 2013;1836(2):321–335. doi: 10.1016/j.bbcan.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Kucerova L, Matuskova M, Hlubinova K, Altanerova V, Altaner C. Tumor cell behaviour modulation by mesenchymal stromal cells. Mol Cancer. 2010;9:129. doi: 10.1186/1476-4598-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li HJ, Reinhardt F, Herschman HR, Weinberg RA. Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E2 signaling. Cancer Discov. 2012;2(9):840–855. doi: 10.1158/2159-8290.CD-12-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F., 3rd Concise review: dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29(1):11–19. doi: 10.1002/stem.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kucerova L, Altanerova V, Matuskova M, Tyciakova S, Altaner C. Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res. 2007;67(13):6304–6313. doi: 10.1158/0008-5472.CAN-06-4024. [DOI] [PubMed] [Google Scholar]
- 18.Kucerova L, Kovacovicova M, Polak S, Bohac M, Fedeles J, Palencar D, Matuskova M. Interaction of human adipose tissue-derived mesenchymal stromal cells with breast cancer cells. Neoplasma. 2011;58(5):361–370. doi: 10.4149/neo_2011_05_361. [DOI] [PubMed] [Google Scholar]
- 19.Kucerova L, Skolekova S, Matuskova M, Bohac M, Kozovska Z. Altered features and increased chemosensitivity of human breast cancer cells mediated by adipose tissue-derived mesenchymal stromal cells. BMC Cancer. 2013;13(1):535. doi: 10.1186/1471-2407-13-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez C, Hofmann TJ, Marino R, Dominici M, Horwitz EM. Human bone marrow mesenchymal stromal cells express the neural ganglioside GD2: a novel surface marker for the identification of MSCs. Blood. 2007;109(10):4245–4248. doi: 10.1182/blood-2006-08-039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kucerova L, Matuskova M, Hlubinova K, Bohovic R, Feketeova L, Janega P, Babal P, Poturnajova M. Bystander cytotoxicity in human medullary thyroid carcinoma cells mediated by fusion yeast cytosine deaminase and 5-fluorocytosine. Cancer Lett. 2011;311(1):101–112. doi: 10.1016/j.canlet.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Baek SJ, Kang SK, Ra JC. In vitro migration capacity of human adipose tissue-derived mesenchymal stem cells reflects their expression of receptors for chemokines and growth factors. Exp Mol Med. 2011;43(10):596–603. doi: 10.3858/emm.2011.43.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooke G, Tong H, Levesque JP, Atkinson K. Molecular trafficking mechanisms of multipotent mesenchymal stem cells derived from human bone marrow and placenta. Stem Cells Dev. 2008;17(5):929–940. doi: 10.1089/scd.2007.0156. [DOI] [PubMed] [Google Scholar]
- 24.Shin SY, Nam JS, Lim Y, Lee YH. TNFalpha-exposed bone marrow-derived mesenchymal stem cells promote locomotion of MDA-MB-231 breast cancer cells through transcriptional activation of CXCR3 ligand chemokines. J Biol Chem. 2010;285(40):30731–30740. doi: 10.1074/jbc.M110.128124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rattigan Y, Hsu JM, Mishra PJ, Glod J, Banerjee D. Interleukin 6 mediated recruitment of mesenchymal stem cells to the hypoxic tumor milieu. Exp Cell Res. 2010;316(20):3417–3424. doi: 10.1016/j.yexcr.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F, Korkaya H, Heath A, Dutcher J, Kleer CG, Jung Y, Dontu G, Taichman R, Wicha MS. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71(2):614–624. doi: 10.1158/0008-5472.CAN-10-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Senst C, Nazari-Shafti T, Kruger S, Honer Zu Bentrup K, Dupin CL, Chaffin AE, Srivastav SK, Worner PM, Abdel-Mageed AB, Alt EU, Izadpanah R. Prospective dual role of mesenchymal stem cells in breast tumor microenvironment. Breast Cancer Res Treat. 2013;137(1):69–79. doi: 10.1007/s10549-012-2321-0. [DOI] [PubMed] [Google Scholar]
- 28.Chaturvedi P, Gilkes DM, Wong CC, Luo W, Zhang H, Wei H, Takano N, Schito L, Levchenko A, Semenza GL. Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. J Clin Invest. 2013;123(1):189–205. doi: 10.1172/JCI64993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 30.Muehlberg FL, Song YH, Krohn A, Pinilla SP, Droll LH, Leng X, Seidensticker M, Ricke J, Altman AM, Devarajan E, Liu W, Arlinghaus RB, Alt EU. Tissue-resident stem cells promote breast cancer growth and metastasis. Carcinogenesis. 2009;30(4):589–597. doi: 10.1093/carcin/bgp036. [DOI] [PubMed] [Google Scholar]
- 31.Bexell D, Gunnarsson S, Tormin A, Darabi A, Gisselsson D, Roybon L, Scheding S, Bengzon J. Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Mol Ther. 2009;17(1):183–190. doi: 10.1038/mt.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desai VD, Hsia HC, Schwarzbauer JE. Reversible modulation of myofibroblast differentiation in adipose-derived mesenchymal stem cells. PLoS One. 2014;9(1):e86865. doi: 10.1371/journal.pone.0086865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ronnov-Jessen L, Petersen OW. A function for filamentous alpha-smooth muscle actin: retardation of motility in fibroblasts. J Cell Biol. 1996;134(1):67–80. doi: 10.1083/jcb.134.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5(12):1640–1646. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]
- 35.Bagley RG, Weber W, Rouleau C, Yao M, Honma N, Kataoka S, Ishida I, Roberts BL, Teicher BA. Human mesenchymal stem cells from bone marrow express tumor endothelial and stromal markers. Int J Oncol. 2009;34(3):619–627. doi: 10.3892/ijo_00000187. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki K, Sun R, Origuchi M, Kanehira M, Takahata T, Itoh J, Umezawa A, Kijima H, Fukuda S, Saijo Y. Mesenchymal stromal cells promote tumor growth through the enhancement of neovascularization. Mol Med. 2011;17(7–8):579–587. doi: 10.2119/molmed.2010.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pricola KL, Kuhn NZ, Haleem-Smith H, Song Y, Tuan RS. Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism. J Cell Biochem. 2009;108(3):577–588. doi: 10.1002/jcb.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korkaya H, Wicha MS. Breast cancer stem cells: we’ve got them surrounded. Clin Cancer Res. 2013;19(3):511–513. doi: 10.1158/1078-0432.CCR-12-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitt E, Bopp T. Amazing IL-9: revealing a new function for an “old” cytokine. J Clin Invest. 2012;122(11):3857–3859. doi: 10.1172/JCI65929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15(6):641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furukawa S, Soeda S, Kiko Y, Suzuki O, Hashimoto Y, Watanabe T, Nishiyama H, Tasaki K, Hojo H, Abe M, Fujimori K. MCP-1 promotes invasion and adhesion of human ovarian cancer cells. Anticancer Res. 2013;33(11):4785–4790. [PubMed] [Google Scholar]
- 42.Jin HJ, Nam HY, Bae YK, Kim SY, Im IR, Oh W, Yang YS, Choi SJ, Kim SW. GD2 expression is closely associated with neuronal differentiation of human umbilical cord blood-derived mesenchymal stem cells. Cell Mol Life Sci CMLS. 2010;67(11):1845–1858. doi: 10.1007/s00018-010-0292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cook D, Genever P. Regulation of mesenchymal stem cell differentiation. Adv Exp Med Biol. 2013;786:213–229. doi: 10.1007/978-94-007-6621-1_12. [DOI] [PubMed] [Google Scholar]
- 44.Oldershaw RA, Hardingham TE. Notch signaling during chondrogenesis of human bone marrow stem cells. Bone. 2010;46(2):286–293. doi: 10.1016/j.bone.2009.04.242. [DOI] [PubMed] [Google Scholar]
- 45.Kurpinski K, Lam H, Chu J, Wang A, Kim A, Tsay E, Agrawal S, Schaffer DV, Li S. Transforming growth factor-beta and notch signaling mediate stem cell differentiation into smooth muscle cells. Stem Cells. 2010;28(4):734–742. doi: 10.1002/stem.319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 14 kb)