Abstract
Bacterial transcriptomics is widely used to investigate gene regulation, bacterial susceptibility to antibiotics, host-pathogen interactions, and pathogenesis. Transcriptomics is crucially dependent on suitable methods to isolate and detect bacterial RNA. Microfluidics offer ways of creating integrated point-of-care systems, analysing a sample from preparation, and RNA isolation to detection. A critical requirement for on-chip diagnostics to deliver on their promise is that mRNA expression is not altered via microfluidic sample processing. This article investigates the impact of the use of microfluidics upon RNA expression of bacteria isolated from blood, a key step towards proving the suitability of such systems for further development.
INTRODUCTION
RNA plays a crucial role in information transfer, catalysis, and gene regulation.1 Many diseases have been associated with gene expression variations, indicating that RNA expression profiles offer new insights into diseases processes and the underlying fundamental molecular biology.2 Similarly, bacterial transcriptomics has aided in the further understanding of antibiotic resistance3–5 and host-pathogen interactions,6–8 as well as the identification of novel drug targets.9–11 The main challenges remain, however, the isolation of low abundance bacteria and removal of inhibitors. Microfluidic systems have been used for high-throughput studies of RNA and their interactions at the single molecule level.1 Recently, systems have been reported measuring multiple mRNA expressions or identifying specific biomarkers.2 However, these systems performed only the detection stage of the on-chip process. Microfluidics has also been utilised for on-chip extraction of viral RNA in blood12 and for bacterial RNA extraction,13 but the RNA expression was not benchmarked against traditional processes.
A major challenge in using RNA expression lies in processing and extracting, in a robust and repeatable manner, RNA from a patient sample before the occurrence of any changes in RNA expression. Expression profiles have been shown to vary, depending upon the chosen extraction method.6 Several microfluidic devices have been reported for the continuous extraction of bacteria from blood, some relying on active forces,14,15 on selective lysis16 while others exploit label-free passive hydrodynamic methods.17–21 However, given that shear stress is a known factor for mRNA deregulation in human22 and bacterial cells,23 it is critical to ensure that microfluidic processing does not impact upon the RNA expression profile.
In this paper, we investigated the impact of microfluidic processing on the RNA expression of bacteria in blood, comparing on-chip sample processing to traditional benchtop methods. The main aim of the study was to determine whether RNA expression was altered by the use of a microfluidic device for the separation of bacteria from human whole blood.
MATERIALS AND METHODS
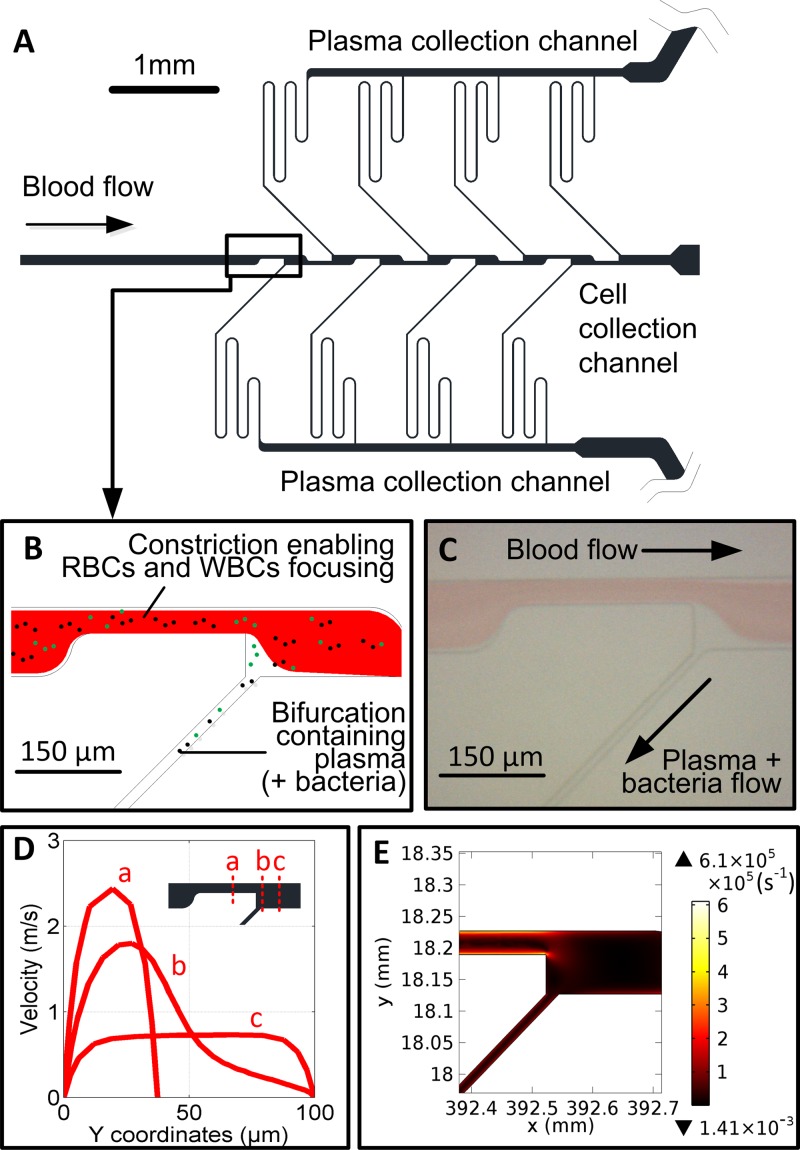
The design and operation of the microfluidic chip used in isolating plasma from blood cells has been reported previously.24 The chip comprises several constrictions enhancing the lateral drift force pushing blood cells (2–30 μm) to the channel centre while plasma is drawn off from cell-free zones through narrower side channels. As this lateral force is smaller for bacterial cells (∼0.25–1 μm), these are relatively marginated, similar to the strategy adopted in Ref. 21 (Figure 1). Device manufacturing was performed by Epigem (Redcar, UK). Flow rates of up to 6 ml/h were tested using syringe pumps (World Precision Instruments, FL, USA).
FIG. 1.
Blood plasma separation device. (a) Schematic of a blood plasma device including constrictions and bifurcations. (b) Lateral lift force in the constriction forces the largest cells including RBCs and WBCs more centrally. The sudden expansion at the end of the constriction creates a cell-free zone, from which plasma can be extracted providing flow rate ratios between the feed channel and daughter channels are correctly set. This allows the relative margination of smaller particles (<1–2 μm) of such platelets and bacterial cells within the cell-free zones found near the wall from which the plasma is extracted. (c) A photograph of a 3:7 (v/v) Blood:PBS flow in the device. Flow rate: 5 ml/h. (d) Simulated velocity profiles at the first constriction and expansion using computational fluid dynamic (CFD) software Comsol 4.4 (Burlington, MA, USA) using a Newtonian model (Inlet flow rate = 5 ml h−1; density = 1025 kg m−3; viscosity = 1750 cP). (e) Simulated shear rate map in the first constriction and expansion. The highest shear rate is situated on the walls of the first constriction. Blood cell induced viscosity and its impact upon shear rate30 was not considered in this model.
Human whole blood was purchased from Seralab (West Sussex, UK). The pooled sample originated from 3 healthy individuals and EDTA K2 added to prevent coagulation. The blood was 10 days old from the point of collection. Escherichia coli CFT 073 was used at a concentration of 105 cfu/ml in blood. Initial microfluidic runs were done with 105 cfu/ml spiked into Luria Bertani (LB) broth. Subsequent experiments used blood cultures in which the organism was grown in blood for 24 h at 37 °C, shaking at 200 rpm. These cultures were then diluted at varying dilutions with phosphate buffered saline (from 10% to 50% blood).
RNA extraction was performed using Qiagen RNA protect (Hilden, Germany) to stabilise the RNA followed by a column based extraction method using the Qiagen RNeasy Mini kit (Hilden, Germany), followed by DNase treatment with Turbo DNA-free (Life Technologies, Carlsbad, USA). RNA yield was quantified using Nanodrop ND-1000 (Thermo Fisher Scientific, Waltham, USA), and RNA quality was determined using an on-chip capillary electrophoresis method, Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, USA).
5 replicates of each of the bench processed and microfluidic processed samples were sent for microarray analysis to determine the RNA expression. Microarray processing was performed by Hologic (Manchester, UK) (Affymetrix protocol). The raw data were analysed by Fios Genomics (Edinburgh, UK). This has been deposited in NCBI Gene Expression Omnibus,25 accessible through GEO Series accession number GSE68064 (http://www.ncbi.nlm.nih.gov/geo/). One way analysis of variance (ANOVA) was applied, and from this, a list of down- and up-regulated genes were transcribed; the function of these genes were determined via comparison with the literature and public gene regulation databases.26–28
RESULTS AND DISCUSSION
The main focus of the sample processing work was to separate bacteria from the blood cells in the blood sample. The rationale behind isolating the bacteria is to reduce the presence of inhibitors present in whole blood, which can lead to poor lysis yield of the bacteria and inhibition of enzymatic steps in the downstream process. Also, prior bacteria separation leads to an RNA extraction that is enriched for bacterial RNA compared to host RNA, hence reducing the noise level during quantification of the expression level. The microfluidic system, based on the axial migration of cells, had been previously demonstrated to successfully isolate plasma from blood samples at a flow rate of several ml/h for blood concentration between 5% and 100%. The flow profile in the constriction and after the constriction (Figure 1(d)), leads to relatively high shear stress on the channel walls (Figure 1(e)), in particular, impacting bacterial cells flowing close to the wall and being drawn out in the plasma channels. Though these rates are high compared to physiological values encountered in the vascular system (40 s−1 and 2000 s−1 in stable laminar flow), only a small percentage of all cells and bacterial cells experience the highest shear rates and this for a short period of time while passing through the constrictions.
The system was initially tested with blood samples that were spiked with bacteria. For this study using commercial banked blood samples, the optimal blood concentration level was determined to 30% and the optimal flow rate was found to be 4 ml/h, enabling processing of a 5 ml sample within 75 min. Bacteria collected from the device output were then lysed, yielding bacterial RNA concentrations of over 16 μg/18 μl with a RNA integrity number (RIN) of 9.4. This compared favourably to the benchtop procedure and ensured microarray analysis was possible as the criteria for microarray processing were a yield of greater than 10 μg/18 μl and a RIN of at least 7, see supplementary Table S1 in Ref. 29.
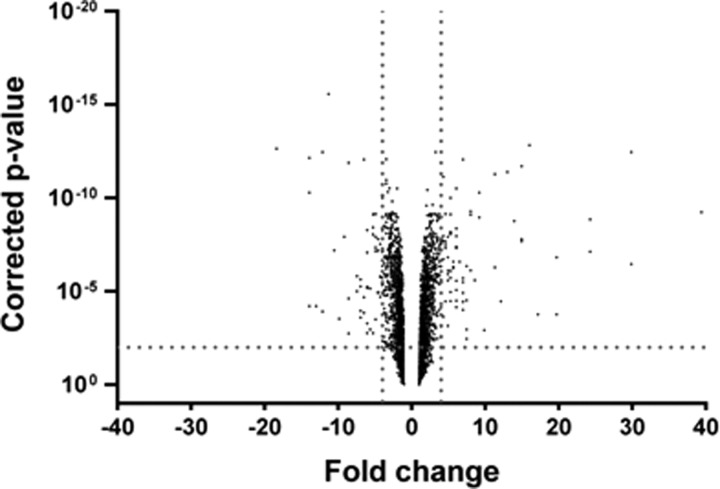
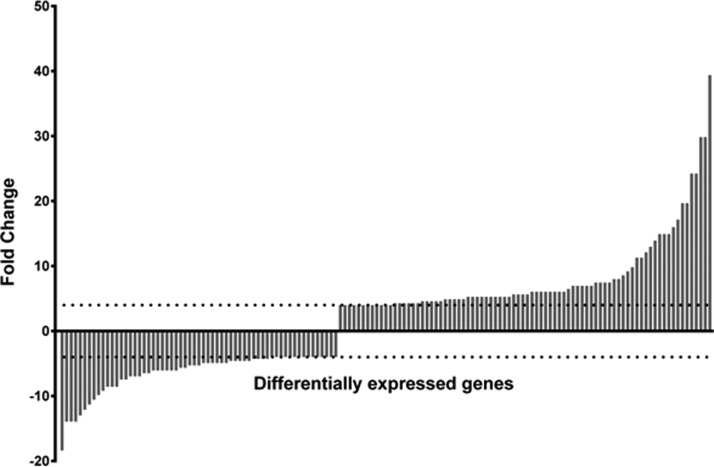
ANOVA analysis was employed to determine any differences in RNA expression between the two conditions (Figure 2). Significant fold change in the gene expression was determined by only selecting genes that were differentially regulated both by ≥4 or ≤−4 fold and with corrected p-value of ≤0.01. Based on these selection criteria, we identified 143 genes that were differentially regulated between the two separation methods. In order to visualise the spread of the genes that were up- and down-regulated, the 143 genes were tabulated into a bar graph, see Figure 3. The 143 genes were then grouped into their functional categories, to visualise the regulatory networks that were affected by the separation method, see supplementary Table S2 in Ref. 29. The main classes of genes which appeared to be differentially regulated belonged to regulatory networks such as metabolism (mainly arginine metabolism/transport), bacteriophages, fimbrial proteins, the SOS response, and DNA replication/repair. The appearance of these networks signifies that the conditions during the sample processing on the microfluidic device led to changes in the metabolic regulation of the organism. The microarray results indicate that microfluidic processing of the samples impacted on the RNA expression. In our study, the differences in gene expression were observed in 143 genes out of the total 5379 genes for E. coli CFT073, a 2.7% change in the overall expression profile.
FIG. 2.
Volcanic plot of fold changes in gene expression of E. coli CFT073 due to separation using the microfluidic method in comparison to the benchtop method. The vertical dotted lines perpendicular to the X-axis delineate the area representing a fold change value of 4 (right line) and −4 (left line). The horizontal dotted line perpendicular to the Y-axis delineates the upper area representing corrected p value of ≤0.01.
FIG. 3.
Indication of the degree of fold change observed across the 143 identified genes. The horizontal dotted lines from the Y-axis depict the fold change value of 4 (top line) and −4 (bottom line).
CONCLUSIONS
This study investigated the impact of microfluidic sample processing on RNA expression to determine the feasibility of lab-on-a-chip based point-of-care diagnostics. Several genes were identified as being up- or down-regulated as a result of the microfluidic processing, though this represented a low percentage of the total expression profile. If RNA biomarkers of interest fall outside the categories identified being susceptible to microfluidic processing, separation methods based on hydrodynamic separation can be considered suitable for diagnostic devices measuring bacterial RNA expression.
ACKNOWLEDGMENTS
The authors would like to acknowledge funding from the MRC Confidence in Concept scheme, MRC/CIC/002. H.B. and M.K.K. would like to acknowledge the Royal Academy of Engineering (RAEng)/EPSRC and RAEng, respectively, for their research fellowships.
References
- 1. Yoshizawa S., “ Micro and nanotechnological tools for study of RNA,” Biochimie 94(7), 1588–1594 (2012). 10.1016/j.biochi.2012.03.018 [DOI] [PubMed] [Google Scholar]
- 2. Wen J., Yang X., Wang K., Tan W., Zhou L., Zuo X., Zhang H., and Chen Y., “ One-dimensional microfluidic beads array for multiple mRNAs expression detection,” Biosens. Bioelectron. 22(11), 2759–2762 (2007). 10.1016/j.bios.2006.11.029 [DOI] [PubMed] [Google Scholar]
- 3. Emerson J. E., Stabler R. A., Wren B. W., and Fairweather N. F., “ Microarray analysis of the transcriptional responses of Clostridium difficile to environmental and antibiotic stress,” J. Med. Microbiol. 57, 757–64 (2008). 10.1099/jmm.0.47657-0 [DOI] [PubMed] [Google Scholar]
- 4. Shaw K. J., Miller N., Liu X., Lerner D., Wan J., Bittner A., and Morrow B. J., “ Comparison of the changes in global gene expression of Escherichia coli induced by four bactericidal agents,” J. Mol. Microbiol. Biotechnol. 5(2), 105–122 (2003). 10.1159/000069981 [DOI] [PubMed] [Google Scholar]
- 5. Kaldalu N., Mei R., and Lewis K., “ Killing by ampicillin and ofloxacin induces overlapping changes in Escherichia coli transcription profile,” Antimicrob. Agents Chemother. 48(3), 890–896 (2004). 10.1128/AAC.48.3.890-896.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boldrick J. C., Alizadeh A. A., Diehn M., Dudoit S., Liu C. L., Belcher C. E., Botstein D., Staudt L. M., Brown P. O., and Relman D. A., “ Stereotyped and specific gene expression programs in human innate immune responses to bacteria,” Proc. Nat. Acad. Sci. 99(2), 972–977 (2002). 10.1073/pnas.231625398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haugen B. J., Pellett S., Redford P., Hamilton H. L., Roesch P. L., and Welch R. A., “ In vivo gene expression analysis identifies genes required for enhanced colonization of the mouse urinary tract by uropathogenic Escherichia coli strain CFT073 dsdA,” Infect. Immun. 75(1), 278–289 (2007). 10.1128/IAI.01319-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kobayashi S. D., Braughton K. R., Whitney A. R., Voyich J. M., Schwan T. G., Musser J. M., and DeLeo F. R., “ Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils,” Proc. Natl. Acad. Sci. 100(19), 10948–10953 (2003). 10.1073/pnas.1833375100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pethe K., Sequeira P. C., Agarwalla S., Rhee K., Kuhen K., Phong W. Y., Patel V., Beer D., Walker J. R., Duraiswamy J., Jiricek J., Keller T. H., Chatterjee A., Tan M. P., Ujjini M., Rao S. P. S., Camacho L., Bifani P., Mak P. A., Ma I., Barnes S. W., Chen Z., Plouffe D., Thayalan P., Ng S. H., Au M., Lee B. H., Tan B. H., Ravindran S., Nanjundappa M., Lin X., Goh A., Lakshminarayana S. B., Shoen C., Cynamon M., Kreiswirth B., Dartois V., Peters E. C., Glynne R., Brenner S., and Dick T., “ A chemical genetic screen in Mycobacterium tuberculosis identifies carbon–source-dependent growth inhibitors devoid of in vivo efficacy,” Nat. Commun. 1, 57 (2010). 10.1038/ncomms1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moir D. T., Di M., Opperman T., Schweizer H. P., and Bowlin T. L., “ A high-throughput, homogeneous, bioluminescent assay for Pseudomonas aeruginosa gyrase inhibitors and other DNA-damaging agents,” J. Biomol. Screen. 12(6), 855–864 (2007). 10.1177/1087057107304729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen B. E., “ Functional linkage between genes that regulate osmotic stress responses and multidrug resistance transporters: Challenges and opportunities for antibiotic discovery,” Antimicrob. Agents Chemother. 58(2), 640–646 (2014). 10.1128/AAC.02095-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bhattacharyya A. and Klapperich C. M., “ Microfluidics-based extraction of viral RNA from infected mammalian cells for disposable molecular diagnostics,” Sens. Actuat. B 129(2), 693–698 (2008). 10.1016/j.snb.2007.09.057 [DOI] [Google Scholar]
- 13. Vulto P., Dame G., Maier U., Makohliso S., Podszun S., Zahn P., and Urban G. A., “ A microfluidic approach for high efficiency extraction of low molecular weight RNA,” Lab Chip 10(5), 610–616 (2010). 10.1039/B913481F [DOI] [PubMed] [Google Scholar]
- 14. Lee J.-J., Jeong K. J., Hashimoto M., Kwon A. H., Rwei A., Shankarappa S. A., Tsui J. H., and Kohane D. S., “ Synthetic ligand-coated magnetic nanoparticles for microfluidic bacterial separation from blood,” Nano Lett. 14(1), 1–5 (2014). 10.1021/nl3047305 [DOI] [PubMed] [Google Scholar]
- 15. Cho Y.-K., Lee J.-G., Park J.-M., Lee B.-S., Lee Y., and Ko C., “ One-step pathogen specific DNA extraction from whole blood on a centrifugal microfluidic device,” Lab Chip 7(5), 565–573 (2007). 10.1039/b616115d [DOI] [PubMed] [Google Scholar]
- 16. Zelenin S., Hansson J., Ardabili S., Ramachandraiah H., Brismar H., and Russom A., “ Microfluidic-based isolation of bacteria from whole blood for sepsis diagnostics,” Biotechnol Lett. 37(4), 825–830 (2015). 10.1007/s10529-014-1734-8 [DOI] [PubMed] [Google Scholar]
- 17. Wu Z., Willing B., Bjerketorp J., Jansson J. K., and Hjort K., “ Soft inertial microfluidics for high throughput separation of bacteria from human blood cells,” Lab Chip 9(9), 1193–1199 (2009). 10.1039/b817611f [DOI] [PubMed] [Google Scholar]
- 18. Mach A. J. and Di Carlo D., “ Continuous scalable blood filtration device using inertial microfluidics,” Biotechnol. Bioeng. 107(2), 302–311 (2010). 10.1002/bit.22833 [DOI] [PubMed] [Google Scholar]
- 19. Raub C. B., Lee C., and Kartalov E., “ Sequestration of bacteria from whole blood by optimized microfluidic cross-flow filtration for Rapid Antimicrobial Susceptibility Testing,” Sens. Actuat. B 210(0), 120–123 (2015). 10.1016/j.snb.2014.10.061 [DOI] [Google Scholar]
- 20. Hansson J., Microfluidic Blood Sample Preparation for Rapid Sepsis Diagnostics ( KTH, Stockholm, 2012). [Google Scholar]
- 21. Wei Hou H., Gan H. Y., Bhagat A. A. S., Li L. D., Lim C. T., and Han J., “ A microfluidics approach towards high-throughput pathogen removal from blood using margination,” Biomicrofluidics 6(2), 024115 (2012). 10.1063/1.4710992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chien S., “ Mechanotransduction and endothelial cell homeostasis: The wisdom of the cell,” Am. J. Physiol. 292(3), H1209–H1224 (2007). 10.1152/ajpheart.01047.2006 [DOI] [PubMed] [Google Scholar]
- 23. Thomas W. E., Nilsson L. M., Forero M., Sokurenko E. V., and Vogel V., “ Shear-dependent ‘stick-and-roll’ adhesion of type 1 fimbriated Escherichia coli,” Mol. Microbiol. 53(5), 1545–1557 (2004). 10.1111/j.1365-2958.2004.04226.x [DOI] [PubMed] [Google Scholar]
- 24. Kersaudy-Kerhoas M., Kavanagh D. M., Dhariwal R. S., Campbell C. J., and Desmulliez M. P. Y., “ Validation of a blood plasma separation system by biomarker detection,” Lab Chip 10, 1587–1595 (2010). 10.1039/b926834k [DOI] [PubMed] [Google Scholar]
- 25. Edgar R., Domrachev M., and Lash A. E., “ Gene expression omnibus: NCBI gene expression and hybridization array data repository,” Nucl. Acids Res. 30(1), 207–210 (2002). 10.1093/nar/30.1.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang D. W., Sherman B. T., and Lempicki R. A., “ Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists,” Nucl. Acids Res. 37(1), 1–13 (2009). 10.1093/nar/gkn923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jensen L. J., Kuhn M., Stark M., Chaffron S., Creevey C., Muller J., Doerks T., Julien P., Roth A., Simonovic M., Bork P., and von Mering C., “ STRING 8—A global view on proteins and their functional interactions in 630 organisms,” Nucleic Acids Res. 37(suppl 1), D412–D416 (2009). 10.1093/nar/gkn760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kanehisa M., Goto S., Sato Y., Kawashima M., Furumichi M., and Tanabe M., “ Data, information, knowledge and principle: Back to metabolism in KEGG,” Nucl. Acids Res. 42(D1), D199–D205 (2014). 10.1093/nar/gkt1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.See supplementary material at http://dx.doi.org/10.1063/1.4921819E-BIOMGB-9-008503 for RNA yield and RIN data as well as functional categorisation of the 143 genes regulated differentially due to microfluidic processing.
- 30. Zhou R., Gordon J., Palmer A. F., and Chang H.-C., “ Role of erythrocyte deformability during capillary wetting,” Biotechnol. Bioeng. 93(2), 201–211 (2006). 10.1002/bit.20672 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- See supplementary material at http://dx.doi.org/10.1063/1.4921819E-BIOMGB-9-008503 for RNA yield and RIN data as well as functional categorisation of the 143 genes regulated differentially due to microfluidic processing.