Abstract
Current research has strongly proposed that contrary to prior beliefs, many ovarian epithelial cancers (OECs) do not, as their name suggests, originate in the ovaries. Recent findings regarding both high-grade and low-grade serous carcinomas has implicated the fallopian tube as a cell source for these OECs, but until now, there has been little insight into the cellular source for clear cell and endometrioid carcinomas. In this commentary review article, we aimed to discuss the new findings that support the possible contribution from the fallopian tube in clear cell and endometrioid carcinomas. Specifically, we have provided results that showcased ovarian surface epithelia (OSE) and ovarian epithelial inclusions (OEIs) as having mesothelial and tubal origins and have strongly recognized the secondary müllerian system and the ability for tubal epithelia to implant upon the ovarian surface as contributing to fallopian tube-derived OEIs (F-OEIs). We have provided initial indications of these F-OEIs and their relationship to endometriosis and then clear cell and endometrioid carcinomas and subsequently offer our new proposal of a probable tubal origin. This new proposal is a paradigm that drastically changes the understanding behind the origin of these OECs and has significant clinical implications in the near future.
Keywords: Fallopian tube, secondary müllerian system, endometriosis, clear cell carcinoma, endometrioid carcinoma
Introduction
Ovarian epithelial cancer (OEC) is categorized into two types depending on its morphological, histological, and genetic composition. Type I ovarian cancers are typically defined by their confinement to the ovary and relative genetic stability. On the other hand, Type II ovarian cancers are defined by their rapid evolution and high rate of TP53 mutations, which, as a result, makes Type II cancers highly aggressive [1]. Serous carcinomas are the most common type of ovarian cancer and are categorized into low-grade and high-grade groups [2]. High-grade serous carcinomas are more common than low-grade serous carcinomas, comprising about 70% of ovarian carcinomas. They are usually diagnosed in advanced stages and are microscopically characterized by their papillary, glandular, and solid growth structures [3]. In contrast, the less common low-grade serous carcinomas have higher genetic stability, show mutations in the KRAS and BRAF genes, and are believed to advance through a stepwise phase involving ovarian cortical inclusions cysts (CICs) or ovarian epithelial inclusions (OEIs), benign cystadenomas, and borderline tumors [1]. In addition, other types of ovarian carcinomas include mucinous, endometrioid, and clear cell carcinomas [3].
For years, studies have aimed to understand the cell origin and pathogenesis of these OECs in an effort to better diagnose and treat patients. Recently, there have been increasing studies linking the fallopian tube as the cell origin for many of the OECs. There has been evidence that high-grade serous carcinomas arise from tubal secretory epithelial cells that implant on the ovarian surface before concluding in metastatic disease. Studies observed the presence of serous tubal intraepithelial carcinomas (STICs) in women who were at high-risk for serous carcinomas and patients who already had dispersive high-grade serous carcinomas [4]. Similarly, there has been evidence that low-grade serous carcinomas also have their cell origin in the fallopian tube [1,3,5]. Using specific morphologic characteristics and immunohistochemical markers, it was found that there are two types of ovarian surface epithelia (OSE), one mesothelial in nature and the other of tubal origin. There are also two types of OEIs corresponding to mesothelium and tubal epithelium respectively. Since most OEIs show a significantly higher resemblance to the fallopian tube rather than the typical mesothelium-derived OSE, we have concluded that the fallopian tube is the main cellular source for low-grade serous carcinomas [6]. Additionally, the cell origin for mucinous ovarian carcinomas has been highly controversial and unclear, though preliminary data has shown a relationship with gastrointestinal mucosa, also indicating a non-ovarian origin [1]. As a result, these studies have prompted exploration into the cell origin of clear cell and endometrioid carcinomas. It has been previously believed that the cell origin of both clear cell and endometrioid carcinomas is derived from ovarian endometriosis, which is believed to originate from the endometrium through the process of retrograde menstruation [7,8]. However, based upon current clinicopathological observations and recent experimental results, a new proposed paradigm has emerged, linking the fallopian tube as another cellular source of ovarian clear cell and endometrioid carcinomas. Since many researchers have already published on the tubal origin of ovarian serous cancers, including low-grade serous carcinomas [1,3,5], this commentary review article will instead focus on the recent findings of the tubal origin underlying clear cell and endometrioid carcinomas.
The secondary müllerian system
Prior to discussing the cell origin of ovarian clear cell and endometrioid carcinoma, it is necessary to first give a brief overview of the secondary müllerian system, since it is both conceptually and histologically linked to our understanding of the carcinogenesis behind these carcinomas. During embryonic development, portions of the two distal müllerian ducts, also known as the primary müllerian system, fuse to form the uterus, cervix, and upper third of the vagina, with the remaining ducts then staying separated to become the fallopian tubes [9]. It has been observed that müllerian tissue structures can also be found outside of the derivatives of the primary müllerian system, a phenomenon that has been termed the “secondary müllerian system” [10,11]. The ectopic presence of müllerian-derived tissue-a phenomenon that encompasses endosalpingiosis, endometriosis, and endocervicosis-is referred to as müllerianosis [12]. Endosalpingiosis and endometriosis, defined as the ectopic presence of tubal and endometrial epithelia respectively, are the most common cases of müllerianosis. However, all three components of the secondary müllerian system can be morphologically interchangeable-from endosalpingiosis to endometriosis or endocervicosis-via a probable metaplastic change, as seen in Figure 1. This morphologically interchangeable phenomenon of müllerianosis was well observed and emphasized by Lauchlan about 40 years ago [10,11]. These various müllerian differentiations are able to provide outstanding interpretations of how different types of OECs develop [13].
Figure 1.
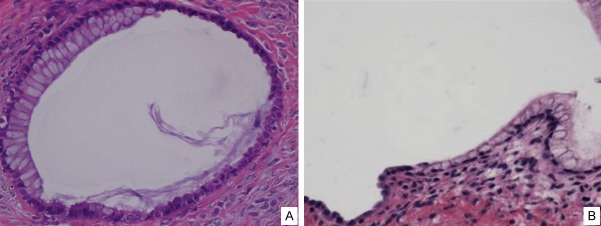
Morphologic transitions between endosalpingiosis and endocervicosis. A single ovarian epithelial inclusion (A) shows mucinous epithelial cells (endocervicosis) on the left and serous epithelia (endosalpingiosis) on the right. A similar finding of such a transition between endosalpingiosis (left) and endocervicosis (right) was found in another ovary (B).
It has been previously believed that the OSE were comprised entirely of mesothelial cells. As a result, because it was also hypothesized that OEIs are formed from the invagination of OSE, it was believed that OEIs exclusively had OSE origins [10]. It was further believed that the invagination of OSE occurred prior to the cells undergoing “müllerian metaplasia”, leading to the formation of the secondary müllerian system [14]. However, the müllerian metaplastic process for OSE-derived OEIs is purely a hypothesis without supporting scientific evidence. As a result, we carried out a study that looked into OSE and OEIs in an effort to provide clear evidence of the cell origin of both.
We designed a study with immunohistochemical staining that used tubal-specific vs. mesothelia-specific markers: PAX-8, a member of the PAX gene family [15], is specific to the secretory tubal epithelia [16] while calretinin is a recognized mesothelial cell marker [17]. We observed a total of 856 cases of OEIs from the ovaries of 45 patients and found that 667 (78%) were of tubal origin (calretinin-/PAX-8+) while the remaining 22% were of ovarian surface mesothelial origin [6]. The morphologic observations corresponded to the immunohistochemical results, as the tubal OEIs (F-OEIs) were observed to be comprised of secretory and ciliated cells, which are characteristic of tubal epithelia, while mesothelium-derived OEIs (M-OEIs) were comprised of flat, non-ciliated cells, which is characteristic of the mesothelial OSE [6]. Representative pictures of the immunohistochemical stainings on both F-OEI and M-OEI are presented in Figure 2. Additionally, immunohistological stainings, morphologic indicators, and entire ovarian sectioning also provided evidence of two types of OSE as opposed to one: those that display a mesothelial phenotype (96%) and those that display a tubal phenotype (4%). Though the presence of tubal OSE was low, the results showed that it was possible for benign tubal epithelia to implant upon the ovarian surface and microscopically mimic “OSE” [18], as shown in Figure 3. The basis behind this implantation can be traced back to the cellular nature of tubal cells. Tubal epithelial cells are prone to easy detachment due to the scarce amount of stroma cells that is present within the tubal fimbria [19]. This tendency for cells to slough off easily can result in easy implantation onto the ovarian surface due to the close anatomical relationship between the two, as the fallopian tube lies in close proximity to the ovary, with the fimbriated ends of the fallopian tube reaching almost every portion of the ovary [20]. In other words, the close relationship between the tubal fimbria and the ovary, as well as the easy detachment of tubal epithelial cells, provides the basis of tubal type “OSE” formation, which truly represents the so-called endosalpingiosis (ectopic tubal epithelia) (Figure 3). This involves a process of tubal detachment through commonly observed activities such as ovulation, inflammation, surface adhesion, and dynamic ovarian stromal modulation [1,6,9], adhesion to the ovarian surface, and invagination into the ovarian cortex. Regarding the interpretation of the small percentage of tubal type epithelia on the ovarian surface and the large amount of F-OEIs within the ovarian cortex, we think that tubal epithelia have a bigger potential to grow than the mesothelial cells and M-OEI cells [18], although the exact mechanism remains to be clarified. In summary, recent results have shown that contrary to previous beliefs, there are two types of OSE: those that originate from the sloughing of tubal epithelia onto the ovarian surface and those that are comprised of mesothelial cells on the ovarian surface. Additionally, from our understanding of the two types of OSE, we can now conclude that there are, consequently, two types of OEI: M-OEI (approximately 20%) and F-OEI (approximately 80%).
Figure 2.
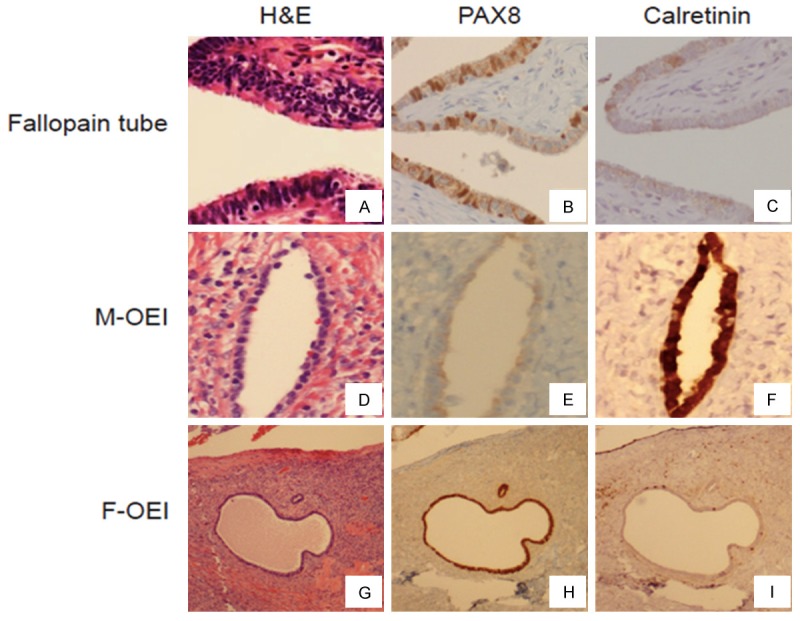
Comparison of immunophenotype between OEIs and the fallopian tube. The fallopian tube and two types of OEIs (A, D, G) were immunohistochemically stained with PAX8 and calretinin. The mesothelium-derived OEI (M-OEI) was negative for PAX8 (E) but positive for calretinin (F). Conversely, the fallopian tube-derived OEI (F-OEI) was positive for PAX8 (H) but negative for calretinin (I), a result that is identical for the stainings of the tubal secretory cells (B and C).
Figure 3.
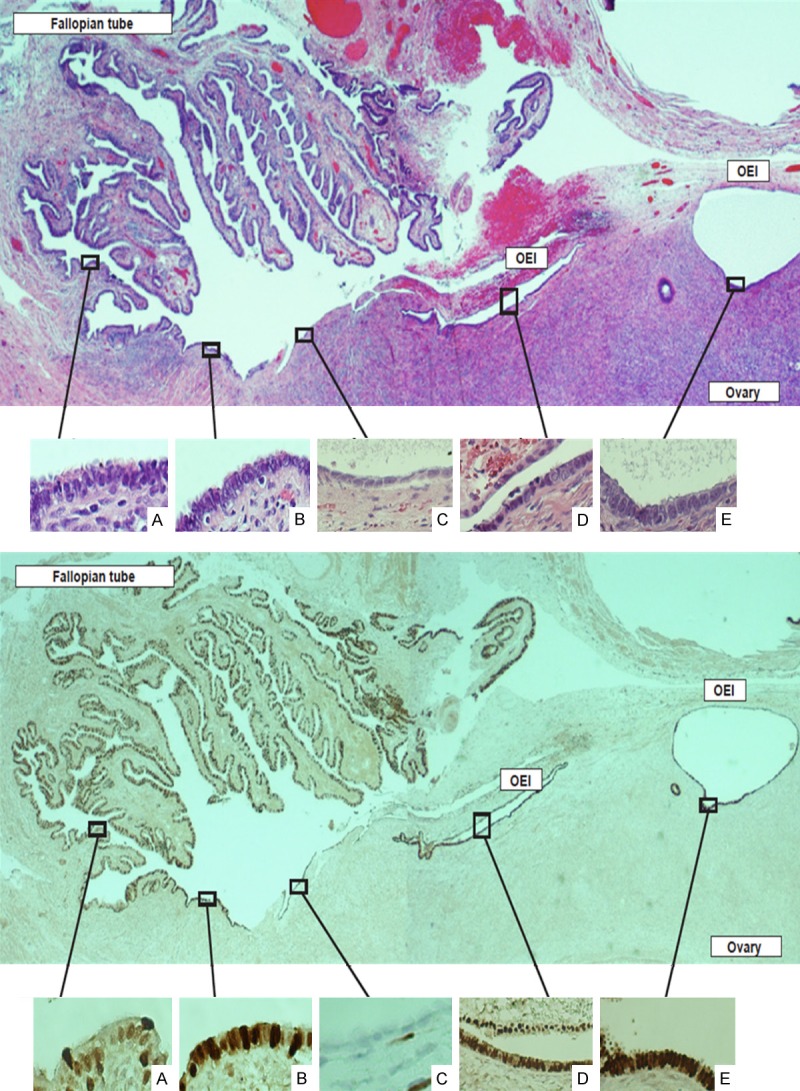
Tubal ovarian adhesions serve as a common process to spread tubal epithelia on the ovarian surface before forming endosalpingiosis. Tubal epithelial cells are commonly seen on the ovarian surface, particularly when tubal ovarian adhesions are present (top panel). This panoramic picture shows magnified tubal epithelia (A), adherent tubal epithelia (B) on the ovarian surface, residual original ovarian surface epithelia that is mesothelial in nature (C), a newly formed ovarian epithelial inclusion (OEI) with surface adhesions (D), and a better formed and dilated OEI (E), which dynamically depicts the process of fallopian tube-derived OEI formation. Morphologically, the epithelial cells on the ovarian surface (B) are identical to tubal epithelia (A). The cells in panels (D and E) show more secretory cells and less ciliated cells compared with the original tubal epithelia (A). This is in contrast to the residual mesothelia (C), which are flat without any cilia and is apparently different from the tubal epithelia. PAX8 staining provides further clarification of the epithelial cells on the ovarian surface as well as the process of OEI formation (bottom panel). The tubal derived epithelial cells are positive for PAX8 (A, B, D, E) while the residual mesothelium on the ovarian surface is negative (C). Interestingly, the top half of the OEI (far right side) shows negative for PAX8 staining, indicative that part of the epithelial cells were entrapped into the OEI during the process of OEI formation due to adhesion.
In addition to our recent findings about the two types of OSE and OEI, an associative relationship has been identified between endosalpingiosis and endometriosis [21]. According to a study by Heinig et al., 38% of patients with endosalpingiosis also had endometriosis [22], showcasing a relatively common phenomenon of the combination of both endometriosis and endosalpingiosis. Since endosalpingiosis is often found alongside endometriosis [21,22], we believe that endosalpingiosis has the ability to convert into endometriosis. In addition, endosalpingiosis can also lead to endocervicosis, but its relationship to neoplastic change is less certain and has not been significantly observed in the endocervical types of OECs, and thus will not be discussed further. While the underlying mechanism behind how endosalpingiosis converts to endometriosis is still unclear and is in the process of being studied [19], F-OEIs are believed to play a part in the emergence of endometriosis.
Cell origin of endometriosis
Since the first dependable observation of endometriosis in the mid-1800s [23], scientists have tried to better understand the cell origin of endometriosis in an effort to treat this disease. Though many theories have been proposed, there is no consensus regarding the cell origin of endometriosis due to the difficulty of in vivo models and varied hormonal responsiveness [19]. One such theory, the coelomic metaplasia theory, has been believed to be a legitimate theory on the cell origin of endometriosis, yet the lack of morphologic evidence would indicate otherwise. In an effort to test this theory of metaplasia, we aimed to identify the earliest signs of morphologic changes of endometriosis within the ovaries. We first proposed that the earliest morphological indicator of endometriosis is initial endometriosis (IE), which is described as lesions showing direct transitions from endosalpingiosis or F-OEI to areas of minimal formation of endometriosis and then to areas of full-blown endometriosis, as illustrated in Figure 4 [24]. IE lesions were classified into two groups: Type I IE is found on the ovarian surface while Type II IE is found within the ovarian cortex [24]. In our 2005 study [24], 34 IE cases were identified out of 110 cases of ovarian endometriosis. Type II IE was found in 25 (73.5%) of cases, with Type I IE comprising 6 (17.6%) and cases with both Type I and Type II comprising 3 (8.8%) [24]. A majority of the IE cases studied were thus derived from OEIs. Specifically, we believe that these are mostly F-OEIs rather than M-OEIs due to the clear presence of ciliated cells (Figure 5). Logically speaking, a majority of these IE within the ovary, and therefore subsequent cases of ovarian endometriosis, seem to have their cell origin in the fallopian tube. However, it took us eight years to consider this option [19]. When this paper was published in 2005, limitations on understanding pointed to the idea that IE showcased a developing metaplastic process through its gradual transformation between OEI and manifestation of endometriosis, assuming that these OEIs were derived from mesothelial cells on the OSE [24]. This was simply because at the time, almost everybody in the field had the concept that all OEIs originated from OSE, which are mesothelial in nature, before being converted into müllerian type cells through a metaplastic process. However, this is in contrast to what has been currently observed, where, as discussed earlier, the majority of OEIs are derived directly from tubal epithelial cells. Furthermore, the formation of such F-OEIs does not require metaplasia to convert mesothelial cells into müllerian epithelia. Rather, direct morphologic evidence of gradual change from F-OEI to IE is readily observed in the majority of specimens with ovarian endometriosis or endometrioma, thus suggesting that IE is likely converted through a direct transition from endosalpingiosis. Such transitional changes are believed to be a metaplastic process, although the detailed mechanism remains to be clarified.
Figure 4.
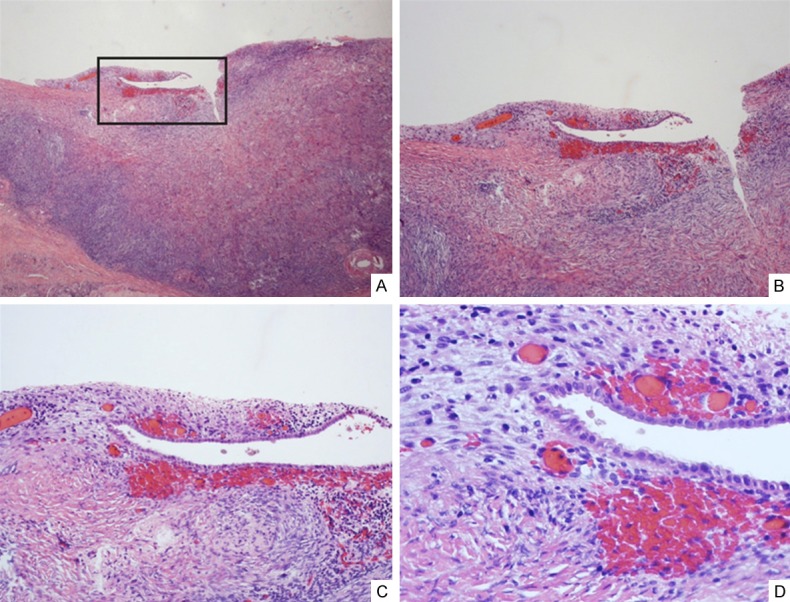
Initial endometriosis. Initial endometriosis is the earliest morphologic indicator of endometriosis and can be seen near the surface of the ovary (A-D). These lesions are commonly seen from the transition of OEI or endosalpingiosis to the initial formation of endometriosis, which illustrates the stromal cellular changes from spindle to oval or round in shape and significantly increased microvessel density immediately adjacent to the epithelia, which are similar to those tubal derived cells. Additionally, the early onsets of eventual OEI formation can be seen by the deep invagination near the ovarian surface (arrows in panel B). Original magnifications: 100 × (A, B) and 200 × (C, D).
Figure 5.
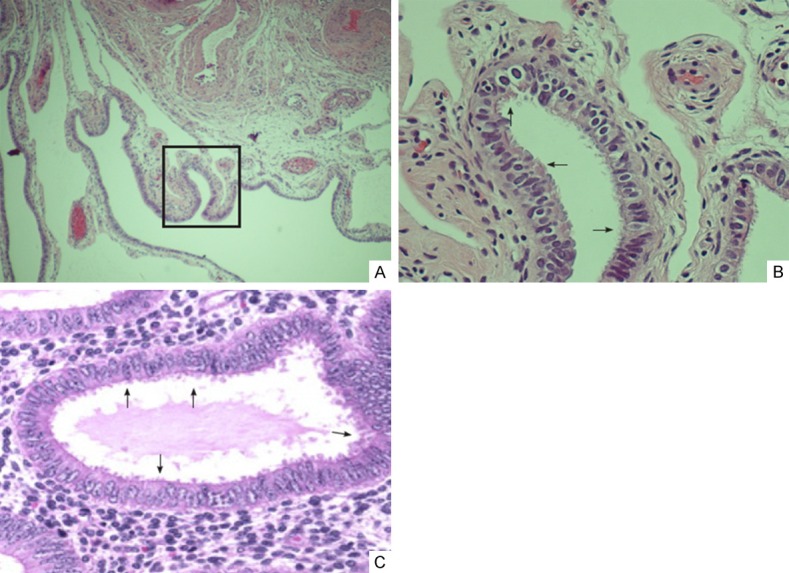
Ciliated epithelia seen in endometriosis are similar to tubal mucosa cells. There are two types of cells within the fallopian tube: ciliated cells and secretory cells (non ciliated). Part of the tubal fimbria (A) and magnified picture (B) from the boxed area of A clearly show ciliations (arrows) in the luminal borders. Ciliated cells are also seen in a case of well-formed endometriosis (arrows, C).
Furthermore, in order to show scientific evidence of the tubal origin of endometriosis, we have to demonstrate that endometriosis is not-or is unlikely to be-derived from the endometrium. Starting about five years ago, we designed a series of experiments to address this important question. To do this, we looked for distinct markers that are able to link the fallopian tube with endometriosis at the exclusion of the endometrium. Using a differential gene array process, we identified a set of novel genes that are either highly expressed in the fallopian tube or in the endometrium. Understanding these unique gene markers allowed us to study the protein expression in endometriosis and compare it to the protein expressions found within the fallopian tube and the endometrium. It was found that about 60% of the ovarian endometriosis studied was likely to be derived from the fallopian tube, with the remaining 40% likely originating from the endometrium [19]. Using these observations, we recognize the fallopian tube as a cellular contributor to endometriosis. While this new theory does not 100% exclude the endometrium as the cell origin for endometriosis, there is support that more than 50% of endometriosis is derived from the fallopian tube. The new evidence supporting a tubal origin for endometriosis is crucial to finding a new effective way to prevent and treat ovarian endometriosis, which has a significant impact on many women in their reproductive age.
Endometriosis as a precursor of clear cell and endometrioid carcinomas
Both ovarian clear cell and endometrioid carcinomas are strongly linked to endometriosis, which is regarded as a precursor for these endometriosis-associated ovarian cancers (EAOCs) [1,25]. Although endometriosis has been found in 21-54% of cases of clear cell and endometrioid carcinomas [26-30], there is a possibility that a majority of these carcinomas, if not all, are derived from endometriosis [31-33].
Although endometriosis is a relatively common benign gynecologic disorder, affecting approximately 10% of women of reproductive age [23], it has the potential to undergo malignant transformation in 0.7-1.0% of patients with endometriosis [34,35]. Though the molecular events behind this transformation are unclear, studies have shown a correlation between clear cell and endometrioid carcinomas and the presence of genetic mutations, particularly mutations in the ARID1A gene, a gene that encodes proteins that participate in chromatin remodeling [36,37]. ARID1A has been recently identified as a tumor suppressor gene, with associated mutations found in about 50% of clear cell carcinomas and about 30% of endometrioid carcinomas [36]. Subsequent studies have shown ARID1A mutations in atypical endometriosis, indicating that these somatic mutations have a specific role in the pathogenesis of OECs [38]. A case of endometriosis, then, has the potential to develop into more dangerous neoplastic processes such as ovarian clear cell and endometrioid carcinomas, as seen in Figure 6. Understanding the potential tubal origin of endometriosis and endometriosis as a precursor to clear cell and endometrioid carcinomas will certainly focus the spotlight on the fallopian tube within the field of ovarian cancer research and clinical practice.
Figure 6.
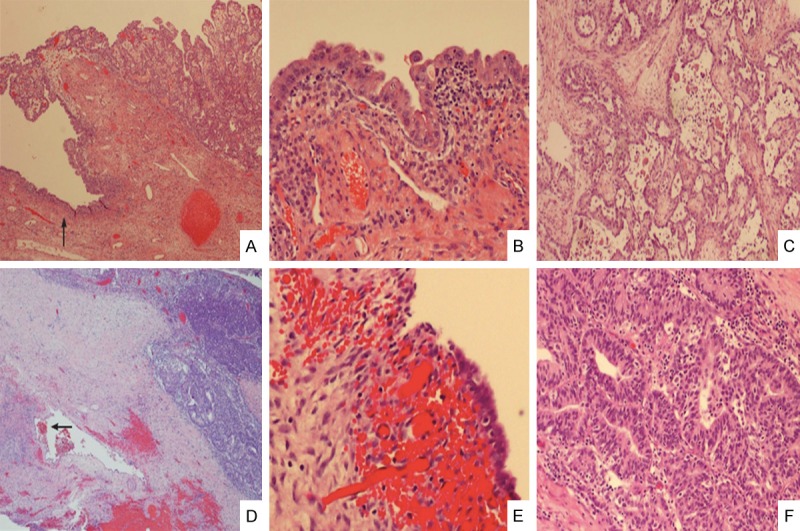
Clear cell and endometrioid carcinomas are associated with endometriosis and atypical endometriosis. A representative sample of an ovarian clear cell carcinoma (upper right corner, A) shows atypical endometriosis in adjacent area (left side with a single arrow, A). The area of atypical endometriosis is better viewed under 200 × magnification (B) and the clear cell carcinoma is magnified 100 × in panel (C) An example of endometrioid carcinoma (upper right corner, (D) also shows endometriosis (mid-left with arrow, (D). The area of endometriosis is magnified in (E) (200 ×) and the endometrioid carcinoma is magnified in (F) (200 ×).
Proposal of tubal origin for clear cell and endometrioid carcinomas
Traditionally, it was believed that the cellular source for OECs was, logically, the ovaries. However, within recent years, mounting evidence has surfaced that OECs, particularly the serous cancers, are tubal instead of ovarian in origin. This novel theory has largely recognized the contributions of the secondary müllerian system to ovarian epithelial carcinogenesis, specifically through tubal epithelial implantation onto the ovarian surface before acting as a direct transition into F-OEIs. The pathogenesis behind these OECs, specifically clear cell and endometrioid carcinomas, showcases a morphologic transformation beginning with endosalpingiosis and F-OEIs before ending with atypical endometriosis and subsequent neoplastic transformation. A holistic diagram of this theory is presented in Figure 7.
Figure 7.

Proposed model of the development of clear cell and endometrioid carcinoma from tubal-derived endometriosis. BTE-benign tubal epithelia; OSE-ovarian surface epithelia; OEIs-ovarian epithelial inclusions; M-OEI-mesothelium-derived OEI; F-OEI-fallopian tube-derived OEI. It is the F-OEI, not M-OEI, which has the potential to develop into clear cell or endometrioid carcinoma through multi-steps of endometriosis.
This paradigm regarding the tubal origin of clear cell and endometrioid carcinomas contains enormous implications for the future of preventative care of these cancers. Though the fallopian tube cannot be said to be the only cell origin of these ovarian carcinomas, there is evidence that the majority of these carcinomas are derived from the fallopian tube. Additionally, more research has to be done in order to gather a deeper understanding of the mechanisms linking these morphologic transformations. While the molecular mechanisms behind the appearance of ovarian epithelial carcinomas has been researched, the understanding of the cell origin of ovarian epithelial carcinomas currently lies on shifting tides, as previous limits in understanding give way to a new model that implicates the fallopian tube as a probable cellular source for at least some of the clear cell and endometrioid cancers. Additionally, since these cancers are closely associated with endometriosis, our observations about the tubal origin of endometriosis may have a significant impact on the clinical management and prevention of this common disease in women.
In summary, numerous studies have shown that so-called ovarian carcinomas, contrary to what their name suggests, barely originate in the ovaries. Instead, the fallopian tube is now being recognized as the main contributor to these OECs. HGSC of the ovary has been essentially confirmed to have tubal origins while LGSC of the ovary most likely has tubal origins. In this commentary review article, we have provided preliminary evidence of the possible tubal origin for at least some of the ovarian clear cell and endometrioid carcinomas. In contrast, there is barely any evidence supporting the notion that OECs are derived from the ovary or the OSE, although the cell origin of ovarian mucinous and Brenner tumors is completely unclear and remains to be determined. Our findings of the possible tubal origin of endometriosis and then clear cell and endometrioid carcinomas will further heat up research in every aspect of the fallopian tube and its relation to cell differentiation and cancer development. These bench works are expected to have a significant clinical translational impact in the near future.
Disclosure of conflict of interest
None.
References
- 1.Kurman RJ, Shih IeM. The Origin and Pathogenesis of Epithelial Ovarian Cancer- a Proposed Unifying Theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer G, Kurman RJ, Chang HW, Cho SK, Shih IeM. Diverse Tumorigenic Pathways in Ovarian Serous Carcinoma. Am J Pathol. 2002;160:1223–1228. doi: 10.1016/s0002-9440(10)62549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prat J. New Insights into Ovarian Cancer Pathology. Ann Oncol. 2012;23:111–117. doi: 10.1093/annonc/mds300. [DOI] [PubMed] [Google Scholar]
- 4.Perets R, Wyant GA, Muto KW, Bijron JG, Poole BB, Chin KT, Chen JY, Ohman AW, Stepule CD, Kwak S, Karst AM, Hirsch MS, Setlur SR, Crum CP, Dinulescu DM, Drapkin R. Transformation of the Fallopian Tube Secretory Epithelium Leads to High-Grade Serous Ovarian Cancer in Brca; Tp53; Pten Models. Cancer Cell. 2013;24:751–765. doi: 10.1016/j.ccr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng W, Fadare O. Fallopian tube as main source for ovarian and pelvic (nonendometrial) serous carcinomas. Int J Clin Exp Pathol. 2012;5:182–186. [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Abushahin N, Pang S, Xiang L, Chambers SK, Fadare O, Kong B, Zheng W. Tubal origin of ‘ovarian’ low-grade serous carcinoma. Mod Pathol. 2011;24:1488–1499. doi: 10.1038/modpathol.2011.106. [DOI] [PubMed] [Google Scholar]
- 7.Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. American J Obstet Gynecol. 1927;14:422–469. [Google Scholar]
- 8.Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151–154. [PubMed] [Google Scholar]
- 9.Katre R, Morani AK, Prasad SR, Surabhi VR, Choudhary S, Sunnapwar A. Tumors and Pseudotumors of the Secondary Mullerian System: Review With Emphasis on Cross-Sectional Imaging Findings. AJR Am J Roentgenol. 2010;195:1452–1459. doi: 10.2214/AJR.10.4302. [DOI] [PubMed] [Google Scholar]
- 10.Lauchlan SC. The secondary mullerian system. Obstet Gynecol Surv. 1972;27:133–146. doi: 10.1097/00006254-197203000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Lauchlan SC. The secondary mullerian system revisited. Int J Gynecol Pathol. 1994;13:73–79. doi: 10.1097/00004347-199401000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Batt RE, Smith RA, Buck Louis GM, Martin DC, Chapron C, Koninckx PR, Yeh J. Mullerianosis. Histol Histopathol. 2007;22:1161–1166. doi: 10.14670/HH-22.1161. [DOI] [PubMed] [Google Scholar]
- 13.Berretta R, Patrelli TS, Faioli R, Mautone D, Gizzo S, Mezzogiorno A, Giordano G, Bacchi Modena A. Secondary Mullerian System: An Atypical Case of Tumor Originating From Vestigial Mullerian Cells Embedded in the Peritoneum. Clin Genitourin Cancer. 2013;11:365–369. doi: 10.1016/j.clgc.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 14.Scully RE. Pathology of Ovarian Cancer Precursors. J Cell Biochem. (Suppl 1995; 23):208–218. doi: 10.1002/jcb.240590928. [DOI] [PubMed] [Google Scholar]
- 15.Lang D, Powell SK, Plummer RS, Young KP, BA R. PAX genes: roles in development, pathophysiology, and cancer. Biochem Pharmacol. 2007;73:1–14. doi: 10.1016/j.bcp.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 16.Bowen NJ, Logani S, Dickerson EB, Kapa LB, Akhtar M, Benigno BB, McDonald JF. Emerging roles for PAX8 in ovarian cancer and endosalpingeal development. Gynecol Oncol. 2007;104:331–337. doi: 10.1016/j.ygyno.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 17.Attanoos RL, Webb R, Dojcinov SD, Gibbs AR. Value of mesothelial and epithelial antibodies in distinguishing diffuse peritoneal mesothelioma in females from serous paillary carcinoma of the ovary and peritoneum. Histopathology. 2002;40:237–244. doi: 10.1046/j.1365-2559.2002.01352.x. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Fadare O, Xiang L, Kong B, Zheng W. Ovarian serous carcinoma: recept concepts on its origin and carcinogenesis. J Hematol Oncol. 2012;5:8. doi: 10.1186/1756-8722-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan Z, Wang Y, Cragun JM, Chambers SK, Zheng W. Cell origin of endometriosis: contribution by the fallopian tube epithelium. Am J Clin Exp Obstet Gynecol. 2013;1:37–42. [Google Scholar]
- 20.Donnez J, Casanas-Roux F, Caprasse J, Ferin J, Thomas K. Cyclic changes in ciliation, cell height, and mitotic activity in human tubal epithelium during reproductive life. Fertil Steril. 1985;43:554–559. doi: 10.1016/s0015-0282(16)48496-7. [DOI] [PubMed] [Google Scholar]
- 21.deHoop TA, Mira J, Thomas MA. Endosalpingiosis and chronic pelvic pain. J Reprod Med. 1997;42:613–616. [PubMed] [Google Scholar]
- 22.Heinig J, Gottschalk I, Cirkel U, Diallo R. Endosalpingiosis: an under estimated cause of chronic pelvic pain or an accidental finding? A retrospective study of 16 cases. Eur J Obstet Gynecol Reprod Biol. 2002;103:75–78. doi: 10.1016/s0301-2115(02)00020-9. [DOI] [PubMed] [Google Scholar]
- 23.Giudice LC, Kao LC. Endometriosis. Lancet Oncol. 2004;362:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 24.Zheng W, Li N, Wang J, Ulukus EC, Ulukus M, Arici A, Liang SX. Initial Endometriosis Showing Direct Morphologic Evidence of Metaplasia in the Pathogenesis of Ovarian Endometriosis. Int J Gynecol Pathol. 2005;24:164–172. doi: 10.1097/01.rct.0000157091.37057.b4. [DOI] [PubMed] [Google Scholar]
- 25.Prowse AH, Manek S, Varma R, Liu J, Godwin AK, Maher ER, Tomlinson IP, Kennedy SH. Molecular genetic evidence that endometriosis is a precursor of ovarian cancer. Int J Cancer. 2006;119:556–562. doi: 10.1002/ijc.21845. [DOI] [PubMed] [Google Scholar]
- 26.Fakunaga M, Nomura K, Ishikawa E, Ushigome S. Ovarian atypical endometriosis: its close association with malignant epithelial tumours. Histopathology. 1997;30:249–255. doi: 10.1046/j.1365-2559.1997.d01-592.x. [DOI] [PubMed] [Google Scholar]
- 27.Sainz de la Cuesta R, Eichhorn JH, Rice LW, Fuller AF Jr, Nikrui N, Goff BA. Histologic transformation of benign endometriosis to early epithelial ovarian cancer. Gynecol Oncol. 1996;60:238–244. doi: 10.1006/gyno.1996.0032. [DOI] [PubMed] [Google Scholar]
- 28.Yoshikawa H, Jimbo H, Okada S, Matsumoto K, Onda T, Yasugi T, Taketani Y. Prevalence of endometriosis in ovarian cancer. Gynecol Obstet Invest. 2000;50:S11–S17. doi: 10.1159/000052873. [DOI] [PubMed] [Google Scholar]
- 29.Jimbo H, Yoshikawa H, Onda T, Yasugi T, Sakamoto A, Taketani Y. Prevalence of ovarian endometriosis in epithelial ovarian cancer. Int J Gynaecol Obstet. 1997;59:245–250. doi: 10.1016/s0020-7292(97)00238-5. [DOI] [PubMed] [Google Scholar]
- 30.Vercellini P, Parazzini F, Bolis G, Carinelli S, Dindelli M, Vendola N, Luchini L, Crosignani PG. Endometriosis and ovarian cancer. Am J Obstet Gynecol. 1993;169:181–182. doi: 10.1016/0002-9378(93)90159-g. [DOI] [PubMed] [Google Scholar]
- 31.Bell DA. Origins and molecular pathology of ovarian cancer. Mod Pathol. 2005;18:S19–S32. doi: 10.1038/modpathol.3800306. [DOI] [PubMed] [Google Scholar]
- 32.Jiang X, Morland SJ, Hitchcock A, Thomas EJ, Campbell IG. Allelotyping of endometriosis with adjacent ovarian carcinoma reveals evidence of a common lineage. Cancer Res. 1998;58:1707–1712. [PubMed] [Google Scholar]
- 33.Wang V, Li C, Lin M, Welch W, Bell D, Wong YF, Berkowitz R, Mok SC, Bandera CA. Ovarian cancer is a heterogenous disease. Cancer Genet Cytogenet. 2005;161:170–173. doi: 10.1016/j.cancergencyto.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 34.Corner GW, Hu CY, Hertig AT. Ovarian carcinoma arising in endometriosis. Am J Obstet Gynecol. 1950;59:760–774. [Google Scholar]
- 35.Lauslahti K. Malignant external endometriosis: a case of adenocarcinoma of umbilical endometriosis. Acta Pathol Microbiol Scand Suppl. 1972;233:98–102. [PubMed] [Google Scholar]
- 36.Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, Yang W, Heravi-Moussavi A, Giuliany R, Chow C, Fee J, Zayed A, Prentice L, Melnyk N, Turashvili G, Delaney AD, Madore J, Yip S, McPherson AW, Ha G, Bell L, Fereday S, Tam A, Galletta L, Tonin PN, Provencher D, Miller D, Jones SJ, Moore RA, Morin GB, Oloumi A, Boyd N, Aparicio SA, Shih IeM, Mes-Masson AM, Bowtell DD, Hirst M, Gilks B, Marra MA, Huntsman DG. ARID1A Mutations in Endometriosis-Associated Ovarian Carcinomas. New Engl J Med. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones S, Wang TL, Shih IeM, Mao TL, Nakayama K, Roden R, Glas R, Slamon D, Diaz LA Jr, Vogelstein B, Kinzler KW, Velculescu VE, Papadopoulos N. Frequent Mutations of Chromatin Remodeling Gene ARID1A in Ovarian Clear Cell Carcinoma. Science. 2010;330:228–231. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guan B, Mao TL, Panuganti PK, Kuhn E, Kurman RJ, Maeda D, Chen E, Jeng YM, Wang TL, Shih leM. Mutation and loss of expression of ARID1A in uterine low-grade endometrioid carcinoma. Am J Surg Pathol. 2011;35:625–632. doi: 10.1097/PAS.0b013e318212782a. [DOI] [PMC free article] [PubMed] [Google Scholar]


