Abstract
Squamous cell carcinoma of the head and neck region (HNSCC), which is related to an infection with human papilloma virus (HPV), responds better to simultaneous radio-chemotherapy with Cisplatin based regimens than HPV-negative tumors. The underlying molecular mechanisms for this clinical observation are not fully understood. Therefore, the response of four HPV-positive (HPV+) (UM-SCC-47, UM-SCC-104, 93-VU-147T, UPCI:SCC152) and four HPV-negative (HPV-) (UD-SCC-1, UM-SCC-6, UM-SCC-11b, UT-SCC-33) HNSCC cell lines to x-irradiation ± Cisplatin incubation in terms of clonogenic survival, cell cycle progression, protein expression (cyclin A2, cyclin E2, E6, E7, p53) and induction of apoptosis, was investigated. HPV+ cells were more radio- and chemosensitive and were more effectively sensitized to x-irradiation by simultaneous Cisplatin incubation than HPV- cell lines. HPV+ cell lines revealed an increased and prolonged G2/M arrest after irradiation, whereas Cisplatin induced a blockage of cells in S phase. In comparison to irradiation only, addition of Cisplatin significantly enhanced apoptosis especially in HPV+ cell lines. While irradiation alone increased the amount of HPV E6 and E7 proteins, both were down-regulated by Cisplatin incubation either alone or in combination with x-rays, which however did not increase the expression of endogenous p53. Our results demonstrate that cell cycle deregulation together with downregulation of HPV E6 and E7 proteins facilitating apoptosis after Cisplatin incubation promote the enhanced sensitivity of HPV+ HNSCC cells to simultaneous radio-chemotherapy. Combined effects of irradiation and Cisplatin appear to be relevant in mediating the enhanced therapeutic response of HPV-related HNSCC and are indicative of the benefit of combined modality approaches in future treatment optimization strategies.
Keywords: Head and neck cancer, radio-chemotherapy, HPV E6/E7 protein, p53, apoptosis
Introduction
Squamous cell carcinoma of the head and neck region (HNSCC) belongs to the sixth most frequent cancers worldwide [1]. Known classical risk factors for developing HNSCC include alcohol and tobacco. Since recently, persistent infection with high-risk human papilloma virus (HPV), mainly type 16 [2] was recognized as an independent risk factor for these tumors, especially if located in the oropharynx, where about 50% of tumors harbor the virus [3-5]. Clinical observations provide evidence that the prevalence of such HPV-related disease is increasing, especially in Europe and North America [6]. Patients with HPV-related tumors tend to be younger, are often diagnosed with lower T- and higher N-stage but importantly, have a better prognosis as compared to HPV-unrelated tumors [4,6,7]. These observations led to classification of HPV-positive (HPV+) tumors as a distinct tumor entity with differing carcinogenesis and mutational background compared to HPV-unrelated HNSCC (HPV-) [8].
At present, primary or adjuvant radiochemotherapy with Cisplatin-based regimes are standard of care in advanced HNSCC irrespective of the HPV-status [9,10]. Treatment strategies adapted to the improved clinical treatment response of HPV-related HNSCC are recently investigated in on-going clinical trials [11]. This attempt however is complicated by the facts that the underlying molecular mechanisms for the differing treatment response are only partly understood, that preclinical data concerning cellular radiosensitivity are not consistent and that preclinical data on combined effects of radiation and cytostatic drugs are missing [12-15].
Approximately 80% [13] of HPV-unrelated tumors as well as HPV- HNSCC cell lines show mutations in TP53, causing loss of function of p53 and p53-dependent pathways, beneath multiple mutations in other tumor suppressor genes as well as proto-oncogenes such as CDKN2A, PIK3CA and NOTCH [16-18]. In contrast, in HPV-related tumors carcinogenesis is mainly driven by the viral oncogenes E6 and E7, which cause proteasomal degradation and ubiquitination of p53 (E6) and Rb (E7) tumor suppressor proteins [5,18,19]. In addition, many other cellular pathways are altered by E6 and E7 [20] resulting in a tumor promoting phenotype that strongly depends on HPV proteins instead of mutations in tumor suppressors or oncogenes [13,21-23]. Although different types of cancer cells and keratinocytes can be sensitized to therapy by transfection of either E6 or E7 in vitro [24], little is known about the molecular mechanisms sensitizing HPV+ HNSCC cells with integrated viral genome to radio-chemotherapy.
We therefore investigated the combined effects of Cisplatin and x-irradiation in HPV+ and HPV- cell lines focusing on combined effects in terms of clonogenic survival, cell cycle regulation, apoptosis and regulation of E6/E7. Such best reflects investigation of current treatment concepts in a well-defined in vitro model. The study aims to elucidate mechanism explaining the differing treatment response of HPV+ and HPV- HNSCC, which is prerequisite to developing alternative HNSCC treatment concepts specific in regard to the underlying mechanism of carcinogenesis, related genomic patterns and activated or inactivated pathways.
Material and methods
Cell lines and culture conditions
All cell lines were grown in RPMI1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 1% non-essential amino acids and 0.1% gentamicin in humidified air (5% CO2) at 37°C. Detailed characteristics of all cell lines were previously published [23]. UD-SCC-1 (HPV-, p53mut (FS/Wt) [25] were provided by T. Hoffmann, University of Düsseldorf, Germany in 2012; UM-SCC-6 (HPV-, p53wt, [25]), UM-SCC-11b (HPV-, p53mut (C242S), [26]), UM-SCC-47 (HPV-16 pos., p53wt, [27]) and UM-SCC-104 (HPV-16 pos., p53wt, [28]) were provided by T.E. Carey, University of Michigan, United States in 2012, UT-SCC-33 (HPV-., p53mut (R282W) [27]), were provided by R.A. Grenman, Turku University, Finland in 2012, UPCI:SCC152 (HPV-16 pos., p53wt [29], were provided by S.M. Gollin, University of Pittsburgh, United States in 2012 and 93-VU-147T (HPV-16 pos., p53mut (L257R/Wt), [30,31]) were provided by J.P. de Winter, VU Medical Center, Amsterdam in 2012.
HPV status of each cell line was confirmed by PCR using the MY09/11 and GP5/6+ primers (data upon request) and expression of HPV-16 E6 and E7 transcripts in qPCR [23]. Identity of all cell lines was proven using Single Nucleotide Polymorphism (SNP) profiles and Short Tandem Repeats (STR) analysis [32].
Colony formation assay
Exponentially growing cells were seeded in increasing numbers (200-24000 cells per 6 cm petri dish) at least 16 h before treatment to achieve comparable numbers of colonies despite dose escalation. After 11-20 d (depending on the cell line), cells were fixed (10% formaldehyde) and stained (0.1% crystal violet) for colony counting (colonies ≥ 50 cells). The surviving fraction was normalized to the plating efficiency of non-treated controls and clonogenic surviving fractions were calculated. Survival curves were fitted to the linear-quadratic equation (SF = exp-[α*D+β*D2]) according to a least squares fit (GraphPad Prism 5.0 software).
Western blot analysis
Whole cell extracts were generated using lysis buffer (RIPA, protease inhibitor cocktail and PMSF (AppliChem, Darmstadt)). Lysates were resolved in SDS-PAGE sample buffer (25 mM Tris-HCl, pH 6.8; 10% glycerol, 2% SDS, 2.5% β-mercaptoethanol, 0.005% bromphenol blue), following protein separation on 8% (cyclins) or 12% (E6, E7, p53) SDS-Page gels. Proteins were blotted onto Immobilon-PVDF membrane (Millipore) and the membrane was probed with primary antibodies against: Cyclin E2, (#4132, 1:1000), Cyclin A2 (clone: BF683, #4656, 1:2000, Cell Signaling), HPV 16 E7 (ED17, 1:200, SCBT) or HPV 16 E6 (1E-6F4, 1:1000; Euromedex). HRP-conjugated anti-rabbit/mouse IgG HRP (horseradish peroxidase)-linked antibodies (Millipore diluted at 1:5000) and ECL™ chemiluminescent substrate (Amersham) were used for visualization at a ChemoCam Imager 3.2 (Intas, Potsdam, Germany).
Cell cycle analysis
Cells were incubated with Cisplatin (20 μM) and/or irradiated with 6 Gy and harvested after 12-48 h. Media, washing buffer and cells dissociated with accutase were collected, fixed overnight (70% ice-cold ethanol) and then incubated in PBS containing 200 µg/ml RNAse A, 0.1% Triton X-100 and 20 µg/ml propidium iodide (30 min, room temperature). At least 20.000 cells were analyzed by flow cytometry (LSR II flow cytometer, Becton Dickinson). Data were processed using FlowJo V7.6.1 software (Tree Star Inc., San Carlos, CA, USA).
Detection of apoptosis
Cells were seeded 24 h before irradiation with 6 Gy and/or incubation with Cisplatin (10 μM). Flow cytometric analysis was done 24 h and 72 h after treatment using the Annexin V-FITC Detection kit (Promokine, Heidelberg) according to the manufacturer’s instructions. Cells were dissolved with accutase, collected together with washing buffer and media, centrifuged, and stained with Annexin V-FITC and propidium iodide (PI) in a CA2+ binding buffer. A minimum of 20.000 cells was analysed, measuring early and late apoptosis by quadrant statistics using FlowJo V7.6.1 software. Results are shown as sum of upper (late apoptotic cells, Annexin V-FITC + PI-positive cells, double positive) and lower right quadrant (early apoptotic cells, Annexin V-FITC positive cells) normalized to the control as previously described [33].
Treatments
Stock solutions of Cisplatin (0.33 mg/ml, Teva GmbH, Ulm, Germany) were prepared by the Center for Cytostatics Preparation, University Hospital Giessen and Marburg, Marburg, Germany and diluted in culture medium to generate indicated concentrations.
Cell monolayers were irradiated in a PMMA-phantom at room temperature with 6 MeV photons using a linear accelerator (Elekta Supernova, Elekta Oncology Systems Ltd., Crawley, West Sussex, UK) with a dose rate of 4 Gy/min.
In case of combined treatments, Cisplatin was added to the culture medium. If not mentioned otherwise, Cisplatin was removed from petri dishes by growth medium change 24 h after irradiation.
Statistical analysis
Statistical significance was tested by calculating the mean ± standard deviation (SD) from all HPV+ and all HPV- cell lines to generate two grouped mean values, one for HPV+ and one for HPV- cell lines using the two-tailed Student’s t-test with a significance level of p < 0.05 (GraphPad Software). Each experiment was done in triplicate with a minimum of three independent repetitions. Data are presented as mean ± standard deviation (SD) if not mentioned otherwise.
Results
HPV+ cell lines are more treatment sensitive
As shown in our previous work [23], HPV+ cells are more radiosensitive than HPV- cells, exhibiting significantly lower surviving fractions (SF) at 1 Gy (p = 0.03) and 2 Gy (p = 0.01; Figure 1A-H). Colony formation assay proved that HPV+ cell lines are also more chemosensitive (0.1-5 μM) than HPV- cell lines (Figure 2A). All HPV+ cell lines showed lower IC50 values for Cisplatin than HPV- cell lines. Comparison of the mean IC50 values revealed a statistically significant difference between the group of HPV+ and HPV- cell lines (p = 0.003; Figure 2B).
Figure 1.
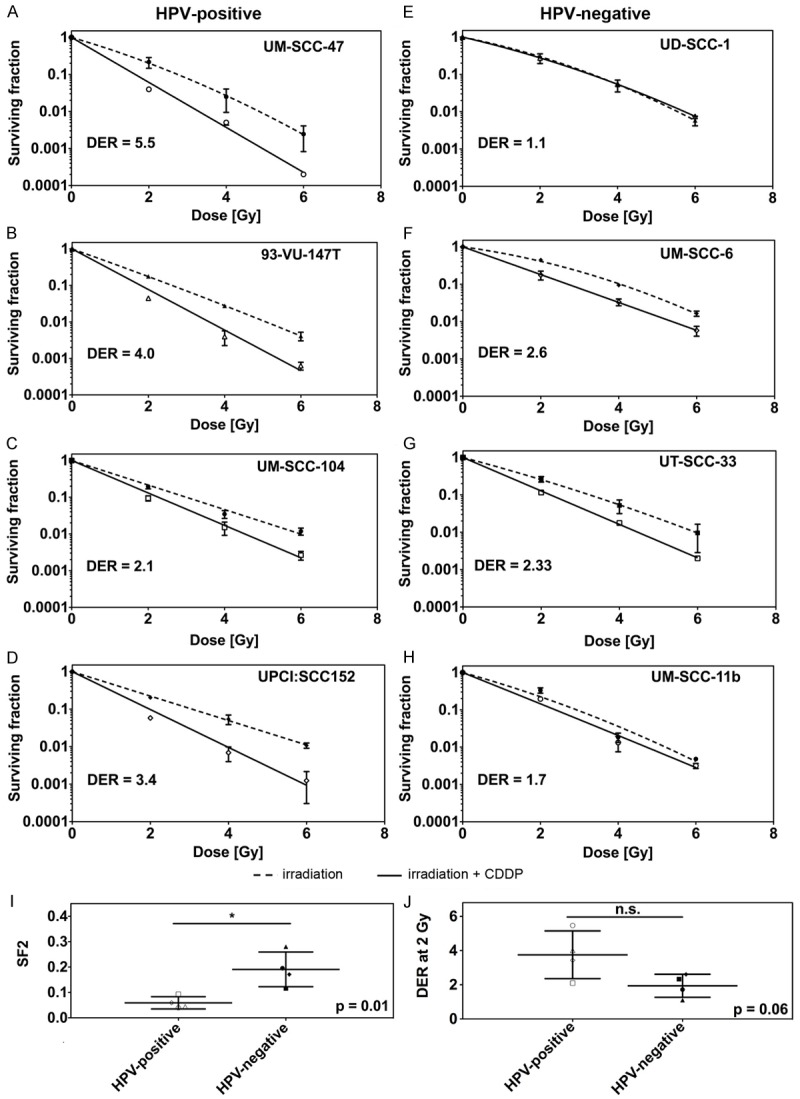
A-H. Clonogenic survival of each HPV+ (left panel) and HPV- (right panel) HNSCC cell line tested after x-irradiation alone or in a combination with Cisplatin including dose enhancement ratios at 2 Gy (DER2). I. Comparison of the average surviving fraction at 2 Gy of HPV+ (open symbols) and HPV- (filled symbols) cell lines after combined treatment with x-rays and Cisplatin (p = 0.01). J. Comparison of the dose enhancement ratio at 2 Gy for HPV+ (open symbols) and HPV- (filled symbols) cell lines (p = 0.06).
Figure 2.
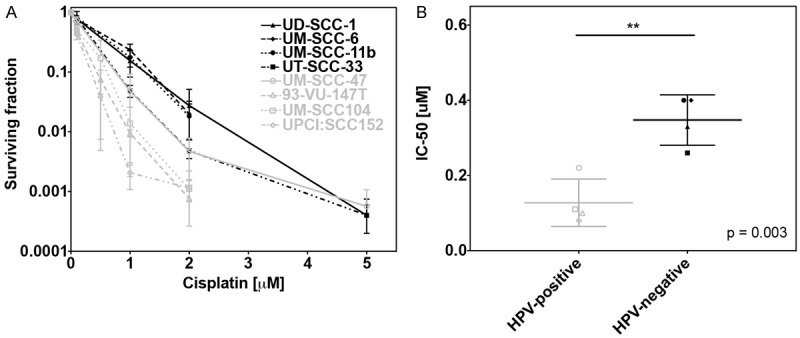
A. Clonogenic survival of all cell lines after treatment with increasing doses of Cisplatin for 24 h. B. Grouped analysis of colony forming assay showing the mean IC50 values for HPV+ (open symbols) and HPV- (filled symbols) cell lines (p = 0.003).
Combined treatment with Cisplatin (0.5 μM) and x-rays (2-6 Gy) led to an even enhanced cytotoxic effect in all cell lines (Figure 1A-H) but UD-SCC-1 cells. Again, HPV+ cell lines were significantly more sensitive than HPV- cell lines (SF2: p = 0.01; Figure 1I). The radiosensitizing effect of Cisplatin was more pronounced in the group of HPV+ cells leading to higher dose enhancement ratios (DER) at 2, 4 and 6 Gy (DER2: 3.8 ± 0.7 vs. 1.9 ± 0.3; p = 0.06; DER4: 5.4 ± 1.2 vs. 2.1 ± 0.5 p = 0.04; DER6: 7.4 ± 1.2 vs. 2.6 ± 1.0 p = 0.02; for HPV+ vs. HPV- cell lines; Figure 1J) as compared to HPV- cell lines.
Deregulated cell-cycle progression in HPV+ cell lines
Progression through the division cycle was investigated in UM-SCC-47 and 93-VU-147T cells (HPV+) and UM-SCC-6 and UM-SCC-11b cells (HPV-) (Figure 3A, 3B). Irradiation with 6 Gy led to a significantly enhanced and prolonged G2/M arrest in HPV+ cells (cells in G2/M at 24 h: p = 0.009 for HPV+ vs. HPV-), which was present until 48 h after irradiation. This effect was associated with a more pronounced decline of G1 phase cells, especially after 10 h (Figure 3C).
Figure 3.
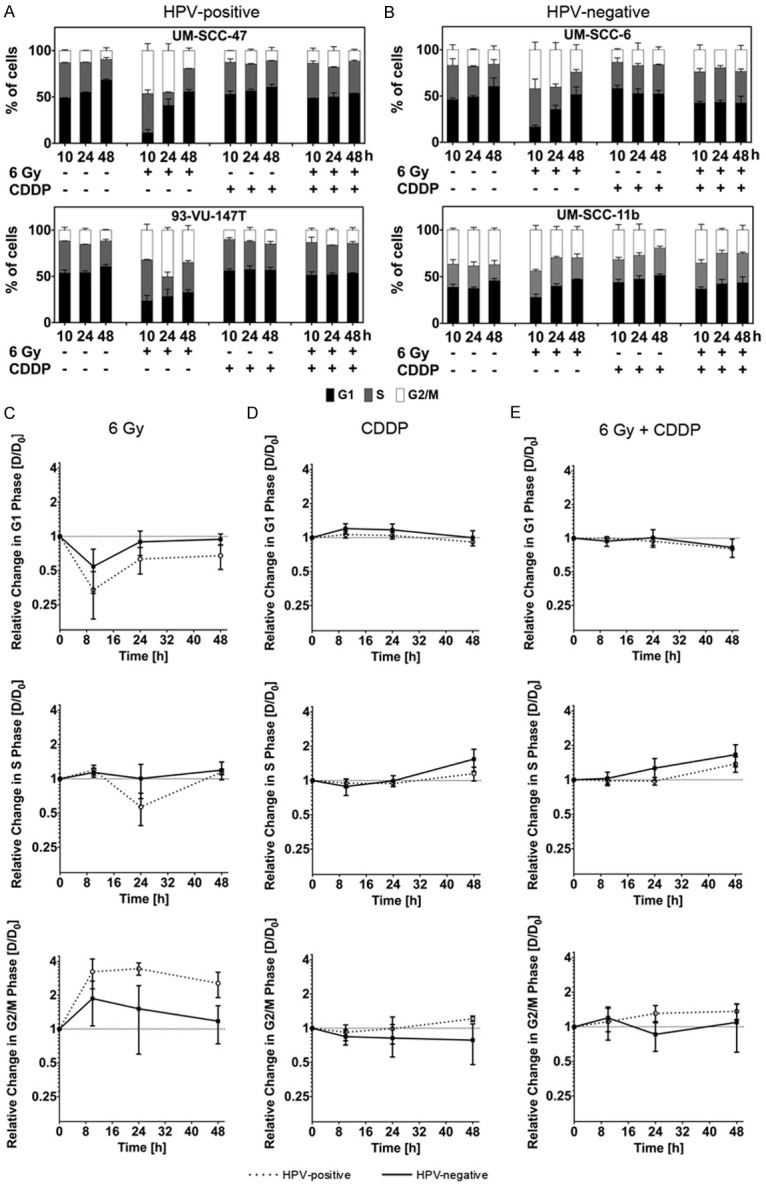
(A, B) Cell cycle progression in UM-SCC-47 and 93-VU147T cells (HPV+; A) and UM-SCC-6 and UM-SCC-11b cells (HPV-; B) 10-48 h after x-irradiation and/or incubation with Cisplatin (control: no treatment; G1 phase: black bar; S phase: grey bar; G2/M phase: white bar). (C-E) Grouped analysis of cells treated with x-irradiation (C) or Cisplatin-incubation (D), or x-irradiation and Cisplatin (E). Comparison of cell cycle alteration in HPV+ (spotted) and HPV- (black) cell lines. Figure shows relative changes in the proportion of cells in the G1, S, and G2/M phases (log2-scale).
Cisplatin treatment (20 μM) alone led to an arrest of all cell lines in S phase. The proportion of cells arrested in S phase increased during a time course of 48 h without significant difference between the HPV+ and HPV- cell lines (Figure 3D).
In cells receiving x-rays and Cisplatin, we found a slight increase in G2/M phase cells in HPV+ cell lines, which was however less pronounced as compared to the effect after irradiation only. The amount of S phase cells was increased and rose until 48 h after treatment, regardless of the HPV-status (Figure 3E).
Cells in subG1 phase with fragmented DNA indicate late stage cell death [34]. We found more cells in subG1 phase in the HPV+ cell lines as compared to the HPV- cell lines after all treatment modalities (Figure 4A, 4B). In HPV+ cell lines, the number of cells in subG1 phase was significantly increased 24 h after Cisplatin incubation and after the combined treatment as compared to irradiation (IR) alone (cells in subG1 phase at 24 h: p = 0.02 for IR vs. CDDP; p = 0.009 for IR vs. CDDP + IR). In contrast, Cisplatin incubation did not lead to significantly more cells in subG1 phase in HPV- cell lines. In all cell lines the combination of Cisplatin and irradiation did not increase subG1 phase cells as compared to Cisplatin incubation alone.
Figure 4.
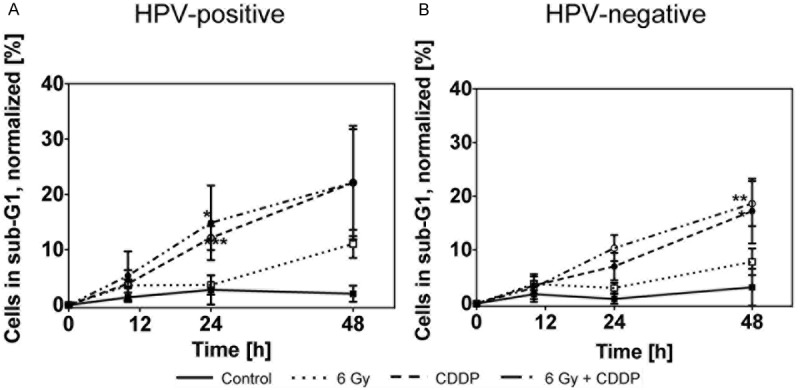
Percentage of cells in subG1 phase (< 2n DNA) in UM-SCC-47 and 93-VU-147T (HPV+; A) and UM-SCC-6 and UM-SCC-11b (HPV-; B) cells after x-irradiation and/or Cisplatin incubation.
Differential expressions of cell cycle-dependent cyclins in HPV+ and HPV- cells
As treatments led to prominent differences in the cell-cycle progression, we further investigated, whether the expression of Cyclin A2 and Cyclin E2 is affected differently in HPV+ and HPV- cells (Figure 5A). Cyclin A2 and Cyclin E2 expression was normalized to their respective level of untreated controls in each cell line at each time point.
Figure 5.
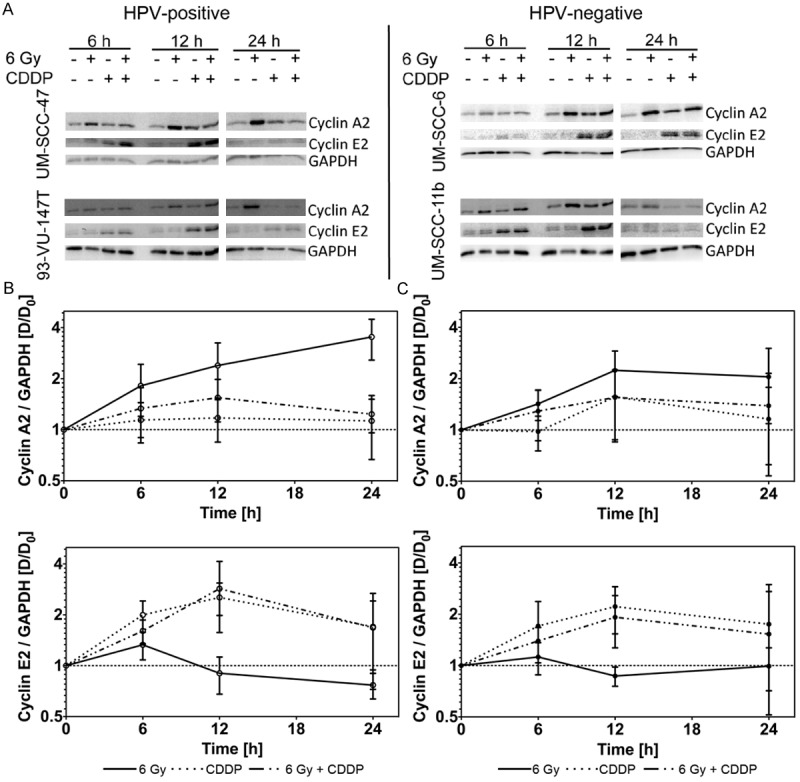
(A) Western blot analysis of cyclin A2, cyclin E2 and GAPDH in UM-SCC-47 and 93-VU-147T (HPV+; left panel) and UM-SCC-6 and UM-SCC-11b (HPV-; right panel) at indicated time points after treatment (x-irradiation and/or Cisplatin). (B/C) Grouped analysis comparing the relative change in the amount of cyclin A2 (B/C, upper panels) and cyclin E2 (B/C, lower panels) in HPV+ (B) and HPV- (C) HNSCC cells (Log2 scale). Values are normalized to the GAPDH control and the untreated control group.
After irradiation with 6 Gy, Cyclin A2 expression was higher in HPV+ cells and rose until 24 h after irradiation, while in HPV- cell lines, Cyclin A2 declined after 12 h (Figure 5C). Cyclin E2 rose in all cell lines until 6 h after irradiation but declined to control levels or even below at later time points (Figure 5C).
After incubation with Cisplatin (20 μM), the expression of Cyclin E2 increased in all cell lines with marginally higher expression in HPV+ cells (Figure 5B, 5C). In Cisplatin treated HPV+ cells, Cyclin A2 slightly increased until 6 h after treatment but then stayed stable until 24 h after treatment. In contrast, in HPV- cell lines, peak levels of Cyclin A2 occurred at 12 h after treatment (Figure 5B, 5C).
After irradiation (6 Gy) and Cisplatin incubation (20 μM) levels of Cyclin E2 were comparable to the levels after Cisplatin incubation alone. Cyclin A2 levels were in between the levels after irradiation and Cisplatin only treated cells (Figure 5B, 5C). Although we found enormous treatment dependant differences in the Cyclin expression, there was no significant difference between HPV+ and HPV- cell lines.
Combined treatment enhances apoptosis in HPV+ cell lines
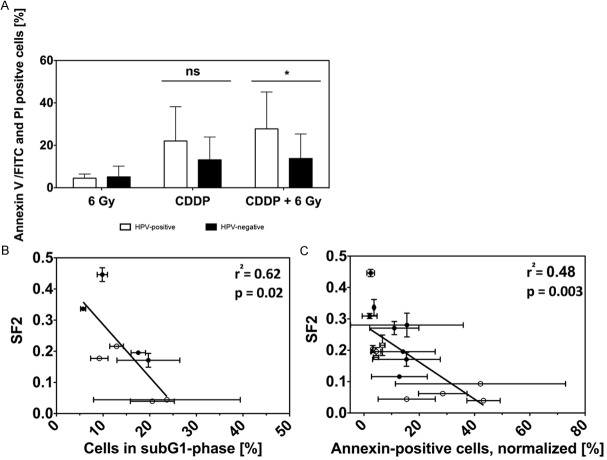
Measuring apoptosis by means of Annexin V-FITC/PI double staining revealed the least amount of apoptosis after irradiation. Twenty four hours after x-rays we did not find a significant difference between HPV+ and HPV- cell lines (p = 0.7; Figure 6A), whereas 72 h after irradiation, the amount of apoptotic cells was significantly higher in HPV+ cell lines (p = 0.04). Cisplatin incubation as well as the combination of Cisplatin (10 μM) with 6 Gy x-rays led to higher levels of early and late apoptotic cells in all cell lines, especially in HPV+ cells up to 24 h after treatment (Figure 6A). The combination of x-irradiation and Cisplatin significantly enhanced the number of Annexin V-positive cells in HPV+ cell lines after 24 h (p = 0.04). After 72 h, the amount of apoptotic cells was comparably high in HPV+ and HPV- cell lines.
Figure 6.
(A) Flow cytometric measurement of early and late apoptosis 24 h after treatment with irradiation and/or Cisplatin. Analysis of mean values for HPV+ and HPV- cell lines showing a statistically significant increase of apoptosis in HPV+ cells 24 h after the combined treatment (p = 0.03). (B) Correlation of surviving fraction at 2 Gy (see Figure 1I) and proportion of cells in subG1 phase (see Figure 3A and 3B); p = 0.02; rr = 0.62. (C) Correlation of surviving fraction at 2 Gy and amount of Annexin-V positive cells 24 h after treatment with 6 Gy x-rays and/or 10 μM Cisplatin (see Figure 3C); p = 0.003; r2 = 0.48. HPV+ cell lines: open symbols; HPV- cell lines: filled symbols.
We found a statistically significant inverse correlation between the increased subG1 phase and the enhanced sensitivity to chemo- and radiotherapy (represented by SF2 values) for both HPV+ and HPV- cell lines (p = 0.02; r2 = 0.62; Figure 6B). Additionally, a significant inverse correlation of Annexin V-positive cells and the sensitivity to Cisplatin and irradiation was found in all cells (p = 0.003; r2 = 0.48; Figure 6C).
Cisplatin reduces expression of HPV-16 encoded E6 and E7
To investigate, whether x-rays (6 Gy) and/or Cisplatin incubation (20 μM) influences the expression of the HPV-16 oncoproteins E6 and E7, we collected protein samples of HPV+ cell lines (UM-SCC-47; 93-VU147T) 6-24 h after treatment (Figure 7A). Specificity of the antibodies against HPV-type 16 E6 and E7 proteins was confirmed in HPV+ and HPV- HNSCC cell lines and cervical cancer cells positive for either HPV-type 16 or 18.
Figure 7.
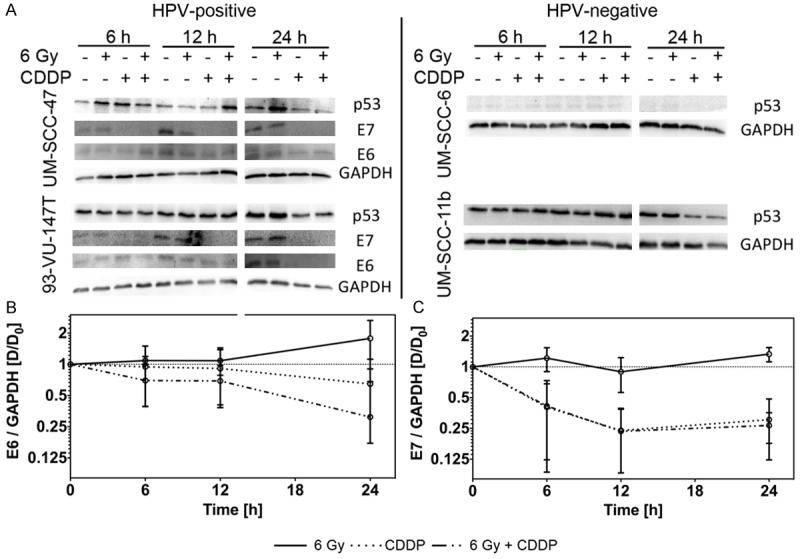
(A) Western blot analysis of p53 and GAPDH in UM-SCC-47 and 93-VU-147T (HPV+; left panel) and UM-SCC-6 and UM-SCC-11b (HPV-; right panel) at indicated time points after treatment. For HPV+ cell lines the amount of HPV-16 E6 and E7 is additionally shown. (B/C) Dot plots comparing the relative change of E6 (B) and E7 (C) after treatment in HPV+ cell lines. All values are normalized to the GAPDH control and to the control group (Log2 scale).
Levels of both proteins, E6 and E7, were slightly increased 6 h after x-irradiation and kept rising until 24 h after treatment. Cisplatin incubation alone led to an early and stable decrease of both proteins; an effect, which was more pronounced for the E7 than for the E6 protein (Figure 7B, 7C).
The E6 protein expression was even more suppressed through the combined treatment of x-rays and Cisplatin, whereas the decrease of the E7 expression was similar after Cisplatin and the combined treatment (Figure 7B, 7C). This decrease of expression of both proteins was significant in comparison to control (E7 at 24 h: p = 0.0005; E6 at 24 h: p = 0.03; for IR+CDDP vs. control).
The decrease of the E6 and E7 expression did not lead to a stable increase in the expression of endogenous p53 in the HPV+ cells. Equally, we found no significant change of p53 protein levels in the HPV- cell lines (Figure 7A).
Discussion
Understanding of the molecular basis of the improved clinical treatment response of HPV-related HNSCC as compared to HPV-unrelated tumors is prerequisite to adapt current treatment strategies aiming at individualized, risk adapted approaches. As combined modality treatments in particular simultaneous radio- and chemotherapy using Cisplatin based regimens are current treatment standard [9,35], studying combined effects is of special interest in this context. With this understanding, it might moreover be possible to target characteristic features of HPV-related HNSCC to further improve treatment outcome.
Intrinsic sensitivity to irradiation and/or Cisplatin
Beside extrinsic factors e.g. immune response, the intrinsic cellular sensitivity of HPV-related tumors to radiochemotherapy is a major factor determining treatment response. Current in vitro data investigating the intrinsic sensitivity are inconclusive [13,23,36-38]. We show on average an enhanced radiosensitivity of the group of HPV+ cell lines confirming our earlier report but noticed that radiosensitivity was diverse among cell lines in both groups and partly overlapping [13,23,36,38]. Similarly, sensitivity against Cisplatin is reported heterogeneous [37,39,40] and possibly related to p53 mutation status rather than to HPV-status [41]. Our study clearly indicates increased sensitivity of the group of HPV+ cells to Cisplatin. The conflicting results concerning radio- and Cisplatin-sensitivity may be attributed to the small number of HPV+ cell lines available and tested, to differences in methodology as well as to the fact, that some authors used cell lines, which were transfected with E6/E7 but not derived from HPV-related tumors. In addition, increasing evidence exists, that the group of HPV-related HNSCC is heterogeneous [42,43] as are the investigated cell lines. Specifically, Lechner et al. [43] found in patient samples that HPV+ tumors had a distinct epigenetic signature in which two main sub-groups could be distinguished, which also distinguished patients in terms of outcome. Also, in the group of HPV-related tumors, the presence of additional risk factors like alcohol and smoking impacts on prognosis [4,42], which seems to be related to a higher mutational burden [39]. Furthermore HPV+ tumors with higher chromosome instability show an unfavorable prognosis [44]. For all HPV+ cell lines used in this study we recently mapped HPV16 DNA integration sites and showed aneuploidy indicating that these cell lines represent a suitable in vitro model [45].
Importantly, the combined effect of irradiation and Cisplatin on cell survival was significantly higher in the group of HPV+ cells. Cisplatin therefore sensitized HPV+ cell lines far more to radiation than HPV- cell lines. Combined therapy like used in this study best reflects clinical treatment regimes. To our knowledge this is the first study showing this effect in vitro, which is in accordance to the clinical observation that patients with HPV-related tumors better respond to radiochemotherapy [4]
Compromised cell cycle arrest and induction of apoptosis
The HPV-encoded oncogene E7 mainly acts through inhibition of the retinoblastoma tumor suppressor protein (pRB) and herewith abrogates the G1/S-checkpoint at the level of cyclin dependant kinase inhibitors and promotes active replication regardless of cell damage and environment [39,46]. We therefore investigated cell cycle progression and cell cycle regulating cyclins after irradiation and/or Cisplatin treatment
Indeed, after irradiation HPV+ cells progressed faster into S phase and showed an enhanced and prolonged G2/M arrest, which was congruent to the far higher and stable up regulation of Cyclin A2. This finding is supported by recent results proving that HPV+ cells have an impaired double strand break repair and tend to accumulate double-strand breaks during the cell cycle without repair before entering the S phase [23,36].
In our study all cell lines failed to arrest in G1 phase, even after Cisplatin treatment, which often induces both, S and G1 arrest [47]. Instead, the number of cells in sub G1-phase was enhanced after Cisplatin treatment indicating late stage cell death. Fojer et al. [48] showed that cells lacking a sufficient G1 arrest progress through S and G2 phase before cell death occurs. As the investigated cell lines indeed showed an increased S phase arrest and prolonged G2 arrest, especially in HPV+ cells, this mechanism seems likely for the studied HPV+ HNSCC cells.
The role of apoptosis in HPV+ cells was additionally confirmed by correlation of the amount of subG1 phase cells as well as Annexin V-FITC/PI positive cells with the SF2 value after irradiation only and after x-ray and Cisplatin treatment. Thus we could prove that a higher rate of apoptotic cell death correlates to a lower SF2 value. We herewith confirmed that enhanced apoptotic cell death contributes to the differential response of HPV+ and HPV- cell lines next to an impaired double-strand break repair [23,36,49,50].
Noya et al. [49] and Nguyen et al. [50] reported that HPV E7 increases the expression of cyclins E and A and by this promotes malignant transformation [51]. Furthermore, dysregulated Cyclin E expression induces chromosomal instability and initiates apoptosis [52,53]. Both cyclins as part of the cyclin-dependent-kinase-cyclin (CDK-cyclin) complex represent key proteins in the transition from G1 to S phase (Cyclin E2), during S phase (Cyclin E2/A2) and from S to G2 phase (Cyclin A2). By correlating Cyclin E2 expression with apoptosis we were able to partly confirm this mechanism in the investigated cell lines. The mechanism, how Cyclin E expression contributes to the enhanced cell death after Cisplatin treatment remains elusive [54]. We were able to prove that Cyclin A2 expression was enhanced in HPV+ cell lines. It is however unclear if Cyclin A2 influences cell death pathways itself and reveals functions besides cell cycle regulation. To further investigate the role of cyclins in HNSCC cell lines, co-immunoprecipitation and knock-out experiments investigating Cdk-cyclin complexes as well as downstream proteins are needed.
Expression of oncoproteins HPV E6, E7 and of p53
In HPV- tumors, p53 is usually disrupted by mutations, whereas most HPV+ HNSCC harbour wild type p53, which can be reactivated by various treatments [5,55]. We were able to show for the first time in HNSCC cells that radiation enhances expression of HPV E6 and E7, which is in accordance to observations in HPV+ cervical cancer cells [56,57]. This up-regulation of oncoproteins due to x-irradiation associated with an improved response seems paradox. However, recent studies found that specifically high E7 expression leads to a delayed DNA damage repair, and higher rates of γH2AX foci [58]. By this mechanism, E7 can promote genomic instability and cell death due to unrepaired DNA double-strand breaks. An increased amount of γH2AX foci, reflecting unrepaired DSB, and correlation to survival in HPV+ cells has already been described [23,30]. Therefore, enhanced expression of E6 and E7 impairs DNA repair and by this leads to higher sensitivity in HPV+ cells against irradiation [59].
On the contrary, Cisplatin reduces HPV E7 and E6 expression, an effect that is also known in HPV+ cervical cancer cells [60] but has not been described in HNSCC before. The combined effect of irradiation and Cisplatin even more effectively reduces E7 and E6 expression. This might explain the higher rate of apoptosis of HPV+ cells, as down regulation of E6 and E7 has been shown to increase apoptosis in HNSCC cells [61]. Li et al. [5] proved that down-regulation of E6 by siRNA can retrieve p53 function promoting apoptosis and cell cycle arrest [5]. However, we noticed no change of the endogenous p53 level, even if combined treatment strictly decreased E6 and E7 levels. This observation could be due to the fact that Cisplatin does not only reduce E6 and E7 expression but rather acts via DNA-adduct formation in HNSCC [62] and by this influences expression of far more proteins than the specific action of siRNA against HPV genes.
In conclusion, cell cycle dysregulation together with down-regulation of HPV E6 and E7 proteins leading to enhanced rates of apoptosis seem to be the basis for the enhanced sensitivity of HPV+ HNSCC cells to the combined effect of x-irradiation and Cisplatin. Functional investigations of p53 and p53- dependent and independent pathways will address the question, whether this effect is p53 dependent. Current discussions are ongoing whether chemotherapy can be avoided especially in the adjuvant setting aiming at reduction of side effects. Our investigations however point at combined effects of irradiation and Cisplatin to be relevant in mediating the enhanced therapeutic response of HPV-related HNSCC. Our data speak in favor of a possible reduction of the total absorbed radiation dose rather than at avoiding Cisplatin. Thus, combined effects of irradiation and cytostatics but especially targeted drugs should be further investigated.
Declaration of conflict of interest
The authors declare that they do not have any financial and personal relationship with other people or organizations that could inappropriately influence (bias) the work reported, including employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 3.Ang KK, Sturgis EM. Human papillomavirus as a marker of the natural history and response to therapy of head and neck squamous cell carcinoma. Semin Radiat Oncol. 2012;22:128–142. doi: 10.1016/j.semradonc.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Sethi S, Ali-Fehmi R, Franceschi S, Struijk L, van Doorn LJ, Quint W, Albashiti B, Ibrahim M, Kato I. Characteristics and survival of head and neck cancer by HPV status: a cancer registry-based study. Int J Cancer. 2012;131:1179–1186. doi: 10.1002/ijc.26500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li C, Johnson DE. Liberation of functional p53 by proteasome inhibition in human papilloma virus-positive head and neck squamous cell carcinoma cells promotes apoptosis and cell cycle arrest. Cell Cycle. 2013;12:923–934. doi: 10.4161/cc.23882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehanna H, Beech T, Nicholson T, El-Hariry I, McConkey C, Paleri V, Roberts S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35:747–755. doi: 10.1002/hed.22015. [DOI] [PubMed] [Google Scholar]
- 7.O’Sullivan B, Huang SH, Perez-Ordonez B, Massey C, Siu LL, Weinreb I, Hope A, Kim J, Bayley AJ, Cummings B, Ringash J, Dawson LA, Cho BC, Chen E, Irish J, Gilbert RW, Hui A, Liu FF, Zhao H, Waldron JN, Xu W. Outcomes of HPV-related oropharyngeal cancer patients treated by radiotherapy alone using altered fractionation. Radiother Oncol. 2012;103:49–56. doi: 10.1016/j.radonc.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 9.Pignon JP, le Maitre A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Bourhis J, Sire C, Graff P, Gregoire V, Maingon P, Calais G, Gery B, Martin L, Alfonsi M, Desprez P, Pignon T, Bardet E, Rives M, Geoffrois L, Daly-Schveitzer N, Sen S, Tuchais C, Dupuis O, Guerif S, Lapeyre M, Favrel V, Hamoir M, Lusinchi A, Temam S, Pinna A, Tao YG, Blanchard P, Auperin A. Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (GORTEC 99-02): an open-label phase 3 randomised trial. Lancet Oncol. 2012;13:145–153. doi: 10.1016/S1470-2045(11)70346-1. [DOI] [PubMed] [Google Scholar]
- 11.Urban D, Corry J, Rischin D. What is the best treatment for patients with human papillomavirus-positive and -negative oropharyngeal cancer? Cancer. 2014;120:1462–1470. doi: 10.1002/cncr.28595. [DOI] [PubMed] [Google Scholar]
- 12.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimple RJ, Smith MA, Blitzer GC, Torres AD, Martin JA, Yang RZ, Peet CR, Lorenz LD, Nickel KP, Klingelhutz AJ, Lambert PF, Harari PM. Enhanced radiation sensitivity in HPV-positive head and neck cancer. Cancer Res. 2013;73:4791–4800. doi: 10.1158/0008-5472.CAN-13-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quon H, Forastiere AA. Controversies in treatment deintensification of human papillomavirus-associated oropharyngeal carcinomas: should we, how should we, and for whom? J. Clin. Oncol. 2013;31:520–522. doi: 10.1200/JCO.2012.46.7746. [DOI] [PubMed] [Google Scholar]
- 15.Dobrosotskaya IY, Bellile E, Spector ME, Kumar B, Feng F, Eisbruch A, Wolf GT, Prince ME, Moyer JS, Teknos T, Chepeha DB, Walline HM, McHugh JB, Cordell KG, Ward PD, Byrd S, Maxwell JH, Urba S, Bradford CR, Carey TE, Worden FP. Weekly chemotherapy with radiation versus high-dose cisplatin with radiation as organ preservation for patients with HPV-positive and HPV-negative locally advanced squamous cell carcinoma of the oropharynx. Head Neck. 2013;36:617–623. doi: 10.1002/hed.23339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C, McKenna A, Shefler E, Ramos AH, Stojanov P, Carter SL, Voet D, Cortes ML, Auclair D, Berger MF, Saksena G, Guiducci C, Onofrio RC, Parkin M, Romkes M, Weissfeld JL, Seethala RR, Wang L, Rangel-Escareno C, Fernandez-Lopez JC, Hidalgo-Miranda A, Melendez-Zajgla J, Winckler W, Ardlie K, Gabriel SB, Meyerson M, Lander ES, Getz G, Golub TR, Garraway LA, Grandis JR. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichols AC, Chan-Seng-Yue M, Yoo J, Xu W, Dhaliwal S, Basmaji J, Szeto CC, Dowthwaite S, Todorovic B, Starmans MH, Lambin P, Palma DA, Fung K, Franklin JH, Wehrli B, Kwan K, Koropatnick J, Mymryk JS, Boutros P, Barrett JW. A Pilot Study Comparing HPV-Positive and HPV-Negative Head and Neck Squamous Cell Carcinomas by Whole Exome Sequencing. ISRN Oncol. 2012;2012:809370. doi: 10.5402/2012/809370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 19.Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010;10:550–560. doi: 10.1038/nrc2886. [DOI] [PubMed] [Google Scholar]
- 20.Rautava J, Syrjanen S. Biology of human papillomavirus infections in head and neck carcinogenesis. Head Neck Pathol. 2012;6(Suppl 1):S3–15. doi: 10.1007/s12105-012-0367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lechner M, Frampton GM, Fenton T, Feber A, Palmer G, Jay A, Pillay N, Forster M, Cronin MT, Lipson D, Miller VA, Brennan TA, Henderson S, Vaz F, O’Flynn P, Kalavrezos N, Yelensky R, Beck S, Stephens PJ, Boshoff C. Targeted next-generation sequencing of head and neck squamous cell carcinoma identifies novel genetic alterations in HPV+ and HPV- tumors. Genome Med. 2013;5:49. doi: 10.1186/gm453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klussmann JP, Mooren JJ, Lehnen M, Claessen SM, Stenner M, Huebbers CU, Weissenborn SJ, Wedemeyer I, Preuss SF, Straetmans JM, Manni JJ, Hopman AH, Speel E. Genetic signatures of HPV-related and unrelated oropharyngeal carcinoma and their prognostic implications. Clin Cancer Res. 2009;15:1779–1786. doi: 10.1158/1078-0432.CCR-08-1463. [DOI] [PubMed] [Google Scholar]
- 23.Arenz A, Ziemann F, Mayer C, Wittig A, Dreffke K, Preising S, Wagner S, Klussmann JP, Engenhart-Cabillic R, Wittekindt C. Increased radiosensitivity of HPV-positive head and neck cancer cell lines due to cell cycle dysregulation and induction of apoptosis. Strahlenther Onkol. 2014;190:839–846. doi: 10.1007/s00066-014-0605-5. [DOI] [PubMed] [Google Scholar]
- 24.Wittekindt C, Wagner S, Mayer CS, Klussmann JP. Basics of tumor development and importance of human papilloma virus (HPV) for head and neck cancer. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2012;11:Doc09. doi: 10.3205/cto000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballo H, Koldovsky P, Hoffmann T, Balz V, Hildebrandt B, Gerharz CD, Bier H. Establishment and characterization of four cell lines derived from human head and neck squamous cell carcinomas for an autologous tumor-fibroblast in vitro model. Anticancer Res. 1999;19:3827–3836. [PubMed] [Google Scholar]
- 26.Lin CJ, Grandis JR, Carey TE, Gollin SM, Whiteside TL, Koch WM, Ferris RL, Lai SY. Head and neck squamous cell carcinoma cell lines: established models and rationale for selection. Head Neck. 2007;29:163–188. doi: 10.1002/hed.20478. [DOI] [PubMed] [Google Scholar]
- 27.Lansford CD, Grenman R, Bier H, Somers KD, Kim SY, Whiteside TL, Clayman GL, Welkoborsky HJ, Carey TE. Head and neck cancers. In: Masters J, Palsson B, editors. Human Cell Culture. Cancer Cell Lines. Part 2. Dordrecht: Kluwer Academic Press; 1999. pp. 185–255. [Google Scholar]
- 28.Tang AL, Hauff SJ, Owen JH, Graham MP, Czerwinski MJ, Park JJ, Walline H, Papagerakis S, Stoerker J, McHugh JB, Chepeha DB, Bradford CR, Carey TE, Prince ME. UM-SCC-104: a new human papillomavirus-16-positive cancer stem cell-containing head and neck squamous cell carcinoma cell line. Head Neck. 2012;34:1480–1491. doi: 10.1002/hed.21962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White JS, Weissfeld JL, Ragin CC, Rossie KM, Martin CL, Shuster M, Ishwad CS, Law JC, Myers EN, Johnson JT, Gollin SM. The influence of clinical and demographic risk factors on the establishment of head and neck squamous cell carcinoma cell lines. Oral Oncol. 2007;43:701–712. doi: 10.1016/j.oraloncology.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rieckmann T, Kriegs M, Nitsch L, Hoffer K, Rohaly G, Kocher S, Petersen C, Dikomey E, Dornreiter I, Dahm-Daphi J. p53 modulates homologous recombination at I-SceI-induced double-strand breaks through cell-cycle regulation. Oncogene. 2013;32:968–975. doi: 10.1038/onc.2012.123. [DOI] [PubMed] [Google Scholar]
- 31.Steenbergen RD, Hermsen MA, Walboomers JM, Joenje H, Arwert F, Meijer CJ, Snijders PJ. Integrated human papillomavirus type 16 and loss of heterozygosity at 11q22 and 18q21 in an oral carcinoma and its derivative cell line. Cancer Res. 1995;55:5465–5471. [PubMed] [Google Scholar]
- 32.Castro F, Dirks WG, Fahnrich S, Hotz-Wagenblatt A, Pawlita M, Schmitt M. High-throughput SNP-based authentication of human cell lines. Int J Cancer. 2013;132:308–314. doi: 10.1002/ijc.27675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greve B, Dreffke K, Rickinger A, Konemann S, Fritz E, Eckardt-Schupp F, Amler S, Sauerland C, Braselmann H, Sauter W, Illig T, Schmezer P, Gomolka M, Willich N, Bolling T. Multicentric investigation of ionising radiation-induced cell death as a predictive parameter of individual radiosensitivity. Apoptosis. 2009;14:226–235. doi: 10.1007/s10495-008-0294-6. [DOI] [PubMed] [Google Scholar]
- 34.Gong J, Traganos F, Darzynkiewicz Z. A selective procedure for DNA extraction from apoptotic cells applicable for gel electrophoresis and flow cytometry. Anal Biochem. 1994;218:314–319. doi: 10.1006/abio.1994.1184. [DOI] [PubMed] [Google Scholar]
- 35.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, Peyrade F, Benasso M, Vynnychenko I, De Raucourt D, Bokemeyer C, Schueler A, Amellal N, Hitt R. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 36.Rieckmann T, Tribius S, Grob TJ, Meyer F, Busch CJ, Petersen C, Dikomey E, Kriegs M. HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother Oncol. 2013;107:242–246. doi: 10.1016/j.radonc.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Spanos WC, Nowicki P, Lee DW, Hoover A, Hostager B, Gupta A, Anderson ME, Lee JH. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135:1137–1146. doi: 10.1001/archoto.2009.159. [DOI] [PubMed] [Google Scholar]
- 38.Gupta AK, Lee JH, Wilke WW, Quon H, Smith G, Maity A, Buatti JM, Spitz DR. Radiation response in two HPV-infected head-and-neck cancer cell lines in comparison to a non-HPV-infected cell line and relationship to signaling through AKT. Int J Radiat Oncol Biol Phys. 2009;74:928–933. doi: 10.1016/j.ijrobp.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pogorzelski M, Ting S, Gauler TC, Breitenbuecher F, Vossebein I, Hoffarth S, Markowetz J, Lang S, Bergmann C, Brandau S, Jawad JA, Schmid KW, Schuler M, Kasper S. Impact of human papilloma virus infection on the response of head and neck cancers to anti-epidermal growth factor receptor antibody therapy. Cell Death Dis. 2014;5:e1091. doi: 10.1038/cddis.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagel R, Martens-de Kemp SR, Buijze M, Jacobs G, Braakhuis BJ, Brakenhoff RH. Treatment response of HPV-positive and HPV-negative head and neck squamous cell carcinoma cell lines. Oral Oncol. 2013;49:560–566. doi: 10.1016/j.oraloncology.2013.03.446. [DOI] [PubMed] [Google Scholar]
- 41.Bradford CR, Zhu S, Ogawa H, Ogawa T, Ubell M, Narayan A, Johnson G, Wolf GT, Fisher SG, Carey TE. P53 mutation correlates with cisplatin sensitivity in head and neck squamous cell carcinoma lines. Head Neck. 2003;25:654–661. doi: 10.1002/hed.10274. [DOI] [PubMed] [Google Scholar]
- 42.Dalianis T. Human papillomavirus and oropharyngeal cancer, the epidemics, and significance of additional clinical biomarkers for prediction of response to therapy (Review) Int J Oncol. 2014;44:1799–1805. doi: 10.3892/ijo.2014.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lechner M, Fenton T, West J, Wilson G, Feber A, Henderson S, Thirlwell C, Dibra HK, Jay A, Butcher L, Chakravarthy AR, Gratrix F, Patel N, Vaz F, O’Flynn P, Kalavrezos N, Teschendorff AE, Boshoff C, Beck S. Identification and functional validation of HPV-mediated hypermethylation in head and neck squamous cell carcinoma. Genome Med. 2013;5:15. doi: 10.1186/gm419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mooren JJ, Kremer B, Claessen SM, Voogd AC, Bot FJ, Peter Klussmann J, Huebbers CU, Hopman AH, Ramaekers FC, Speel EJ. Chromosome stability in tonsillar squamous cell carcinoma is associated with HPV16 integration and indicates a favorable prognosis. Int J Cancer. 2013;132:1781–1789. doi: 10.1002/ijc.27846. [DOI] [PubMed] [Google Scholar]
- 45.Olthof NC, Huebbers CU, Kolligs J, Henfling M, Ramaekers FC, Cornet I, van Lent-Albrechts JA, Stegmann AP, Silling S, Wieland U, Carey TE, Walline HM, Gollin SM, Hoffmann TK, de Winter J, Kremer B, Klussmann JP, Speel EJ. Viral load, gene expression and mapping of viral integration sites in HPV16-associated HNSCC cell lines. Int J Cancer. 2014;136:E207–18. doi: 10.1002/ijc.29112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Demers GW, Halbert CL, Galloway DA. Elevated wild-type p53 protein levels in human epithelial cell lines immortalized by the human papillomavirus type 16 E7 gene. Virology. 1994;198:169–174. doi: 10.1006/viro.1994.1019. [DOI] [PubMed] [Google Scholar]
- 47.Cepeda V, Fuertes MA, Castilla J, Alonso C, Quevedo C, Perez JM. Biochemical mechanisms of cisplatin cytotoxicity. Anticancer Agents Med Chem. 2007;7:3–18. doi: 10.2174/187152007779314044. [DOI] [PubMed] [Google Scholar]
- 48.Foijer F, te Riele H. Check, double check: the G2 barrier to cancer. Cell Cycle. 2006;5:831–836. doi: 10.4161/cc.5.8.2687. [DOI] [PubMed] [Google Scholar]
- 49.Noya F, Chien WM, Broker TR, Chow LT. p21cip1 Degradation in differentiated keratinocytes is abrogated by costabilization with cyclin E induced by human papillomavirus E7. J Virol. 2001;75:6121–6134. doi: 10.1128/JVI.75.13.6121-6134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen CL, Munger K. Direct association of the HPV16 E7 oncoprotein with cyclin A/CDK2 and cyclin E/CDK2 complexes. Virology. 2008;380:21–25. doi: 10.1016/j.virol.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zubillaga-Guerrero MI, Illades-Aguiar B, Leyva-Vazquez MA, Flores-Alfaro E, Castaneda-Saucedo E, Munoz-Valle JF, Alarcon-Romero LC. The integration of HR-HPV increases the expression of cyclins A and E in cytologies with and without low-grade lesions. J Cytol. 2013;30:1–7. doi: 10.4103/0970-9371.107504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caldon CE, Sergio CM, Burgess A, Deans AJ, Sutherland RL, Musgrove EA. Cyclin E2 induces genomic instability by mechanisms distinct from cyclin E1. Cell Cycle. 2013;12:606–617. doi: 10.4161/cc.23512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mazumder S, Plesca D, Almasan A. A jekyll and hyde role of cyclin E in the genotoxic stress response: switching from cell cycle control to apoptosis regulation. Cell Cycle. 2007;6:1437–1442. doi: 10.4161/cc.6.12.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bedrosian I, Lee C, Tucker SL, Palla SL, Lu K, Keyomarsi K. Cyclin E-associated kinase activity predicts response to platinum-based chemotherapy. Clin Cancer Res. 2007;13:4800–4806. doi: 10.1158/1078-0432.CCR-07-0142. [DOI] [PubMed] [Google Scholar]
- 55.Caicedo-Granados E, Lin R, Fujisawa C, Yueh B, Sangwan V, Saluja A. Wild-type p53 reactivation by small-molecule Minnelide in human papillomavirus (HPV)-positive head and neck squamous cell carcinoma. Oral Oncol. 2014;50:1149–1156. doi: 10.1016/j.oraloncology.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Santin AD, Hermonat PL, Ravaggi A, Chiriva-Internati M, Pecorelli S, Parham GP. Radiation-enhanced expression of E6/E7 transforming oncogenes of human papillomavirus-16 in human cervical carcinoma. Cancer. 1998;83:2346–2352. doi: 10.1002/(sici)1097-0142(19981201)83:11<2346::aid-cncr14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 57.Abdulkarim B, Sabri S, Deutsch E, Chagraoui H, Maggiorella L, Thierry J, Eschwege F, Vainchenker W, Chouaib S, Bourhis J. Antiviral agent Cidofovir restores p53 function and enhances the radiosensitivity in HPV-associated cancers. Oncogene. 2002;21:2334–2346. doi: 10.1038/sj.onc.1205006. [DOI] [PubMed] [Google Scholar]
- 58.Park JW, Nickel KP, Torres AD, Lee D, Lambert PF, Kimple RJ. Human papillomavirus type 16 E7 oncoprotein causes a delay in repair of DNA damage. Radiother Oncol. 2014;113:337–344. doi: 10.1016/j.radonc.2014.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 60.Butz K, Geisen C, Ullmann A, Spitkovsky D, Hoppe-Seyler F. Cellular responses of HPV-positive cancer cells to genotoxic anti-cancer agents: repression of E6/E7-oncogene expression and induction of apoptosis. Int J Cancer. 1996;68:506–513. doi: 10.1002/(SICI)1097-0215(19961115)68:4<506::AID-IJC17>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 61.Gubanova E, Brown B, Ivanov SV, Helleday T, Mills GB, Yarbrough WG, Issaeva N. Downregulation of SMG-1 in HPV-positive head and neck squamous cell carcinoma due to promoter hypermethylation correlates with improved survival. Clin Cancer Res. 2012;18:1257–1267. doi: 10.1158/1078-0432.CCR-11-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martens-de Kemp SR, Dalm SU, Wijnolts FM, Brink A, Honeywell RJ, Peters GJ, Braakhuis BJ, Brakenhoff RH. DNA-bound platinum is the major determinant of cisplatin sensitivity in head and neck squamous carcinoma cells. PLoS One. 2013;8:e61555. doi: 10.1371/journal.pone.0061555. [DOI] [PMC free article] [PubMed] [Google Scholar]