Abstract
Epithelial-mesenchymal transition (EMT) is considered as the most important mechanism that underlies the initiation of cancer metastasis. Here we report that the naturally existing flavonoid, hispidulin is capable of preventing human colorectal cancer cells from hypoxia-induced EMT. The treatment of the cells with hispidulin reversed the EMT-related phenotype that has the morphological changes, down-regulation of E-cadherin, and hypoxia-induced cell migration and invasion. The effect was mediated at least in part by inhibiting the mRNA and protein expressions of HIF-1α via modulation of PTEN/PI3K/Akt pathway. In addition, we found that hispidulin-mediated prevention of the E-cadherin down-regulation and cell motility involved blockade of the hypoxia-induced up-regulation of Snail, Slug and Twist. Hispidulin was also effective in increasing expression of E-cadherin mRNA in HT29 colorectal cancer xenografts implanted in the nude mice. In summary, this study showed that hispidulin can prevent EMT induced by hypoxia, the environment that commonly exists in the center of a solid tumor. Given the low toxicity of hispidulin to the healthy tissues, our study suggests that hispidulin can serve as a safe therapeutic agent for suppressing cancer metastasis.
Keywords: Hispidulin, EMT, Hypoxia, PI3K/Akt, PTEN
Introduction
Colon cancer, ranking as the third most common malignancy worldwide, accounts for nearly 1 million newly diagnosed cases and half a million deaths each year [1]. Clinical studies showed that in a vast majority of the patients colorectal cancer is metastasized and therefore it becomes incurable [2,3]. Cancer metastasis encompasses a series of highly coordinated cellular events that promote detaching of a tumor cell from the primary site. The escaped cell invades the surrounding blood or lymphatic vessels, survives in the blood and extravasates into a distant tissue, engages a blood supply and starts to grow as secondary tumor [4]. Epithelial cells constituting the surface and cavities of the body and organs are immobile and firmly glued together. However, for metastasis to occur, epithelial cells are known to transition to mesenchymal cells. As a normal process of embryonic development, EMT involves transformation of the epithelial cells to mesenchymal cells which are highly mobile. Cancer cells have been found to utilize this mechanism of converting immobile epithelial cells into movable mesenchymal cells for their spread [5]. Thus, as a consequence of EMT, specific morphological changes leading to loss of cell-cell tight contact occur in the epithelial cells. The transformed cells acquire characteristics of mesenchymal cells which facilitates their mobility and hence promotes invasiveness into the surrounding tissue and/or distant organs [6]. The epithelial protein E-cadherin is down regulated and mesenchymal proteins, such as vimentin and N-cadherin are up-regulated. Moreover, the cells that have undergone EMT are found to express matrix metalloproteases, the major enzymes that participate in the migration, spreading, tissue invasion and metastasis of the tumor cell [7].
Mounting evidence exists to suggest that hypoxic environment of the solid tumors including colorectal cancer is responsible for their poor response to treatment [8,9]. The hypoxic environment in most of the rapidly growing solid tumors results from the poor development of angiogenic vessels which leads to insufficient supply of oxygen [10,11]. Weinmann et al [12] have shown that hypoxic microenvironment plays a key role in the progression of metastasis. Moreover, accumulating data suggest that hypoxia inducible factors (HIFs), especially HIF-1, promote EMT by regulating the expression and activity of major transcription factors including TWIST, Snail, Slug, SIP1 and ZEB1 [13-16]. Given the fact that hypoxia-induced EMT plays a key role in metastasis, it appears that EMT can be used as a promising target for developing new and effective anti-cancer therapy.
In the recent years, the use of natural products in cancer prevention and control has attracted an increasing amount of attention. Epidemiological studies have shown that high consumption of flavonoids decreased the risk of several types of cancer, suggesting the cancer controlling potential of these compounds [17]. Hispidulin (4’,5,7-trihydroxy-6-methoxyflavone), an active flavonoid isolated from a number of traditional Chinese medicinal herbs e.g., S. Involucrata [18] has been shown to possess a variety of pharmacological activities including antifungal, anti-inflammatory, antioxidant, anti-thrombosis, antiepileptic, neuroprotective and anti-osteoporotic [19-27]. Hispidulin has also been found to inhibit the in vitro growth of human cancer cells including pancreatic, gastric, and ovarian and glioblastoma [28-31]. Previously, we evidenced the apoptotic effect of hispidulin in hepatocellular carcinoma cells [32]. In the present study, we attempted to investigate the effect of hispidulin on hypoxia-induced EMT in colorectal cancer cells which has not been studied before.
Materials and methods
Cell line and culture conditions
The human colon cancer cell line HT-29 was obtained from Centre for Cell Resources of Shanghai Institutes for Life Sciences, Chinese Academy of Sciences (Shanghai, China). The cells were cultured and maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 mg/ml streptomycin, and 25 mg/ml amphotericin B. Cells were incubated at 37°C in a humidified incubator with 5% CO2 and 95% air, and regularly examined under an inverted microscope.
For treatments, cells were seeded in 6-well plate at a density of 5 × 104 cells/cm2 and cultured in normoxic conditions for 24 hours to allow them to adhere to the substratum. The medium was then replaced with new medium supplemented with Hispidulin at indicated concentrations. In experiments designed to evaluate the role of hypoxia, cells were seeded in normoxic conditions and grown to 65-70% confluency and then they were incubated in strictly controlled hypoxic conditions (1% O2) for the indicated period of time.
Viability assay
For cell viability assay, the HT-29 cells (1.2 × 105 cells/ml) were cultured in 96-well plates and after 24 hours of plating, they were treated with the drugs. Antineoplastic effects of the drugs were examined after treatment for the indicated time by the nonradioactive cell proliferation assay using a commercial kit (Promega Corporation, Madison, WI). The MTT-based method was conducted following the manufacturer’s instructions and metabolic conversion of tetrazolium salt to formazan was measured by reading the absorbance at 570 nm.
Wound scratch assay
Each well of 24-well tissue culture plate was seeded with the cells to a final density of 100,000 cells and the plated were incubated at 37°C in 5% CO2 for 24 hours to permit their adhesion formation of a confluent monolayer. Then a scratch of approximately 0.4-0.5 mm in width was made on these cells with a sterile pipette tip. Cell surface was then washed with serum-free culture medium for three times to remove dislodged cells. Wound closure was monitored by capturing digitized images with an inverted microscope (MOTIC CHINA GROUP CO., Xiamen, China) and digital camera (Nikon, Tokyo, Japan) at 0, 12 and 24 hours of the scratching. The images were then analyzed using Image-J software.
Cell invasion assay
The 24-well plates with Transwell filters coated with Matrigel (8-μm pore size; BD Biosciences, San Jose, California) were used for the cell invasion assays [33]. Equal numbers (1 × 105) of cells, untransfected or stably transfected with pEGFP-N1-SOX2 or pEGFP-N1 were plated and starved overnight in serum-free medium. Then they were trypsinized and washed three times in DMEM containing 1% FBS. A total of 1 × 105 cells were then resuspended in 500 μl DMEM containing 1% FBS and added to the upper chamber of the well, while MEM with 10% FBS was added to the lower chamber as a chemoattractant. For controls, medium containing 1% FBS was added to the lower chamber. After 24 hours of incubation, Matrigel and the cells remaining in the upper chamber were removed by cotton swabs. The cells on the lower surface of the membrane were fixed in formaldehyde and stained with hematoxylin. The cells were photographed and counted in at least five randomly selected microscopic fields (magnification, ×200).
Quantitative real-time PCR
Total RNA was extract from the cells using a simple Total RNA Kit (TIANGEN Co., Beijing, China) and 3 µg of RNA was converted into cDNA using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA). Real-time PCR was carried out in MX3000p PCR system (Stratagene, Europe). Reaction was performed using KAPASYBR Green fast PCR master mix PCR kit (It contains all the PCR components along with SYBR Green dye). The synthesis of the primers used for SYBRGreen reverse transcription-PCR (qRT-PCR) was based on the published sequence [34]. Data were analyzed with comparative ΔCt method (ABPrism software, Applied Biosystems, Foster City, CA) using GAPDH as an internal normalization control.
Western blotting
Western blot analysis was performed using a standard protocol. The cell lysate samples (30-50 μg) were mixed with sample buffer, boiled for 5 minutes, electrophoresed in 10% sodium dodecyl sulfate polyacrylamide gel and then transferred to PVDF membranes. The membrane was then blocked in PBS containing 5% bovine serum albumin (BSA) for 1 hour at room temperature. Then incubated at 4°C overnight with primary antibodies against, N-cadherin, E-cadherin, vimentin, fibronectin, α-SMA, HIF-1α, PTEN, PI3K, Akt, Snail, Slug and Twist (1:2000 dilution) and p-Akt (1:1000 dilution) (Cell Signaling Technology, Danvers, Massachusetts) in Tris-buffered saline. After washing, the membranes were incubated with HRP-conjugated secondary antibodies (Beyotime Institute of Biotechnology, Shanghai, China). ECL reagent (7Sea Biotech., Shanghai, China) was used for detection of the signals according to manufacturer’s instructions.
Immunofluorescence staining and confocal microscope imaging
HT-29 cells were cultured in chambered slides (Thermo Scientific Nunc Lab-Tek Chamber Slides, USA) and after adherence, they were treated with DMEM containing the drugs or vehicle (0.01% DMSO) for 24 hours. Following the treatment, the cells were fixed and permeabilized with methanol/acetone (1:1). Cells were then incubated with monoclonal antibodies of E-cadherin (Cell Signaling Technology, Inc., Beverly, MA, USA, 1:100) or β-catenin (Cell Signaling Technology, Inc., Beverly, MA, USA, 1:100). Next, the cells were incubated with Cy3-labeled secondary antibody (Beyotime Institute of Biotechnology, Shanghai, China, 1:100) at room temperature for 1 hour in the dark, followed by incubation with DAPI (Biosharp Biotech., Hefei, China, 1:1000) for 5 minutes. After washing three times with PBS to remove excessive staining solution, the cells were examined under a laser scanning confocal microscope (Olympus FV1000S-SIM/IX81, Tokyo, Japan).
Luciferase reporter assay
Luciferase reporter assay was performed as described previously [35]. Briefly, the HT-29 cells were seeded into 96-well plates and cultured to 80% confluence. Cells were then transfected with plasmid containing HIF-responsive elements (HRE) and renilla luciferase-pGL3 as internal control for transfection efficiency using Lipofectamine 2000 reagent (Invitrogen, CA) following manufacturer’s instructions. After transfection, the luciferase activity was measured using a commercial kit (Promega Corp., Madison, MI) according to manufacturer’s manual.
Silencing HIF-1α with shRNA and overexpression HIF-1α
For silencing HIF-1α, the HIF-1α-targeting shRNA was obtained from Origene (Rockville, MD). Scrambled shRNA was used as a negative control. Transfection of the cells with shRNA was performed following the standard protocol. Briefly, the cells were plated at a density of 5 × 103 cells/well in a 6-well culture plate and incubated to allow 70-80% confluence (about 24 h). The cells were then starved in serum-free culture for 1 hour. The transfection mixture containing HIF-1α-targeting shRNA and Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) was incubated for 20 minutes at room temperature. The cells were then incubated with the above mixture for 5 hours at 37°C in a humidified atmosphere containing 5% CO2. Subsequently, the cells were washed with PBS and maintained in DMEM containing 10% FBS for 48 hours. The expression of HIF-1α was detected by western blot analysis.
For HIF-1α overexpression, the HT29 cells were grown to sub-confluent densities and transfected with pcDNA3-EGFP empty or pcDNA3-HA-HIF-1α vector constructs using the transfection reagent TransIT-LT-1 and following manufacturer’s instructions (Mirus Bio Corp., Madison, WI). Overexpression of HIF-1α was confirmed by Western blotting and real-time PCR analysis.
The in vivo experiments to measure anti-metastasis activity of hispidulin
Animal experiments were approved by the Animal Care and Use Committee of Qingdao University. The HT-29 cells were injected into the nude mice. Tumor appearance was inspected twice per week. Once the tumor masses became established and palpable, animals were randomly allocated to 4 groups (n = 15 per group) to receive intraperitoneal (IP) injections of hispidulin dissolved in 0.9% sodium chloride in 1% DMSO. Group (A) received vehicle whereas groups (B), (C) and (D) were administered with 10 mg/kg, 20 mg/kg or 40 mg/kg of hispidulin respectively per day. Tumor volume was calculated as = (length × width2)/2, where length and width were measured as the longest and the shortest orthogonal axes respectively. At the end of the experiment, the animals were sacrificed and tumors were harvested to prepare the RNA and measure the expression of E-cadherin using qRT-PCR.
Statistical analysis
Data are expressed as means ± SD. Analysis of variance (ANOVA) followed by Dunnett’s t test were performed to determine if the difference between groups was significant. Values of P < 0.05 were considered statistically significant.
Results
Hispidulin was more effective in inhibiting the viability of the cells grown in hypoxia than those in normoxic conditions
Before investigating the effect of hispidulin on cells grown under hypoxia, we determined the sensitivity of the cells to hispidulin under normoxic conditions. As shown in Figure 1A, hispidulin exhibited a dose- and time-dependent anti-proliferative effect as evaluated by MTT assay. Treatment with different doses of Hispidulin (0, 12.5 and 50 μM) for 12 hours reduced the cell proliferation by 8.3 ± 3.2, 14.5 ± 5.2 and 27.2 ± 6.7%, respectively.
Figure 1.
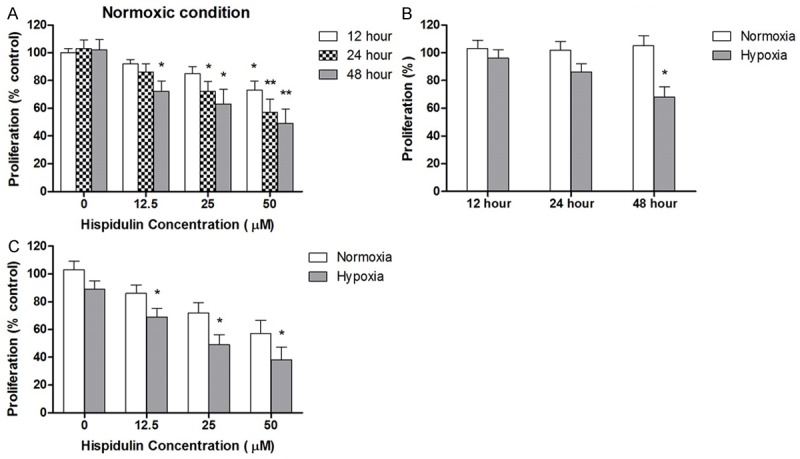
Hispidulin inhibits the proliferation of HT-19 cells dose- and time-dependently. HT-29 cells grown under normoxia or hypoxia were challenged with different doses of hispidulin at the indicated time points. Cell proliferation was evaluated by MTT assay. Data are presented as mean ± SD of three independent experiments. *P < 0.05 vs. control, **P < 0.01 vs. control.
After 24-hour of incubation with hispidulin at 0, 12.5 and 50 μM concentrations, cell viability was decreased by 13.3 ± 6.2, 27.2 ± 7.3 and 42.3 ± 9.6%, respectively. When incubation was prolonged to 72 hours, further decrease in cell viability was observed as shown in Figure 1A. Then we determined the hispidulin sensitivity of the cells grown in hypoxia. The viability of HT-29 cells exposed to 1% O2 for 12, 24 or 48 hours, was determined by the MTT-based Cell Titer Promega assay. As shown in Figure 1B, the cell viability was not significantly affected with an exposure to hypoxia for 24 hours, however, after 48 hours the viability was reduced by 32.3 ± 5.4%. Therefore, in the subsequent experiments, the cells were exposed to hypoxia for 24 hours. The cells were cultured under normoxia or hypoxia and treated with hispidulin for 24 hours to determine its antineoplastic effect. As shown in Figure 1C, the inhibitory effect of hispidulin on viability was significantly higher on the cells grown under hypoxia than those cultured under normoxia.
Hispidulin prevented the hypoxia-induced migration and invasion of HT-29 cells
Next, we examined the effect of hispidulin on hypoxia-induced cell migration and invasion. The cell migration was determined by using the wound healing assay. As shown in Figure 2A, hypoxia caused a 2.65-fold increase in the cell migration which was significantly suppressed with hispidulin treatment. Furthermore, the hypoxia-induced invasiveness of HT-29 cells detected with transwell assay was substantially abolished with hispidulin (Figure 2B).
Figure 2.
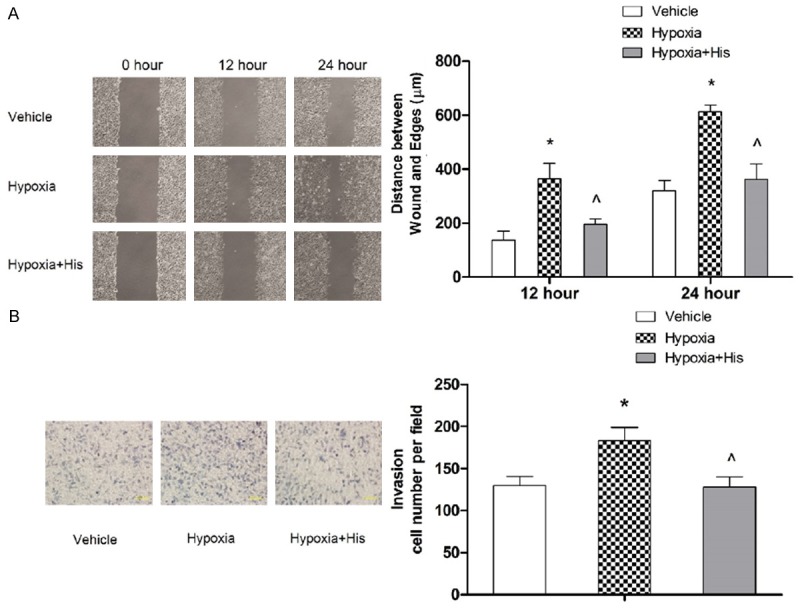
Hispidulin suppresses hypoxia-induced migration (A) and invasion (B) in HT-29 cells. HT-29 cells growing in hypoxia were treated with hispidulin (25 μM) for 24 hours. Cell migration and invasion were assessed by wound scratch and Transwell assays, respectively. The results represent mean ± SD from three independent experiments. *P < 0.05 vs. control, ^P < 0.05 vs. hypoxia.
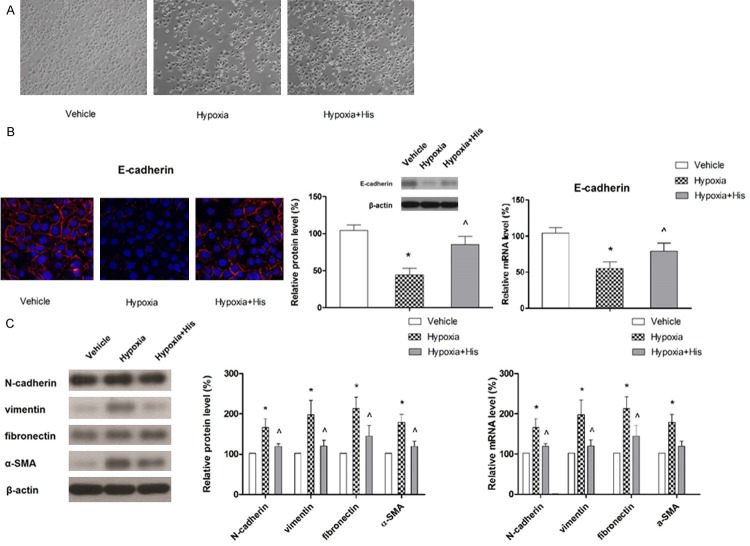
Hispidulin affected the early changes in hypoxia-induced EMT markers in HT-29 cells
Next we sought to determine if hispidulin mediated the aforementioned effects through modulation of hypoxia-induced EMT. Thus, we examined for the changes in cell morphology and molecular markers 24 hours after exposure to hypoxia. Epithelial cells normally exhibit an apical-basal polarity, express high levels of E-cadherin, and form adhesive junctions with adjacent cells. By contrast, mesenchymal cells lack cell polarity, exhibit a spindle-like morphology and overexpress mesenchymal markers, such as N-cadherin and vimentin [36]. As shown in Figure 3A, exposure to hypoxia resulted in a change in the morphology and detachment of the cells. The expression of epithelial marker E-cadherin examined by immunoflorescence, was found to be decreased with exposure to hypoxia while hispidulin treatment reversed this effect (Figure 3B). Similarly, western blot and qRT-PCR analyses demonstrated that suppression of E-cadherin expression caused by hypoxia was restored with hispidulin treatment. In order to further confirm the effect of hispidulin on hypoxia-induced EMT, the expressions of mesenchymal markers, N-cad-herin, vimentin, fibronectin and α-SMA were also examined by western blotting. As shown in Figure 3C, hypoxia led to a marked up-regulation of N-cadherin, vimentin, fibronectin and α-SMA and the effect was suppressed with hispidulin. Taken together, these results clearly indicate that hispidulin inhibited the hypoxia-induced cellular transition from epithelial to mesenchymal phenotype.
Figure 3.
Hispidulin prevents hypoxia-induced EMT. HT-29 cells growing in hypoxia were treated with hispidulin (25 μM) for 24 hours. A. Effect of hispidulin on cell morphology. The images represent three independent experiments. B. The effect of hispidulin on the expression of E-cadherin examined by florescence staining, western blotting and qRT-PCR. The images and blot are representative of three independent experiments. C. The effect of hispidulin on the expressions of N-cadherin, vimentin, fibronectin and α-SMA determined by western blotting and qRT-PCR. The immunoblot represents three independent experiments. Data are presented as mean ± SD. *P < 0.05 vs. control, ^P < 0.05 vs. hypoxia.
Hispidulin blocked the hypoxia-induced up-regulation of Snail, Slug and Twist
Transcriptional repressors Snail, Slug and Twist are known to regulate the expression of mesenchymal and epithelial markers and hence play a crucial role in EMT [37]. Therefore, in order to completely understand the mechanism of hispidulin’s action, we investigated the effect of hispidulin on these regulatory molecules. As shown in Figure 4, exposure to hypoxia caused a significant increase in the protein and mRNA expressions of Snail, Slug and Twist. Upon treatment with hispidulin, the hypoxia-caused up-regulation of these transcription repressors was inhibited at both protein and mRNA levels. The results indicate that hispidulin exerted its inhibitory effect on mesenchymal markers by modulating the expression of these transcriptional repressors.
Figure 4.
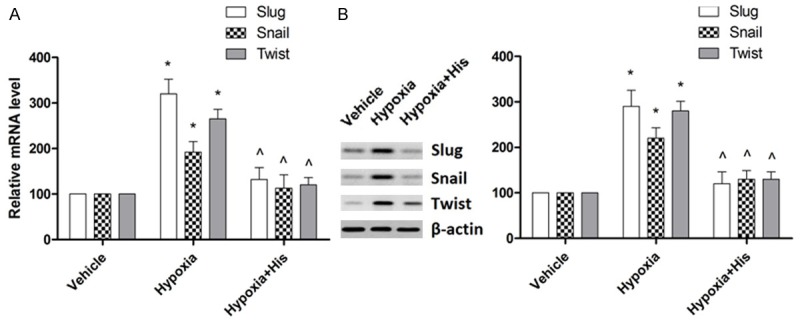
Effect of hispidulin on the expressions of mRNA (A) and protein (B) of Snail, Slug and Twist. HT-29 cells were treated with hispidulin (25 μM) for 24 hours. The mRNA and protein expressions were determined by qRT-PCR and western blotting respectively. The immunoblot represents three independent experiments. Data are presented as mean ± SD from three independent experiments. *P < 0.05 vs. control, ^P < 0.05 vs. hypoxia.
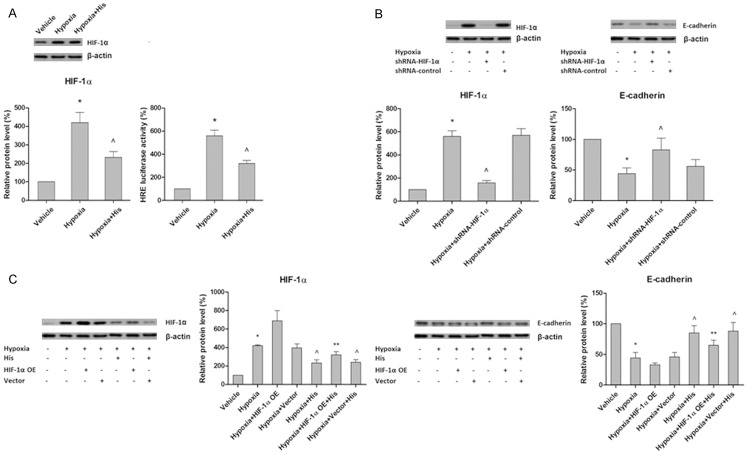
Inhibition of HIF-1α is involved in the prevention of hypoxia-induced EMT by hispidulin
Accumulating evidence suggests the involvement of HIF, especially HIF-1α, as a major upstream regulator of Snail, Slug and Twist expressions in the process of EMT [38,39]. Hence, we attempted to explore whether the preventing effect of hispidulin against hypoxia-induced EMT involves modulation of the HIF-1α expression. As shown in Figure 5A, hispidulin treatment of the cells attenuated the hypoxia-triggered accumulation of HIF-1α. To further verify the role of HIF-1α in hypoxia-mediated EMT, we silenced HIF-1α with shRNA. As it was expected, blocking of HIF-1α mimicked the action of hispidulin and inhibited the suppression of hypoxia-caused E-cadherin (Figure 5B). Furthermore, the inhibitory effect of hispidulin on EMT was not complete in the cells overexpressing HIF-1α, as shown in Figure 5C. Taken together, our results suggest that hispidulin prevented the hypoxia-induced EMT by inhibiting HIF-1α.
Figure 5.
HIF-1α inhibition is involved in the prevention of hypoxia-induced EMT by hispidulin. HT-29 cells were incubated under hypoxic conditions in the presence or absence of hispidulin (25 μM). A. Hispidulin suppressed the hypoxia-induced expression of HIF-1α. B. Silencing of HIF-1α with shRNA restored hypoxia-induced reduction in E-cadherin expression. C. Overexpression of HIF-1α partially blocked the inhibitory effect of hispidulin on hypoxia-induced E-cadherin expression. The immunoblots represent three independent experiments. Data are presented as mean ± SD from three independent experiments. *P < 0.05 vs. control, ^P < 0.05 vs. hypoxia, **P < 0.05 vs. hypoxia + His.
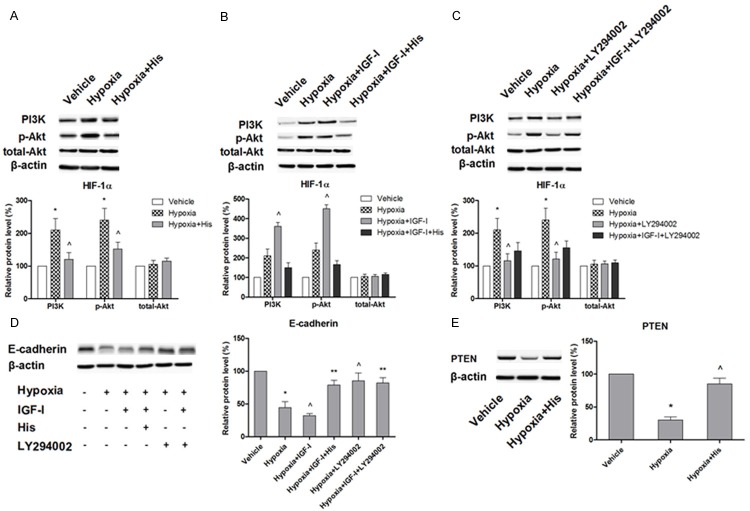
Hispidulin-mediated blockade of HIF-1α and EMT involved PI3K/Akt pathway
The crucial role of PI3K/Akt signaling in stabilizing HIF-1α in hypoxia has been evidenced [40]. It has also been reported that activation of PI3K/Akt signaling increased the expression of HIF-1α by promoting the translation without altering the mRNA transcription [41,42]. Therefore, we attempted to investigate the effect of hispidulin on PI3k/Akt signaling to tease out the involvement of this signaling in modulating the HIF-1α expression and function. As shown in Figure 6A, exposure of HT29 cells to hypoxia for 24 hours induced a substantial increase in the expression of phosphorylated Akt and PI3K but that of total Akt remained unchanged suggesting the effect to be post-translational. When treated with hispidulin for 24 hours, the increase in levels of phosphorylated Akt and PI3K was reversed whereas total Akt levels remained unaffected. Next, we aimed to further elucidate the mechanism of hispidulin-mediated modulation of PI3k/Akt signaling and treated the cells with IGF-1, the activator of PI3k/Akt in the presence and absence of hispidulin. As shown in Figure 6B, treatment of the cells with 10 ng/ml of IGF-I for 2 hours, significantly augmented the hypoxia-stimulated expression of Phosphorylated PI3K and Akt but not of total PI3K/Akt. In the presence of hispidulin, the effect was blocked. Then we utilized a pharmacological inhibitor of PI3K/Akt, LY294002, to further verify the involvement of this signaling pathway. We found that under hypoxic conditions, treatment with 20 μM of LY294002 for 24 hours produced the same results as hispidulin, that is, it decreased the expression of HIF-1α (Figure 6C). Also, when HT29 cells pretreated with LY294002 for 24 hours were incubated with a combination of LY294002 and IGF-I for 2 hours the IGF-I-induced up-regulation of HIF-1α was inhibited. In parallel, LY294002 also inhibited the expressions of phosphorylated PI3K/Akt and blocked their up-regulation by hypoxia. Taken together, these results suggest that hispidulin-caused suppression of HIF-1α was most probably mediated via post-translational down regulation of PI3K/Akt signaling.
Figure 6.
Western blot analysis to show the effect of hispidulin on: A. hypoxia-induced phosphorylated/total Akt and PI3K expressions. HT-29 cells were grown under hypoxic conditions in the presence or absence of hispidulin (25 μM). B. IGF-1-up-regulated expression of phosphorylated Akt and PI3K. HT-29 cells were incubated under hypoxia in the presence or absence of hispidulin then treated with IGF-I (10 ng/ml) for 2 hours. C. Effect of LY294002 on the expressions of phosphorylated/total PI3k and Akt in the presence and absence of IGF-1. B. HT-29 cells were incubated under hypoxia in the presence or absence of hispidulin then treated with IGF-I (10 ng/ml) for 2 hours. D. E-cadherin expression in response to hispidulin, IGF-1 and LY294002. C & D. HT-29 cells were grown under hypoxia in the presence or absence of LY294002 then treatment with IGF-I (10 ng/ml) for 2 hours. E. Effect of hispidulin on hypoxia-inhibited expression of PTEN. The immunoblots represent three independent experiments. Data are presented as mean ± SD from three independent experiments. *P < 0.05 vs. control, ^P < 0.05 vs. hypoxia, **P < 0.05 vs. hypoxia + IGF-I.
Next, we explored the link of PI3K/Akt signaling pathway with hypoxia-induced EMT and effect of hispidulin on it. As shown in Figure 6D, IGF-1 significantly augmented the hypoxia-induced inhibition of E-cadherin expression which was significantly blocked with hispidulin pretreatment. Similarly, IGF-1 suppressed the E-ca-dherin expression whereas pretreatment of the cells with LY294002E for 24 hours blocked this effect suggesting the implication of PI3k/Akt in EMT. This observation also proposes that LY294002 mimicked the action of hispidulin. Furthermore, these results clearly suggest that hispidulin prevented the hypoxia-induced EMT by modulating the HIF-1α expression/activity via inhibition of PI3K/Akt signaling.
Hispidulin inhibited PI3K/Akt signaling by regulating PTEN
Phosphatase and tensin homolog (PTEN), one of the most common suppressors of malignancy, antagonizes the PI3K signaling by removing the phosphate group from the D3 position of phosphatidylinositol trisphosphate and phosphatidylinositol bisphosphate [43]. Recently, a growing body of evidence has indicated that PTEN plays a role of hypoxia-responsive factor [44]. Hence, we examined the effect of hispidulin on PTEN expression. As shown in Figure 6E, the hypoxia-induced significant decrease in the expression of PTEN was reversed with hispidulin. Combining these observations with aforementioned results, we suggest that hispidulin attenuated hypoxia-induced EMT by modulating the PTEN/PI3k/Akt/HIF-1α pathway.
Hispidulin increased the in vivo expression of E-cadherin mRNA
After obtaining the encouraging results from in vitro experiments, we sought to investigate the in vivo effect of hispidulin. The nude mice were grafted with HT29 colorectal cancer cells and the tumors were allowed to grow to 100 mm3. Then the mice were treated with different doses of hispidulin, 40 mg, 20 mg, and 10 mg/kg/day. The effectiveness of the treatment was evaluated by measuring the tumor volume. As shown in Figure 7A, hispidulin inhibited the growth of tumor dose-dependently and the anti-tumor effect was significant at the dose of 20 mg/kg/day (P < 0.05 vs. vehicle). Moreover, the PCR results showed that hispidulin caused a marked and significant increase in the transcription of E-cadherin mRNA (P < 0.05), suggesting the in vivo effectiveness of this compound in controlling EMT (Figure 7B).
Figure 7.
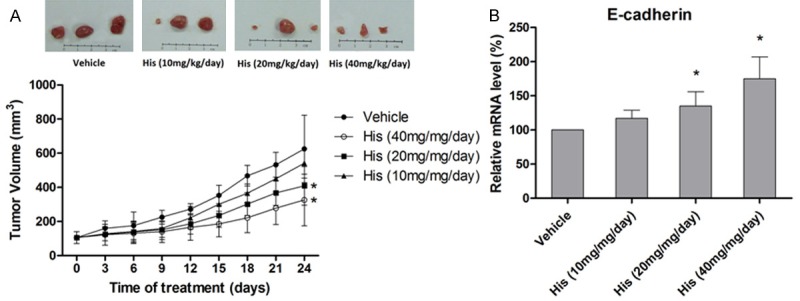
In vivo effect of hispidulin on E-cadherin expression. A. Tumors representing each of the experimental groups (n = 15 per group). Relative tumor volumes are expressed as mean ± SD. B. Effect of hispidulin on gene transcription of E-cadherin measured by qRT-PCR in the extracts of tumors. Data are presented as mean ± SD of three independent experiments. *P < 0.05 vs. vehicle.
Discussion
Hispidulin, an active constituent of traditional Chinese medicine, has been used for treating fungal infection and inflammation for thousands of years in Asia [19]. The compound has also been reported to display anti-cancer effect in vivo and in vitro [45]. However, the effect of hispidulin on EMT, a phenomenon that plays a vital role in cancer progression and metastasis, has not been studied before. The hypoxic microenvironment in the central region of solid tumors is known to induce EMT and promote invasiveness of cancer cells [46]. One of the characteristics of EMT is that instead of epithelial cell marker, E-cadherin, the cells express mesenchymal markers (vimentin and N-cad-herin) [37]. In the context of colorectal cancer, hypoxia has also been known to affect the stromal cells, a factor associated with poor prognosis [47]. The main finding of the present study is that hispidulin can effectively block the hypoxia-promoted EMT in colorectal cancer cells by inhibiting the PTEN/PI3k/Akt/HIF-1α signaling pathway, at least in part.
The adaptive mechanism triggered in response to hypoxia is known to involve the recruitment/stabilization of HIF-1α [16]. HIF-1α in turn, dimerizes with HIF-1β, translocates to the nuclei and binds with hypoxia-responsive element in the promoter of several hypoxia-dependent target genes. As a result, transcription of the genes required for sustaining the cells in hypoxic conditions is activated [48]. Thus, an up-regulation of HIF-1α has been suggested to occur in many solid tumors. Moreover, the increased HIF-1α expression has been correlated with angiogenesis, cell invasiveness metastasis and a poor prognosis of the disease [49,50]. In particular, HIF-1α has been shown to play an essential role in executing EMT in an earlier study by Cannito et al [46]. Accordingly, HIF-1α appears to be a suitable target for suppressing metastasis and developing anti-cancer therapy. In this study, we found that hispidulin significantly attenuated the hypoxia-induced increase in HIF-1α level, thus suppressing the adaptive mechanism of cancer cells for survival under hypoxic conditions. Furthermore, with experimental manipulations, such as silencing and overexpression of HIF-1α, we found that hispidulin effectively blocked metastasis of HT29 cells mainly by inhibiting HIF-1α expression.
Under hypoxia, accumulation of HIF-1α results from shutdown of the proteasomal degradation system due to the lack of oxygen. However, HIF-1α expression has also been found to be modulated by a number of signaling pathways, including AMPK, PI3k/Akt, and extracellular signal-regulated kinase (ERK) [51]. Previously, we showed that hispidulin-induced apoptosis in hepatocellular carcinoma cells was mediated at least in part by inhibiting PI3k/Akt signaling pathway [32]. Therefore, we postulated that in HT29 cells also, hispidulin might be involved in the regulation of HIF-1α expression via inhibiting PI3k/Akt signaling. In fact, utilizing the activator and inhibitor of PI3k/Akt signaling, we confirmed that hispidulin exerted inhibition of HIF-1α expression involved this pathway in cells grown under hypoxia. Previously, Cannito et al [46] showed that hypoxia-triggered ROS release from mitochondria was mainly responsible for phosphorylating and activating Akt. On the other hand, we observed that in normoxic conditions, hispidulin treatment resulted in ROS release and mitochondrial dysfunction indicating that mechanism of Akt inhibition by hispidulin varies according to the prevailing conditions [32]. In any case, further work is needed to find out if role of ROS generation is involved in hispidulin-mediated anti-cancer effect of hispidulin.
The role of tumor suppressor gene PTEN in regulating HIF-1α through PTEN-PI3K axis has recently been emphasized in cancer models [52-54]. Moreover, flavonoids have been reported to enhance the expression of PTEN. For instance, Xie et al [55] have evidenced genistein-mediated decrease of DNA methylation in the promoter region of multiple tumor suppressor genes (TSGs), such as, ataxia telangiectasia mutated (ATM), adenomatous polyposis coli (APC), and PTEN. Similarly, Park et al [56] demonstrated that pharmacological inhibition of AMPK attenuated the PTEN expression and genistein upregulated PTEN by activating AMPK. Since hispidulin has been shown to reverse the hypoxia-triggered inactivation of AMPK signaling in a number of cancer cell lines by us and other groups [57], it is highly possible that stimulation of PTEN expression is also AMPK-dependent.
In conclusion, hispidulin prevented hypoxia-induced EMT in colorectal cancer cells in vitro and in vivo mainly by inhibiting HIF-1α expression via modulation of PTEN/PI3k/Akt signaling pathway. This finding provides with a novel insight into the mechanism of hispidulin-mediated inhibition of EMT and cancer metastasis.
Acknowledgements
This work was supported by National Natural Science Foundation (No. 81473384 and 31470570), Chongqing Natural Science Foundation (No. cstc2014jcyjA80013), Science Foundation of Chongqing Education Commission (No. kj1400534), and funds from Qingdao University (No. 14-2-3-50-nsh, 13-1-3-74 and 600201304).
Disclosure of conflict of interest
We declare that we have no conflict of interest.
References
- 1.Center MM, Jemal A, Smith RA, Ward E. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59:366–78. doi: 10.3322/caac.20038. [DOI] [PubMed] [Google Scholar]
- 2.McMillan DC, McArdle CS. Epidemiology of colorectal liver metastases. Surg Oncol. 2007;16:3–5. doi: 10.1016/j.suronc.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N. Colorectal cancer. Lancet. 2010;375:1030–1047. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 4.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 5.Garber K. Epithelial-to-mesenchymal transition is important to metastasis, but questions remain. J Natl Cancer Inst. 2008;100:232–233. 239. doi: 10.1093/jnci/djn032. [DOI] [PubMed] [Google Scholar]
- 6.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Xu Y, Chen Y, Chen S, Jia X, Sun T, Liu Y, Li X, Xiang R, Li N. SOX2 promotes tumor metastasis by stimulating epithelial-to-mesenchymal transition via regulation of WNT/beta-catenin signal network. Cancer Lett. 2013;336:379–389. doi: 10.1016/j.canlet.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 8.Meyer T, Hart IR. Mechanisms of tumour metastasis. Eur J Cancer. 1998;34:214–221. doi: 10.1016/s0959-8049(97)10129-0. [DOI] [PubMed] [Google Scholar]
- 9.Baba Y, Nosho K, Shima K, Irahara N, Chan AT, Meyerhardt JA, Chung DC, Giovannucci EL, Fuchs CS, Ogino S. HIF1A overexpression is associated with poor prognosis in a cohort of 731 colorectal cancers. Am J Pathol. 2010;176:2292–301. doi: 10.2353/ajpath.2010.090972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajaganeshan R, Prasad R, Guillou PJ, Poston G, Scott N, Jayne DG. The role of hypoxia in recurrence following resection of Dukes’ B colorectal cancer. Int J Colorectal Dis. 2008;23:1049–55. doi: 10.1007/s00384-008-0497-x. [DOI] [PubMed] [Google Scholar]
- 11.Lunt SJ, Chaudary N, Hill RP. The tumor microenvironment and metastatic disease. Clin Exp Metastasis. 2009;26:19–34. doi: 10.1007/s10585-008-9182-2. [DOI] [PubMed] [Google Scholar]
- 12.Weinmann M, Belka C, Plasswilm L. Tumour hypoxia: impact on biology, prognosis and treatment of solid malignant tumours. Onkologie. 2004;27:83–90. doi: 10.1159/000075611. [DOI] [PubMed] [Google Scholar]
- 13.Hill RP, Marie-Egyptienne DT, Hedley DW. Cancer stem cells, hypoxia and metastasis. Semin Radiat Oncol. 2009;19:106–11. doi: 10.1016/j.semradonc.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Kim WY, Perera S, Zhou B, Carretero J, Yeh JJ, Heathcote SA, Jackson AL, Nikolinakos P, Ospina B, Naumov G, Brandstetter KA, Weigman VJ, Zaghlul S, Hayes DN, Padera RF, Heymach JV, Kung AL, Sharpless NE, Kaelin WG Jr, Wong KK. HIF2alpha cooperates with RAS to promote lung tumorigenesis in mice. J Clin Invest. 2009;119:2160–70. doi: 10.1172/JCI38443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, Teng SC, Wu KJ. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10:295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 16.Zhou G, Dada LA, Wu M, Kelly A, Trejo H, Zhou Q, Varga J, Sznajder JI. Hypoxia-induced alveolar epithelial-mesenchymal transition requires mitochondrial ROS and hypoxia-inducible factor 1. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1120–30. doi: 10.1152/ajplung.00007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clere N, Faure S, Martinez MC, Andriantsitohaina R. Anticancer properties of flavonoids: roles in various stages of carcinogenesis. Cardiovasc Hematol Agents Med Chem. 2011;9:62–77. doi: 10.2174/187152511796196498. [DOI] [PubMed] [Google Scholar]
- 18.Way TD, Lee JC, Kuo DH, Fan LL, Huang CH, Lin HY, Shieh PC, Kuo PT, Liao CF, Liu H, Kao JY. Inhibition of epidermal growth factor receptor signaling by Saussurea involucrata, a rare traditional Chinese medicinal herb, in human hormone-resistant prostate cancer PC-3 cells. J Agric Food Chem. 2010;58:3356–65. doi: 10.1021/jf903793p. [DOI] [PubMed] [Google Scholar]
- 19.Yin Y, Gong FY, Wu XX, Sun Y, Li YH, Chen T, Xu Q. Anti-inflammatory and immunosuppressive effect of flavones isolated from Artemisia vestita. J Ethnopharmacol. 2008;120:1–6. doi: 10.1016/j.jep.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 20.Kavvadias D, Sand P, Youdim KA, Qaiser MZ, Rice-Evans C, Baur R, Sigel E, Rausch WD, Riederer P, Schreier P. The flavone hispidulin, a benzodiazepine receptor ligand with positive allosteric properties, traverses the blood-brain barrier and exhibits anticonvulsive effects. Br J Pharmacol. 2004;142:811–20. doi: 10.1038/sj.bjp.0705828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan RX, Lu H, Wolfender JL, Yu TT, Zheng WF, Yang L, Gafner S, Hostettmann K. Mono- and sesquiterpenes and antifungal constituents from Artemisia species. Planta Med. 1999;65:64–7. doi: 10.1055/s-1999-13965. [DOI] [PubMed] [Google Scholar]
- 22.Nagao T, Abe F, Kinjo J, Okabe H. Antiproliferative constituents in plants 10. Flavones from the leaves of Lantana montevidensis Briq. and consideration of structure-activity relationship. Biol Pharm Bull. 2002;25:875–9. doi: 10.1248/bpb.25.875. [DOI] [PubMed] [Google Scholar]
- 23.Chen YT, Zheng RL, Jia ZJ, Ju Y. Flavonoids as superoxide scavengers and antioxidants. Free Radic Biol Med. 1990;9:19–21. doi: 10.1016/0891-5849(90)90045-k. [DOI] [PubMed] [Google Scholar]
- 24.Bourdillat B, Delautier D, Labat C, Benveniste J, Potier P, Brink C. Mechanism of action of hispidulin, a natural flavone, on human platelets. Prog Clin Biol Res. 1988;280:211–4. [PubMed] [Google Scholar]
- 25.Niu X, Chen J, Wang P, Zhou H, Li S, Zhang M. The effects of hispidulin on bupivacaine-induced neurotoxicity: role of AMPK signaling pathway. Cell Biochem Biophys. 2014;70:241–9. doi: 10.1007/s12013-014-9888-5. [DOI] [PubMed] [Google Scholar]
- 26.Zhou R, Wang Z, Ma C. Hispidulin exerts anti-osteoporotic activity in ovariectomized mice via activating AMPK signaling pathway. Cell Biochem Biophys. 2014;69:311–7. doi: 10.1007/s12013-013-9800-8. [DOI] [PubMed] [Google Scholar]
- 27.Nepal M, Choi HJ, Choi BY, Yang MS, Chae JI, Li L, Soh Y. Hispidulin attenuates bone resorption and osteoclastogenesis via the RANKL-induced NF-κB and NFATc1 pathways. Eur J Pharmacol. 2013;715:96–104. doi: 10.1016/j.ejphar.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Yang JM, Hung CM, Fu CN, Lee JC, Huang CH, Yang MH, Lin CL, Kao JY, Way TD. Hispidulin sensitizes human ovarian cancer cells to TRAIL-induced apoptosis by AMPK activation leading to Mcl-1 block in translation. J Agric Food Chem. 2010;58:10020–6. doi: 10.1021/jf102304g. [DOI] [PubMed] [Google Scholar]
- 29.Lin YC, Hung CM, Tsai JC, Lee JC, Chen YL, Wei CW, Kao JY, Way TD. Hispidulin potently inhibits human glioblastoma multiforme cells through activation of AMP-activated protein kinase (AMPK) J Agric Food Chem. 2010;58:9511–7. doi: 10.1021/jf1019533. [DOI] [PubMed] [Google Scholar]
- 30.He L, Wu Y, Lin L, Wang J, Wu Y, Chen Y, Yi Z, Liu M, Pang X. Hispidulin, a small flavonoid molecule, suppresses the angiogenesis and growth of human pancreatic cancer by targeting vascular endothelial growth factor receptor 2-mediated PI3K/Akt/mTOR signaling pathway. Cancer Sci. 2011;102:219–25. doi: 10.1111/j.1349-7006.2010.01778.x. [DOI] [PubMed] [Google Scholar]
- 31.Yu CY, Su KY, Lee PL, Jhan JY, Tsao PH, Chan DC, Chen YL. Potential Therapeutic Role of Hispidulin in Gastric Cancer through Induction of Apoptosis via NAG-1 Signaling. Evid Based Complement Alternat Med. 2013;2013:518301. doi: 10.1155/2013/518301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao H, Wang H, Peng J. Hispidulin induces apoptosis through mitochondrial dysfunction and inhibition of P13k/Akt signalling pathway in HepG2 cancer cells. Cell Biochem Biophys. 2014;69:27–34. doi: 10.1007/s12013-013-9762-x. [DOI] [PubMed] [Google Scholar]
- 33.Liao W, Liu W, Yuan Q, Liu X, Ou Y, He S, Yuan S, Qin L, Chen Q, Nong K, Mei M, Huang J. Silencing of DLGAP5 by siRNA Significantly Inhibits the Proliferation and Invasion of Hepatocellular Carcinoma Cells. PLoS One. 2013;8:e80789. doi: 10.1371/journal.pone.0080789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhat FA, Sharmila G, Balakrishnan S, Arunkumar R, Elumalai P, Suganya S, Raja Singh P, Srinivasan N, Arunakaran J. Quercetin reverses EGF-induced epithelial to mesenchymal transition and invasiveness in prostate cancer (PC-3) cell line via EGFR/PI3K/Akt pathway. J Nutr Biochem. 2014;25:1132–9. doi: 10.1016/j.jnutbio.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Cao J, Weng Q, Wu R, Yan Y, Jing H, Zhu H, He Q, Yang B. Suppression of hypoxia-inducible factor 1α (HIF-1α) by tirapazamine is dependent on eIF2α phosphorylation rather than the mTORC1/4E-BP1 pathway. PLoS One. 2010;5:e13910. doi: 10.1371/journal.pone.0013910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 37.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen H, Shen A, Zhang Y, Chen Y, Lin J, Lin W, Sferra T, Peng J. Pien Tze Huang inhibits hypoxia-induced epithelial-mesenchymal transition in human colon carcinoma cells through suppression of the HIF-1 pathway. Exp Ther Med. 2014;7:1237–1242. doi: 10.3892/etm.2014.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo D, Wang J, Li J, Post M. Mouse snail is a target gene for HIF. Mol Cancer Res. 2011;9:234–45. doi: 10.1158/1541-7786.MCR-10-0214. [DOI] [PubMed] [Google Scholar]
- 40.Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–5. [PubMed] [Google Scholar]
- 41.Feldser D, Agani F, Iyer NV, Pak B, Ferreira G, Semenza GL. Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res. 1999;59:3915–8. [PubMed] [Google Scholar]
- 42.Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joshi S, Singh AR, Durden DL. MDM2 regulates hypoxic hypoxia-inducible factor 1alpha stability in an E3 ligase, proteasome, and PTEN-phosphatidylinositol 3-kinase-AKT-dependent manner. J Biol Chem. 2014;289:22785–22797. doi: 10.1074/jbc.M114.587493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen F, Zhuang M, Zhong C, Peng J, Wang X, Li J, Chen Z, Huang Y. Baicalein reverses hypoxia-induced 5-FU resistance in gastric cancer AGS cells through suppression of glycolysis and the PTEN/Akt/HIF-1alpha signaling pathway. Oncol Rep. 2015;33:457–463. doi: 10.3892/or.2014.3550. [DOI] [PubMed] [Google Scholar]
- 45.Gao H, Jiang Q, Han Y, Peng J, Wang C. Hispidulin Potentiates the Antitumor Effect of Sunitinib Against Human Renal Cell Carcinoma in Laboratory Models. Cell Biochem Biophys. 2015;71:757–64. doi: 10.1007/s12013-014-0260-6. [DOI] [PubMed] [Google Scholar]
- 46.Cannito S, Novo E, Compagnone A, Valfrè di Bonzo L, Busletta C, Zamara E, Paternostro C, Povero D, Bandino A, Bozzo F, Cravanzola C, Bravoco V, Colombatto S, Parola M. Redox mechanisms switch on hypoxia-dependent epithelial-mesenchymal transition in cancer cells. Carcinogenesis. 2008;29:2267–78. doi: 10.1093/carcin/bgn216. [DOI] [PubMed] [Google Scholar]
- 47.Liu L, Zhu XD, Wang WQ, Shen Y, Qin Y, Ren ZG, Sun HC, Tang ZY. Activation of beta-catenin by hypoxia in hepatocellular carcinoma contributes to enhanced metastatic potential and poor prognosis. Clin Cancer Res. 2010;16:2740–50. doi: 10.1158/1078-0432.CCR-09-2610. [DOI] [PubMed] [Google Scholar]
- 48.Bocca C, Bozzo F, Cannito S, Parola M, Miglietta A. Celecoxib inactivates epithelial-mesenchymal transition stimulated by hypoxia and/or epidermal growth factor in colon cancer cells. Mol Carcinog. 2012;51:783–95. doi: 10.1002/mc.20846. [DOI] [PubMed] [Google Scholar]
- 49.Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001;7:345–350. doi: 10.1016/s1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- 50.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5. [PubMed] [Google Scholar]
- 51.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 52.Ma J, Han LZ, Liang H, Mi C, Shi H, Lee JJ, Jin X. Celastrol inhibits the HIF-1α pathway by inhibition of mTOR/p70S6K/eIF4E and ERK1/2 phosphorylation in human hepatoma cells. Oncol Rep. 2014;32:235–42. doi: 10.3892/or.2014.3211. [DOI] [PubMed] [Google Scholar]
- 53.Priebe A, Tan L, Wahl H, Kueck A, He G, Kwok R, Opipari A, Liu JR. Glucose deprivation activates AMPK and induces cell death through modulation of Akt in ovarian cancer cells. Gynecol Oncol. 2011;122:389–95. doi: 10.1016/j.ygyno.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 54.Park JH, Lee JY, Shin DH, Jang KS, Kim HJ, Kong G. Loss of Mel-18 induces tumor angiogenesis through enhancing the activity and expression of HIF-1α mediated by the PTEN/PI3K/Akt pathway. Oncogene. 2011;30:4578–89. doi: 10.1038/onc.2011.174. [DOI] [PubMed] [Google Scholar]
- 55.Xie Q, Bai Q, Zou LY, Zhang QY, Zhou Y, Chang H, Yi L, Zhu JD, Mi MT. Genistein inhibits DNA methylation and increases expression of tumor suppressor genes in human breast cancer cells. Genes Chromosomes Cancer. 2014;53:422–31. doi: 10.1002/gcc.22154. [DOI] [PubMed] [Google Scholar]
- 56.Park CE, Yun H, Lee EB, Min BI, Bae H, Choe W, Kang I, Kim SS, Ha J. The antioxidant effects of genistein are associated with AMP-activated protein kinase activation and PTEN induction in prostate cancer cells. J Med Food. 2010;13:815–20. doi: 10.1089/jmf.2009.1359. [DOI] [PubMed] [Google Scholar]
- 57.Shin DH, Choi YJ, Park JW. SIRT1 and AMPK mediate hypoxia-induced resistance of non-small cell lung cancers to cisplatin and doxorubicin. Cancer Res. 2014;74:298–308. doi: 10.1158/0008-5472.CAN-13-2620. [DOI] [PubMed] [Google Scholar]