Abstract
Cancer cell growth is complicated progression which is regulated and controlled by multiple factors including cell cycle, migration and apoptosis. In present study, we report that TADs, a novel derivative of taspine, has an essential role in resisting hepatocellular carcinoma growth (including arrest cell cycle) and migration, and inducing cell apoptosis. Our findings demonstrated that the TADs showed good inhibition on the hepatoma cell growth and migration, and good action on apoptosis induction. Using genome-wide microarray analysis, we found the down-regulated growth and apoptosis factors, and selected down-regulated genes were confirmed by Western blot. Knockdown of a checkpoint c-Myc by siRNA significantly attenuated tumor inhibition and apoptosis effects of TADs. Moreover, our results indicated TADs could simultaneously increase cyclin D1 protein levels and decrease amount of cyclin E, cyclin B1 and cdc2 of the cycle proteins, and also TADs reduced Bcl-2 expression, and upregulated Bad, Bak and Bax activities. In conclusion, these results illustrated that TADs is a key factor in growth and apoptosis signaling inhibitor, has potential in cancer therapy.
Keywords: TADs, hepatocellular carcinoma, apoptosis, cell cycle, c-Myc
Introduction
Natural products play an important role in the discovery of novel lead compounds and new chemical entities. Taspine which was originally identified from a screen of Radix et Rhizoma leonticis (Hong Mao Qi in Chinese) [1]. We previously found that taspine displayed anticancer and antiangiogenesis properties [2,3]. TADs (Figure 1) was a novel derivative of taspine. Previous reports showed TADs displayed significant inhibitory activity on proliferation of several different cancer cell lines including breast cancer cell, non-small cell lung cancer [4,5]. Present research works indicated TADs showed obviously inhibition on hepatoma carcinoma cell.
Figure 1.
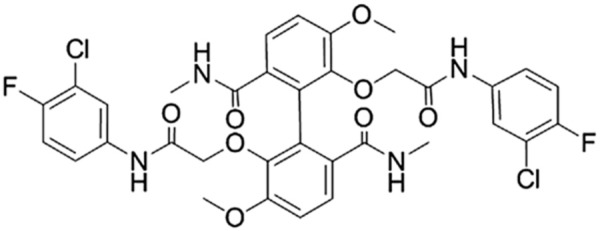
Chemical structure of TADs.
Hepatocellular carcinoma (HCC) is the fifth most common solid tumor in the world and the third most common cause of cancer mortality [6]. Despite significant advances in early detection and therapy, HCC is still one of the leading causes for cancer-related death worldwide [7]. Tumor recurrence in HCC can occur as metastases, whereas more than 90% of HCC-related deaths are the result of secondary local or distant diseases. Recently, drugs targeting key pathways have generated new perspectives in the field of the treatment of HCC. There is an urgent need for more effective agents for the clinical management of HCC. TADs could inhibit hepatocellular carcinoma activity and might be a useful therapeutic candidate for HCC intervention. Tumor cell proliferation is closely related to the cell cycle, cell apoptosis and tumor cell metastasis, and therefore induction of cell cycle arrest and apoptosis are an effective method of controlling tumor cell growth [8].
Cell cycle arrest and apoptosis represent two effective mechanisms involved in the induction of cell death [9]. There are various molecular players involved in the proliferation, cell cycle, and cell apoptosis pathway, such as the, cyclins, cyclin-dependent kinases (CDKs), Bcl-2 family, etc. These signaling pathways regulate important cellular functions including cellular proliferation, migration, cell cycle and apoptosis [10,11].
Myc (c-Myc) is a regulator gene that codes for a transcription factor. The protein encoded by this gene is a multifunctional, nuclear phosphoprotein that plays a role in cell cycle progression, apoptosis and cellular transformation. Malfunctions in c-Myc have also been found in carcinoma. c-Myc is thus viewed as a promising target for anti-cancer drugs [12,13]. PLG (Plasminogen) plays an essential role in the proteolytically degradation of extracellular matrix and the basement membrane surrounding the primary tumor, which is a major determinant for cancer invasion and metastasis. Recent studies indicate that PLG affects adhesion, angiogenesis, differentiation and proliferation [14]. Protein tyrosine phosphatase, receptor type, C (PTPRC), is a member of the protein tyrosine phosphatase (PTP) family. PTPs are known to be signaling molecules that regulate a variety of cellular processes including cell growth, differentiation, mitotic cycle, and apoptosis [15,16].
The present study aimed to extend the previous study of TADs and to evaluate its inhibition on HCC growth through arrest cell cycle and inducing apoptosis using proliferation, colony formation, transwell assay and siRNA assay. The potential targets were analyzed by microarray analysis. In addition, the key role of c-Myc in TADs-induced apoptosis and migration in human hepatocellular carcinoma cell was also investigated.
Materials and methods
Reagents
Cell culture mediums (DMEM, RPMI 1640), trypsin, MTT, Dimethylsulfoxide (DMSO), RNase, Propidium iodide (PI), Annexin-V-FITC, Hoechst 33258 was purchased from Sigma (St. Louis, MO, USA). Protease inhibitor cocktail was from Roche (CA, USA). Fetal bovine serum (FBS) was purchased from Excell Bio (Shanghai, China). Cyclin B1 rabbit polyAb, cdc2 rabbit polyAb, cyclin D1 mouse mAb and cyclin E rabbit polyAb, bad rabbit polyAb, bak rabbit polyAb, bax, bcl-2 rabbit polyAb, c-Myc rabbit polyAb, PLG rabbit polyAb, PTPRC rabbit polyAb, HRP-conjugated GAPDH mAb were from Proteintech Group (USA). BCA protein assay reagent kit and enhanced chemiluminescent (ECL) plus reagent kit were obtained from Pierce (Pierce Biotech, USA). Total RNA extracted kit was from Fastagen (Fastagen, China). Revert AIDTM. first strand cDNA synthesis kit was from Fermentas (Hanover, Lithuania). RNAi was from Fermentas (Hanover, Lithuania). Other reagents used were analytical grade.
Cell culture
SMMC-7721, BEL-7402 and Hep G2 cancer cells from Shanghai Institute of Cell Biology in the Chinese Academy of Sciences were maintained in RPMI1640 and DMEM with 10% FBS, and incubated at 37°C with 5% CO2. All cells were used in experiments during the linear phase of growth.
Cell viability assay
Exponentially growing cells (SMMC-7721, BEL-7402 and HepG2 cells or siRNA cells of SMMC-7721 were cultured in 96-well plates) were plated into 96-well plate (Costar, USA), 24 hours after seeding, cells were incubated in the absence or presence of TADs for 48 h. The cell viability was evaluated using MTT assay, as described previously [17]. The cell numbers were analyzed for the dynamic proliferation rate of cells. The experiment was repeated three times independently.
Colony formation assay
SMMC-7721, BEL-7402 and HepG2 cells were cultured in 6-well plates and fresh medium with or without TADs, was added for 10~15 days. Colonies with cell numbers of > 50 cells per colony were photographed and counted after staining with 0.01% crystal violet solution. All the experiments were performed in triplicate wells in three independent experiments [18].
Microarray analysis
The RNA for the microarray experiments was extracted from SMMC-7721 cells. Microarray experiments were performed in the whole human genome oligo microarray (Agilent Technologies, 4 × 44K, CA, USA), which represents more than 41000 human genes and transcripts. It was used in this study to further systematically screen the differentially expressed genes between control and TADs-treated cells at the Shanghai Biotechnology Corporation as described previously [19,20]. Data were normalized by the Quantile method. All microarray datasets were submitted to the “Gene Expression Omnibus” with an accession number of GSE52554.
Analyses of the cell cycle
SMMC-7721 cells were cultured in six-well culture plates for 24 h and incubated for 24 h after the medium was changed to serum-free medium. Cells were incubated for another 48 h with TADs at various concentrations respectively. The cells were trypsinized, collected, and washed in PBS, then cells were subsequently fixed in 70% ethanol, incubated with RNase (50 μg/ml) and PI (60 μg/ml) protect from light at room temperature for 30 min. The cell cycle was assessed according to the percentage of cells with DNA using the PI staining technique as described previously [14,21].
Cell apoptosis analysis
SMMC-7721 cells were treated with different concentrations of TADs for 48 h. The cells were then collected, washed, and re-suspended in phosphate buffered saline (PBS). The apoptotic cell death rate was examined with Annexin V-FITC and PI double staining using the Annexin V-FITC apoptosis detection kit according to the manufacturer’s instructions. After staining the cells with Annexin V-FITC/PI, flow cytometric analysis was performed and data was analyzed using Cell Quest software [22,23].
Western blotting
The SMMC-7721 cells treated with or without TADs for 48 h were prepared by extracting proteins with RIPA lysis buffer containing protease inhibitor cocktail on ice. Cell lysates were analyzed for western blot analysis with primary antibodies, followed by enhanced chemiluminescence. Blots were reprobed with GADPH to compare protein load in each lane [14].
siRNA transfection
For in vitro knockdown experiments, a smart pool of double-stranded siRNA against c-Myc as well as nonspecific siRNA were obtained from Shanghai GenePharma Co., Ltd. For transfection, siRNA was delivered at a final concentration of 80 nM using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions [24,25]. Designed and synthesized a double stranded siRNA oligonucleotide were: CCAAGGUAGUUAUCCUUAATT and UUAAGGAUAACUACCUUGGTT. The sense and antisense sequences were: 5’-ACACATCAgCACAACTACg-3’, 5’-CgCCTCTTgACATTCTCC-3’. We incubated the cells for 24 h to allow knockdown of c-Myc. These cells were used for proliferation, wound healing and Hoechst staining assays.
Wound healing assay
SMMC-7721 cells were planted into 12-well plate and allowed to grow to 70% confluency in complete medium. Cells were then serum starved for 24 h. Adherent cells were scraped off the bottom of a culture plate using a pipette tip to create a cell-free area. The cell culture was washed with PBS to remove cell debris and then incubated in the absence or presence of TADs for another 48 h. Cell migration into the wound surface and the average distance of migrating cells were determined under an inverted microscope after scratching [26,27].
Hoechst staining assay
Cells were treated with different concentrations of TADs in 6-well plates for 48 h. Cells were then incubated with Hoechst 33258 stain for 10 min at 37°C according to the manufacturer’s instructions, and examined under fluorescence microscope [28].
Statistical analysis
Data were given as mean ± S.D. in quantitative experiments. Statistical analyses of differences between the groups were performed with ANOVA by Student’s t tests. Results were considered statistically significant if the P value was < 0.05.
Results
TADs inhibited liver cancer cell viability and colony formation
To evaluate the effect of TADs on liver cancer cells, we observed its action on cell viability and colony formation. The results showed that TADs significantly inhibited cell viability in SMMC-7721, BEL-7402 and Hep G2 cells, respectively (Figure 2A). In colony formation assay, upon 10~15 days continuous culture, TADs suppressed colony formation of SMMC-7721, BEL-7402 and Hep G2 cells (Figure 2B). TADs showed good inhibition on the cell viability and colony formation of SMMC-7721 cells related to cells of BEL-7402 and Hep G2 cells (Figure 2A and 2C). These findings indicate that TADs has potential anti-tumor properties in hepatocarcinogenesis.
Figure 2.
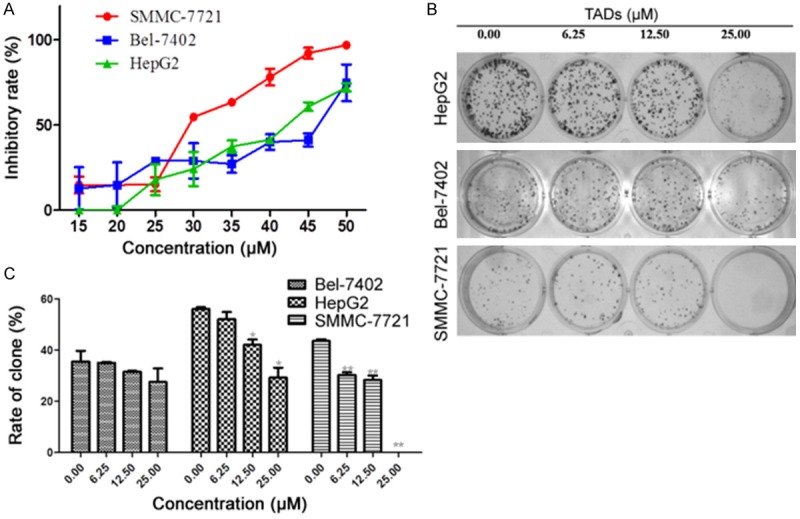
TADs suppressed liver cancer cell proliferation and colony formation. (A) Cells were treated by TADs for 48h at 6.25, 12.50, 25.00 μM. TADs inhibited SMMC-7721, BEL-7402 and Hep G2 cells growth in vitro. (B) Effect on colony formation of SMMC-7721, BEL-7402 and Hep G2 by TADs. The representative colony formation picture of SMMC-7721, BEL-7402 and Hep G2 cell. (C) Quantitation data of (B), TADs showed better inhibition on the colony formation of SMMC-7721 than BEL-7402 and Hep G2 cells. Data were expressed as means ± SD (n = 5). *p < 0.05, **p < 0.01 vs. the untreated control group.
Differentially expressed genes in control and TADs-treated cells
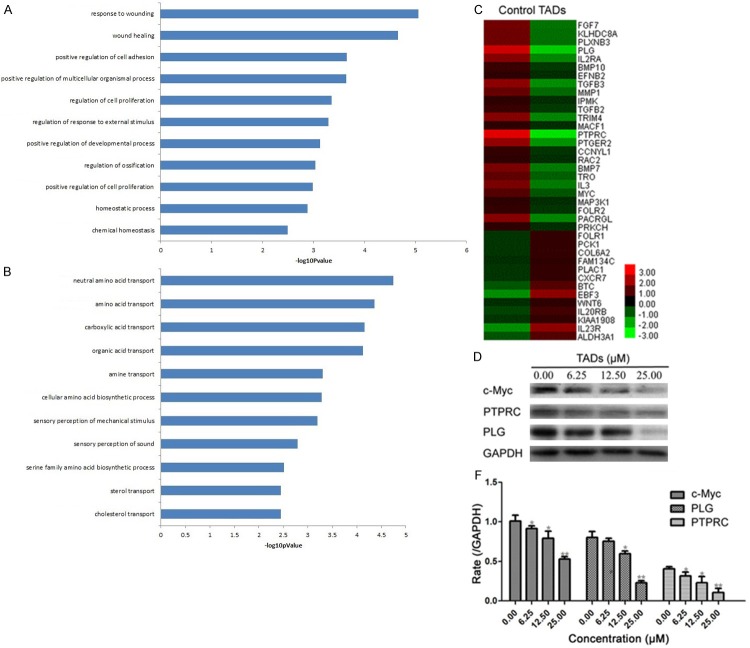
To gain insights into the mechanisms of TADs on inhibition to HCC, SMMC-7721 cells were cultivated in the absence or presence of 25.0 μM TADs for 48 h, followed by the Agilent Whole Human Genome Oligo Microarray. A group of differentially expressed genes obtained from the primary analysis were analyzed by GO enrichment and decrease pathway analysis, respectively. All the down-regulated genes in TADs-treated cells were shown in Figure 3A. The GO enrichment analysis revealed that the main GO categories for the up-regulated genes in TADs-treated cells as summarized in Figure 3B A heat map with two-dimensional hierarchical clustering revealed 38 genes differentially expressed between the two groups, which were down-regulated and related to growth, proliferation and metastasis, as illustrated in Figure 3C. The total genes were found to be differentially expressed between control and TADs-treated cells at p < 0.05. In this experiment, the genes responsible for growth, migration and apoptosis were most important to match our research goal. The analysis of our microarray data showed that c-Myc genes, associated with growth, migration and apoptosis, were down-regulated in TADs-treated cells as compared with the control cells. And also, we determined the PLG, RTPRC and c-Myc protein expressions of growth, migration and apoptosis for the confirmation of microarray assay by Western blot (Figure 3D). It indicated TADs could downregulate PLG, RTPRC and c-Myc expression in dose-effect relationship.
Figure 3.
The genetically different populations by TADs for SMMC-7721 cells. A: The GO category for the down-regulated genes in TADs group compared with control group. B: The GO category for the up-regulated genes in the TADs group compared with the control group. –logPvalue ≥ 2 was used as a cut-off threshold to select significant GO categories. The higher the enrichment, the more significant the biological processes. C: Hierarchical cluster analysis of 38 differentially down-regulated expressed genes for cell growth and apoptosis in samples. Each column represents one sample, and each gene was depicted by one row, where red denotes an increase in gene expression and green denotes a decrease in gene expression as compared with the other group. D: Comparison of c-Myc, PLG and PTPRC gene expression levels between control and TADs group by western blot, which confirmed the microarray analysis. Data were expressed as means ± SD (n = 3). *p < 0.05, **p < 0.01 vs. the control group.
TADs arrested cell cycle and regulated related cycle proteins
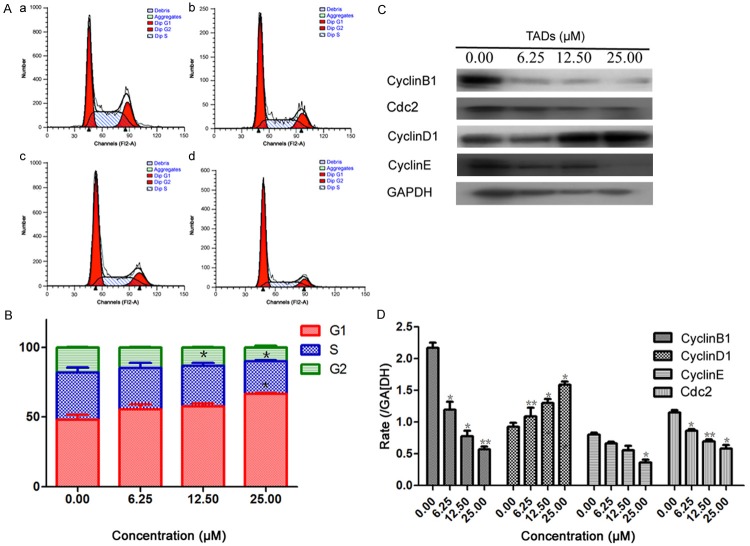
The effect of TADs on cell cycle profile was analyzed using PI staining and flow cytometry analysis. Cells were treated without TADs or with TADs at 6.25, 12.50, 25.00 μM for 48 h and stained with propidium iodide. As shown in Figure 4A and 4B, treatment of TADs induced an accumulation of SMMC-7721 cells in the G1 phase of the cell cycle. The population of phase G1 was increased with the increased concentrations of TADs. A decrease in the population in the S phases was also observed. To elucidate the specific cell cycle regulatory proteins responsible for the cell cycle arrest, we explored the effect of TADs on regulatory protein molecules including cyclin D1, cyclin E and cyclin B1, which are cyclins required for advance from G1 to S, S to G2, G2 to G1 respectively. Meanwhile, we investigated the effect of TADs on cyclin-dependent kinases cdc2. As shown in Figure 4C and 4D, the levels of cyclin D1 protein gradually increased with TADs at the concentrations of 6.25-25.00 μM. In contrast, the amount of cyclin E, cyclin B1 and cdc2 was significantly down-regulated in TADs-treated cells. These data suggest that these cell cycle regulatory molecules are involved in TADs-induced changes in cell cycle progression.
Figure 4.
Effect of TADs on SMMC-7721 cell cycle and cycle-related molecules. Cells were treated with TADS for 48 h and stained with PI. The cells were then analyzed by FACs. (A) TADs arrested SMMC-7721 cell cycle at the G1 phase. (a) Control. (b) 6.25 μM. (c) 12.50 μM. (d) 25.00 μM. (B) Quantitation data of (A). Data were expressed as percentage of cell population in G0-G1, S and G2-M phases of the cell cycle (n = 3). (C) Effect of TADs on cycle proteins expression of Cyclin D1, Cyclin E, Cyclin B1 and Cdc2 by western blot analysis. (D) Quantitation data of (C). TADs decreased the protein expressions of Cyclin E, Cyclin B and Cdc2 expressions, and increased Cyclin D1 expression in a dose-dependent manner compared to the untreated control. Data were expressed as means ± SD (n = 3). *p < 0.05, **p < 0.01 vs. the control group.
TADs induced cell apoptosis and regulated related apoptosis proteins
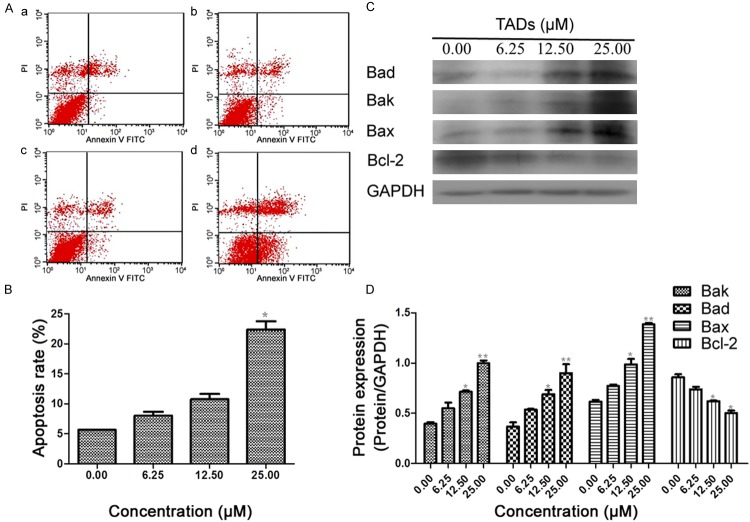
Apoptotic SMMC-7721 cells were measured by FACS. Annexin-V staining combined with PI staining was done in cells in the control group and cells treated with TADs at different concentrations, and then analyzed by flow cytometry. Early apoptosis was in the right quadrant of a dot-plot graph using annexin-V-FITC versus PI. After treatment with TADs for 48 h, the percentage of cells undergoing apoptosis were increased with the increased concentrations, (Figure 5A and 5B). Since Bcl-2 family members controlled cytochrome c release in mitochondria apoptosis pathway, we examined whether Bcl-2 family members were involved in the TADs -induced apoptosis. To test this, we analyzed the expressions of Bcl-2 family proteins (Bcl-2, Bad, Bax and Bak) in SMMC-7721 cells treated with increasing concentration of TADs (6.25, 12.50, 25.00 μM) for 48 h by western blot analysis. Treatment with TADs led to a notable decrease in Bcl-2 expression and a significant up-regulation in Bak, Bad and Bax expression (Figure 5C and 5D).
Figure 5.
Effects of TADs on SMMC-7721 cell apoptosis and apoptosis-related molecules. Cells were cultured overnight in 6-well plates and treated in triplicate with TADs for 48 h. (A) Flow cytometric analysis of TADs-induced apoptosis in SMMC-7721 cells. The proportion of apoptotic cells was determined by double-staining with Annexin V/FITC and PI. The flow cytometry profile presents Annexin V-FITC (x-axis) and PI staining (y-axis). The values represent the percentage of cells in each of the four quadrants (lower left quadrant, viable cells; upper left quadrant, necrotic or dead cells; lower right quadrant, early-stage apoptotic cells; and upper right quadrant, late-stage apoptotic cells). (B) Quantitation data of (A). (C) Effect of TADs on apoptosis proteins expression of Bax, Bad, Bak and Bcl-2 by western blot analysis. (D) Quantitation data of (C). Data are presented as the mean ± standard error of the mean (n = 3). *p < 0.05, **p < 0.01 vs. the control group.
Effect of c-Myc on cell viability, migration and apoptosis
In order to clarify key regulation molecular on the HCC growth by TADs, we carried out the siRNA assay on the c-Myc gene in SMMC-7721 cells (Figure 6A-C). We also compared the proliferation, migration and apoptosis between normal cells and knockdown of c-Myc in SMMC-7721 cells, and found that there was an obvious difference in the two groups, knockdown of c-Myc significantly reduced the inhibition of TADs on the growth of SMMC-7721 cells, as compared with the control groups transfected by a negative control shRNA (shRNA-NC) (P < 0.05, Figure 6D). At the same time, treatment of TADs significantly decreased the migration ability by wound healing assay at the concentrations of 6.25-25.00 μM, respectively, and the knockdown of c-Myc significantly reduced the inhibition on the migration of SMMC-7721 cells, as compared with the control groups (P < 0.05, Figure 6E-G). In the Hoechst staining assay, the same change rule appeared between normal cells and knockdown of c-Myc in SMMC-7721 cells (Figure 6H and 6I). It indicates c-Myc is a key factor in the cell viability, migration and apoptosis by TADs.
Figure 6.
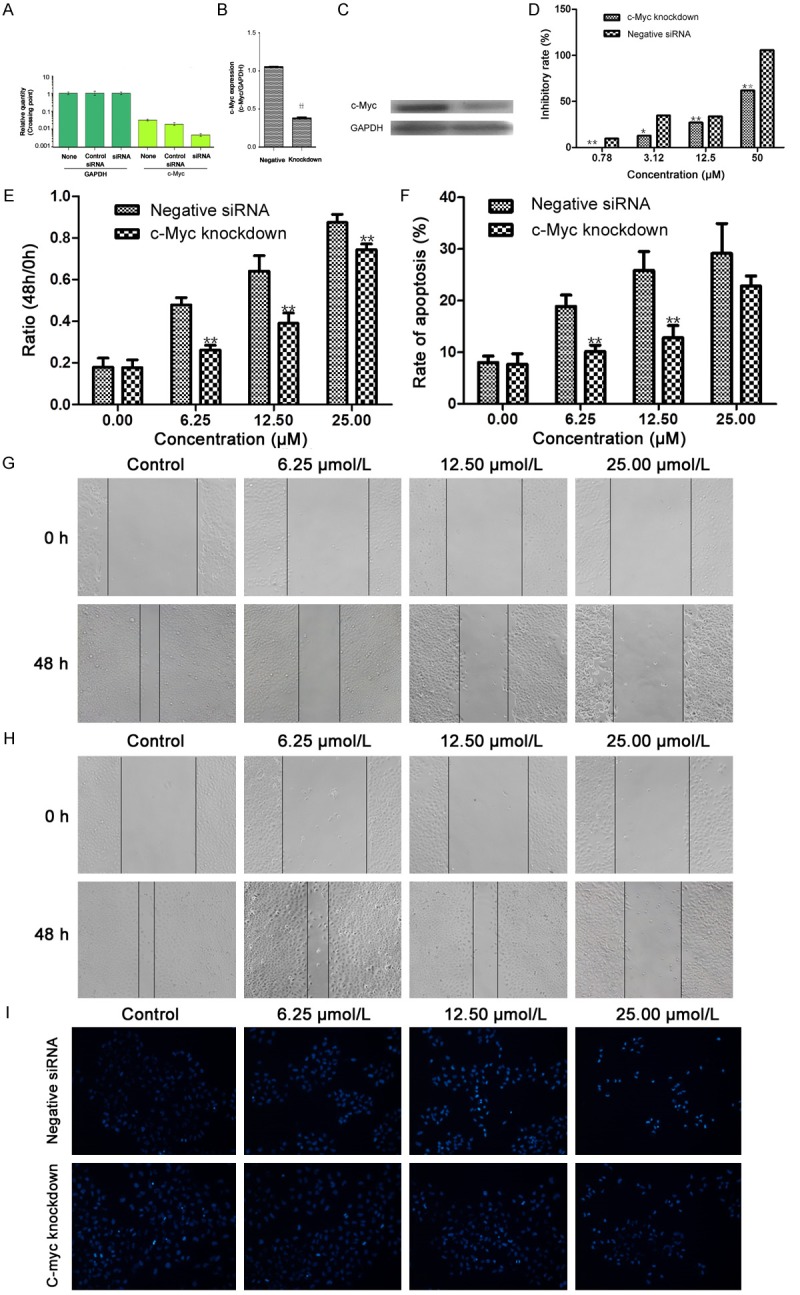
c-Myc plays a key role in TADs-inhibited cell growth and migration, and TADs-induced apoptosis in SMMC-7721 cell. c-Myc mRNA expression (A) and protein expression (B and C) in SMMC-7721 cells transfection with 80 nM c-Myc siRNA using Lipofectamine 2000 reagent and SMMC-7721 cells (wild type) were determined by RT-PCR analysis and western blot assay. Transfection with a control siRNA construct served as a negative control. Data represents the means ± SD (n = 3). *p < 0.05, **p < 0.01 versus the c-Myc expression in SMMC-7721 cells. (D) Effect of TADs on cell proliferation in SMMC-7721 cells (wild type) and knockdown cells by MTT assay. After treated with c-Myc siRNA for 24 h, cells were treated with TADs for 48 h. Data represents the means ± SD from three repeated experiments. Five wells were treated in each experiment. (E, F) Representative micrographs of the scratch wound healing assay in wild type SMMC-7721 cells (E) and knockdown cells (F) in the absence or presence of TADs at 48 h. Bar = 100 μm. Confluent cells were assayed for migration into the wound at 48 h after scratching. Migrating cells were measured by average distance of migrating. G: Representative micrographs of Hoechst 33258 staining in wild type SMMC-7721 cells and knockdown cells by TADs at 48 h. H: Quantitation data of (E and F). (I) Quantitation data of (G). The results are expressed as the means ± SD. The data shown are representative of three independent experiments. *p < 0.05, **p < 0.01 versus the normal SMMC-7721 cells.
Discussion
Conventional chemotherapy often encounters drug malfunction after a long time treatment. To overcome this problem, novel therapeutic strategies are in an urgent need to be developed, and one of the attractive strategies is to develop effective new drugs in chemotherapy. Here, TADs has showed good momentum have not been investigated, and its exact mechanism of action is largely unknown. The current study focuses on the inhibition evaluation of TADs on HCC growth through migration inhibition, cell cycle arrest and apoptosis. In this study, TADs had a good inhibitory effect on liver cancer growth. The potential targets are c-Myc, PLG and PTPRC, and mechanism which TADs affect migration, cell cycle and apoptosis involves the regulation of cycle proteins, proliferation and cell apoptosis signaling molecules.
Through cell proliferation and colony formation assays in vitro (Figure 2), TADs displayed good inhibition on HCC tumor growth. To gain insights into the mechanisms of TADs on inhibition to HCC, microarray analysis of Agilent microarray-based gene expression profiling was used to screen the potential targets and signaling pathways. The major findings in microarray analysis were that the differentially expressed genes associated with growth and migration were obtained, such as c-Myc, PLG and PTPRC, the selected down-regulated genes were confirmed by western blot (Figure 3D). The genes responsible for growth, migration cell cycle and apoptosis were most important to match our research goal. So we carried out potential signaling molecules analysis by TADs.
Cell cycle progression is tightly related to various cyclins and cyclin-dependent kinases (Cdks), such as cyclin B1, cyclin D1, cyclin E and cdk2, as well as cell proliferation [14,29]. Induction of cell cycle arrest is an effective method of controlling tumor cell proliferation. Cdks are evolutionarily conserved proteins that are essential for cell cycle control, and distinct pairs of cyclins and cdks regulate progression in different stages of the cell cycle. Cdks activity is regulated by cyclins, which bind to and activate the cdk [30]. Flow cytometry revealed treatment of TADs induced an accumulation of SMMC-7721 cells in the G1 phase of the cell cycle and a decrease in the population of cells in the G2-GM and S phase. Western blot assay showed the levels of cyclin D1 protein gradually increased with TADs at the concentrations of 6.25-25.0 μM. In contrast, the amount of cyclin E, cyclin B1 and cdc2 was significantly down-regulated in TADs-treated cells. These data suggest that these cell cycle regulatory molecules are involved in TADs-induced changes in cell cycle progression. It indicates TADs could inhibit cell growth by arresting cell cycle caused by regulating cyclin D1, cyclin E, cyclin B1 and cdc2 expressions.
Apoptosis is the major form of programmed cell death plays critical roles in cancer characterized by a series of molecular features. It can be triggered by diverse intracellular signals that act upon the Bcl-2 protein family. The Bcl-2 family proteins, such as Bcl-2 and Bax, are the best-characterized regulators of apoptosis [31-33]. The bcl-2 family of homologous proteins represents a critical checkpoint within most apoptotic pathways, acting upstream of such irreversible damage to cellular constituents, includes both anti-apoptotic and pro-apoptotic proteins such as Bcl-2 and Bax respectively. The balance between these two classes of proteins is critical for determines how cells respond to apoptotic or survival signals. The data here show that TADs-induced apoptosis is associated with up-regulation of Bad, Bak and Bax expressions and down-regulation of Bcl-2 expression. These results support the relationship that TADs induces apoptosis by regulating the expression of bcl-2 family proteins and c-Myc.
c-Myc is activated upon various mitogenic signals. By modifying the expression of its target genes, c-Myc activation results in numerous biological effects. The first to be discovered is its capability to drive cell proliferation and upregulate cyclins, but it also plays a very important role in regulating cell growth and apoptosis. c-Myc is a very strong proto-oncogene and it is very often found to be upregulated in many types of cancers [12,34,35]. Knockdown of c-Myc significantly reduced the inhibition on the proliferation and migration of SMMC-7721 cells by TADs (Figure 6D-G). At the same time, the apoptosis rate (Figure 6H, 6I) of SMMC-7721 cells also decreased. So, our results indicated TADs downregulated c-Myc gene and protein expressions, which contributed to HCC cell cycle arrest and apoptosis. In fact, there is an interrelation in the signaling molecules of cell cycle and apoptosis with c-Myc, PLG and PTPRC. These results support the relationship that TADs induces apoptosis by regulating the expression of bcl-2 family proteins and c-Myc, PLG and PTPRC. We, therefore, concluded that the inhibiting effect of TADs on HCC was through synthetically regulating cell cycle, migration and cell apoptosis signaling molecules.
In summary, results presented in this work demonstrate that TADs inhibits HCC growth by downregulating the signaling of c-Myc, PLG and PTPRC, simultaneously increasing cyclin D1 protein levels and decreasing amount of cyclin E, cyclin B1 and cdc2 of the cycle proteins. Furthermore, TADs reduced Bcl-2 expression, and upregulated Bad, Bak and Bax activities. These results support the likelihood that TADs affects HCC growth by interfering in two essential cellular processes for tumor development: cell proliferation (including arrest cell cycle) and cell apoptosis. These data support the development of TADs as a novel, multiple action growth and migration signaling inhibitor and apoptosis inducer.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant 81370088 and 81227802), the Fundamental Research Funds for the Central Universities, the project of shaanxi star of science and technology (2012KJXX-06), and supporting plan of education ministry’s new century excellent talents (NCET-13-0467).
Disclosure of conflict of interest
None.
References
- 1.Li YP, He LC. Establishment of the model of vascular endothelial cell membrane chromatography and its preliminary application. Chinese Science Bulletin. 2007;52:922–928. [Google Scholar]
- 2.Zhan Y, Zhang Y, Chen Y, Wang N, Zheng L, He L. Activity of taspine isolated from Radix et Rhizoma Leonticis against estrogen-receptor-positive breast cancer. Fitoterapia. 2011;82:896–902. doi: 10.1016/j.fitote.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, He L, Meng L, Luo W. Taspine isolated from Radix et Rhizoma Leonticis inhibits proliferation and migration of endothelial cells as well as chicken chorioallantoic membrane neovascularisation. Vascul Pharmacol. 2008;48:129–137. doi: 10.1016/j.vph.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Zhan Y, Zhang Y, Liu C, Zhang J, Smith WW, Wang N, Chen Y, Zheng L, He L. A Novel Taspine Derivative, HMQ1611, Inhibits Breast Cancer Cell Growth via Estrogen Receptor and EGF Receptor Signaling Pathways. Cancer Prev Res (Phila) 2012;5:864–873. doi: 10.1158/1940-6207.CAPR-11-0575. [DOI] [PubMed] [Google Scholar]
- 5.Lu W, Dai B, Ma W, Zhang Y. A novel taspine analog, HMQ1611, inhibits growth of non-small cell lung cancer by inhibiting angiogenesis. Oncol Lett. 2012;4:1109–1113. doi: 10.3892/ol.2012.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C, Gao D, Guo K, Kang X, Jiang K, Sun C, Li Y, Sun L, Shu H, Jin G, Sun H, Wu W, Liu Y. Novel synergistic antitumor effects of rapamycin with bortezomib on hepatocellular carcinoma cells and orthotopic tumor model. BMC Cancer. 2012;12:166. doi: 10.1186/1471-2407-12-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen B, Chu ESH, Zhao G, Man K, Wu CW, Cheng JTY, Li G, Nie Y, Lo CM, Teoh N, Farrell GC, Sung JJY, Yu J. PPARgamma inhibits hepatocellular carcinoma metastases in vitro and in mice. Br J Cancer. 2012;106:1486–1494. doi: 10.1038/bjc.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nurse P. A long twentieth century of the cell cycle and beyond. Cell. 2000;100:71–78. doi: 10.1016/s0092-8674(00)81684-0. [DOI] [PubMed] [Google Scholar]
- 9.King KL, Cidlowski JA. Cell cycle regulation and apoptosis. Annu Rev Physiol. 1998;60:601–617. doi: 10.1146/annurev.physiol.60.1.601. [DOI] [PubMed] [Google Scholar]
- 10.Jiang BH, Liu LZ. AKT signaling in regulating angiogenesis. Current Cancer Drug Targets. 2008;8:19–26. doi: 10.2174/156800908783497122. [DOI] [PubMed] [Google Scholar]
- 11.Munoz-Chapuli R, Quesada AR, Medina MA. Angiogenesis and signal transduction in endothelial cells. Cellular and Molecular Life Sciences. 2004;61:2224–2243. doi: 10.1007/s00018-004-4070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li YL, Choi PS, Casey SC, Dill DL, Felsher DW. MYC through miR-17-92 Suppresses Specific Target Genes to Maintain Survival, Autonomous Proliferation, and a Neoplastic State. Cancer Cell. 2014;26:262–272. doi: 10.1016/j.ccr.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavalheiro GR, Matos-Rodrigues GE, Gomes AL, Rodrigues PMG, Martins RAP. c-myc Regulates Cell Proliferation during Lens Development. PLoS One. 2014;9:e87182. doi: 10.1371/journal.pone.0087182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Zhan Y, Zhang D, Dai B, Ma W, Qi J, Liu R, He L. Eupolyphaga sinensis walker displays inhibition on hepatocellular carcinoma through regulating cell growth and metastasis signaling. Sci Rep. 2014;4:5518. doi: 10.1038/srep05518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williamson AJK, Pierce A, Jaworska E, Zhou C, Aspinall-O’Dea M, Lancashire L, Unwin RD, Abraham SA, Walker MJ, Cadecco S, Spooncer E, Holyoake TL, Whetton AD. A Specific PTPRC/CD45 Phosphorylation Event Governed by Stem Cell Chemokine CXCL12 Regulates Primitive Hematopoietic Cell Motility. Mol Cell Proteomics. 2013;12:3319–3329. doi: 10.1074/mcp.M112.024604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xue J, Gao XQ, Fu CY, Cong Z, Jiang H, Wang W, Chen T, Wei Q, Qin C. Regulation of galectin-3-induced apoptosis of Jurkat cells by both O-glycans and N-glycans on CD45. Febs Letters. 2013;587:3986–3994. doi: 10.1016/j.febslet.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 17.Zhang YM, Dai BL, Zheng L, Zhan YZ, Zhang J, Smith WW, Wang XL, Chen YN, He LC. A novel angiogenesis inhibitor impairs lovo cell survival via targeting against human VEGFR and its signaling pathway of phosphorylation. Cell Death Dis. 2012;3:e406. doi: 10.1038/cddis.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ge GF, Yu CH, Yu B, Shen ZH, Zhang DL, Wu QF. Antitumor effects and chemical compositions of Eupolyphaga sinensis Walker ethanol extract. J Ethnopharmacol. 2012;141:178–182. doi: 10.1016/j.jep.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 19.Hou Y, Zou Q, Ge R, Shen F, Wang Y. The critical role of CD133(+)CD44(+/high) tumor cells in hematogenous metastasis of liver cancers. Cell Res. 2012;22:259–272. doi: 10.1038/cr.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng J, Xie HY, Xu X, Wu J, Wei X, Su R, Zhang W, Lv Z, Zheng S, Zhou L. NDRG1 as a biomarker for metastasis, recurrence and of poor prognosis in hepatocellular carcinoma. Cancer Lett. 2011;310:35–45. doi: 10.1016/j.canlet.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Zheng L, Wang X, Luo W, Zhan Y, Zhang Y. Brucine, an effective natural compound derived from nux-vomica, induces G1 phase arrest and apoptosis in LoVo cells. Food Chem Toxicol. 2013;58:332–339. doi: 10.1016/j.fct.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 22.Linger RM, Cohen RA, Cummings CT, Sather S, Migdall-Wilson J, Middleton DH, Lu X, Baron AE, Franklin WA, Merrick DT, Jedlicka P, DeRyckere D, Heasley LE, Graham DK. Mer or Axl receptor tyrosine kinase inhibition promotes apoptosis, blocks growth and enhances chemosensitivity of human non-small cell lung cancer. Oncogene. 2013;32:3420–3431. doi: 10.1038/onc.2012.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Jiang Q, Wang N, Dai B, Chen Y, He L. Effects of taspine on proliferation and apoptosis by regulating caspase-3 expression and the ratio of Bax/Bcl-2 in A431 cells. Phytother Res. 2011;25:357–364. doi: 10.1002/ptr.3268. [DOI] [PubMed] [Google Scholar]
- 24.Akaogi K, Nakajima Y, Ito I, Kawasaki S, Oie SH, Murayama A, Kimura K, Yanagisawa J. KLF4 suppresses estrogen-dependent breast cancer growth by inhibiting the transcriptional activity of ERalpha. Oncogene. 2009;28:2894–2902. doi: 10.1038/onc.2009.151. [DOI] [PubMed] [Google Scholar]
- 25.Kortylewski M, Swiderski P, Herrmann A, Wang L, Kowolik C, Kujawski M, Lee H, Scuto A, Liu Y, Yang C, Deng J, Soifer HS, Raubitschek A, Forman S, Rossi JJ, Pardoll DM, Jove R, Yu H. In vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nat Biotechnol. 2009;27:925–932. doi: 10.1038/nbt.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim SY, Ahn SH, Park H, Lee J, Choi K, Choi C, Choi JH, Park EM, Choi YH. Transcriptional regulation of adrenomedullin by oncostatin M in human astroglioma cells: Implications for tumor invasion and migration. Sci Rep. 2014;4:6444. doi: 10.1038/srep06444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang H, Wang B, Wang T, Xu L, He C, Wen H, Yan J, Su H, Zhu X. Toll-Like Receptor 4 Prompts Human Breast Cancer Cells Invasiveness via Lipopolysaccharide Stimulation and Is Overexpressed in Patients with Lymph Node Metastasis. PLoS One. 2014;9:e109980. doi: 10.1371/journal.pone.0109980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasul A, Ding C, Li X, Khan M, Yi F, Ali M, Ma T. Dracorhodin perchlorate inhibits PI3K/Akt and NF-kappaB activation, up-regulates the expression of p53, and enhances apoptosis. Apoptosis. 2012;17:1104–1119. doi: 10.1007/s10495-012-0742-1. [DOI] [PubMed] [Google Scholar]
- 29.Yen CC, Huang YH, Liao CY, Liao CJ, Cheng WL, Chen WJ, Lin KH. Mediation of the inhibitory effect of thyroid hormone on proliferation of hepatoma cells by transforming growth factor-beta. J Mol Endocrinol. 2006;36:9–21. doi: 10.1677/jme.1.01911. [DOI] [PubMed] [Google Scholar]
- 30.Morgan DO. Principles of Cdk Regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 31.Brosseau C, Colston K, Dalgleish AG, Galustian C. The immunomodulatory drug lenalidomide restores a vitamin D sensitive phenotype to the vitamin D resistant breast cancer cell line MDA-MB-231 through inhibition of BCL-2: potential for breast cancer therapeutics. Apoptosis. 2012;17:164–173. doi: 10.1007/s10495-011-0670-5. [DOI] [PubMed] [Google Scholar]
- 32.Tischner D, Manzl C, Soratroi C, Villunger A, Krumschnabel G. Necrosis-like death can engage multiple pro-apoptotic Bcl-2 protein family members. Apoptosis. 2012;17:1197–1209. doi: 10.1007/s10495-012-0756-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strasser A, Cory S, Adams JM. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. Embo Journal. 2011;30:3667–3683. doi: 10.1038/emboj.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finver SN, Nishikura K, Finger LR, Haluska FG, Finan J, Nowell PC, Croce CM. Sequence-Analysis of the Myc Oncogene Involved in the T(8-14)(Q24-Q11) Chromosome-Translocation in a Human-Leukemia T-Cell Line Indicates That Putative Regulatory Regions Are Not Altered. Proc Natl Acad Sci U S A. 1988;85:3052–3056. doi: 10.1073/pnas.85.9.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denis N, Kitzis A, Kruh J, Dautry F, Corcos D. Stimulation of Methotrexate Resistance and Dihydrofolate-Reductase Gene Amplification by C-Myc. Oncogene. 1991;6:1453–1457. [PubMed] [Google Scholar]