Abstract
Our recent study observed that the expression of Musashi-2 (MSI2), a member of the Musashi family, was up-regulated in hepatitis B virus (HBV) related hepatocellular carcinoma parenchymal cells. Using quantitative PCR, tissue microarray (TMA) and immunohistochemical staining, we evaluated MSI2 mRNA and protein levels in tumor tissues from patients with different stages of hepatocellular carcinoma with paired adjacent noncancerous sample sets. The following techniques were used to further investigate MSI2 function and its potential molecular mechanism: RNAi, wound healing assay, Transwell assay, quantitative PCR and western blot analysis. Immunohistochemical detection of MSI2 on a TMA containing 106 paired specimens showed that increased cytoplasmic and nuclear MSI2 staining was significantly associated with tumor size, tumor differentiation, recurrence, TNM stage, vessel invasion and Ki-67 proliferative index. Patients with MSI2-positive tumors had a significantly higher disease recurrence rate and poorer survival than patients with MSI2-negative tumors after radical surgery. Based on univariate analysis, MSI2 expression showed an unfavorable influence on both disease-free survival and overall survival. Multivariate analysis revealed that higher MSI2 expression, together with tumor size, tumor differentiation, tumor thrombus, and Ki-67 expression were independent predictors of overall survival. With MSI2 knockdown, hepatoma cell migration and invasion were inhibited and the expression of β-catenin, T cell factor (TCF) and lymphoid enhancer factor (LEF) were dysregulated. Thus, we propose that MSI2 may predict unfavorable outcomes in hepatitis B virus related hepatocellular carcinoma and promote cancer progression via the Wnt/β-catenin signaling pathway.
Keywords: Musashi-2, hepatocellular carcinoma, progression, tissue microarray, Wnt/β-catenin pathway
Introduction
Human hepatocellular carcinoma (HCC) is highly malignant and is the second cause of cancer-related death in China [1]. The 5-year survival rates of HBV-related HCC range from 15% to 26% after diagnosis [2]. The clinical efficacy of the current therapies and available targeted therapies for HCC are limited [3]. The long-term prognosis of HCC remains poor, primarily because of its frequent recurrence caused by multi-centric carcinogenesis and intrahepatic metastasis [4]. Although various genetic and epigenetic changes leading to HCC have been revealed, the molecular mechanisms underlying tumorigenesis are not fully elucidated [5].
Several signaling pathways have been found to be dysregulated in HCC. Among them, dysregulation of the Wnt/β-catenin pathway, which plays an important role in normal liver development [6], is by far the most complex to treat [7,8]. However, its aberrant activation is involved in carcinogenesis of primary HCC [9,10]. The nuclear β-catenin/T cell factor (TCF)/Lymphoid enhancer factor (LEF) transcription complex is the most important complex involved in the Wnt/β-catenin pathway, also called as the canonical Wnt signaling pathway [11,12]. Therefore, over expression and/or under expression of any marker in this pathway may be early molecular events during hepatocarcinogenesis [13].
MSI2 is preferentially expressed in the hematopoietic system, which affects asymmetric cell division, stem cell function and cell fate determination in various somatic tissues [14]. Recently, MSI2 has been shown to play an important role in hematopoietic malignancies, being associated with a worse clinical prognosis in acute myeloid leukemia (AML) [15]. Similarly, upregulation of MSI2 has been demonstrated to correlate with a higher risk of CML relapse [16]. Further, MSI2 expression is deregulated during tumorigenesis in different adult tissues [14], including glioblastoma [17], esophageal [17], colon [18], pulmonary [19], breast [20], gastric [21], liver [7], and bladder [22] cancers. Interestingly, MSI2 has been reported to be involved in several signaling pathways, such as Numb/notch signaling [23], MAPK signaling [24], and the EMT process [7]. Thus, the relevant mechanisms of MSI2 remain obscure.
Data obtained from the present clinical analysis suggest that MSI2 might predict an unfavorable outcome in hepatitis B virus related hepatocellular carcinoma and promote cancer progression via the Wnt/β-catenin signaling pathway. The present study evaluated MSI2 mRNA and protein levels in tumor tissues from patients with different stages of hepatocellular carcinoma with paired adjacent noncancerous sample sets. Applying RNAi, wound healing assay, Transwell assay, quantitative PCR and western blot analysis, we further investigated MSI2 function and its potential molecular mechanisms.
Materials and methods
Tissue specimens
All patient-derived specimens were collected and archived under protocols approved by the institutional review boards of Shandong University affiliated Shandong Provincial Qianfoshan Hospital Medical Center. Formalin-fixed, paraffin-embedded (FFPE) samples for immunohistochemistry were obtained from 106 patients with primary HBV-related HCC who had undergone radical hepatectomy at the above referred Hospital Hepatobiliary Cancer Center between January 2005 and December 2007. The diagnosis was confirmed by at least 2 pathologists. Our patient population comprised 94 men and 12 women with a mean age of 54 years old (range, 38-72 years old) at the time of operation. Our tissue microarray (TMA) is comprised of primary HCC tissue paired with adjacent noncancerous tissues. Detailed patient demographic information is presented in Table 1. All patients provided informed consent according to a protocol approved by the Institutional Review Board of Shandong University affiliated Shandong Provincial Qianfoshan Hospital.
Table 1.
Correlation between MSI2 expression and clinicopathologic characteristics in 106 HCC patients
| Variables | n | MSI2 protein expression (n) | P value | |
|---|---|---|---|---|
|
| ||||
| Negative | Positive | |||
| Age (years)a | ||||
| ≤ 50 | 38 | 16 | 22 | 0.836 |
| > 50 | 68 | 26 | 42 | |
| Gendera | ||||
| Male | 94 | 36 | 58 | 0.535 |
| Female | 12 | 6 | 6 | |
| HBV infection | ||||
| No | 0 | Undetermined | ||
| Yes | 106 | 38 | 68 | |
| Cirrhosis | ||||
| No | 0 | Undetermined | ||
| Yes | 106 | 38 | 68 | |
| Tumor size (cm)a | ||||
| ≤ 5 | 46 | 24 | 22 | 0.028* |
| > 5 | 60 | 18 | 42 | |
| Tumor capsulea | ||||
| No | 52 | 20 | 32 | 0.845 |
| Yes | 54 | 22 | 32 | |
| Tumor differentiationa | ||||
| High/moderate | 80 | 37 | 43 | 0.020* |
| Low | 26 | 5 | 21 | |
| Tumor thrombusa | ||||
| No | 82 | 36 | 46 | 0.105 |
| Yes | 24 | 6 | 18 | |
| Recurrencea | ||||
| No | 52 | 28 | 24 | 0.005* |
| Yes | 54 | 14 | 40 | |
| TNM stageb | ||||
| I♀ | 84 | 30 | 54 | 0.027* |
| II | 10 | 10 | 0 | |
| III | 12 | 2 | 10 | |
| AFP (ng/ml)b | ||||
| ≤ 20 | 2 | 2 | 0 | 0.126 |
| > 20 | 104 | 36 | 68 | |
| vessel invasionb | ||||
| No | 84 | 38 | 46 | 0.027* |
| Yes | 22 | 4 | 18 | |
| Ki-67 expression | ||||
| Negative | 31 | 26 | 5 | < 0.001* |
| Positive | 75 | 16 | 59 | |
P values are based on
χ2 test;
Fisher’s exact test;
Significant difference;
I+II~III.
Follow-up after surgery
The 106 patients who underwent a hepatectomy were subjected to close clinical observations; including chest/abdominal/pelvic computed tomographic (CT) imaging or abdominal color Doppler ultrasound, AFP level, and blood testing at 2- to 3-month intervals. Follow-up was in accord with the National Comprehensive Cancer Network (NCCN) Practice Guidelines for HCC. Disease-free survival (DFS) was defined as the interval from the initial surgery to clinically or radiologically proven recurrence/metastasis and tumor-related death, while overall survival (OS) rates was defined as survival from the initial surgery to tumor-related death. The end date of the follow-up study for conducting the analysis was December 2012.
TMA construction
For TMA construction, formalin-fixed, paraffin-embedded samples containing primary tumors and paired adjacent noncancerous tissues were used. All of the 106 specimens were retrieved from the archives in the Department of pathology of our Hospital. Representative areas of tissue were established by microscopic review of H&E stained slides and 2.0 mm diameter cores were punched from the paraffin blocks. Two cores from each of primary cancer and adjacent normal tissue at a distance of at least 2 cm from the tumor were arrayed. TMAs were created using a Tissue Microarrayer (Beecher Instruments, Sun Prairie, WI, USA). All specimens were examined by at least two pathologists to prevent bias.
Immunohistochemistry
MSI2 and Ki-67 expression were detected on the TMAs following citrate buffer (pH 6.0) antigen retrieval using standard methodology and a primary antibody against MSI2 (1:80 dilution, Abcam, USA) or Ki-67 (1:50 dilution, Dako Cytomation, Copenhagen, Denmark).Tissue sections were counterstained with Mayer’s Hematoxylin. Positive staining was scored by two independent investigators without the knowledge of patient outcomes (double-blind) according to the stained area. The evaluation was based on the staining intensity and extent of staining as previously described [25]. The specimens were divided into negative and positive groups according to their overall scores. Representative images are shown for MSI2 staining in the nuclear and cytoplasmic compartments as the literature depicted [15] (Figure 1). Ki-67 index was scored as previously described [26].
Figure 1.
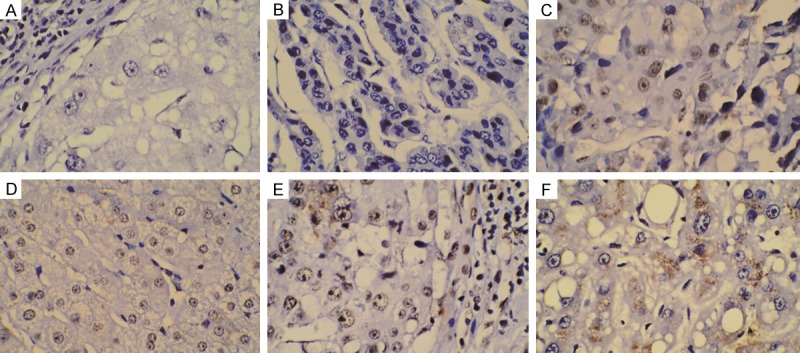
Immunohistochemical staining of Musashi-2(MSI2) expression in normal tissue and hepatocellular carcinoma. (A, B) Negative MSI2 expression in normal hepatic tissue (A) and primary tumor tissue (B). (D, E) Positive MSI2 staining in normal hepatic tissue (D) and poorly differentiated tumor tissue (E). Strongly Positive MSI-2 staining in nucleus (C) and cytoplasm (F) of poorly differentiated tumors. Original magnification × 400.
RNA extraction, reverse transcription PCR and quantitative real-time PCR
Total RNA was extracted according to the manufacturer’s instructions (TRIzol, Invitrogen, USA). First-strand cDNA was synthesized from 1 µg of total RNA according to the manufacturer’s instructions (Promega, USA). 20 ng of cDNA was used as a template for the specific PCR reactions. The sense primer and antisense primer used to amplify the MSI2, β-catenin, LEF-1, and TCF-4 gene were shown in Table 2. Quantitative PCR was performed using a Mastercycler ep Realplex (Eppendorf) using the IQTM SYBR Green Supermix Kit (BIO-RAD) according to the manufacturer’s protocol. The cycling conditions were as follows: 95°C for 10 min, and then 40 cycles of 95°C for 15 s, 60°C for 45 s, and 60°C for 15 s, with a final extension at 60°C for 1 min. PCR products were separated on 1.5% agarose gels and then visualized using an ultraviolet imaging system (FuRi Co., Shanghai, China). GAPDH was used as the internal control for all samples, and the relative quantification was given by the Ct values, MSI2ΔCt [ΔCt = Ct (MSI2) - Ct (GAPDH)] values were calculated for each group.
Table 2.
The sense primer and antisense primer used to amplify the MSI2, β-catenin, LEF-1 and TCF-4 gene in Quantitative Real-time PCR were shown
| Gene name | Sense primer | Size (bp) |
|---|---|---|
|
| ||
| Antisense primer | ||
| MSI2 | 5’TTCGCAGACCCAGCAAGTG 3’ | 154 |
| 5’TCGCAGATAACCCGCCTAC 3’ | ||
| β-catenin | 5’GGTTTCCCATTGGTTCAC 3’ | 246 |
| 5’CATAAATCCCGCCTAACG 3’ | ||
| LEF-1 | 5’CTCTGTCTTTCCTGCTGTTG 3’ | 215 |
| 5’CTAAATCGCCTTCCTCTTCG 3’ | ||
| TCF-4 | 5’CAGTCTTCCTCCGATGTC 3’ | 115 |
| 5’CCCGCTTCCTCTATTTGC 3’ |
bp base pair; LEF-1 Lymphoid enhancer factor-1; TCF-4 T cell transcription factor-4.
Cell culture, reagents
The SMMC-7721 and Hep3B human HCC cell lines (Center of Shanghai Institutes for Biological Sciences, Type Culture Collection of Chinese Academy of Sciences) were cultured at 37°C, high humidity, and 5% CO2 in RPMI 1640 medium (Hyclone) supplemented with 10% FBS (Gibco), 1% streptomycin and penicillin.
Si-RNA transfection
A short interfering RNA (si-RNA) targeting MSI2 sequence (5’-AGATAGCCTTAGAGACTATTT-3’) was synthesized. A scrambled si-RNA (5’- CCAGCAAGTGTAGATAAAGTA-3’) with no homology with the mammalian mRNA sequences was used as a negative control. Cells were transfected with si-RNA using LipofectamineTM2000 (Invitrogen) according to the manufacturer’s instructions. HCC SMMC-7721 and Hep3B mock-group cells were mock-transfected with OligofectamineTM2000 alone.
Tumor cell wound healing assay
Cells were seeded into 24-well tissue culture plates at a density that reached 70% to 80% confluence as a monolayer after 24 h of growth. The monolayer was scratched with a pipette tip across the center to create a cross in each well. The well was washed twice with medium to remove the detached cells. The cells were grown for additional 48 h in fresh medium. Four different views of each well were documented and the cell migration ability was represented by the gap distance quantitatively evaluated using WCIF ImageJ software. Each experiment was performed in triplicate.
Tumor cell invasion assay
The invasion assay was conducted in a modified 24-well Boyden chamber using an uncoated membrane and a membrane coated with Matrigel (BD Biosciences, San Jose, CA, USA). Briefly, cells (6 × 104) prepared in serum-free medium (500 mL) were loaded in the upper well, and medium supplemented with 10% fetal bovine serum was placed in the lower wells as a chemo-attractant stimulus. The noninvasive cells on the upper chamber of the filter were removed with a cotton swab after 24-h incubation. Cells that migrated to the bottom surface of the filter were fixed, stained, and counted under a microscope in 4 different randomly selected fields at a × 200 magnification.
Protein isolation and western blot analysis
Total cytoplasmic protein was extracted using a Protein Extraction Kit (JRDUN. Biotech, Shanghai, China).For Western blot studies, denatured proteins from either cancer or noncancerous tissues or cells were subjected to SDS–PAGE, transferred to PVDF membranes, and incubated at 4°C overnight with rabbit anti-MSI2 antibody (1:800; Abcam, USA), rabbit anti-β-catenin antibody (1:1000; CST, USA), rabbit anti-LEF-1 antibody (1:30000; Abcam), mouse monoclonal anti-TCF-4 antibody (1:1000; Millipore, USA) and mouse monoclonal anti-GAPDH antibody (1:1500; Fermentas, USA). After washing in Tris buffered saline with 0.05% Tween 20 (TBS-Tween), blots were incubated with horseradish peroxidase-conjugated antibody. Finally, blots were developed using the enhanced Chemiluminescence system (ECL Plus, Amersham Pharmacia Biotech, UK).
Statistical analysis
Chi-square and Fisher exact tests were used to determine the statistical significance of the differences between experimental groups. Correlation between MSI2 and Ki-67 proliferative index was performed using Kappa correlation coefficient (k). Multiple group comparisons were achieved by one-way analysis of variance followed by the Bonferroni post-hoc test. When a significant difference was apparent between groups, multiple comparisons of means were performed using the Bonferroni procedure with type I error adjustment. The Kaplan–Meier survival rate curve was used to analyze HCC patients’ cumulative survival rates. A Cox proportional hazards regression model was used to calculate univariate and multivariate hazard ratios for the study variables. All analyses were performed with the SPSS 15.0 software (SPSS Inc., Chicago, IL, USA).A value of P < 0.05 was considered statistically significant.
Results
MSI2 expression in human HBV-related HCC
We first examined MSI2 expression in tumor tissues compared with paired adjacent noncancerous tissues in 10 patient samples using qRT-PCR. Our data showed a significant upregulation of MSI2 expression level in HCC tissues compared with the corresponding noncancerous tissues (Figure 2).
Figure 2.
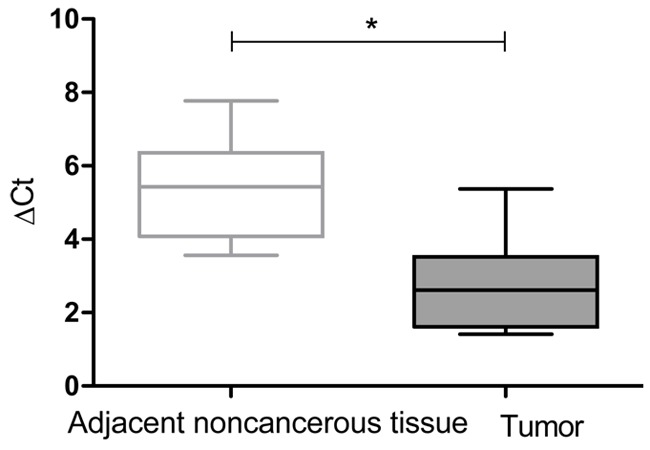
Real-time PCR analysis of MSI2 mRNA expression in 10 paired hepatocellular carcinoma samples and adjacent noncancerous tissues. For each sample, the relative MSI2 mRNA level was normalized using GAPDH expression. Data are presented as the median (line) ΔCt value with boxed 25th and 75th percentiles. The data range is represented by the upper and lower bars. *P < 0.01.
To further ascertain the clinicopathologic significance of MSI2 expression, immunohistochemistry was performed to detect the expression of MSI2 in a tissue array containing 106 cases of primary HBV-related HCC paired with adjacent noncancerous tissues. As shown in Figure 1, MSI2 protein was localized to the nucleus and cytoplasm of the cancer cells, with minimal staining in the normal tissue. MSI2 was significantly up-regulated in 60.4% (64 of 106) of primary HBV-related HCC lesions, whereas weakly positive staining was only found in 30.2% (32 of 106) of cases when compared with adjacent noncancerous tissues. Immuno-histochemical staining of MSI2 was mostly found in the nucleus of HCC cells which was in 71.9% (46 of 64) positive cases (Figure 1), with much more than that staining in cytoplasm and total cancer cells suggesting a possible nuclear translocation role of MSI2 involved in the differentiation and is a typical of HCC.
There was a significant association between MSI2 immunoreactivity and clinicopathologic variables among the HBV-related HCC patients (Table 1). MSI2 expression demonstrated a positive correlation with tumor size (P = 0.028), tumor differentiation (P = 0.020), TNM stage (P = 0.027) and vessel invasion (P = 0.027).Additionally, there was a significant difference between the MSI2-positive and MSI2-negative groups and the number of patients who developed early recurrence from primary HCC after radical hepatectomy. More patients with MSI2-positive tumors subsequently developed recurrence than did those with MSI2-negative tumors (P = 0.005) (MSI2-positive, 40 of 54 patients [74.1%]; MSI2-negative, 24 of 52 [46.2%]). In order to disclose the relationship between MSI2 and HCC cell proliferation, we used the Kappa’s correlation coefficient to determine the correlation between MSI2 and Ki-67 proliferative index and showed a significant correlation between MSI2 expression and the presence of Ki-67 (κ = 0.401, P < 0.001, Table 3).
Table 3.
The correlation between MSI2 and Ki-67 proliferative index in 106 HBV-related HCC samples
| Ki-67 expression | MSI2 expression | P value | k | |
|---|---|---|---|---|
|
| ||||
| Positive | Negative | |||
| Positive | 55 | 20 | < 0.001 | 0.401 |
| Negative | 9 | 22 | ||
k Kappa’s correlation coefficient.
High MSI2 expression predicts unfavorable clinical outcome in HBV-related HCC
The 5-year OS rate of the 106 patients with primary HBV-related HCC was 60.4% (64/106), with 42 deaths observed during the follow-up period. The 5-year DFS rate was 71.7% (76/106), with 30 events observed during follow-up.
We used Kaplan–Meier analysis to show that the expression of MSI2 was significantly correlated with disease-free survival and overall survival in HBV-related HCC patients (log-rank test, P = 0.002 vs. P < 0.001, Figure 3). Using univariate analysis, it was demonstrated that patients whose focal HCC were MSI2-positive had a significantly lower 5-year DFS than those with MSI2-negative tumors (59.4 vs. 90.5%; HR 3.62; 95% CI (1.47-8.90), Figure 3A, Table 4). The 5-year OS was also significantly lower in patients with MSI2-positive tumors than in those with MSI2-negative tumors (43.8 vs. 85.7%; HR 3.79; 95% CI (1.75-8.22), Figure 3B, Table 5). In addition, Ki-67 (P < 0.05), tumor size (P < 0.05) and tumor differentiation (P < 0.001) were associated with OS and DFS (Tables 3, 4).
Figure 3.
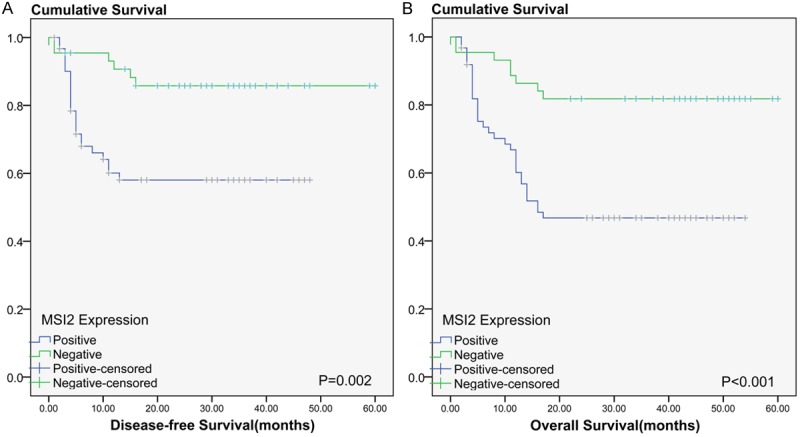
Disease-free survival (DFS) and overall survival (OS) rates were estimated by the Kaplan–Meier method. Both the (A) DFS rate and (B) OS rate of patients with MSI2 positive primary tumors was significantly lower than that of patients with MSI2 negative primary tumors (log-rank test, A: P = 0.002, B: P < 0.001).
Table 4.
Univariate and multivariate analysis of disease-free survival after surgery of 106 HCC patients
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | CI (95%) | P value | HR | CI (95%) | P value | |
| Tumor size (cm) | 2.35 | 1.09-5.02 | 0.028* | 3.20 | 1.17-8.70 | 0.023* |
| Tumor capsule | 1.13 | 0.55-2.32 | 0.735 | 1.84 | 0.81-4.19 | 0.144 |
| Tumor differentiation | 4.49 | 2.18-9.24 | < 0.001* | 4.07 | 1.84-9.02 | 0.001* |
| Tumor thrombus | 1.23 | 0.55-2.76 | 0.618 | 3.18 | 1.02-9.94 | 0.046* |
| Recurrence | 0.76 | 0.35-1.62 | 0.471 | 0.92 | 0.38-2.24 | 0.855 |
| TNM stage | 1.49 | 0.66-3.34 | 0.338 | 2.58 | 0.93-7.15 | 0.070 |
| AFP (ng/ml) | 0.05 | 0-1845.26 | 0.573 | - | - | 0.981 |
| vessel invasion | 2.08 | 0.97-4.45 | 0.059 | 0.51 | 0.14-1.83 | 0.303 |
| MSI2 expression | 3.62 | 1.47-8.90 | 0.005* | 2.54 | 0.98-6.59 | 0.056 |
| Ki-67 expression | 4.68 | 1.42-15.47 | 0.011* | 5.02 | 1.21-20.78 | 0.026* |
HR, hazard ratio; CI, confidence interval;
P < 0.05 indicates a significant association between variables.
Table 5.
Univariate and multivariate analysis of overall survival after surgery of 106 HCC patients
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | CI (95%) | P value | HR | CI (95%) | P value | |
| Tumor size (cm) | 2.29 | 1.21-4.36 | 0.011* | 2.33 | 1.08-5.03 | 0.031* |
| Tumor capsule | 1.15 | 0.63-2.11 | 0.653 | 1.73 | 0.89-3.35 | 0.106 |
| Tumor differentiation | 3.22 | 1.74-5.94 | < 0.001* | 3.39 | 1.72-6.67 | < 0.001* |
| Tumor thrombus | 1.41 | 0.72-2.76 | 0.314 | 2.95 | 1.11-7.82 | 0.030* |
| Recurrence | 1.43 | 0.78-2.62 | 0.247 | 1.45 | 0.71-2.96 | 0.311 |
| TNM stage | 1.34 | 0.66-2.73 | 0.422 | 2.37 | 0.96-5.83 | 0.061 |
| AFP (ng/ml) | 0.048 | 0-372.73 | 0.506 | - | - | 0.983 |
| vessel invasion | 1.83 | 0.94-3.58 | 0.077 | 0.65 | 0.22-1.95 | 0.441 |
| MSI2 expression | 3.79 | 1.75-8.22 | 0.001* | 2.44 | 1.07-5.58 | 0.034* |
| Ki-67 expression | 3.94 | 1.55-10.04 | 0.004* | 4.36 | 1.47-12.9 | 0.008* |
HR, hazard ratio; CI, confidence interval;
Significant difference.
Multivariate analysis was performed using the Cox proportional hazards model for all significant variables in the univariate analysis. The results from the multivariate analysis showed that Ki-67 (P = 0.026), tumor size (P = 0.023), tumor thrombus (P = 0.046) and tumor differentiation (P = 0.001) were independent prognostic factors for DFS (Table 4). Moreover, on Cox proportional hazard analyses of OS, MSI2 (P = 0.034), Ki67 (P = 0.008), tumor size (P = 0.031), tumor thrombus (P = 0.030), and tumor differentiation (P < 0.001) emerged as significant independent prognostic factors to predict patients’ clinical outcome (Table 5).
MSI2 knockdown inhibited tumor cell migration and invasion
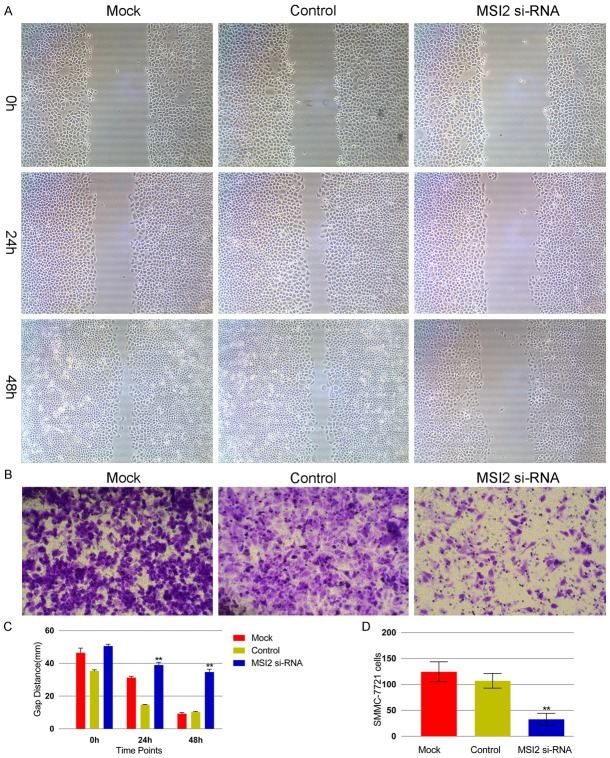
There was no significant difference in MSI2 expression at the mRNA level between SMMC-7721 and Hep3B cell lines (Figure 6). The SMMC-7721 cell line was selected as the human HCC cell line in the following assay as the literature described [7]. The effect of MSI2 knockdown on migration potency in SMMC-7721 cells was assayed using a wound healing test to relatively reflect the migration distance by the gap width. The MSI2 si-RNA group exhibited a significantly reduced migratory ability compared with the control group (P < 0.001, Figure 4A, 4C). We also assessed the effect of MSI2 depletion on tumor invasion using the Transwell assay and demonstrated that disruption of endogenous MSI2 expression inhibits the invasive potential of MSI2 si-RNA HCC cells compared with the control group (P < 0.001, Figure 4B, 4D). All the above experiments were performed with Hep3B cell line.
Figure 6.
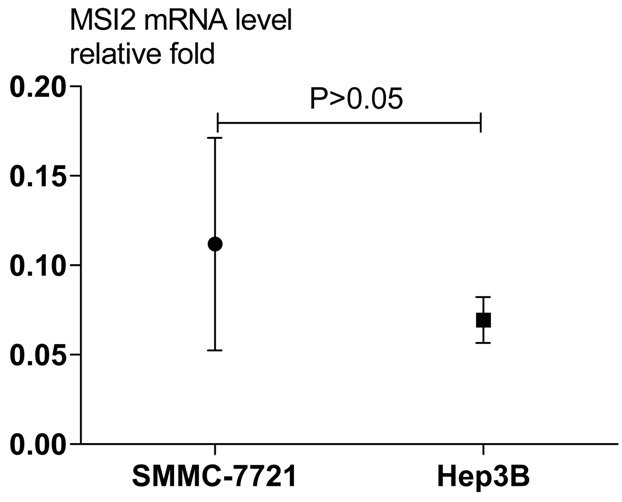
Real-time PCR analysis of MSI2 mRNA expression in HCC cell lines SMMC-7721 and Hep3B. The relative MSI2 mRNA level was normalized using GAPDH expression. The data range is represented by the upper and lower bars (P > 0.05).
Figure 4.
MSI2 inhibition suppresses the migration and invasive ability of hepatocellular carcinoma cells by wound healing assay (A) and Transwell assay (B). (A) After scratches were made, SMMC-7721 cells were allowed to proliferate for another 24 h and 48 h in the absence or presence of different concentrations of MSI2. (B) Representative images show invasion of SMMC-7721 cells through Transwells with Matrigel. Magnification, × 200. Columns C and D represent the mean of three individual experiments performed in triplicate; error bars represent SD, ΔP < 0.001, vs. control group.
MSI2 knockdown decreases the mRNA and protein levels of β-catenin and relative genes of the Wnt/β-catenin signaling pathway
In our study, we first examined the β-catenin and LEF-1/TCF-4 protein levels in SMMC-7721 and Hep3B cell lines with MSI2 knockdown by western blot. As a result, the protein levels of β-catenin and LEF-1/TCF-4 were decreased in the above cell lines, and their expression levels had significant differences compared with the control group (Figure 5A-D).
Figure 5.
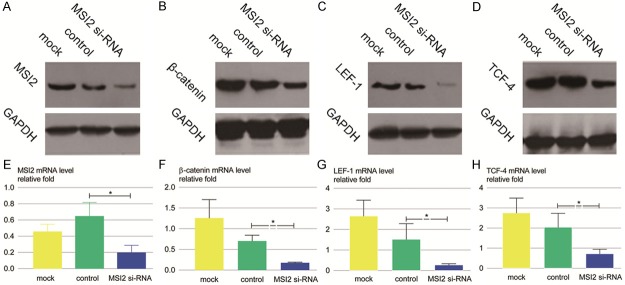
Effect of MSI2 knockdown on Wnt/β-catenin signaling pathway of SMMC-7721 cells. SMMC-7721 cells were treated with MSI2 si-RNA, control si-RNA and Oligofectamine alone (mock) for 48 h, following which cells were harvested for analysis of MSI2, β-catenin,LEF-1, and TCF-4 at mRNA (E-H) and protein (A-D) level. Relative quantification of mRNA is presented as mean values ± SD relative to control transfectants of 3 independent experiments (*P < 0.01).
Since MSI2 knockdown repressed β-catenin and LEF-1/TCF-4 protein levels, we decided to examine if MSI2 played a similar role at a transcriptional level. For this purpose, the mRNA levels of β-catenin and its downstream target genes were examined by qRT-PCR after transfection of MSI2 si-RNA. As expected, the mRNA expression of β-catenin and LEF-1/TCF-4 was verified to be down-regulated. Corresponding to the β-catenin gene, two downstream genes of the Wnt/β-catenin signaling pathway, LEF-1 and TCF-4 were also decreased at the post-transcriptional level compared with the control group (Figure 5E-H).
Discussion
In this study, we examined the expression of MSI2 in a panel of HBV-related HCC tissue samples and cells and found that MSI2 is highly expressed in the majority of cancer tissues at both the protein level and mRNA level. We further investigated the role of MSI2 in HCC cancer development using RNAi in HCC cancer cell lines. We found that knockdown of MSI2 inhibited HCC cell migration and invasion. The above data suggests that MSI2 may contribute to the progression of HCC carcinogenesis.
MSI2 has recently been shown to be critical in HSC proliferation and differentiation, with upregulation of mRNA leading to increased proliferation of undifferentiated cells in cell culture [27]. Consistently, we found that MSI2 was expressed at higher levels in low differentiated HCC tissues in comparison with high/moderate differentiated tissues with significant correlation. Furthermore, MSI2 was predominantly observed to be expressed in the nucleus, indicating that it might mediate transcriptional control and tumor development. Our data also showed a significant association between tumor MSI2 expression and the Ki-67 index. Taken together, these findings indicate that MSI2 performs as an oncogenic factor with potential cancer progression in HCC.
To date, valid prognostic biomarkers for HBV-related HCC have not been established [28]. In the present study, patients with high tumor MSI2 expression had an increased risk of tumor recurrence and shorter survival. To further define increased MSI2 expression as an independent factor influencing tumor prognosis, a multivariate Cox regression analysis was performed. As a result, no significant difference was found in DFS (P = 0.056) between the negative and positive MSI2 expression groups of patients. These results are in contrast to recent published studies [7], which may need to be explained by future research with an expanded sample set. However, significant differences in OS were detected in HCC patients with MSI2 dysregulation. These data indicate that MSI2 expression levels may be useful in stratifying HBV-related HCC patients for novel therapeutic strategies together with other potential biomarkers.
Migration and invasive capacity of cells are two of the most important features of malignant cell behavior [29]. Intriguingly, down-regulation of MSI2 significantly inhibited HCC cell migration and invasion by MSI2 knockdown. Consistent with our present data, Lu He [7] et al found that knockdown of MSI2 significantly reduced the invasive abilities of two independent HCC cell knockdown clones. Taken together, our results in combination with the findings from others, suggests that MSI2 plays an important role in HCC progression by promoting cell migration and invasion.
The mechanism by which MSI2 contributes to tumorigenesis is not well understood. MSI2 proteins are RNA-binding proteins that bind to mRNAs and inhibit transcription [30]. Based on the previous study of the literature, deregulation of the Wnt/β-catenin pathway is an early event in hepatocarcinogenesis and has been associated with an aggressive HCC phenotype, since it is implicated both in cell survival, proliferation, migration and invasion [13]. Thus, component proteins identified in this pathway are potential candidates for pharmacological intervention. The β-catenin protein is the crucial molecule in the Wnt/β-catenin pathway which can translocate to the nucleus where it serves as a transcription activator to form a transcriptional complex with the TCF-4/LEF-1 proteins followed by the activation of target genes that up regulate cell-proliferation, migration, invasion, cell cycle progression and metastasis [31]. As expected, in the present study, β-catenin and TCF-4/LEF-1 expression are both significantly down-regulated with MSI2 knockdown in SMMC-7721 and Hep3B tumor cell lines. To date, there has been no report about the mechanism of the Wnt/β-catenin signaling pathway correlating with MSI2. Based on these data, we speculated that the binding ligand molecule may exist among the Wnt/β-catenin pathway proteins, deducing that a similar RNA binding mechanism is induced by MSI2. Certainly, further studies are needed to investigate the target genes and elucidate the exact mechanism. However, we present the first report that MSI2 expression might promote HCC progression via the Wnt/β-catenin pathway.
In conclusion, this is the first study to highlight the clinical significance and underlying molecular mechanism of MSI2 in HBV-related HCC. We propose that MSI2 could serve as a biomarker to predict prognosis in HBV-related HCC patients who underwent curative hepatectomy. Our study preliminarily unveils that MSI2 promotes the migration and invasion of HCC cells via the Wnt/β-catenin signaling pathway. Our results also reveal a novel regulatory effect of MSI2 on β-catenin and TCF-4/LEF-1. These results may open up new possibilities for future therapeutic interventions. However, these preliminary findings need to be verified in a larger, prospective, and controlled clinical study.
Acknowledgements
We are grateful for funding support from: Shandong Natural Science Foundation (Grant Number: 2009GG20002070).
Disclosure of conflict of interest
None.
References
- 1.Yang WL, Wei L, Huang WQ, Li R, Shen WY, Liu JY, Xu JM, Li B, Qin Y. Vigilin is overexpressed in hepatocellular carcinoma and is required for HCC cell proliferation and tumor growth. Oncol Rep. 2014;31:2328–2334. doi: 10.3892/or.2014.3111. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen VT, Law MG, Dore GJ. Hepatitis B-related hepatocellular carcinoma: epidemiological characteristics and disease burden. J Viral Hepat. 2009;16:453–463. doi: 10.1111/j.1365-2893.2009.01117.x. [DOI] [PubMed] [Google Scholar]
- 3.Leong TY, Leong AS. Epidemiology and carcinogenesis of hepatocellular carcinoma. Hpb (Oxford) 2005;7:5–15. doi: 10.1080/13651820410024021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurokohchi K, Takaguchi K, Kita K, Masaki T, Kuriyama S. Successful treatment of advanced hepatocellular carcinoma by combined administration of 5-fluorouracil and pegylated interferon-alpha. World J Gastroenterol. 2005;11:5401–5403. doi: 10.3748/wjg.v11.i34.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El–Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 6.Tan X, Yuan Y, Zeng G, Apte U, Thompson MD, Cieply B, Stolz DB, Michalopoulos GK, Kaestner KH, Monga SP. β-Catenin deletion in hepatoblasts disrupts hepatic morphogenesis and survival during mouse development. Hepatology. 2008;47:1667–1679. doi: 10.1002/hep.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He L, Zhou X, Qu C, Hu L, Tang Y, Zhang Q, Liang M, Hong J. Musashi2 predicts poor prognosis and invasion in hepatocellular carcinoma by driving epithelial-mesenchymal transition. J Cell Mol Med. 2014;18:49–58. doi: 10.1111/jcmm.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312–1327. doi: 10.1002/hep.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nejak-Bowen KN, Thompson MD, Singh S, Bowen WC Jr, Dar MJ, Khillan J, Dai C, Monga SP. Accelerated liver regeneration and hepatocarcinogenesis in mice overexpressing serine-45 mutant β-catenin. Hepatology. 2010;51:1603–1613. doi: 10.1002/hep.23538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiquro H, Fujita M, Tokino T, Sasaki Y, Imaoka S, Murata M, Shimano T, Yamaoka Y, Nakamura Y. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24:245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- 11.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 12.Cavard C, Colnot S, Audard V, Benhamouche S, Finzi L, Torre C, Grimber G, Godard C, Terris B, Perret C. Wnt/β-catenin pathway in hepatocellular carcinoma pathogenesis and liver physiology. Future Oncol. 2008;4:647–660. doi: 10.2217/14796694.4.5.647. [DOI] [PubMed] [Google Scholar]
- 13.Pez F, Lopez A, Kim M, Wands JR, Caron de Fromentel C, Merle P. Wnt signaling and hepatocarcinogenesis: Molecular targets for the development of innovative anticancer drugs. J Hepatol. 2013;59:1107–1117. doi: 10.1016/j.jhep.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Okano H, Kawahara H, Toriya M, Nakao K, Shibata S, Imai T. Function of RNA-binding protein Musashi-1 in stem cells. Exp Cell Res. 2005;306:349–356. doi: 10.1016/j.yexcr.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 15.Byers RJ, Currie T, Tholouli E, Rodig SJ, Kutok JL. MSI2 protein expression predicts unfavorable outcome in acute myeloid leukemia. Blood. 2011;118:2857–2867. doi: 10.1182/blood-2011-04-346767. [DOI] [PubMed] [Google Scholar]
- 16.Ito T, Kwon HY, Zimdahl B, Congdon KL, Blum J, Lento WE, Zhao C, Lagoo A, Gerrard G, Foroni L, Goldman J, Goh H, Kim SH, Kim DW, Chuah C, Oehler VG, Radich JP, Jordan CT, Reya T. Regulation of myeloid leukaemia by the cell-fate determinant Musashi. Nature. 2010;466:765–768. doi: 10.1038/nature09171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toda M, Lizuka Y, Yu W, Imai T, Ikeda E, Yoshida K, Kawase T, Kawakami Y, Okano H, Uyemura K. Expression of the neural RNA-binding protein Musashi1 in human gliomas. Glia. 2001;34:1–7. doi: 10.1002/glia.1034. [DOI] [PubMed] [Google Scholar]
- 18.Todaro M, Francipane MG, Medema JP, Stassi G. Colon cancer stem cells: promise of targeted therapy. Gastroenterology. 2010;138:2151–2162. doi: 10.1053/j.gastro.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 19.Moreira AL, Gonen M, Rekhtman N, Downey RJ. Progenitor stem cell marker expression by pulmonary carcinomas. Modern Pathology. 2010;23:889–895. doi: 10.1038/modpathol.2010.68. [DOI] [PubMed] [Google Scholar]
- 20.MacNicol AM, Wilczynska A, MacNicol MC. Function and regulation of the mammalian Musashi mRNA translational regulator. Biochem Soc Trans. 2008;36:528–530. doi: 10.1042/BST0360528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emadi-Baygi M, Nikpour P, Mohammad-Hashem F, Maracy MR, Haghjooy-Javanmard S. MSI2 expression is decreased in grade II of gastric carcinoma. Pathol Res Pract. 2013;209:689–691. doi: 10.1016/j.prp.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Nikpour P, Baygi ME, Steinhoff C, Hader C, Luca AC, Mowla SJ, Schulz WA. The RNA binding protein Musashi1 regulates apoptosis, gene expression and stress granule formation in urothelial carcinoma cells. J Cell Mol Med. 2011;15:1210–1224. doi: 10.1111/j.1582-4934.2010.01090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pereira JK, Traina F, Machado-Neto JA, Duarte Ada S, Lopes MR, Saad ST, Favaro P. Distinct expression profiles of MSI2 and NUMB genes in myelodysplastic syndromes and acute myeloid leukemia patients. Leukemia Res. 2012;36:1300–1303. doi: 10.1016/j.leukres.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Tan S, Wang J, Chen S, Quan J, Xian J, Zhang Ss, He J, Zhang L. Musashi2 modulates K562 leukemic cell proliferation and apoptosis involving the MAPK pathway. Exp Cell Res. 2014;320:119–127. doi: 10.1016/j.yexcr.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Li D, Yan D, Tang H, Zhou C, Fan J, Li S, Wang X, Xia J, Huang F, Qiu G, Peng Z. IMP3 is a novel prognostic marker that correlates with colon cancer progression and pathogenesis. Ann Surg Oncol. 2009;16:3499–3506. doi: 10.1245/s10434-009-0648-5. [DOI] [PubMed] [Google Scholar]
- 26.Liu AW, Cai J, Zhao XL, Xu AM, Fu HQ, Nian H, Zhang SH. The clinicopathological significance of BUBR1 overexpression in hepatocellular carcinoma. J Clin Pathol. 2009;62:1003–1008. doi: 10.1136/jcp.2009.066944. [DOI] [PubMed] [Google Scholar]
- 27.De Andres-Aquayo L, Varas F, Kallin EM, Wurst W, Floss T, Graf T. Musashi 2 is a regulator of the HSC compartment identified by a retroviral insertion screen and knockout mice. Blood. 2011;118:554–564. doi: 10.1182/blood-2010-12-322081. [DOI] [PubMed] [Google Scholar]
- 28.Zhan P, Ji YN. Prognostic significance of TP53 expression for patients with hepatocellular carcinoma:a meta-analysis. Hepatobiliary Surg Nutr. 2014;3:11–17. doi: 10.3978/j.issn.2304-3881.2014.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang HJ, Yao DF, Yao M, Huang H, Wang L, Yan MJ, Yan XD, Gu X, Wu W, Lu SL. Annexin A2 silencing inhibits invasion, migration, and tumorigenic potential of hepatoma cells. World J Gastroenterol. 2013;19:3792–3801. doi: 10.3748/wjg.v19.i24.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbouti A, Hoglund M, Johansson B, Lassen C, Nilsson PG, Hagemeijer A, Mitelman F, Fioretos T. A novel gene, MSI2, encoding a putative RNA-binding protein is recurrently rearranged at disease progression of chronic myeloid leukemia and forms a fusion gene with HOXA9 as a result of the cryptic t (7; 17)(p15; q23) Cancer Res. 2003;63:1202–1206. [PubMed] [Google Scholar]
- 31.Ge WS, Wang YJ, Wu JX, Fan JG, Chen YW, Zhu L. β-catenin is overexpressed in hepatic fibrosis and blockage of Wnt/β-catenin signaling inhibits hepatic stellate cell activation. Mol Med Rep. 2014;96:2145–2151. doi: 10.3892/mmr.2014.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]