Abstract
Glioma is the world’s commonest primary brain malignancy with much of its biology relating to translational and post-translational events still unknown. In this study, we investigated the clinicopathological significance of N-linked β1-6-GlcNAc branches and GnT-V enzyme in the development and progression of astrocytic glioma. Expression of GnT-V and its GlcNAc-β1-6 oligosaccharides by-product together with Con-A binding sugars were assessed immunohistochemically on tissue microarrays of 16 normal brain and 159 tissue samples of astrocytomas of variable grades and histology. Although tissues of both grade I astrocytomas and normal brain showed considerably higher GnT-V expression, GlcNAc-β1-6 expression was significantly high only in tissues of grade I astrocytomas (p < 0.001), which is attributable to elevated levels of the precursor Con-A binding sugar moieties (p < 0.001). The activity of GnT-V enzyme was found to be dependent on the degree of glioma pathogenesis, as the GlcNAc-β1-6 branched expression diminished with every progressive grade of glioma, reaching minimum in glioblastoma (p < 0.001). Having biphasic activity in gliomagenesis, the role of GnT-V in glioma was deciphered by generating different ectopic GnT-V expressions in U-87 cells, which showed the highest GnT-V expression among selected glioma cell lines. Transient GnT-V rescue was achieved in knockdown clones by transfection with GnT-V expression vector. Suppression of GnT-V in U-87 cells slowed cell proliferation with G0/G1 cell cycle phase arrest. Reduced tumorigenicity, invasiveness and cell-ECM interactions were also associated with suppressed in vitro GnT-V activity suggesting GnT-V may act as an oncoprotein. We report for the first time that GnT-V products are involved in early gliomagenesis but their reduced expression, correlating with low Con-A binding sugars level found in high tumor grades predicts the loss of total N-glycosylation in glioma development and may be of potential diagnostic and/or prognostic value in astrocytoma.
Keywords: N-Acetylglucosaminyltransferase V (GnT-V), GlcNAc-β1-6 linkage, Con-A (Concanavalin A), astrocytic glioma, early gliomagenesis
Introduction
Biochemical changes in carbohydrate portion of proteins and lipids are hallmarks of tumor progression and neoplastic transformation [1]. Comparative studies of Asparagine linked (N-linked) oligosaccharides in transformed cells from normal cells reveal an increase of tri and tetra antennary complex type glycan, containing N-acetylglucosamine (beta 1,6) mannose (GlcNAc-β1-6 man) linkages [2,3]. These tumor associated glycans often arises from the changes in the expression of glycosyltransferase residing in Golgi compartments [4]. One of the highly expressed glycosyltransferase in tumors, N-acetyl glucosamine glycosyltransferase (GlcNAc-T-V or GnT-V), encoded by the MGAT5 gene, is involved in the synthesis of GlcNAc-β1-6-Man linkage on N-glycans in median Golgi [2,5]. GnT-V catalyzes the sugar associated with transformation and malignancy, and is therefore an important glycosyltransferase attracting extensive studies in attempt to elucidate the mechanisms underlying its involvement in many different cancers.
High expression of GnT-V is associated with poor clinical outcomes of gastric, colorectal and endometrial cancers [6-8]. Several proto-oncoproteins including ETS and c-MYB have their binding sites on the promoter region of MGAT5 [9], whose transcription can also be induced by several oncogenes including Her2/neu, H-ras and v-sis/PDGF [10,11]. Interestingly, high expression of this enzyme has been reported to be associated with good prognosis in bladder cancer [12] and non-small cell lung carcinoma [13]. In 2000, Yamamoto et al. showed the association of β1-6-GlcNAc branching with glioma invasion [14] while Taniguchi’s group [15] found high expression of MGAT5 to favor the survival of neuroblastoma patients, presumably by escaping PARP cleavage- induced apoptosis. The functional diversity of the GnT-V enzyme is underpinned by its gene’s sequence which employs multiple promoter systems for its transcription [9] rendering it prone to control and influence of several cell signalling pathways. The expression and activity of GnT-V is also known to be tissue specific. Whereas its expression is elevated in high grade mucinous types of ovarian tumors, non-mucinous types of epithelial ovarian tumors showed lower expression compared to benign and borderline tumors [16]. It is also known that optimum decoration of GlcNAc-β1-6 branches is required for change in adhesive properties of cells, Guo and colleagues in 2002 reported that, HT1080 transfected clones with intermediate levels of GnT-V overexpression exhibited greater alteration in their adhesion properties compared to that of clones with higher levels of GnT-V overexpression.
In view of the multi-functional involvement of GnT-V in different cancers, we hypothesize that GnT-V and its product may play a crucial role in gliomagenesis. Gliomas are the most common subtypes of primary brain tumors derived from glial cells and commonly found in the anterior cerebral hemisphere [17]. Unlike other solid tumors, gliomas rarely metastasize outside the CNS but are neurologically destructive, highly invasive and considered among the most lethal human carcinomas [18]. Patients with glioblastomas (GBM), the most aggressive manifestation of astrocytic glioma, have a median survival of 12 months. In spite of satisfactory advancement in treatment and increasing understanding of glioma biology at the molecular level, little has changed in the outcome for patients with the disease.
In this study we have examined the expression of GnT-V in different astrocytic glioma grades and found an unfamiliar correlation. We found that GnT-V expression and its product β1-6-GlcNAc branches are positively correlated in the early stage gliomas while their expression decreased with glioma progression and reached lowest levels in glioblastoma. We further studied the in vitro functional behaviours of the enzyme and its product in tumorigenesis and gliomagenesis
Materials and methods
Cell lines and cell culture
U-87 MG and U-251 MG cell lines were kindly provided by Dr Jianhai Jiang (Fudan University, Shanghai, China); year 2012. U-118 MG glioma cell line was purchased from Chinese Academy of Sciences Committee on Type Culture Collection Cell Bank, Shanghai Institutes for Biological Sciences in 2012. GnT-V/shRNA and Mock as control were established from U-87 cells. All experiments were performed within 10 passages. U-87 MG cell line was checked for authentication by STR analysis. Cells were cultured at 37°C, 5% CO2 and routinely maintained in DMEM (Gibco) with 10% fetal bovine serum (FBS) and 0.5% penicillin and streptomycin (Beyotime, China).
RNA extraction and semi-quantitative RT-PCR
Total RNA was prepared with RNAiso Plus (Takara, Dalian, China), and treated with DNase 1 (AMPD1 Sigma). cDNA was synthesized from 2 µg total RNA using M-MLV, (AccuPower RT PreMix, Bioneer). Gene expression was quantified by rTaq (Takara, Dalian, China). The primer pair specific for GnT-V (forward, 5’-GCAATGGCTCTCTTCACTCC-3’ and reverse, 5’-GCTGTCATGACTCCAGCGTA-3’) and β-actin (forward, 5’GGAGTCCTGTGGCATCCACG-3’ and reverse 5’-CTAGAAGCATTTGCGGTGGA-3’) were used. PCR products were separated on 1.5% agarose gel and band intensities were measured by Gel ProAnalyzer software.
Western blot assay
Protein concentration in the lysates was determined by BCA kit (KeyGen Biotech, China). Equal amounts of proteins were separated by 10% SDS-PAGE and transferred onto nitrocellulose membrane. The membrane was blocked with 5% skim milk (BD Difco) overnight in TBST at 4°C, then probed with mouse anti-GnT-V monoclonal antibody (Abnova), and rabbit anti-GAPDH (Bio world) at room temperature for three hours. Membranes were incubated for 30 minutes with the appropriate peroxidase-conjugated secondary antibodies. Bands on the membranes were visualized using ECL kit (GE healthcare).
Lectin flow cytometry
Cells were harvested using non enzymatic cell dissociation solution (M&C gene technology), and permeabilized by 0.1% triton X-100 for 7 minutes at room temperature. Direct immunofluorescence flow cytometry, was performed by incubating 1 × 106 cells with 3 ug/ml of FITC labelled PHA-L lectin (vector) in 3% BSA at 4°C for 30 minutes. The cells were finally washed and suspended in 500 ul of ice cold PBS. In the indirect flow cytometry, prior to the incubation with primary antibody, cells were blocked with 3% BSA, and 1.5 × 106 cells were incubated with 1.5 ug/ml of biotinylated PHA-L (vector). APC streptavidin (Biolegend) of final concentration of 1:500 was used, and incubated for 20 minutes at room temperature in the dark. Cells were suspended in cold PBS for final analysis after washing with 1% BSA. All the centrifugations were done at 1500 rpm for 4 minutes each. Fluorescent analyses were performed using a FACSCalibur (Becton Dickinson). Only live cells were established as gates. Unstained cells served as controls for direct flow cytometry and cells stained with only conjugated APC streptavidin acted as a control in indirect flow cytometry.
Construction of shRNA & generation of different ectopic GnT-V expressions in U-87
Short hairpin interfering oligonucleotides specific for GnT-V with nucleotide sequence 5’-GCATGATGCTTCTGCACTTCA-3’ was purchased from the GenePharma (Shanghai, China). Oligonucleotides were annealed and then ligated into pLL3.7 vector (Invitrogen). To obtain the lentiviruses, packaging plasmids were transfected simultaneously with our constructed plasmid (pLL3.7-shRNA GnT-V) into human embryonic kidney 293T cells using Lipiofectamine2000 transfection reagent (Invitrogen). Mock vector controls were generated using only the empty pLL3.7 vector similarly packaged as in the above. Lentiviruses were transduced in U-87 cells in the presence of 8 ug/ml Polyberene (Sigma). Stable shRNA/GnT-V and the control mock cell lines were established by the single cell dilution method. Wild type GnT-V and GnT-V/mutant expression vectors were a kind gift from Dr Jianguo Gu (Tohoku Pharmaceutical University, Miyangi, Japan). Transient transfection was done to rescue the knockdown of GnT-V using Lipofectamine 2000.
Cell proliferation assay
Cell proliferation was determined using tetrazolium WST-8 dye (KeyGen BioTech, China), according to manufacturer’s instruction. Briefly, 1 × 104 cells from each group were seeded into 96 well culture plate in triplicate and maintained under optimum culture condition. Growth rate of each group was measured on 24, 48 and 72 h, after seeding of cells in 96 well plates. Absorbance (Optical density), measured at 450 nm using Microplate reader at 24, 48 and 72 h, was determined and used to estimate the rate of cellular activity and growth among groups.
Soft agar colony formation assay
Soft agar colony formation assay was performed in 6 cm tissue culture dishes using 2-Hydroxyethylagaros type VII, low gelling temperature (Sigma) Volume 5 mls of 0.75% agarose in complete medium was prepared and layered at the bottom of the dish. About 5 × 103 cells were suspended in Volume 3 mls of 0.36% of agarose in the same medium and this suspension was served as top layer. Cells were incubated for 2 weeks in a humidified incubator under optimum culture conditions. Finally, cells were stained with crystal violet and scanned by photography (Olympus IX71). The number of established colonies was quantified using the CellSens software (Olympus).
Adhesion assay
Different wells on 96 well plates were coated with 20 ug/ml of Fibronectin, Laminin and Collagen IV (Sigma) overnight for incubation at 4°C. 1% BSA and Poly-L lysine were used as negative and positive controls, respectively. The coating was blocked for 1hr with 1% BSA at 37°C. Cells were harvested, washed in PBS and then suspended in serum free medium with 0.1% BSA. 2 × 103 cells were seeded into separate wells of the three surface coatings and incubated for 1 h. Non-adherent cells were removed by washing with PBS and attached cells stained with 0.2% crystal violet for 20 minutes. Cells were lysed in 2% SDS after removal of excess dye with DDH2O. Absorbance was determined at 570nm using Multskan Microplate reader.
In vitro cell invasion and migration assay
12 well Transwell membrane plates (Corning Costar Corporation) of pore size 8um and 6.5mm in diameter was used to assess the invasive and migratory ability of cells. For invasion assay, transwells were coated with 500 ug/ml of Matrigel (BD Biosciences-Discovery Labware) as a basement matrix for invasion assay. About 50 × 103 cells were seeded into the separate upper wells of pre-coated transwell in DMEM without FBS. The lower chambers were filled with same medium containing 10% FBS. Cells were incubated for 36 h under optimum culture conditions. The cells were then fixed with methanol and 3% paraformaldehyde, and stained with 0.5% crystal violet. Unattached cells in the upper chamber of transwell were removed with cotton swabbing and allowed to dry. Lastly, transwell membranes were scanned and photographs of microscope field (at X200) taken from Olympus IX71 inverted microscope. Invasiveness was estimated as mean number of cells per 10 randomly selected microscope fields per group. For migration assay; transwells were remain uncoated and 25 × 103 cells were allowed to migrate for 24 hrs. Rest of the method was followed as same as mentioned above for invasion assay.
Cell cycle analysis
Growing cells were harvested and washed with PBS. About 1 × 106 cells per group were collected and fixed separately in 70% ethanol for at least 24 h. fixed cells were washed in PBS and then treated with 50 ug/ml propidium iodide and 100 ug/ml of RNase A solution and incubated in the dark at 37°C for 45 minutes. Cell cycle analysis was performed with BD Accuri C6 flow cytometer. Generated data was analyzed by Flowjo software.
Clinical samples
Clinical samples in the form of tissue microarrays (GL2083a) were purchased from Xi’an Alena Biotechnology Co. Ltd., China (Agent for US Biomax). The microarray consisted of 192 different brain cancers and 16 normal brain tissues. Of the tumor samples, astrocytic glioma contributed 157, out of which 23, 19, 65, 20 and 32 were grade I, grade I-II, grade II, grade III and grade IV (glioblastoma), respectively. The remaining tumor samples belonged to other brain cancer types not included in this study due to lack of a reasonable representable size of the population that was needed to frame the analysis of each group. The total population of patients consisted of 71 females and 104 males with a mean array age of 44.6 years (range, 9-70 years). In order to have representable populations of every grade, samples of grade I-II were merged with grade I group, as they were more closer in appearance to grade I in comparison with grade II astrocytoma (Figure 1B).
Figure 1.
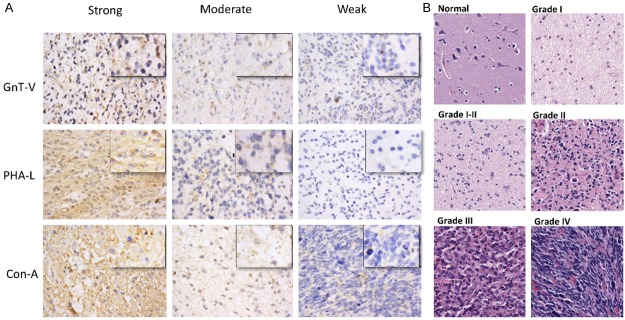
Immuno-staining of different histological stages of astrocytomas. A. Representative micrographs of immunohistochemical staining of astrocytic glioma tissue samples with GnT-V antibody, PHA-L and Con-A lectins. Staining intensities are divided into three tired grading systems for each group, independently. The staining intensity of every group was calculated by scanning each core of tissue microarray by Aperio image scope and the final positivity of each sample was calculated by the formula [A + B + C / Z+ (A + B + C)], where A = Total Intensity of weak positive, B = Total Intensity of Moderate Positive, C = Total intensity of Strong Positives, Z = Total intensity of negative. Original magnification was (X400; Leica DM400B microscope). B. Representative micrographs of H&E stained tissue samples of different grades of astrocytic glioma and normal brain tissue. Original magnification was X200. Tumor grades were determined according to the 2007 WHO grading system by the providers.
Immunohistochemistry & lectin histochemistry
Tissues on microarray slides were pre-heated at 60°C, deparaffinised with xylene and rehydrated in descending grades of alcohol. Antigen retrieval was subsequently achieved by boiling in citrate buffer of pH 6.0 in a pressure cooker. Endogenous peroxidase activity in tissue samples were silenced with 0.3% H2O2, whereas non-specific binding was controlled by blocking with 10% normal horse serum. Slide used to detect Con-A binding sugars was further blocked by avidin/biotin blocking kit (Vector Labs). Arrays were then incubated with GnT-V monoclonal antibody (1:75; Abnova), biotinylated PHA-L (1:200; Vector Labs) and biotinylated Con-A (1:200; Vector Labs) separately on each replicate array. Primary lectin staining was followed by incubation with horseradish peroxidase conjugated streptavidin (Vector Labs), while arrays incubated with primary GnT-V antibody was followed by 2-step plus polymer HRP anti-mouse/rabbit IgG detection system (PV9000; Beijing Zhongshan Jinqiao Biotechnology Co). Color development was performed using freshly prepared working solution of Diaminobenzidine (DAB) followed by counterstaining with Mayer hematoxylin. Sections were then dehydrated in ascending grades of alcohol, cleared in xylene, and mounted with neutral balsam. Immunostaining was verified by two independent pathologists. Images were taken from Leica DM400B microscope and the staining intensities were quantified with Aperio Image Scope. Scoring was performed by 3 tired grading systems.
Statistical analysis
The difference between experimental groups for the categorical data was computed by Chi-square test. Fisher Exact test was used when appropriate. Student’s t -test was used to test the significant differences between two groups for continuous variables and one-way analysis of variance (ANOVA) for more than two groups. All statistical tests were two sided and P-value < 0.05 was considered statistically significant. Graph pad prism version 5 was used for all statistical analysis. All in vitro experiments were performed at least twice and in triplicate. Data are presented as mean ± SEM or SD where appropriate.
Results
GnT-V enzyme, β1-6-GlcNAc branches and other N-glycans are involved in gliomagenesis
Clinical samples were categorised into groups as low, intermediate and strong on the basis of staining intensity for each targeted antigens i.e. GnT-V, PHA-L and Con-A reactive oligosaccharides (Figure 1A). We found that expression of PHA-L recognizing sugars, i.e [Gal (β1, 4) GlcNAc β1-6 Man α1-6], had strong correlation with astrocytic glioma (p = 0.00). Grade I tumors showed significant positive correlation with the strong PHA-L staining intensity. 24 out of 42 samples, representing 57%, stained strong while only 2 (4.8%) samples stained weak (Table 1). Grade III astrocytoma and grade IV glioblastoma; however, presented a strikingly different expression pattern for PHA-L, as only 1 out of 32 (3.1%) grade IV (glioblastoma) samples showed strong staining while 62.5% stained weak and similar with the observed staining pattern among Grade III tumor samples. Adjusted residual of Table 1 did not reveal any significant correlation of Grade II astrocytoma with any of PHA-L staining. Also, there was variable expression of GlcNAcβ-1-6 branched oligosaccharides in different regions of normal brain sample. The significant increase in expression of GlcNAcβ-1-6 branches in grade 1 tumor tissues compared with that of normal brain tissues is potential hallmark of early gliomagenesis.
Table 1.
Clinicopathological characteristics of astrocytic glioma according to expression of GlcNAc β-1-6 branches, GnT-V and Con-A recognizing sugar
| Factors | Cases | PHA-L staining | P-value | GnT-V | P-value | Con-A | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||||
| Weak | Moderate | Strong | Weak | Moderate | Strong | Weak | Moderate | Strong | |||||
| Histologic Grades of Astrocytoma & Normal Brain | 175 | 0.00† | 0.00† | 0.00† | |||||||||
| Grade I~ | 42 | 2 (4.8%)b | 16 (38.1%) | 24 (57.1%)a | 3 (7.1%)b | 12 (28.6%) | 27 (64.3%)a | 1 (2.4%)b | 4 (9.5%) | 37 (88.1%)a | |||
| Grade II | 65 | 26 (40%) | 25 (38.5%) | 14 (21.5%) | 22 (33.8%) | 24 (36.9%) | 19 (29.2%) | 16 (24.6%) | 22 (33.8%) | 27 (41.5%) | |||
| Grade III | 20 | 13 (65%)a | 6 (30%) | 1 (5%)b | 5 (25%) | 7 (35%) | 8 (40%) | 6 (30%) | 8 (40%) | 6 (30%) | |||
| Grade IV (Glioblastoma) | 32 | 20 (62.5%)a | 11 (34.4%) | 1 (3.1%)b | 17 (53.1%)a | 11 (34.4%) | 4 (12.5%)b | 16 (50%)a | 9 (28.1%) | 7 (21.9%)b | |||
| Normal | 8 | 2 (25%) | 4 (50%) | 2 (25%) | 1 (12.5%) | 0 (0%) | 7 (87.5%)a | 1 (12.5%) | 4 (50%) | 3 (37.5%) | |||
| Normal (cancer adjacent) | 8 | 2 (25%) | 4 (50%) | 2 (25%) | 2 (25%) | 3 (37.5%) | 3 (37.5%) | 2 (25%) | 5 (62.5%) | 1 (12.5%) | |||
| Sex | 0.247† | 0.545† | 0.866† | ||||||||||
| Male | 104 | 41 (39.4%) | 34 (32.7%) | 29 (27.9%) | 31 (29.8%) | 31 (29.8%) | 42 (40.4%) | 25 (24%) | 32 (30.8%) | 47 (45.2%) | |||
| Female | 71 | 22 (31%) | 32 (45.1%) | 17 (23.9%) | 19 (26.8%) | 26 (36.6%) | 26 (36.6%) | 16 (22.5%) | 20 (28.2%) | 35 (49.3%) | |||
| Age | 0.134† | 0.748† | 0.701† | ||||||||||
| ≤ 50 | 114 | 35 (30.7) | 47 (41.2%) | 32 (28.1%) | 34 (29.8%) | 38 (33.8%) | 42 (36.8%) | 26 (22.8%) | 32 (28.1%) | 56 (49.1%) | |||
| > 50 | 61 | 28 (45.9%) | 19 (31.1%) | 14 (23%) | 16 (26.2%) | 19 (31.1%) | 26 (42.6%) | 15 (24.6%) | 20 (32.8%) | 26 (42.6%) | |||
Phaseolus vulgaris Leukoagglutinin (PHA-L), which recognizes GlcNAc-β1-6 branches; N-acetylglucosaminyl transferase V (GnT-V); Concanavalin A binding sugar (Con-A);
denotes chi-square test;
statistically significant positive correlation found within the cell of table;
signifies inverse correlation of individual cell of table.
Significant differences within the cell was calculated by (Adjusted Residuals) of chi-square table.
19 out of 42 samples of grade I were in between grade I and grade II of astrocytoma in terms of histopathological architecture.
Some investigators have reported the lack of consistency in the correlation between GnT-V enzyme expression and that of its β1-6 branched products [19]. To further probe this, we examined levels of GnT-V expression on replicate arrays that were used for PHA-L staining. We found that GnT-V staining pattern was similar to that of PHA-L staining in Grade I and Grade IV astrocytic gliomas (Table 1). In total, 64.3% and 7.1% of samples stained strong and weak, respectively for GnT-V in Grade I astrocytoma, while in glioblastoma tissues, 53.1% of the samples had weak staining and only 4 out of 32 (< 13%) presented with strong GnT-V staining. No significant correlation was noted in any group of GnT-V staining within grade II and grade III astrocytoma samples. Unlike in the PHA-L staining, the sustained decrement in GnT-V staining intensity with tumor progression was notably absent and only grade I and grade IV tumors had GnT-V expression correlate with the expression of β1-6 branches. High expression of GnT-V was also found in normal brain tissues, 7 out of 8 samples stained strong for GnT-V. It is therefore suggestive, per the observed PHA-L and GnT-V expression pattern in normal brain tissues and grade I astroglioma that, the expression of GlcNAc-β1-6 branches in grade I could be induced by other factors apart from the direct influence of elevated GnT-V expression.
Expression of β1-6 branched oligosaccharides is dependent on the expression of both the GnT-V enzyme and its precursor sugar residues [19]. We further assessed the expression of Con-A reactive oligosaccharides on other replicate arrays and found sufficiently high positive association of Con-A binding sugars with grade I astrocytomas. Out of 42 grade I samples, 37 (88.1%) stained strong with only one staining weak for Con-A lectin. The number of strong Con-A staining within groups decreased with worsening tumor stage or grade and was least in glioblastomas samples (Table 1). Con-A reactive oligosaccharides showed no particular expression pattern in normal brain tissues, but the significant increase in expression of Con-A binding lectin in grade I astroglioma was observed to have correlated with higher expression in β1-6-GlcNAc branches. There was also no positive association between age and/or sex of the patients and any of the above-mentioned lectins or GnT-V antibody (Table 1). Collectively, these results showed an increased expression of GnT-V products in low grade astrocytoma tissues as compared to those in high grade tumor and normal brain samples. These results suggest that β1-6-GlcNAc branched expression on the cell surface is associated with early gliomagenesis and along with Con-A lectin staining may be of diagnostic and/or prognostic value as a biomarker in astrocytoma management and grading.
U-87 MG cell line showed highest expression of endogenous GnT-V
In order to elucidate the effect of GnT-V in astroglioma, we screened three glioma cell lines, U-87 MG, U-251MG and U-118MG for its expression. Among the cell lines, U-87 MG had the highest expression of GnT-V at both the mRNA and protein levels (Figure 2A and 2B). To determine whether the level of β1-6 branched oligosaccharides correlated with the expression of GnT-V, we performed direct flow cytometry with FITC-PHAL lectin. Fluorescent intensity was computed as the median value of each staining. U-87 cell line showed the highest expression of PHA-L recognizing sugars (Figure 2C) and associated enzyme among the cell lines, hence was chosen to develop different ectopic GnT-V expression models. As the U-87 MG cell line is derived from Grade IV glioblastoma patient and could not represent the holistic picture of the nature of GnT-V enzyme if used as model cell line, as the GnT-V activity was found to be higher in early stages of glioma (Table 1). Nonetheless, the high expression of GnT-V in U-87 reveals that the compensatory channels or pathways for the suppression of GnT-V enzyme has not been activated and the manipulation of GnT-V in U-87 cells can depicts the functional nature of the enzyme in astro-glioma.
Figure 2.
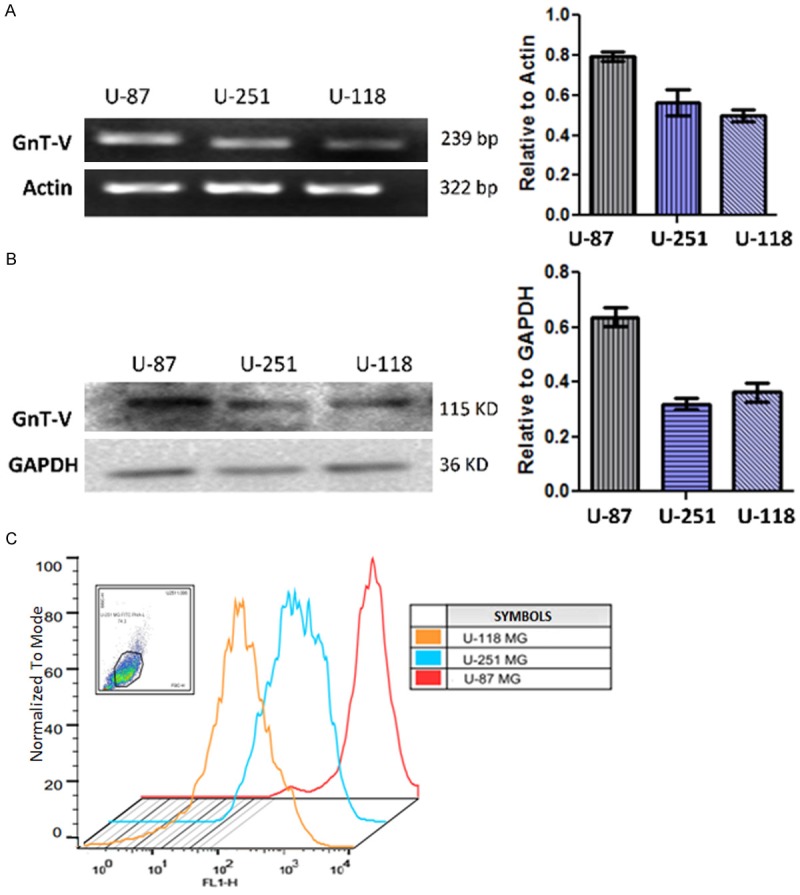
U-87 glioma cells has the highest endogenous GnT-V expression among other selected glioma cell lines. Endogenous expression of GnT-V and its GlcNAc-β1-6 branch products screened in three glioma cell lines. U-87MG cell line has the highest expression of GnT-V not only at mRNA and protein level but also its product which can be detected by PHA-L lectins. Data is represented as mean ± SEM; N ≥ 3. A. Semi-quantitative RT-PCR results comparing GnT-V mRNA expression among cell lines. Actin was used as internal standard and loading control. B. Quantification of endogenous GnT-V protein by western blotting with GDPAH as loading control. C. Direct flow cytometry with FITC-PHA-L. Graphs were merged to show the differences in the fluorescence intensity among cell lines. Fluorescent intensity of each cell line was calculated independently of each other and expressed as the median value after normalizing with their respective negative control.
In vitro GnT-V knockdown induces morphological changes in U-87 cells
To study the functional significance of GnT-V enzyme, U-87 cell line was chosen as suitable cell line for in vitro model generation by MGAT5 gene silencing. Cells were separately transduced with lentiviral particles carrying GnT-V shRNA and mock plasmid. Knockdown clones were established by a single cell dilution of EGFP expressing cells. Knockdown success of more than 90% was achieved and was confirmed at both the RNA and protein level by RT-PCR and western blotting, respectively (Figure 3B, 3C). Indirect Lectin flow cytometry revealed a decrease in the expression of GlcNAc-β1-6 branching in GnT-V/shRNA cells compared with mock control cells (Figure 3D). Knockdown of GnT-V in cells resulted in the alteration of cell morphology. GnT-V/shRNA cells assumed a more flattened and spreading shape, suggestive of possibly activated cytoskeletal remodelling going on within the cells (Figure 3A). The observed changes in cell morphology subsequent to GnT-V knockdown has previously been reported by Yamamoto and colleagues [14]. In their work, GnT-V activity in U-373 MG glioma cells was indirectly muted by the overexpression of enzyme GnT-III which catalyzes the bisecting GlcNAc branches. This resulted in reduced expression of GnT-V products with the concomitant cell morphological changes suggestive of enhanced cytoskeletal remodelling activity.
Figure 3.
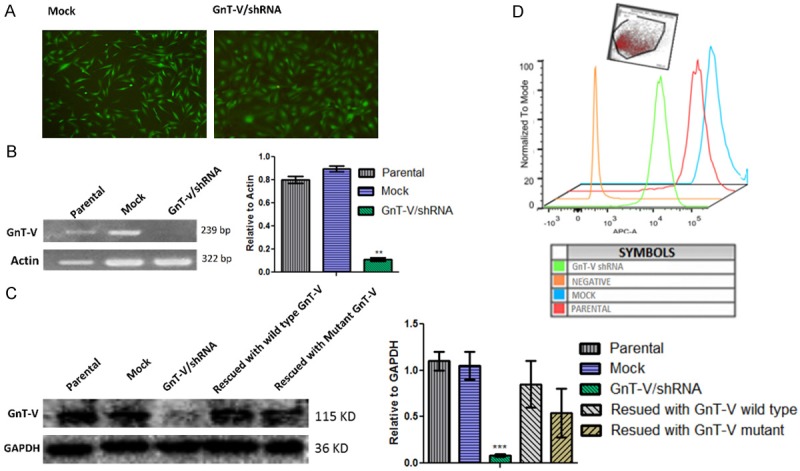
Construction of different ectopic expression for GnT-V in U-87 cells. Parental (wild type U-87) cells were infected with shRNA/GnT-V lentiviral vector and mock (pLL3.7) vector for control group. Data is represented as mean ± SEM; (***p-value < 0.001). A. Representative images are of EGFP expressing mock and knockdown GnT-V cells. GnT-V knockdown cells were well spread and morphologically more flattened on the culture dish as compared to the control. Images were taken at (X40) magnification from Olympus IX71 inverted fluorescent microscope. B. Successful GnT-V knockdown confirmed at the mRNA level by semi-quantitative RT-PCR; (N ≥ 3) C. GnT-V/shRNA cell line was rescued by GnT-V wild type expression vector and GnT-V mutant vector. Western blot showed GnT-V knockdown at the protein level and the successful GnT-V rescue in GnT-V/shRNA cell line; (N ≥ 3). D. Indirect Flowcytometry using APC conjugated streptavidin to probe GlcNAc β-1-6 branch expression following GnT-V gene knockdown. Median fluorescent intensity was reduced to half in GnT-V/shRNA cells as compared to the mock and/or parental type (N = 2).
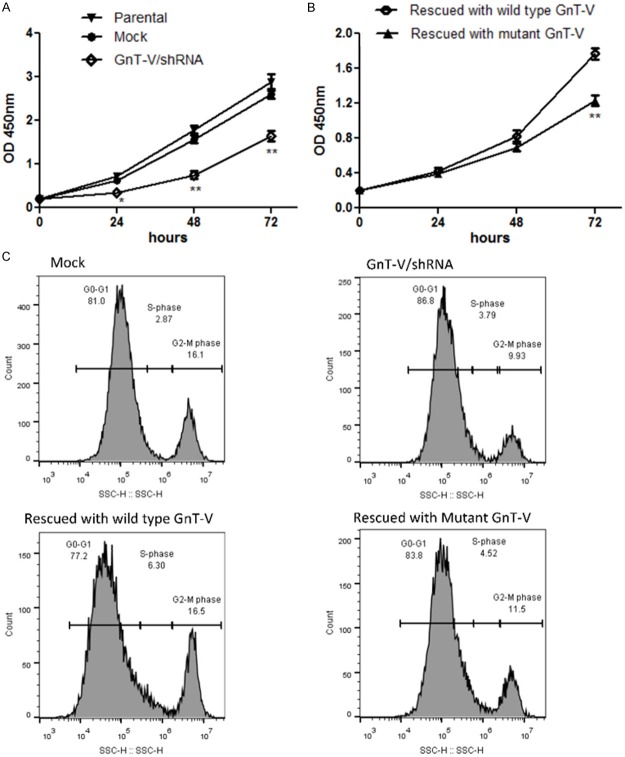
GnT-V depletion inhibits U-87 cell proliferation by promoting G0/G1 growth arrest
We first studied the growth properties of GnT-V/shRNA cells and estimated their growth rate based on dehydrogenase activity on tetrazolium salt which produces a water soluble formazan dye when reduced. Rate of cell growth and proliferation among GnT-V/shRNA cells was significantly reduced when compared with that of cells of mock control group or their parental types (Figure 4A). There was however, impressive recovery in cell growth rate following GnT-V/shRNA cell rescue with wild type GnT-V expression vector (Figure 4B). Both graphs in (Figure 4) are shown as separate figures, and not as single graph. This is because of the additional treatment that has done to achieve transient rescue of GnT-V and its mutant type (Figure 4B), and this lipofectamine treatment had further slowed down the cell proliferation of rescue wild type GnT-V/shRNA, which could suitably be comparable with restoration of GnT-V/shRNA with mutant type. Successful GnT-V rescue and mutant GnT-V type was confirmed at the protein level by western blotting (Figure 3C). From the above observations, it is speculative that the decrease in growth rate in GnT-V/shRNA cells was of GnT-V enzyme knockdown-induced. We further explored the consequence of GnT-V depletion on the cell growth cycle. We observed from cell cycle analyses that, blocking GnT-V expression induced cell arrest at G0 and G1 phases of the cell division cycle. Intriguingly, GnT-V rescue led to the release of the suppressed cell cycle by reversing the growth arrest. This was evidenced by higher G2/M peaks shown on flow cytometry outputs for cell cycle analyses for GnT-V/shRNA rescue cells compared with control cells (Figure 4C). In summary, we are convinced that GnT-V expression partly regulates the cell cycle and is particularly useful during phase transition of G0/G1 to G2/M. This is consistent with an existing report that in hepatoma cells the activity of GnT-V was highest at G2/M phase while the expression remained fairly constant in the other phases of the cell cycle [20].
Figure 4.
GnT-V knockdown reduced proliferation of U-87 cells and promoted G0/G1 phase arrest. Cell cycle Experiments were performed after 96 hours post transfection with wild type and mutant GnT-V expression vector while cell proliferation assay was performed after 48 hours of post transfection. (A and B) show the growth curve for experimental groups detected by WST-8 dye, as described in material and method. Absorbance was taken after 24, 48 and 72 hours of seeding cells in 96 well plates. GnT-V/shRNA had the lowest growth rate compared with control (A). Rescuing with wild type of GnT-V resulted in growth rate restoration compared to rescue with mutant GnT-V. Data is represented as mean ± SEM (*p-value < 0.05, **p-value < 0.01). (C) Cell cycle analysis by propidium iodide staining showed that GnT-V knockdown produced a shorter peak of G2/GM compared to its mock, while GnT-V rescue with wild type GnT-V showed the release of cells from G0/G1 phase to S and G2/M phase of cell cycle.
GnT-V contributes glial cells to transform, invade and migrate
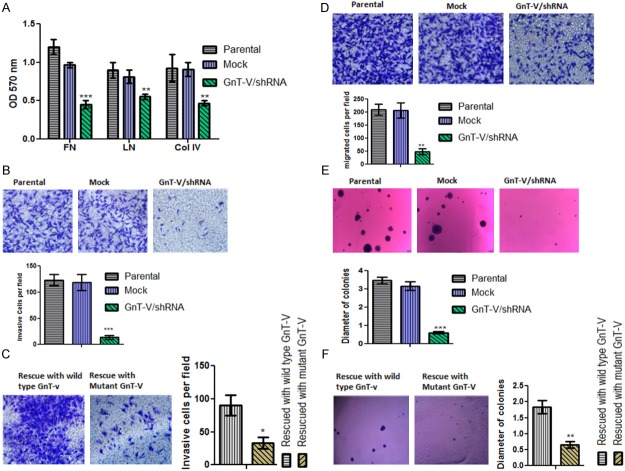
Currently, there exist no developed models for glioma invasion, although the underlying principles have been conceptualized and put into discrete steps which include (1) adhesion of cells to surrounding brain parenchyma, (2) degradation of extracellular matrix (ECM) to allow passage and (3) glioma cell movement. We investigated the involvement of GnT-V enzyme and its β1-6-GlcNac branched products in any of the steps involved in glioma invasion. Firstly, we studied the adhesion characteristics of GnT-V/shRNA cells with three main extracellular matrix (ECM) components; Fibronectin (Fn), Laminin (Ln) and Collagen IV (Col IV). We observed that the ability of cells attachment to the named ECM components waned in GnT-V/shRNA cells compared to control cells (Figure 5A) suggesting that decreased GnT-V expression hampers the interaction between glial cells and the surrounding parenchyma. Next, we studied the basement membrane degradation characteristics of knockdown GnT-V cells using Matrigel. We found that decreased in GnT-V expression resulted in a significantly lowered invasive potential of cells (Figure 5B). There was a restoration of invasive potential to pre-knockdown levels upon GnT-V rescue in GnT-V/shRNA cells (Figure 5C). We also assessed cell movement or migration ability by Transwell assay, based on chemotaxis and again observed that GnT-V/shRNA cells were poor in migration compared to the mock control or parental cells (Figure 5D). Our work produced evidence that contradicts existing reports of weakened cell migration ability with enhanced cell adhesion to ECM. In our case, GnT-V/shRNA cells were poor in attachment and migration compared to controls indicating that optimum level of adhesion is required for the cells to migrate [21,22].
Figure 5.
Silencing of GnT-V altered cell to ECM adhesion (A), decrease invasive (B, C), migratory (D) and anchorage independence (E, F) of glioma cells. (A) Effects of GnT-V knockdown on cell adhesion ability; GnT-V knockdown reduced the ability of cells to adhere to the ECM components; fibronectin (FN), laminin (LN) and collagen IV (Col IV). Data is represented as mean ± SEM and p-value signifies the difference between mock and GnT-V/shRNA group. (**p-value < 0.01, ***p-value < 0.001; N = 4). (B & C) represents Matrigel/Transwell invasion assay, invasive ability of GnT-V/shRNA U-87 cell group was greatly reduced compared to that of control cell groups. Invasive ability in GnT-V/shRNA cells were regained after successful rescue of GnT-V expression using wild type GnT-V expression vector. Images were taken at a magnification of (X200; Olympus IX71). Data are the mean ± S.E of three independent experiments (*p-value < 0.05, ***p-value < 0.001). (D) Migration rate of U-87 glioma cells is attributed to the activity of GnT-V enzyme. Migration ability of cells were checked by transwell chamber. GnT-V/shRNA cells showed decreased in number of migrated cells per field compared to controls. Images were taken at a magnification of (X200; Olympus IX71) (**p-value < 0.01, N = 3). (E) To assess the transforming ability in GnT-V/shRNA stable cell line, soft agar colony formation assay was performed. Ability to form clones in knockdown GnT-V was greatly reduced compared to control group. Original magnification images was X40 (Olympus IX71). The graph represents the differences in the mean clonal diameters of controls and GnT-V/shRNA cell line. Data represented as mean ± S.E of two independent experiments (***p-value < 0.001). (F) In order to reassess the loss of transforming ability of knockdown GnT-V, cells were rescued by wild type GnT-V vector. Mutant GnT-V expression vector was used as control. GnT-V/shRNA cells regain their transforming ability when GnT-V is rescued by wild type GnT-V gene. Plates were scanned under light microscope (at x40) and images were taken from hand digital camera. Data represented as mean ± S.E of two independent experiments (**p-value < 0.01).
As the GnT-V expression is controlled by many oncogenes which are important for cellular transformations [10,11], we further investigated the effect(s) of GnT-V silencing on the transforming ability of U-87 cells. For this purpose, soft agar colony formation assay was performed to assess the clonogenic potential of U-87 cells. As shown in (Figure 5E), colony number and size of GnT-V knockdown cells were diminished compared with those of controls indicating the important role of GnT-V in oncogenic transformation of cells. In fact, GnT-V/shRNA cells did not form colonies larger than 20 cells. Rescuing the expression of GnT-V restored anchorage independent growth ability in knockdown GnT-V cell line (Figure 5F).
Discussion
The dynamic function and expression of N-acetyl glucosamine glycosyltransferase-V (GnT-V) in different tissues and its role in certain human cancers said to be of prognostic significance remains controversial. In this study we, for the first time, have pointed out that the activity of GnT-V and its β1-6-GlcNAc sugar products change through the course of gliomagenesis. We found that PHA-L staining of GnT-V products was strongest in early gliomas as 57% and 4.8% of the total grade I astrocytic glioma samples respectively stained strong and weak for PHA-L lectin. The reactivity of PHA-L progressively reduced in the higher tumor grades reaching 3% of strong staining in glioblastomas.
This study is unable to confirm any correlation between GnT-V enzyme expression and its catalytic β1-6 branched oligosaccharide products, in normal brain tissues. GnT-V expression remained high in early gliomas and normal brain tissues but the expression of its product elevated only significantly in grade I astroglioma. This suggests other possible triggers for β1-6-GlcNAc branched up-regulated expression in grade I that may include (1) elevated UDP-GlcNAc levels [23] and (2) altered expression of other N-glycans biosynthesis regulating enzymes [24]. Con-A lectin interacts with diverse receptors and binds to complex types of biantennary N-glycans but with greater specificity to those containing mannose carbohydrates and terminal glucose [25]. In our study, 88% of grade I astrocytic tumor tissues stained strong for Con-A lectin while the occurrence of the strong staining among tissues tended to decrease in progressive glioma grades. This observation is in harmony with that of Wang et al [26] who reported of intense Con-A staining in lower grade astrocytomas and weakened expression of mannose residues in high grade astrocytomas. Also, high grade gliomas have been shown to exhibit a loss of WGA and RCA-1 staining which recognize N-acetylglucosamine and N-acetylgalactosamine, respectively [26]. This suggests that there is a constant decrease of total N-glycosylation in the progression of glioma. Loss of WGA and Con-A lectin binding ability is also common in other human cancers has been of prognostic value as a marker in Burkitt’s lymphoma, gastric cancers and urinary bladder cancers [19,27,28]. Altogether, the loss of β1-6-GlcNAc sugar in high grade astrocytomas can be attributed to the loss of overall N-glycosylation in view of the weakened reactivity of Con-A, RCA and WGA lectins.
The etiology of gliomas is not exhaustively known and the mechanisms underlying their development poorly understood. However, loss of function of P53 gene[29], the expression of receptor tyrosine kinase Ligands, platelet derive growth factor (PDGF), and its receptor (PDGFR) have been found to increase in low grade gliomas [30]. Our study has discovered that early grade gliomas are associated with higher GnT-V activity. Partridge et al. [31] showed that in GnT-V-/- mouse tumor cell line derived from polyoma virus middle T (PyMT) oncogene had delayed in tumor growth and the cells were less responsive to PDGF as compared to GnT-V+/+ derived tumor cells. It is possible therefore that, increase in GnT-V activity may potentially favor the increase in the expression of PDGFR and its ligands in gliomagenesis. We also assessed the effect(s) of GnT-V knockdown on the transforming ability of glial cells and found that it resulted in a compromised of the anchorage independent properties of GnT-V/shRNA cells. This shows that the activity of GnT-V is important for the transforming potential of glial cells further in support of our hypothesis of GnT-V involvement in early gliomagenesis.
A characteristic of gliomas is their ability to invade surrounding stromal tissues regardless of grade and/or degree of pathogenesis. Immunohistochemistry has revealed higher expression of matrix metalloproteinases, particularly MMP-2 and MMP-9 in glioblastomas and anaplastic astrocytomas but present at undetectable levels in low grade astrocytomas [32,33]. Other proteases such as cathepsin B and UPA urokinase type plasminogen activator (uPA) reportedly, also show significant expression only in high grade gliomas [18]. Higher β1-6 branched expression is associated with greater invasive and metastatic potentials in many cancers including breast and esophageal carcinomas [34,35]. Our results show that decreased expression led to a drastic reduction in the invasive and migratory prowess of GnT-V/shRNA glioma cell line. Guo et al. [36] showed that higher levels of GnT-V increased the invasive potential in the human fibrosarcoma cell line (HT1080), but without a corresponding increase in the expression levels of matrix metalloproteinases. This supports the argument that increased β1-6 branched expression may potentiate cells of low grade gliomas to invade surrounding tissues without the aid of proteinases.
Other tyrosine receptor kinases such as EGFR, TGF-β and IGF are involved in the regulation of multiple intracellular pathways, and their upregulated expression common in high grade astrocytomas [37,38]. The GnT-V enzyme reportedly modulates the activities of tyrosine receptor kinases as increased GnT-V expression enhances interactions between these kinases and ligand growth factors [39]. In the light of the above argument, the reason for the diminished expression of GnT-V and its β1-6 branched oligosaccharides products observed in high grade gliomas, and the benefit(s) or otherwise to gliomas remain unclear. Predicting an explanation, Dennis and colleagues [40] showed that PyMT driven tumors in mammary fat pads of GnT-V-/- mice underwent a latency period at the early stage but showed accelerated tumor growth in the later stages. And these tumors exhibited increased resistance to Src, EGFR kinase and brefeldin A (a protein transport inhibitor). Clinically, high grade gliomas including anaplastic astrocytoma and glioblastoma have greater resistance to several chemotherapeutic drugs and the progressive decrease in β1-6 branching could be a potential reason. This is supported by reports that upregulated expression of GlcNAc-β1-6 chains in U-373 MG glioma cell line increased their sensitivity to pro-apoptotic drugs [41].
The low expression of GnT-V in high astrocytic glioma having in vitro proto oncogenic nature can also be explained by looking on the studies that has been done on one scaffolding protein, Caveolin-1. Caveolin-1 was described either as a tumor suppressor or as a tumor promoter provided its levels in tumors against non-transformed tissues [42,43]. Ken Sasai et al. showed that the Caveolin-1 limits the appearance of β1-6 branches by causing the reorganization of the Golgi complex [44]. Down regulation of Caveolin-1 by small interfering RNA showed increased in high glycosylated form of CD147 (HG-CD147), which is attributable to the action of GnT-V on CD147. Caveolin-1 is also reported to be over expressed in high grade Glioma [45], which could likely be a participant in the decreased of GnT-V expression and β1-6-GlcNAc branches in high degree gliomas. Interestingly over expression of Caveolin 1 rather than down regulation in U-87 glioma cells induced decreased in cell proliferation, clonogenicity and invasion [46], same like the down regulation of GnT-V was expected to elucidate increased in oncogenic properties of glioma cells rather than the suppression of it. Understanding the relation of GnT-V and GlcNAc-β1-6 branch along with caveolin-1 protein signalling and trafficking in future can unfold the mechanistic explanation of the dual characteristics of GnT-V activity in gliomagenesis.
In conclusion, we have shown that GnT-V enzyme activity is crucial in gliomagenesis. We propose from our in vitro experimentation that the enzyme may function as an onco-protein in glioma cells. The expression of GnT-V and β1-6-GlcNAc sugar products were low in high grade gliomas which may suggest the activation of other compensatory genetic and epigenetic mechanisms, as the expression of one of the β1-6 branched sugar precursor moieties, Con-A recognizing sugars, also reduced in high grade astrocytomas. We believe that the Con-A and PHA-L binding sugars may be of diagnostic and prognostic relevance in gliomas. For early grade gliomas in particular, the GnT-V enzyme presents potential therapeutic benefits and inhibition of its expression could be helpful in mitigating tumor aggression in the early stages of development.
Acknowledgements
Authors would like to thank Prof. Jianhai Jiang (Fudan University) for providing U-87 and U-251 cell lines, Prof. Jianguo Gu (Tohoku Pharmaceutical University) for providing GnT-V overexpression wild type and mutant plasmids, Aisha Sultana (Aga Khan University Hospital), Muhammad Kamran Raja (Institute of Cancer Stem Cell Research, Dalian Medical University) for technical assistance and Benjamin Arko-Boham (Department of Anatomy, Dalian Medical University) for critically reviewing the manuscript. This study was supported by grants from the National Program on Key Basic Research Project (973 Program) (NO. 2012CB822103), the National Natural Science Foundation of China (No. 81330060, 30970648, 31170774) and the Fundamental Research Funds for the Central Universities.
Disclosure of conflict of interest
None.
References
- 1.Hakomori S, Kannagi R. Glycosphingolipids as tumor-associated and differentiation markers. J Natl Cancer Inst. 1983;71:231–251. [PubMed] [Google Scholar]
- 2.Pierce M, Arango J. Rous sarcoma virus-transformed baby hamster kidney cells express higher levels of asparagine-linked tri- and tetraantennary glycopeptides containing [GlcNAc-beta (1,6)Man-alpha (1,6)Man] and poly-N-acetyllactosamine sequences than baby hamster kidney cells. J Biol Chem. 1986;261:10772–10777. [PubMed] [Google Scholar]
- 3.Yamashita K, Ohkura T, Tachibana Y, Takasaki S, Kobata A. Comparative study of the oligosaccharides released from baby hamster kidney cells and their polyoma transformant by hydrazinolysis. J Biol Chem. 1984;259:10834–10840. [PubMed] [Google Scholar]
- 4.Dube DH, Bertozzi CR. Glycans in cancer and inflammation--potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 5.Lowe JB, Marth JD. A genetic approach to Mammalian glycan function. Annu Rev Biochem. 2003;72:643–691. doi: 10.1146/annurev.biochem.72.121801.161809. [DOI] [PubMed] [Google Scholar]
- 6.Tian H, Miyoshi E, Kawaguchi N, Shaker M, Ito Y, Taniguchi N, Tsujimoto M, Matsuura N. The implication of N-acetylglucosaminyltransferase V expression in gastric cancer. Pathobiology. 2008;75:288–294. doi: 10.1159/000151709. [DOI] [PubMed] [Google Scholar]
- 7.Murata K, Miyoshi E, Kameyama M, Ishikawa O, Kabuto T, Sasaki Y, Hiratsuka M, Ohigashi H, Ishiguro S, Ito S, Honda H, Takemura F, Taniguchi N, Imaoka S. Expression of N-acetylglucosaminyltransferase V in colorectal cancer correlates with metastasis and poor prognosis. Clin Cancer Res. 2000;6:1772–1777. [PubMed] [Google Scholar]
- 8.Yamamoto E, Ino K, Miyoshi E, Shibata K, Takahashi N, Kajiyama H, Nawa A, Nomura S, Nagasaka T, Kikkawa F. Expression of N-acetylglucosaminyltransferase V in endometrial cancer correlates with poor prognosis. Br J Cancer. 2007;97:1538–1544. doi: 10.1038/sj.bjc.6604044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saito H, Gu J, Nishikawa A, Ihara Y, Fujii J, Kohgo Y, Taniguchi N. Organization of the human N-acetylglucosaminyltransferase V gene. Eur J Biochem. 1995;233:18–26. doi: 10.1111/j.1432-1033.1995.018_1.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen L, Zhang W, Fregien N, Pierce M. The her-2/neu oncogene stimulates the transcription of N-acetylglucosaminyltransferase V and expression of its cell surface oligosaccharide products. Oncogene. 1998;17:2087–2093. doi: 10.1038/sj.onc.1202124. [DOI] [PubMed] [Google Scholar]
- 11.Guo HB, Zhang QS, Chen HL. Effects of H-ras and v-sis overexpression on N-acetylglucosaminyltransferase V and metastasis-related phenotypes in human hepatocarcinoma cells. J Cancer Res Clin Oncol. 2000;126:263–270. doi: 10.1007/s004320050341. [DOI] [PubMed] [Google Scholar]
- 12.Ishimura H, Takahashi T, Nakagawa H, Nishimura S, Arai Y, Horikawa Y, Habuchi T, Miyoshi E, Kyan A, Hagisawa S, Ohyama C. N-acetylglucosaminyltransferase V and beta1-6 branching N-linked oligosaccharides are associated with good prognosis of patients with bladder cancer. Clin Cancer Res. 2006;12:2506–2511. doi: 10.1158/1078-0432.CCR-05-1938. [DOI] [PubMed] [Google Scholar]
- 13.Dosaka-Akita H, Miyoshi E, Suzuki O, Itoh T, Katoh H, Taniguchi N. Expression of N-acetylglucosaminyltransferase v is associated with prognosis and histology in non-small cell lung cancers. Clin Cancer Res. 2004;10:1773–1779. doi: 10.1158/1078-0432.ccr-1047-3. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto H, Swoger J, Greene S, Saito T, Hurh J, Sweeley C, Leestma J, Mkrdichian E, Cerullo L, Nishikawa A, Ihara Y, Taniguchi N, Moskal JR. Beta1,6-N-acetylglucosamine-bearing N-glycans in human gliomas: implications for a role in regulating invasivity. Cancer Res. 2000;60:134–142. [PubMed] [Google Scholar]
- 15.Inamori K, Gu J, Ohira M, Kawasaki A, Nakamura Y, Nakagawa T, Kondo A, Miyoshi E, Nakagawara A, Taniguchi N. High expression of N-acetylglucosaminyltransferase V in favorable neuroblastomas: Involvement of its effect on apoptosis. FEBS Lett. 2006;580:627–632. doi: 10.1016/j.febslet.2005.12.089. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi N, Yamamoto E, Ino K, Miyoshi E, Nagasaka T, Kajiyama H, Shibata K, Nawa A, Kikkawa F. High expression of N-acetylglucosaminyltransferase V in mucinous tumors of the ovary. Oncol Rep. 2009;22:1027–32. doi: 10.3892/or_00000531. [DOI] [PubMed] [Google Scholar]
- 17.Larjavaara S, Mantyla R, Salminen T, Haapasalo H, Raitanen J, Jaaskelainen J, Auvinen A. Incidence of gliomas by anatomic location. Neuro Oncol. 2007;9:319–325. doi: 10.1215/15228517-2007-016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maher EA, Furnari FB, Bachoo RM, Rowitch DH, Louis DN, Cavenee WK, DePinho RA. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki O, Nozawa Y, Abe M. Loss of L-PHA-, PNA-, or ConA-reactive oligosaccharides is associated with a poor prognosis in human Burkitt’s lymphoma. Oncol Rep. 2007;17:775–779. [PubMed] [Google Scholar]
- 20.Guo HB, Jiang AL, Ju TZ, Chen HL. Opposing changes in N-acetylglucosaminyltransferase-V and -III during the cell cycle and all-trans retinoic acid treatment of hepatocarcinoma cell line. Biochim Biophys Acta. 2000;1495:297–307. doi: 10.1016/s0167-4889(99)00157-3. [DOI] [PubMed] [Google Scholar]
- 21.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 22.Palecek SP, Horwitz AF, Lauffenburger DA. Kinetic model for integrin-mediated adhesion release during cell migration. Ann Biomed Eng. 1999;27:219–235. doi: 10.1114/1.176. [DOI] [PubMed] [Google Scholar]
- 23.Sasai K, Ikeda Y, Fujii T, Tsuda T, Taniguchi N. UDP-GlcNAc concentration is an important factor in the biosynthesis of beta1,6-branched oligosaccharides: regulation based on the kinetic properties of N-acetylglucosaminyltransferase V. Glycobiology. 2002;12:119–127. doi: 10.1093/glycob/12.2.119. [DOI] [PubMed] [Google Scholar]
- 24.Fan J, Wang S, Yu S, He J, Zheng W, Zhang J. N-acetylglucosaminyltransferase IVa regulates metastatic potential of mouse hepatocarcinoma cells through glycosylation of CD147. Glycoconj J. 2012;29:323–334. doi: 10.1007/s10719-012-9414-1. [DOI] [PubMed] [Google Scholar]
- 25.Cummings RD, Etzler ME. Antibodies and Lectins in Glycan Analysis. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. 2nd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2009. The Consortium of Glycobiology Editors, La Jolla, California. [PubMed] [Google Scholar]
- 26.Wang XC, Kochi N, Tani E, Kaba K, Matsumoto T, Shindo H. Lectin histochemistry of human gliomas. Acta Neuropathol. 1989;79:176–182. doi: 10.1007/BF00294376. [DOI] [PubMed] [Google Scholar]
- 27.Langkilde NC, Wolf H, Orntoft TF. Binding of wheat and peanut lectins to human transitional cell carcinomas. Correlation with histopathologic grade, invasion, and DNA ploidy. Cancer. 1989;64:849–853. doi: 10.1002/1097-0142(19890815)64:4<849::aid-cncr2820640415>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 28.Koyama S, Terashima S, Takano Y, Ohori T, Kanno T, Hoshino Y, Inoue H. Wheat germ agglutinin binding is a useful prognostic indicator in stomach cancer. Int $lxfS1$ 1999;4:96–100. [Google Scholar]
- 29.Chung R, Whaley J, Kley N, Anderson K, Louis D, Menon A, Hettlich C, Freiman R, Hedley-Whyte ET, Martuza R, et al. TP53 gene mutations and 17p deletions in human astrocytomas. Genes Chromosomes Cancer. 1991;3:323–331. doi: 10.1002/gcc.2870030502. [DOI] [PubMed] [Google Scholar]
- 30.Heldin CH, Westermark B. Platelet-derived growth factor: mechanism of action and possible in vivo function. Cell Regul. 1990;1:555–566. doi: 10.1091/mbc.1.8.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Partridge EA, Le Roy C, Di Guglielmo GM, Pawling J, Cheung P, Granovsky M, Nabi IR, Wrana JL, Dennis JW. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science. 2004;306:120–124. doi: 10.1126/science.1102109. [DOI] [PubMed] [Google Scholar]
- 32.Nakada M, Nakamura H, Ikeda E, Fujimoto N, Yamashita J, Sato H, Seiki M, Okada Y. Expression and tissue localization of membrane-type 1, 2, and 3 matrix metalloproteinases in human astrocytic tumors. Am J Pathol. 1999;154:417–428. doi: 10.1016/S0002-9440(10)65288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakagawa T, Kubota T, Kabuto M, Sato K, Kawano H, Hayakawa T, Okada Y. Production of matrix metalloproteinases and tissue inhibitor of metalloproteinases-1 by human brain tumors. J Neurosurg. 1994;81:69–77. doi: 10.3171/jns.1994.81.1.0069. [DOI] [PubMed] [Google Scholar]
- 34.Takano R, Nose M, Nishihira T, Kyogoku M. Increase of beta 1-6-branched oligosaccharides in human esophageal carcinomas invasive against surrounding tissue in vivo and in vitro. Am J Pathol. 1990;137:1007–1011. [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandes B, Sagman U, Auger M, Demetrio M, Dennis JW. Beta 1-6 branched oligosaccharides as a marker of tumor progression in human breast and colon neoplasia. Cancer Res. 1991;51:718–723. [PubMed] [Google Scholar]
- 36.Guo HB, Lee I, Kamar M, Akiyama SK, Pierce M. Aberrant N-glycosylation of beta1 integrin causes reduced alpha5beta1 integrin clustering and stimulates cell migration. Cancer Res. 2002;62:6837–6845. [PubMed] [Google Scholar]
- 37.Chakravarti A, Loeffler JS, Dyson NJ. Insulin-like growth factor receptor I mediates resistance to anti-epidermal growth factor receptor therapy in primary human glioblastoma cells through continued activation of phosphoinositide 3-kinase signaling. Cancer Res. 2002;62:200–207. [PubMed] [Google Scholar]
- 38.Kaminska B, Kocyk M, Kijewska M. TGF beta signaling and its role in glioma pathogenesis. Adv Exp Med Biol. 2013;986:171–187. doi: 10.1007/978-94-007-4719-7_9. [DOI] [PubMed] [Google Scholar]
- 39.Cheung P, Dennis JW. Mgat5 and Pten interact to regulate cell growth and polarity. Glycobiology. 2007;17:767–773. doi: 10.1093/glycob/cwm037. [DOI] [PubMed] [Google Scholar]
- 40.Mendelsohn R, Cheung P, Berger L, Partridge E, Lau K, Datti A, Pawling J, Dennis JW. Complex N-glycan and metabolic control in tumor cells. Cancer Res. 2007;67:9771–9780. doi: 10.1158/0008-5472.CAN-06-4580. [DOI] [PubMed] [Google Scholar]
- 41.Dawson G, Moskal JR, Dawson SA. Transfection of 2,6 and 2,3-sialyltransferase genes and GlcNAc-transferase genes into human glioma cell line U-373 MG affects glycoconjugate expression and enhances cell death. J Neurochem. 2004;89:1436–1444. doi: 10.1111/j.1471-4159.2004.02435.x. [DOI] [PubMed] [Google Scholar]
- 42.Quest AF, Leyton L, Parraga M. Caveolins, caveolae, and lipid rafts in cellular transport, signaling, and disease. Biochem Cell Biol. 2004;82:129–144. doi: 10.1139/o03-071. [DOI] [PubMed] [Google Scholar]
- 43.Fiucci G, Ravid D, Reich R, Liscovitch M. Caveolin-1 inhibits anchorage-independent growth, anoikis and invasiveness in MCF-7 human breast cancer cells. Oncogene. 2002;21:2365–2375. doi: 10.1038/sj.onc.1205300. [DOI] [PubMed] [Google Scholar]
- 44.Sasai K, Ikeda Y, Ihara H, Honke K, Taniguchi N. Caveolin-1 regulates the functional localization of N-acetylglucosaminyltransferase III within the golgi apparatus. J Biol Chem. 2003;278:25295–25301. doi: 10.1074/jbc.M301913200. [DOI] [PubMed] [Google Scholar]
- 45.Parat MO, Riggins GJ. Caveolin-1, caveolae, and glioblastoma. Neuro Oncol. 2012;14:679–688. doi: 10.1093/neuonc/nos079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin S, Cosset EC, Terrand J, Maglott A, Takeda K, Dontenwill M. Caveolin-1 regulates glioblastoma aggressiveness through the control of alpha(5)beta(1) integrin expression and modulates glioblastoma responsiveness to SJ749, an alpha(5)beta(1) integrin antagonist. Biochim Biophys Acta. 2009;1793:354–367. doi: 10.1016/j.bbamcr.2008.09.019. [DOI] [PubMed] [Google Scholar]