Abstract
Lymphoid enhancer-binding factor-1 (LEF1) is a key transcription factor mediating Wnt signaling pathway. Our previous studies indicate that LEF1 is highly expressed in androgen-independent prostate cancer (PCa) and enhances invasion ability in androgen-independent PCa cells. However, the molecular mechanism of LEF1 effect on invasion remains largely unknown. Using microRNA profiling analysis comparing androgen-independent LNCaP-AI PCa cells with high levels of endogenous LEF1 to LNCaP-AI cells with LEF1 knockdown by LEF1shRNA, we found miR-181a to be increased 12.3-fold in LNCaP-AI cells. We confirmed a positive correlation between LEF1 and miR-181a expression across multiple PCa cell lines. Additionally, we showed that in PCa cells, overexpression of LEF1 increased miR-181a expression and subsequently induced EMT associated migration and invasion, whereas LEF1 knockdown decreased miR-181a expression and subsequently resulted in inhibition of EMT, migration and invasion. Mechanistically, we demonstrated by chromatin immunoprecipitation assays that LEF1 could enhance miR-181a expression via its binding to the promoter regions of hsa-miR-181a. Overall, this study identified a novel LEF1-miR-181a-EMT axis in regulation of PCa migration and invasion.
Keywords: LEF1, miR-181a, prostate cancer, EMT, invasion
Introduction
Prostate cancer (PCa) is the leading cause of malignancy incidence and one of the leading causes of cancer related death in US men [1]. Although most advanced PCa patients initially respond to androgen deprivation therapy (ADT), virtually all of these cancers will recur as castration-resistant prostate cancer (CRPC) in which few effective treatments are available. Thus, there is an urgent need to identify new therapeutic targets for the treatment of advanced PCa.
Epithelial-mesenchymal transition (EMT) is the physiological process in which epithelial cells undergo cytoskeletal and morphological transformation and become mesenchymal cells [2]. Due to its impact on cell polarity and cell-cell adhesion, EMT also plays a critical role in the process of migration, invasion and subsequent metastasis, characteristics of aggressive PCa [3]. E-cadherin, an epithelial cell marker, is located on the cell surface of epithelial tissues and mediates cell-cell adhesion. Loss of E-cadherin is associated with EMT, tumor cell invasion and metastasis [4-6]. Other mesenchymal markers such as N-cadherin, Vimentin, and Snail are negatively correlated with E-cadherin, and stimulate EMT [4].
MicroRNAs (miRNAs) are small, non-coding RNA molecules that negatively regulate downstream target genes at the posttranscriptional level primarily via specific binding to the 3’-UTR region of the target mRNA. Changes in expression of miRNAs, including miR-181a, have been implicated in various cancers. miR-181a was reported to be upregulated in breast cancer and ovarian cancer, and promotes cancer metastasis induced by TGF-beta [7-10]. From analysis of clinical samples, high miR-181a expression has been shown to be associated with poor survival in patients with colorectal cancer [11], which supports miR-181a as an oncogenic factor. Importantly, miR-181a expression was found to be activated by Wnt/beta-catenin signaling in hepatocellular carcinoma [12], a signaling pathway that is affected by Lymphoid enhancer-binding factor-1 (LEF1) dysfunction [13-16]. LEF1 was found to be necessary for up-regulation of the mesenchymal marker vimentin independent of beta-catenin activation [17]. Taken together, LEF1 may play an important upstream role in tumor progression and EMT transition. Until now, the mechanism of LEF1 in human PCa has not been extensively explored.
In this study, we used a microarray screening approach to identify miRNAs regulated by LEF1 using PCa cell lines expressing high or low levels of LEF1. We demonstrate that LEF1, at least partly, promotes EMT and invasion via direct transcriptional activation of miR-181a. These data reveal a novel regulation of LEF1-miR181a-EMT axis for PCa EMT and invasion. Understanding the molecular pathway and role of LEF1-miR181a-EMT in PCa as crucial steps leading to metastasis is of great importance for the development of improved therapeutic strategies for metastatic PCa patients.
Materials and methods
Cell culture, migration and matrigel invasion assays
LNCaP, LNCaP-LEF1, C4-2B and DU145 cells were maintained in RPMI 1640 (Gibco) and PC3 cells were cultured in 50% RPMI 1640 and 50% F2 (Gibco) with 10% heat-inactivated FBS, 1% penicillin and streptomycin (PS). The androgen-independent LNCaP-AI and LNCaP-AI-LEF1shRNA cells were maintained in RPMI 1640 medium containing 10% charcoal-stripped, heat-inactivated FBS and 1% PS (CSFBS; Hyclone Laboratories, Inc.). RC165 and RC170 were maintained in DMEM with 10% heat-inactivated FBS, 1% PS. BD Matrigel Chamber Assay and migration assay were performed as previously described [18].
miRNA array and miRNA quantification by qPCR
The four cell lines, LNCaP, LNCaP-LEF1, LNCaP-AI and LNCaP-AI-LEF1shRNA, were used for miRNA array analysis. The HTG Molecular qDiscovery miRNA Whole Transcriptome Array (WTA), including 687 human miRNAs, was used to compare the expression profiles of PCa. miRNA hybridization and scanning were performed by HTG [19]. Cell lysis and miRNA profiling was conducted and analyzed by High Throughput Genomics, Inc (www.htgenomics.com) using the HTG platform (miRNA on the qNPA ArrayPlate) with a total of 770 miRNAs.
Total RNA was extracted with mirVana miRNA Isolation kit (AM1560, Ambion). Taqman Micro-RNA Reverse Transcription or RetroScript kit was used for cDNA synthesis with isolated RNA by following the manufacturer’s instructions. PCR was performed using the TaqMan Universal PCR Master Mix or Fast SYBR Green Master Mix and BioRad CFX96 machine. The endogenous reference gene RNU6B (MS00014000) or GAPDH was used for RNA quantification. The PCR primers used were: 5’-GTCTCCTCTGACTTCAACAGCG-3’ and 5’-ACCACCCTGTTGCTGTAGCCAA-3’ (GAPDH); 5’-CTACCCATCCTCACTGTCAGTC-3’ and 5’-GGATGTTCCTGTTTGACCTGAGG-3’ (LEF1). All miRNA TaqMan primers were purchased from Ambion.
Western blot analysis
50 μg whole-cell extract was subjected to 10 % SDS-PAGE gel and transferred to a nitrocellulose membrane for Western blot analysis. Immunoblots were blocked for 30 min with 5% nonfat dry milk and then incubated with primary antibodies (LEF1, 1:200; E-cadherin, 1:1,000; N-cadherin, 1:1,000; anti-GAPDH, 1:5,000; CD44, 1:1,000; Beta-integrin, 1:1,000; Snail, 1:1,000) for 2 h at room temperature and incubated for 1.5 h with the horseradish peroxidase-conjugated secondary antibody (Amersham Biosciences) at 1:5,000 dilution in 5% nonfat dry milk. The protein bands were detected by an enhanced chemiluminescence kit (Amersham Biosciences).
Chromatin immunoprecipitation (ChIP)
ChIP was performed with Pierce Agarose ChIP Kit (Thermo Scientific) according to the manufacturer’s instructions. Briefly, cells were cross-linked for 10 minutes in 1% formaldehyde solution, followed by adding glycine solution to a final concentration of 1X. Cells were washed twice in ice-cold PBS then collected for lysis and MNase digestion. 45 μl of chromatin was used for immunoprecipitation. 5 μg of anti-LEF1 and IgG were used and incubated overnight at 4°C on a rocking platform. Samples were incubated with Protein A/G agarose beads for 2 hours at 4°C on a rocking platform, and washed six times with IP Wash Buffer 2 and 3. Immunoprecipitation samples containing DNA were eluted in IP Elution Buffer and reverse crosslinked at 65°C for 1.5 hours. Finally, 50μl DNA Elution Solution was used for dissolving the pure DNA. Quantitative PCR analysis primers sets were: 5’-TCAACAAAGCTGGCTAATGC-3’ and 5’-AAGCAGCCAAATGGTACACG-3’ (-207); 5’-ACTTTGGCTCTGCAGTGATG-3’ and 5’-TGCCAGGAGCCAAGGTTAT-3’ (-642); 5’-GCAGGATCTCAGCAAAGGAG-3’ and 5’-CACTGCAGAGCCAAAGTCAA-3’ (-759); 5’-CCATTCAAAGACATTTTCTCAGAC-3’ and 5’-TCAATTTGTGTCAGCAGAG-3’ (-1225).
Statistical analysis
The data was shown as the mean ± SEM. An unpaired two-tailed Student’s t test was used for comparisons, with P < 0.05 considered to be significant. *denotes P < 0.05, **denotes P < 0.01, ***denotes P < 0.001.
Results
Identification of miRNAs associated with LEF1 by miRNA array
To identify miRNAs associated with LEF1 expression, we performed miRNA microarray analysis in LNCaP, LNCaP-LEF1, LNCaP-AI and LNCaP-AI-LEF1shRNA cells. LNCaP expresses minimal levels of LEF1 and LNCaP-AI, an androgen-independent cell line derived from parental LNCaP cells, expresses high relative levels of LEF1 [20]. The paired comparison of miRNA expression of LNCaP-AI/LNCaP (left column) and LNCaP-AI/LNCaP-AI-LEF1shRNA by a clustering analysis revealed distinct patterns of miRNAs potentially associated with LEF1 expression (Figure 1A). Of the 770 miRNAs tested when comparing LNCaP-AI to LNCaP, 9 miRNAs showed changes of at least 2-fold, suggesting that these miRNAs were associated with LEF1 expression. miR-181a was increased by 12.36-fold in LNCaP-AI cells. We confirmed the expression levels of the 6 up-regulated miRNAs affected by LEF1 by real time qRT-PCR in LNCaP, LNCaP-LEF1, LNCaP-AI and LNCaP-AI-LEF1shRNA cells. Based on the array data and the literature, miR-181a was selected for further studies (Figure 1A).
Figure 1.
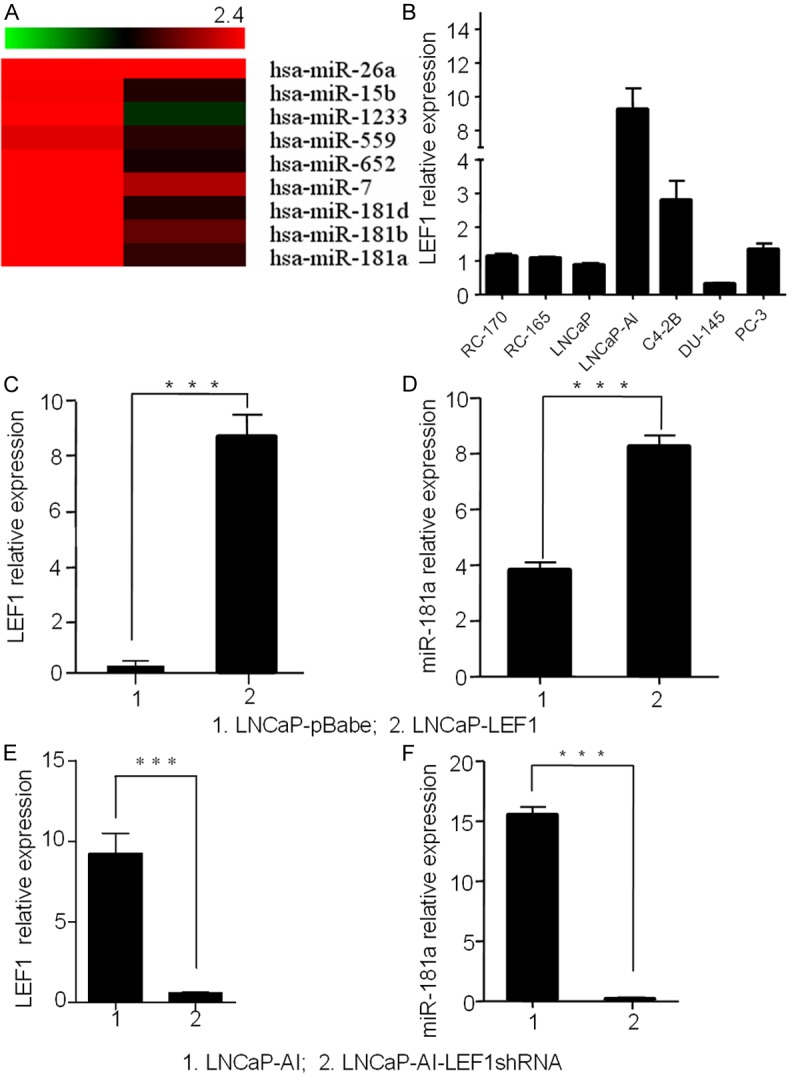
LEF1 has a positive correlation with has-miR-181a. A. A clustering analysis between paired miRNA expression comparison of LNCaP-AI/LNCaP and LNCaP-AI/LNCaP-AI-LEF1shRNA, top 9 up-regulated miRNAs were listed. B. LEF1 expression level in five (LNCaP, LNCaP-AI, C4-2B, DU-145 and PC-3) PCa cell lines and two benign prostate cell lines (RC-170, RC-165). C and D. LEF1 and miR-181a expression in LNCaP cells. LEF1 plasmid transfection increased miR-181a in LNCaP cells. E and F. miR-181a expression decreased with LEF1 knockdown by shRNA in LNCaP-AI cells. GAPDH and U6 were used for normalization.
LEF1 upregulates miR-181a to enhance PCa EMT and invasion
LEF1 positively regulates miR-181a
In our miRNA array analysis, we found a positive relationship between LEF1 and miR-181a (Figure 1A), consistent with a previous report that LEF1 can regulate miR-181a in hepatocellular carcinoma [12]. To confirm LEF1 regulation of miR-181a expression in PCa cells, we examined miR-181a expression in LNCaP cells transfected with LEF1 compared to control LNCaP cells or LNCaP-AI cells with LEF1 knockdown by shRNA compared to control LNCaP-AI cells (Figure 1C and 1E). Our data showed that LEF1 overexpression increased miR-181a level (Figure 1D) in LNCaP cells while LEF1 knockdown reduced miR-181a expression in LNCaP-AI cells (Figure 1F). These data indicate LEF1 positively regulates miR-181a in PCa cells.
LEF1 directly targets miR-181a promoter binding sites
Several studies have indicated that beta-catenin/TCF complex exerts transcriptional regulation via binding to a consensus core TCF/LEF1 binding site (5’A/T A/T CAAAG-3’) within promoter regions [12,21,22]. By reviewing published reports and searching the GenBank database, we found four predicted binding sites in 2kb upstream of the promoter region of hsa-miR-181a-2 [12] (Figure 2A). To confirm LEF1 upregulation of miR-181a via direct binding, we used ChIP to detect LEF1 occupancy at each of four putative binding sites of hsa-miR-181a-2 promoter region in LNCaP-AI cells. Remarkably, LEF1 was significantly recruited to each of the four predicted binding sites within the promoter regions of hsa-miR-181a-2 (Figure 2B and 2C). These results indicate the direct binding of LEF1 protein to the promoter of hsa-miR-181a-2 in the upregulation of miR-181a transcription.
Figure 2.
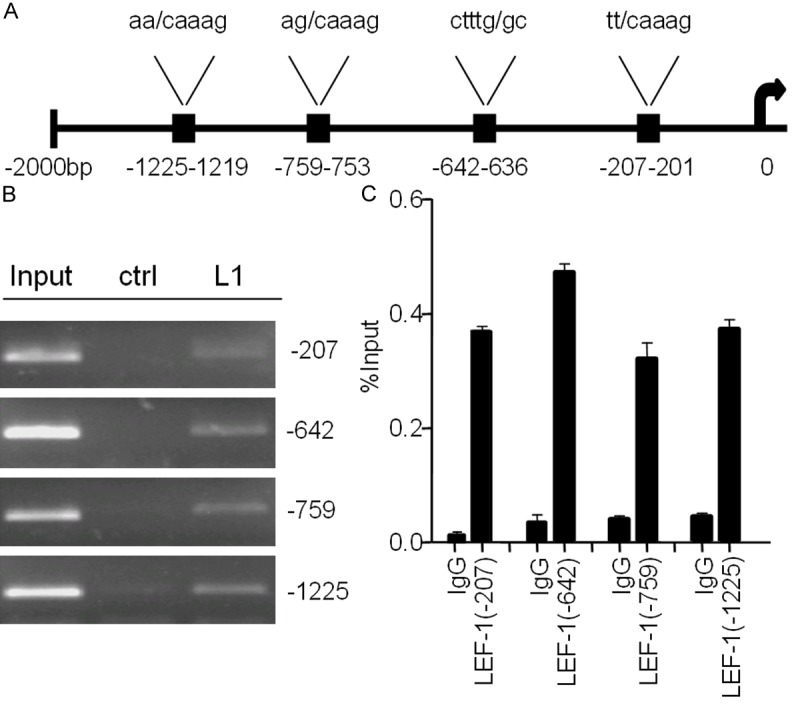
A. Schematics of four binding sites on hsa-miR-181a-2 promoter region. B and C. LEF1 was recruited to the four binding sites by ChIP.
miR-181a regulates EMT in PCa cells
To investigate whether miR-181a induces EMT in PCa cells, we measured changes in E-cadherin and N-cadherin protein expression after transfection with miR-181a mimic or anti-miR-181a in LNCaP, LNCaP-AI and C4-2B cells. Increased N-cadherin and decreased E-cadherin expression was observed with miR-181a overexpression in LNCaP and C4-2B cell lines (Figure 3B and 3C). Anti-miR-181a treatment reduced N-cadherin and increased E-cadherin in LNCaP-AI and C4-2B cells (Figure 3A and 3D). Of note, miR-181a did not regulate LEF1 expression in LNCaP-AI or C4-2B cells (data not shown).
Figure 3.
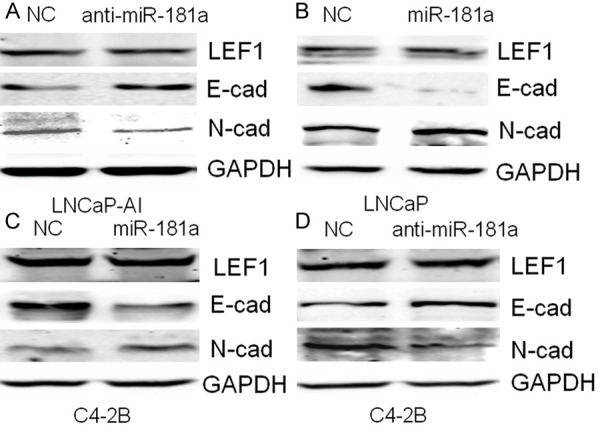
A-D. Measurement of miR-181a regulation of EMT protein marker expression by western blotting.
miR-181a induces invasion ability in PCa
To examine if miR-181a regulates invasion, we performed transwell migration and invasion assays with transient transfection of miR-181a mimic. As shown in Figure 4A and 4B, migration and invasion were significantly increased upon miR-181a mimic transfection in LNCaP cells in a dosage-dependent manner. Similar results were also obtained in C4-2B cells (Figure 4E and 4F). Furthermore, migration and invasion were significantly inhibited upon anti-miR-181a treatment in LNCaP-AI and C4-2B cells (Figure 4C, 4D, 4G and 4H), suggesting that miR-181a promotes migration and invasion in PCa cells.
Figure 4.
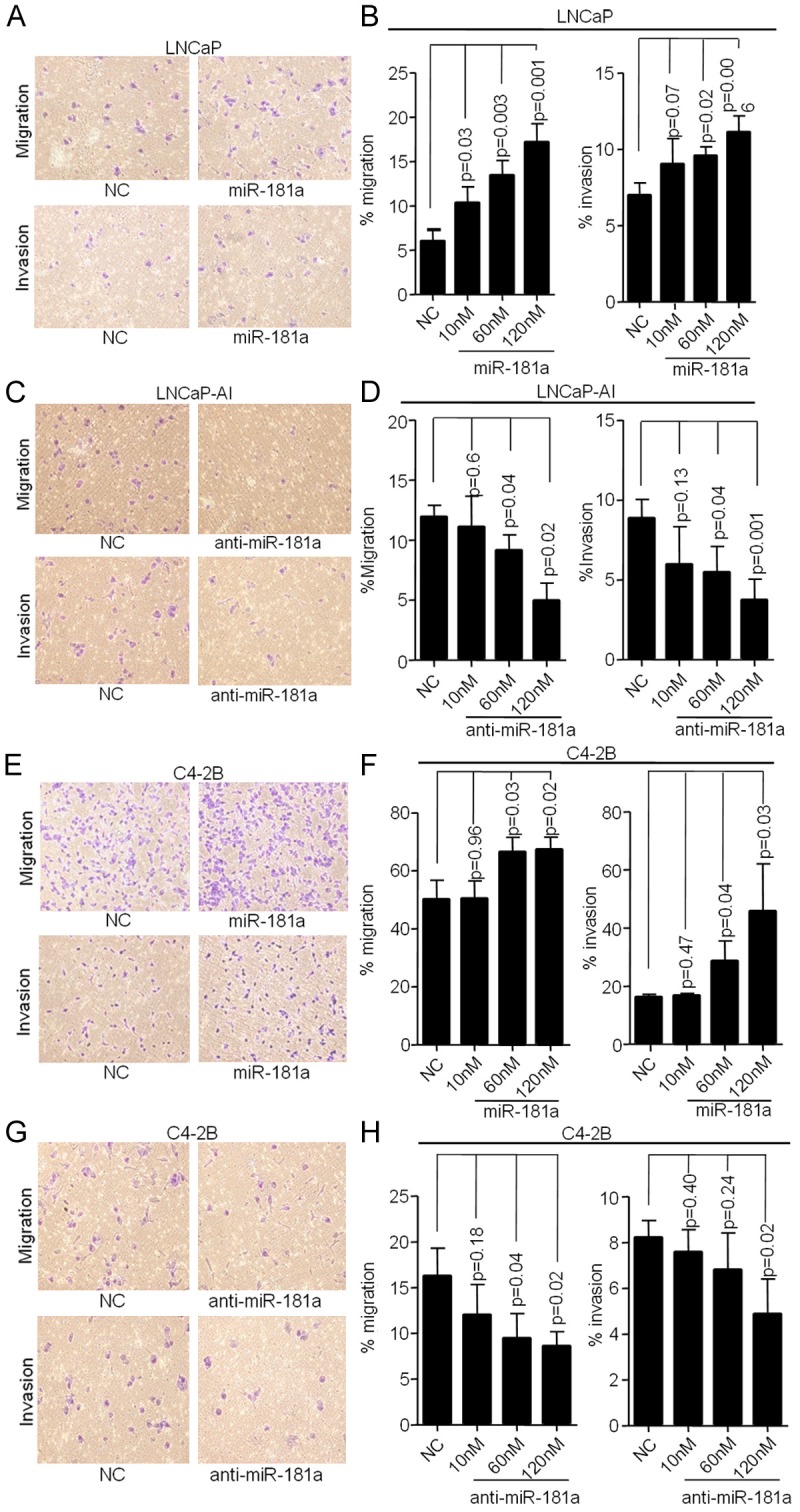
miR-181a regulates migration and invasion. A and B. Migration and invasion increased with miR-181a mimic transfection in LNCaP cells (low miR-181a expression) compared with control cells in a dose-dependent manner. C and D. Transfection with anti-miR-181a decreased migration and invasion in LNCaP-AI cells (high miR-181a expression). E-H. Transfection of miR-181a mimic increased while transfection with anti-miR-181a decreased migration and invasion in C4-2B cells.
LEF1-miR181a-EMT axis in regulating PCa EMT and invasion
Next, we wanted to determine whether increased LEF1 and miR-181a will have additive or synergistic effects on regulating EMT processes evidenced by the loss of E-cadherin expression, an EMT marker. We measured the levels of E-cadherin expression by western blot analysis after transfection of LEF1, miR-181a mimic or co-transfection of both LEF1 and miR-181a mimic. While both miR-181a and LEF1 alone downregulated E-cadherin respectively (Figure 5A-C), co-transfection of both shows a synergistic inhibitory effect on E-cadherin by in all three cell lines (Figure 5A-C).
Figure 5.
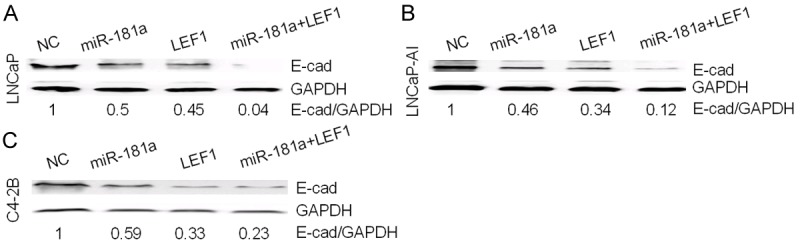
LEF1-miR-181a-EMT axis in PCa. A-C. A synergistic inhibitory effect on E-cadherin protein level by combined overexpression of LEF1 and miR-181a.
Discussion
High expression of LEF1 in PCa cells is associated with EMT and aggressive malignant attributes. In this study, we aimed to identify miRNAs that may be responsible for the phenotype that has been observed upon LEF1 overexpression. Among the miRNAs that we found to be regulated by LEF1, the miR-181a family were selected for further investigation as they were the most highly dysregulated in PCa. miR-181a of the miR-181 family was the highest relative expressed miRNA in the high LEF1 expressing PCa cell lines we examined (Figure 1A).
In terms of the relationship between LEF1 and miR-181a, we first showed LEF1 overexpression increased miR-181a expression in LNCaP cells and confirmed the LEF1 direct regulation of miR-181a by chromatin immunoprecipitation on each of the four putative LEF1 binding sites of the miR-181a-2 promoter region. In reciprocal, knockdown of LEF1 reduces the levels of miR-181a in high miRNA-181a expressing androgen-independent LNCaP-AI and C4-2B cells. The results of these experiments indicate LEF1 directly and positively regulates miR-181a and in turn positively regulates EMT. Taken together, these findings provide a detailed molecular link of LEF1-miR-181a-EMT in PCa, which is consistent to the previous report from Ji et al indicating that LEF1/TCF4 transcriptionally activates miR-181a-2 expression in hepatocellular carcinoma [12].
Although limited knowledge is available regarding the relationship between LEF1 and miR-181a, miR-181a is reported to be overexpressed in several cancers, including breast cancer [9,23], hepatocellular carcinoma [24] and oral squamous cell carcinoma [25]. In this study, we demonstrated miR-181a induces EMT, migration and invasion, at least partly, by direct modulation via LEF1. miR-181a, miR-181b and miR-181d all belong to the miR-181 family which contain similar mature sequences and biological function. The exact mechanism of miR-181a promoting EMT remains to be determined. There are two recent publications suggesting that miR-181a promotes TGF-beta mediated EMT via targeting Smad7 in ovarian cancer, and miR-181a mediates TGF-beta induced hepatocyte EMT in hepatocellular cancer [26,27]. Interestingly, a recent study demonstrated that miR-181a could promote osteoblastic differentiation through repression of TGF-beta signaling molecules [28] which suggested that LEF1 may stimulate osteoblastic lesion through its regulation of miR-181a in both PCa cells and osteoblastic lineage cells in the bone microenvironment. Thus, increased information on the regulation by LEF1-miR-181a could have a significant impact on PCa skeletal metastasis.
Our study indicates miR-181a plays an oncogenic function in PCa, which is in line with previous reports of miR-181a [9,23-25]. However, other groups have demonstrated that miR-181a is downregulated in oral and lung squamous malignancies and may play a role as a tumor suppressor in these types of solid tumors [29,30]. It will be important in the future to determine the expression of miR-181a in tissues from PCa patients.
In summary, we illustrate that the activity of LEF1 in direct transcriptional upregulation of miR-181a to stimulate EMT and contribute to PCa cell migration and invasion. This study provides a novel direct LEF1-miR-181a role in PCa EMT process which specifically modulates subsequent migration and invasion. This further reveals a mechanism of action for initiation of an aggressive PCa phenotype by increased LEF1 expression via altered downstream miRNA activity and potentially direct involvement in PCa metastasis. These results may provide novel therapeutic targets for treatment of aggressive PCa.
Acknowledgements
This work is supported by Wuhan Science and Technology Bureau Foundation (201260523172-6), Hubei Province Key Laboratory of Molecular Imaging Open Foundation (02.03.2013-59) to JQL and JZ, DOD PCRP (PC080010, PC11164), NIH UO1 (1U01CA149556-01) and VA Merit (1I01BX001505-01) to PL, DOD postdoctoral fellowship (PC PC081578) to YRL, National Cancer Institute (CA172894, CA180277) to X. Li, postdoctoral fellowships from NYU Molecular Oncology and Immunology Training grant (T32 CA009161) to GD. This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (Biomedical Laboratory Research and Development).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- 3.Ongkeko WM, Burton D, Kiang A, Abhold E, Kuo SZ, Rahimy E, Yang M, Hoffman RM, Wang-Rodriguez J, Deftos LJ. Parathyroid hormone related-protein promotes epithelial-to-mesenchymal transition in prostate cancer. PLoS One. 2014;9:e85803. doi: 10.1371/journal.pone.0085803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaw SY, Majeed AA, Dalley AJ, Chan A, Stein S, Farah CS. Epithelial to mesenchymal transition (EMT) biomarkers--E-cadherin, beta-catenin, APC and Vimentin--in oral squamous cell carcinogenesis and transformation. Oral Oncol. 2012;48:997–1006. doi: 10.1016/j.oraloncology.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Theys J, Jutten B, Habets R, Paesmans K, Groot AJ, Lambin P, Wouters BG, Lammering G, Vooijs M. E-Cadherin loss associated with EMT promotes radioresistance in human tumor cells. Radiother Oncol. 2011;99:392–397. doi: 10.1016/j.radonc.2011.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye Y, Xiao Y, Wang W, Yearsley K, Gao JX, Shetuni B, Barsky SH. ERalpha signaling through slug regulates E-cadherin and EMT. Oncogene. 2010;29:1451–1462. doi: 10.1038/onc.2009.433. [DOI] [PubMed] [Google Scholar]
- 7.Liang J, Zhang Y, Jiang G, Liu Z, Xiang W, Chen X, Chen Z, Zhao J. MiR-138 induces renal carcinoma cell senescence by targeting EZH2 and is downregulated in human clear cell renal cell carcinoma. Oncol Res. 2013;21:83–91. doi: 10.3727/096504013X13775486749218. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Yu Y, Tsuyada A, Ren X, Wu X, Stubblefield K, Rankin-Gee EK, Wang SE. Transforming growth factor-beta regulates the sphere-initiating stem cell-like feature in breast cancer through miRNA-181 and ATM. Oncogene. 2011;30:1470–1480. doi: 10.1038/onc.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor MA, Sossey-Alaoui K, Thompson CL, Danielpour D, Schiemann WP. TGF-beta upregulates miR-181a expression to promote breast cancer metastasis. J Clin Invest. 2013;123:150–163. doi: 10.1172/JCI64946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikh A, Lee C, Peronne J, Marchini S, Baccarini A, Kolev V, Romualdi C, Fruscio R, Shah H, Wang F, Mullokandov G, Fishman D, D’Incalci M, Rahaman J, Kalir T, Redline RW, Brown BD, Narla G, DiFeo A. microRNA-181a has a critical role in ovarian cancer progression through the regulation of the epithelial-mesenchymal transition. Nat Commun. 2014;5:2977. doi: 10.1038/ncomms3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pichler M, Winter E, Ress AL, Bauernhofer T, Gerger A, Kiesslich T, Lax S, Samonigg H, Hoefler G. miR-181a is associated with poor clinical outcome in patients with colorectal cancer treated with EGFR inhibitor. J Clin Pathol. 2014;67:198–203. doi: 10.1136/jclinpath-2013-201904. [DOI] [PubMed] [Google Scholar]
- 12.Ji J, Yamashita T, Wang XW. Wnt/beta-catenin signaling activates microRNA-181 expression in hepatocellular carcinoma. Cell Biosci. 2011;1:4. doi: 10.1186/2045-3701-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gehrke I, Gandhirajan RK, Kreuzer KA. Targeting the WNT/beta-catenin/TCF/LEF1 axis in solid and haematological cancers: Multiplicity of therapeutic options. Eur J Cancer. 2009;45:2759–2767. doi: 10.1016/j.ejca.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Jeong EG, Lee SH, Yoo NJ. Mutational analysis of Wnt pathway gene LEF1 in common human carcinomas. Dig Liver Dis. 2007;39:287–288. doi: 10.1016/j.dld.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Lee JE, Wu SF, Goering LM, Dorsky RI. Canonical Wnt signaling through Lef1 is required for hypothalamic neurogenesis. Development. 2006;133:4451–4461. doi: 10.1242/dev.02613. [DOI] [PubMed] [Google Scholar]
- 16.Boras-Granic K, Chang H, Grosschedl R, Hamel PA. Lef1 is required for the transition of Wnt signaling from mesenchymal to epithelial cells in the mouse embryonic mammary gland. Dev Biol. 2006;295:219–231. doi: 10.1016/j.ydbio.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 17.Nawshad A, Medici D, Liu CC, Hay ED. TGFbeta3 inhibits E-cadherin gene expression in palate medial-edge epithelial cells through a Smad2-Smad4-LEF1 transcription complex. J Cell Sci. 2007;120:1646–1653. doi: 10.1242/jcs.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Ligr M, McCarron JP, Daniels G, Zhang D, Zhao X, Ye F, Wang J, Liu X, Osman I, Mencher SK, Lepor H, Wang LG, Ferrari A, Lee P. Natura-alpha targets forkhead box m1 and inhibits androgen-dependent and -independent prostate cancer growth and invasion. Clin Cancer Res. 2011;17:4414–4424. doi: 10.1158/1078-0432.CCR-11-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gokey NG, Srinivasan R, Lopez-Anido C, Krueger C, Svaren J. Developmental regulation of microRNA expression in Schwann cells. Mol Cell Biol. 2012;32:558–568. doi: 10.1128/MCB.06270-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Wang L, Zhang M, Melamed J, Liu X, Reiter R, Wei J, Peng Y, Zou X, Pellicer A, Garabedian MJ, Ferrari A, Lee P. LEF1 in androgen-independent prostate cancer: regulation of androgen receptor expression, prostate cancer growth, and invasion. Cancer Res. 2009;69:3332–3338. doi: 10.1158/0008-5472.CAN-08-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamashita T, Budhu A, Forgues M, Wang XW. Activation of Hepatic Stem Cell Marker EpCAM by Wnt-β-Catenin Signaling in Hepatocellular Carcinoma. Cancer Res. 2007;67:10831–10839. doi: 10.1158/0008-5472.CAN-07-0908. [DOI] [PubMed] [Google Scholar]
- 22.Tetsu O, McCormick F. [beta] -Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 23.Ota D, Mimori K, Yokobori T, Iwatsuki M, Kataoka A, Masuda N, Ishii H, Ohno S, Mori M. Identification of recurrence-related microRNAs in the bone marrow of breast cancer patients. Int J Oncol. 2011;38:955–962. doi: 10.3892/ijo.2011.926. [DOI] [PubMed] [Google Scholar]
- 24.Ji J, Yamashita T, Budhu A, Forgues M, Jia HL, Li C, Deng C, Wauthier E, Reid LM, Ye QH, Qin LX, Yang W, Wang HY, Tang ZY, Croce CM, Wang XW. Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology. 2009;50:472–480. doi: 10.1002/hep.22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang CC, Hung PS, Wang PW, Liu CJ, Chu TH, Cheng HW, Lin SC. miR-181 as a putative biomarker for lymph-node metastasis of oral squamous cell carcinoma. J Oral Pathol Med. 2011;40:397–404. doi: 10.1111/j.1600-0714.2010.01003.x. [DOI] [PubMed] [Google Scholar]
- 26.Parikh A, Lee C, Joseph P, Marchini S, Baccarini A, Kolev V, Romualdi C, Fruscio R, Shah H, Wang F, Mullokandov G, Fishman D, D’Incalci M, Rahaman J, Kalir T, Redline RW, Brown BD, Narla G, DiFeo A. microRNA-181a has a critical role in ovarian cancer progression through the regulation of the epithelial-mesenchymal transition. Nat Commun. 2014;5:2977. doi: 10.1038/ncomms3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brockhausen J, Tay SS, Grzelak CA, Bertolino P, Bowen DG, d’Avigdor WM, Teoh N, Pok S, Shackel N, Gamble J, Vadas M, McCaughan GW. miR-181a mediates TGF-β induced hepatocyte EMT and is dysregulated in cirrhosis and hepatocellular cancer. Liver Int. 2014;35:240–53. doi: 10.1111/liv.12517. [DOI] [PubMed] [Google Scholar]
- 28.Bhushan R, Grunhagen J, Becker J, Robinson PN, Ott CE, Knaus P. miR-181a promotes osteoblastic differentiation through repression of TGF-beta signaling molecules. Int J Biochem Cell Biol. 2013;45:696–705. doi: 10.1016/j.biocel.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Shin KH, Bae SD, Hong HS, Kim RH, Kang MK, Park NH. miR-181a shows tumor suppressive effect against oral squamous cell carcinoma cells by downregulating K-ras. Biochem Biophys Res Commun. 2011;404:896–902. doi: 10.1016/j.bbrc.2010.12.055. [DOI] [PubMed] [Google Scholar]
- 30.Gao W, Shen H, Liu L, Xu J, Xu J, Shu Y. MiR-21 overexpression in human primary squamous cell lung carcinoma is associated with poor patient prognosis. J Cancer Res Clin Oncol. 2011;137:557–566. doi: 10.1007/s00432-010-0918-4. [DOI] [PubMed] [Google Scholar]


