Abstract
Epidemiological studies indicate that women have a higher chance of developing muscle invasive bladder cancer (BCa) than men, suggesting that estrogen and estrogen receptors (ERs) may play critical roles in BCa progression. However, the ERs roles in the bladder tumor microenvironment and impacts on BCa progression remain largely unclear. Using IHC staining in human BCa samples, we found that higher ERα expression in the stromal compartment of BCa may be correlated with unfavorable clinical outcomes. Results from cell line studies revealed that co-culturing with fibroblasts could promote BCa T24, UMUC3 and 5637 cells invasion. Importantly, addition of ERα in fibroblasts further enhanced the BCa cell invasion and knock-down of ERα in fibroblasts could then partially reduce the fibroblasts-enhanced BCa invasion. Mechanism dissection suggested that ERα could function through modulating the CCL cytokines expression in fibroblasts to increase the BCa IL-6 expression. An interruption approach using IL-6 neutralizing antibody then reversed the fibroblast ERα-enhanced BCa cell invasion. Together, these data suggest that the higher expression of ERα in fibroblasts may be the result of modulating the CCL1 expression in fibroblasts and/or IL-6 production in BCa cells to enhance BCa cells invasion. Targeting these individual molecules in this newly identified ERα-stimulated CCL1 and IL-6 signal pathways may become an alternative therapy to better suppress the BCa cell invasion.
Keywords: Bladder cancer, fibroblast, CCL1, IL-6, estrogen receptor α
Introduction
Earlier studies suggested that the tumor stroma can regulate tumor development and fibroblasts are one of the most active cell types of the stroma [1,2]. Increasing evidence showed that fibroblasts have a profound influence on the cancer development and progression [1,3,4]. In the tumor microenvironment, cancer associated fibroblasts (CAF) are also the most common cells in the stromal compartment. It has been proven that CAF can be transformed from normal fibroblasts through the stimulation of cancer cells-released growth factors [5]. Additional studies showed that CAF could then increase in population through transforming from normal fibroblasts [6], differentiation from bone marrow-derived mesenchymal stem cells [6], or by epithelial to mesenchymal transition (EMT) [7]. The major functions of CAF include the regulation of deposition of extracellular matrix (ECM), epithelial differentiation, tumor inflammation, and wound healing [8]. These activated fibroblasts can be characterized molecularly by several markers expressed by the fibroblasts in their activated state. Some of the most common CAF markers are α-smooth muscle actin (α-SMA), fibroblast-specific protein 1 (FSP1) and fibroblast activation protein (FAP) [2,9] and these markers could be applied to identify specific subpopulations of fibroblasts. CAF has been demonstrated to promote transformation of immortalized epithelial cells to increase cancer cells population [3,10]. Meanwhile, studies also indicated that CAF can release several types of growth factor families, including the fibroblast growth factor (FGF), the insulin-like growth factor (IGF), the epithelial growth factor (EGF), hepatocyte growth factor (HGF), and the transforming growth factor-beta (TGF-beta) family [11-13], to promote cancer cells proliferation. Ezer et al. demonstrated that CAF could mediate inflammation and angiogenesis by recruiting macrophages, which may then promote tumor growth [14].
Earlier studies showed bladder tumor stromal cells enhanced tumor formation when carcinogen-treated stroma was heterotypically grafted with untreated epithelial cells [15,16]. Blaveri et al. was looking for biomarkers to classify BCa subtype and predict patients outcome, and they concluded that the expressions of stromal related genes increased in high grade bladder tumor samples [17]. These studies support the hypothesis that tumor stroma components promote BCa proliferation and invasion.
Increasing bodies of evidence indicated infiltrated inflammatory cells could promote, but not suppress, tumor progression [18,19]. In addition to immune cells, CAF are also a source of inflammation factors and could promote inflammation [4,20]. Tumors produced inflammation factors include IL-1α, IL-8, IL-10, TNF-α, and CXCL-12, which could suppress anti-tumor immune cells’ function, activate extracellular proteases, induce VEGF-a to promote angiogenesis or recruit other inflammatory cells [21]. In BCa tumors, CCL2 has a positive correlation with tumor progression and patients with high levels of CCL2 in BCa tissue have poor clinical outcome [22]. Another study also showed increased CXCL1 in BCa tissues could be applied as a prognostic marker for predicting the invasive phenotype [23].
There are two major types of ERs, ER alpha and ER beta (ERα and ERβ), which belong to the nuclear receptor superfamily and could mediate estrogen actions to manage physiological functions. Estrogen action regulates transcription of target genes via binding to the estrogen response element (ERE) or non-ERE mediated transactivation, as well as non-genomic regulations [24]. The estradiol production in females is most commonly thought of as an endocrine product of the ovary, however, there are many sources of estrogens in females as well as in males. For example, several tissues have the capacity to synthesize estrogens from androgens [25-27]. In addition, the adipose tissues can produce estrogens and contribute significantly to the circulating pool of estrogens [28]. Supportively, another report also found that estrogen production increased in obese men and there were 30% of male BCa patients with high ERβ expression with a high correlation with worse progression [18]. Thus, estrogen/ERs may also play important roles in male diseases, including BCa.
BCa incidence in males is around three fold higher than in females, but the 5 year survival rate is lower in female BCa patients, suggesting estrogen and estrogen receptors (ERs) may play different roles in BCa initiation and invasion [29,30]. A growing body of evidence also suggest that ERs are highly related to BCa development, but the roles and mechanisms are not yet conclusive [31-34]. Recent results indicated that ERα plays a protective role to prevent initiation and ERβ promotes metastasis of BCa, but it remains to be elucidated whether ERα and ERβ play distinct roles in different types of cells within the BCa tumor microenvironment.
The bladder CAF may play important roles in the regulation of deposition of extracellular matrix (ECM), epithelial differentiation, tumor inflammation, and wound healing [8] to affect BCa development. Miyamoto et al. demonstrated 8.2% of BCa samples show strong ERα expression in tumor stromal tissue, higher than 1.3% in benign tissues [35]. However, the functions of the high expression of ERα in stroma remain largely unknown.
Our results demonstrate the fibroblast ERα and C-C chemokine (CCL) family genes could influence BCa progression and the data may lead to developing new alternative therapy strategies for BCa in the future.
Materials and methods
Cell lines
Three bladder cancer cell lines, T24, UMUC3 and 5637 were purchased from the American Type Culture Collection (ATCC) (Rockville, MD). Fibroblasts were purchased from Cell Systems (Troisdorf, Germany) and immortalized by SV40 large T antigen. All cells were maintained in DMEM media with 10% fetal bovine serum and 1% penicillin/streptomycin.
Invasion assay
The effects of fibroblasts in BCa cells invasion capability was determined by transwell invasion assays. To mimic the BCa stromal-epithelial interaction in the tumor environment, fibroblasts were co-cultured with BCa cells for 48 hrs. Briefly, fibroblasts were seeded into the upper chamber (pore size 0.4 μm) at 105 cells/chamber and co-cultured with BCa cells in the lower chamber at 105 cells/well with DMEM including 10% FBS in 6-wells transwell plates. After co-culture, the BCa cells were trypsinized reseeded on the top of new transwells (with pore size 8 μm) pre-coated with matrigel (0.2 mg/ml; 100 μl/well) for 24 hrs to determine BCa invasion. The BCa cells invaded to the lower surface of the membrane were fixed by 75% ethanol and stained with 1% toluidine blue. Cell numbers were counted in five randomly chosen microscopic fields per membrane. Each data point was performed in triplicate with 2 sets of independent experiments.
Plasmid construction and lentiviral ERα and CCL1 transduction
To express ERα, cDNA of ERα was cloned into the pWPI vector. The 293T packaging cells were transiently transfected with pMD2.G and psPAX2 with pWPI-vector or pWPI-ERα to produce lentiviral particles. The supernatants containing lentiviral particles were collected 48 hrs post-transfection of 293T cells. The lentiviral supernatant was then filtered and used to transduce fibroblast cells for 48 hrs. The viral transduced bladder fibroblasts cells were then subjected to 1 μg/ml puromycin selection. In addition to overexpressing ERα in fibroblasts, we also suppressed ERα or CCL1 expression in fibroblasts by infecting cells with lentiviral shERα or shCCL1 and compared to cells with lentiviral sh Luciferase (shLuc) as control. The shRNA against ERα (PLKO.1-puro-shERα) and CCL1 (PLKO.l-puroshCCL1) were constructed with target sequences: 5’-GTACCAATGACAAGGGAAGT-3’ (for ERα), and 5’-GCAATCCTGTGTTACAGAAAT-3’ (for CCL1).
RNA extraction and quantitative real-time PCR analysis
Total RNA was extracted by Trizol reagent (Invitrogen, CA) according to the manufacturer’s instructions. RNAs (1 μg) were subjected to reverse transcription using Superscript III transcriptase (Invitrogen). The obtained cDNAs were applied for qPCR using a SYBR green Bio-Rad CFX96 system. Primers used for quantitative-PCR are listed in Table 2. Relative RNA expression levels were normalized to the expression of GAPDH.
Table 2.
Primer sequences for quantitative PCR
| Gene | Sequence |
|---|---|
| GAPDH | Forward: 5’-GGAGCGAGATCCCTCCAAAAT-3’ |
| Reverse: 5’-GGCTGTTGTCATACTTCTCATGG-3’ | |
| ERα | Forward: 5’-CCCACTCAACAGCGTGTCTC-3’ |
| Reverse: 5’-CGTCGATTATCTGAATTTGGCCT-3’ | |
| CCL1 | Forward: 5’-CTCATTTGCGGAGCAAGAGAT-3’ |
| Reverse: 5’-GCCTCTGAACCCATCCAACTG-3’ | |
| CCL5 | Forward: 5’-CTGCCTCCCCATATTCCTCG-3’ |
| Reverse: 5’-CACACTTGGCGGTTCTTTCG-3’ | |
| CCL11 | Forward: 5’-CCCCTTCAGCGACTAGAGAG-3’ |
| Reverse: 5’-TCTTGGGGTCGGCACAGAT-3’ | |
| CXCL1 | Forward: 5’-CTCTTCCGCTCCTCTCACAG-3’ |
| Reverse: 5’-GGGGACTTCACGTTCACACT-3’ | |
| CXCL7 | Forward: 5’-GTAACAGTGCGAGACCACTTC-3’ |
| Reverse: 5’-CTTTGCCTTTCGCCAAGTTTC-3’ |
Statistical analysis
Values were expressed as mean ± standard deviation (S.D.). The Student’s t test and one way ANOVA were used to calculate two-sided P values, which were considered statistically significant when P < 0.05.
Results
BCa fibroblast cells promote invasion of BCa cells, not non-malignant urothelial cells
To examine the potential impacts of fibroblast cells on the BCa progression, we applied the transwell chambers to co-culture and perform invasion assay. For co-culture of stromal and epithelial cells, bladder fibroblast cells were seeded on the top insert well, and BCa T24 cells on the bottom well. After two days of co-culture, the BCa cells were reseeded to matrigel-coated insert wells for invasion assays for 24 hrs. The invaded BCa cells were quantified and the results revealed that co-culture of fibroblasts and BCa cells could enhance BCa T24 cell invasion (Figure 1A). In contrast, the co-cultured fibroblasts with non-malignant urothelial SV-HUC cells failed to enhance the non-malignant urothelial SV-HUC cell invasion (Supplemental Figure 1). When we replaced T24 cells with other BCa cells, including UMUC3 (Figure 1B) and 5637 cells (Figure 1C), similar results showed that co-culture of fibroblast cells with BCa cells could enhance the BCa invasion capability.
Figure 1.

Bladder fibroblasts promote BCa cells invasion. To test how bladder fibroblasts affect BCa cells invasion, those 2 types of cells were co-incubated in a transwell setting. Fibroblasts were seeded in the top well of 6-wells transwell plate and three BCa cell lines, the T24, UMUC3, or 5637 cells, were seeded in the bottom transwells (pore size: 0.4 μm). After 2 days of co-culture, BCa cells were re-seeded on matrigel coated transwells (pore size: 8 μm) to determine their invasion rate. All invaded cells were counted in 6 independent fields (magnitude, 200X). Invaded BCa cells were calculated and averaged. BCa cells with fibroblasts co-culture were compared to BCa without fibroblasts co-culture (control). All data were repeated in three independent experiments and analyzed by t-test.
ERα and CCL family genes are selectively increased in fibroblasts after co-culture with BCa cells
To dissect the mechanism(s) by which could allow the co-cultured BCa cells to gain a better invasion capacity, we screened ERα and some selective cytokines/chemokines including CCL1, CCL5, CCL11, CXCL1 and CXCL7 in fibroblast cells after co-culture (Figure 2A). Our results showed both ERα and CCL1 significantly and consistently increased in fibroblasts after co-cultured with UMUC3 and 5637 BCa cells. These results suggest co-culturing fibroblasts with BCa cells may involve the modulation of ERα and some selective cytokines/chemokines, supported by earlier studies showing ERα and the CCL family genes might play key roles to influence the cancer progression [36,37].
Figure 2.
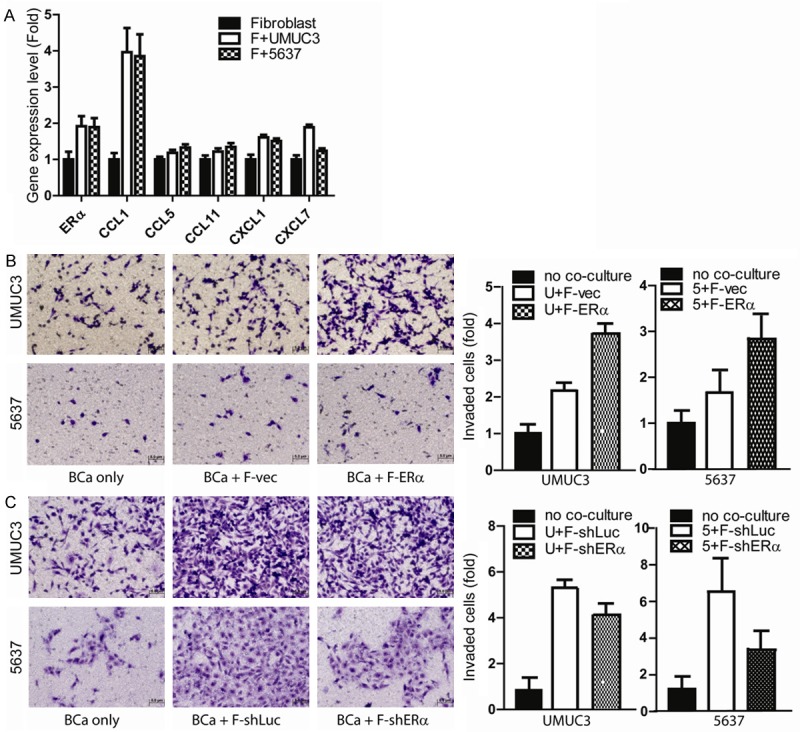
Fibroblasts promote BCa invasion via ERα and CCL1 pathways. (A) ERα and CCL1 increases in fibroblasts after co-culture with BCa UMUC3 and 5637. RNAs were collected from fibroblasts after co-culturing with BCa for subsequent qPCR analysis. (B) Higher ERα expression in fibroblasts could better promote BCa invasion. We used lentiviral-vector (F-vec) and lentiviral-ERα (F-ERα) to transduce fibroblasts. Those fibroblasts were then co-cultured with BCa cells (UMUC3, upper panels; 5637, lower panels) for 2 days in a transwell setting. After co-incubation, BCa cells were then re-seeded for matrigel invasion assay. (C) Suppressed fibroblasts ERα expression could reverse the fibroblast-enhanced BCa 5637 and UMUC3 cell invasion. To further confirm ERα in fibroblast-promoted BCa invasion, lentiviral-shERα was transduced into fibroblast. Fibroblasts with shERα, or control scramble RNA were co-cultured with BCa cells for 2 days. Transwell invasion assay was then used to measure BCa cell invasion rate. Our data showed fibroblast ERα and CCL1 could promote BCa progression. The invasion period is 24 hrs in most conditions except shERα group that we need to extend invasion period to 36 hr due to low endogenous ERα expression and found the difference between co-culture fibroblast with shLuc and shERα.
Addition of ERα in fibroblasts further increased fibroblasts-enhanced BCa cell invasion
To further prove above findings and to reveal the ERα roles for the fibroblasts-enhanced BCa cell invasion, we applied the lentiviral-ERα to ectopically express ERα in the fibroblasts. We next tested the impacts of fibroblasts with high vs. low ERα expression on BCa cell invasion. Our results revealed that fibroblasts enhanced UMUC3 cell invasion, and addition of ERα in fibroblasts further increased the fibroblasts-enhanced UMUC3 cell invasion (Figure 2B). Similar results were also obtained when we replaced UMUC3 cells with 5637 cells (Figure 2B). Importantly, using the opposite approach with lentiviral ERα-shRNA to suppress fibroblast ERα significantly impaired the fibroblast-enhanced 5637 and UMUC3 cell invasion (Figure 2C).
Together, results from Figure 2A-C suggest co-culturing fibroblasts with BCa cells may enhance BCa cell invasion and that could be controled by fibroblast ERα expression.
Higher ERα expression in stromal area surrounding BCa than in non-neoplastic stromal area
We immunohistochemically stained ERα in tissue microarrays consisting of 122 bladder cancers and 76 non-neoplastic urothelial tissues [35] and evaluated its expression in stromal cells. Strong positivity of ERα was found in 8.2% of BCa stromal cells, which was higher than in the non-neoplastic stroma (1.3%) (Table 1).
Table 1.
ERα expression in fibroblast and its correlation to grade and stage
| N | ERa expression in stromal cells adjacent to tumor | P value | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| 0 | 1+ | 2+ | 3+ | 0 vs 1+/2+/3+ | 0/1+ vs 2+/3+ | 0/1+/2+ vs 3+ | ||
| Stroma in benign | 76 | 29 (38.2%) | 30 (39.5%) | 15 (19.7%) | 1 (1.3%) | 0.185 | 0.4 | 0.054 |
| Stroma in tumor | 122 | 60 (49.2%) | 29 (23.8%) | 23 (18.9%) | 10 (8.2%) | |||
ERα up-regulates CCL1 expression in bladder fibroblasts
Our IHC staining of human BCa tissues demonstrated that ERα in bladder stromal fibroblasts has a higher expression (3+) (Table 1). Q-PCR data showed both ERα and CCL family gene expressions are increased in fibroblast cells after co-culture with BCa cells (Figure 2A). To further prove if increased ERα expression in fibroblasts may function through stimulating CCL family gene expression to control BCa invasion, we manipulated fibroblasts ERα expression and co-cultured with BCa cells to examine how stromal ERα regulate the downstream gene expression by Q-PCR. Results showed that higher ERα expression in fibroblasts can selectively up-regulate CCL1, but not CCL5, CCL11, CXCL1 and CXCL7.
Among those CCL family genes, CCL1 expression has profound changes when controlling fibroblast ERα expression. We next applied the interruption approach using CCL1-shRNA to examine its potential impacts on fibroblast ERα-enhanced BCa cell invasion. As shown in Figure 3, fibroblast ERα-enhanced BCa cell invasion was significantly reversed after knocking down CCL1 by the CCL1-shRNA.
Figure 3.
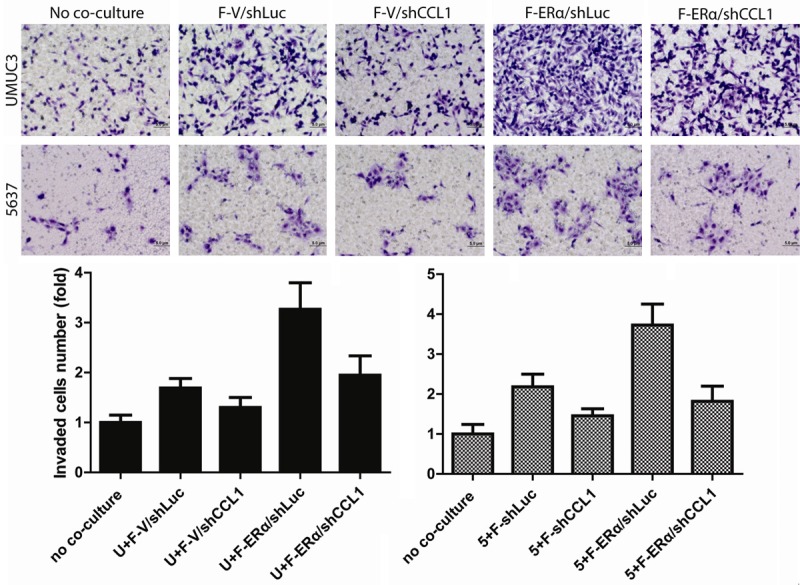
Fibroblast ERα-enhanced BCa cell invasion was partially reversed after knockdown of CCL1. To validate the importance of ERα→CCL1 signal, we transduced lentiviral-CCL1-shRNA in ERα (+) fibroblasts (F). BCa cell invasion capability was determined after co-incubation with fibroblasts. Compared to scramble RNA control (V), our data showed that fibroblast ERα-enhanced BCa cell invasion was partially reversed after knocking down CCL1.
Together, results from Figures 2 and 3 suggest fibroblasts may function through modulation of ERα/CCL1 signals to enhance BCa cell invasion.
Fibroblast ERα enhances BCa cell invasion via modulation of BCa IL-6 expression
To further dissect potential mechanisms by which fibroblast ERα could enhance BCa cell invasion, we then screened some metastasis-related genes in BCa cells after co-culture with fibroblasts. The results revealed that IL-6 expression was increased in both UMUC3 and 5637 cells after co-culture with fibroblasts (Figure 4A), yet IL8 expression was increased only in BCa UMUC3 cells after co-culture with fibroblasts (Figure 4A). Interestingly, we found addition of ERα in fibroblasts further increased fibroblasts-enhanced IL-6 expression in BCa 5637 cells (Figure 4B), suggesting fibroblast ERα may be able to function through modulating the BCa cells IL-6 expression to influence the BCa cell invasion. This conclusion was further proved when we applied the interruption approach using IL-6 neutralizing antibody. As shown in Figure 4C, blocking IL-6 with neutralizing antibody could significantly reverse the fibroblasts-enhanced BCa UMUC3 cells invasion.
Figure 4.
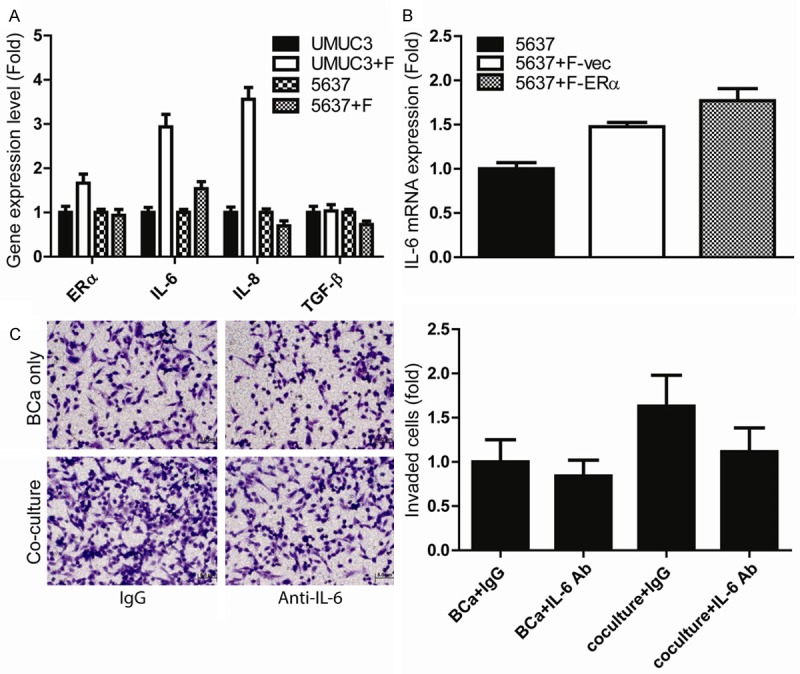
Fibroblasts enhance BCa cell invasion via modulation of BCa IL-6 expression. A. IL-6 expression was increased in both UMUC3 and 5637 cells after co-culture with fibroblasts. We screened some altered metastasis-related genes in BCa cells after co-culture with fibroblasts. Q-PCR results revealed that IL-6 expression was increased in both UMUC3 and 5637 cells after co-culture with fibroblasts and IL-8 expression was increased in only UMUC3 cells after co-culture with fibroblasts. B. Increase of ERα expression in fibroblasts further increased fibroblasts-enhanced IL-6 expression in BCa 5637 cells, suggesting fibroblast ERα may be able to function through modulation of the IL-6 expression to influence the BCa cell invasion. C. Blocking IL-6 with neutralizing antibody led to partially reverse the fibroblasts-enhanced BCa cell invasion. BCa cells were seeded in the transwell pre-coated with martrigel, fibroblasts were seeded on the bottom well, IL-6 anti-body (1:400 dilution) or mock control was added to the co-culture wells for 24 hrs. to determine the invasion rate.
Together, results from Figure 4A-C suggest that fibroblasts ERα may enhance BCa cell invasion via modulating the IL-6 expression in BCa cells.
Discussion
Clinical observations show that females have a lower cancer incidence and higher muscle invasive rate than males, which indicates gender differences may be a factor affecting BCa incidence and invasion [30,38]. This also suggests that estrogen and its receptors in the females might be a critical factor to mediate this gender difference. The IHC staining results suggest that protein expressions of ERα and ERβ are differentially correlated with the BCa stages [35,39]. In the tumor area, our previous study showed expression of ERβ was positively linked to cancer grade, stage, and patient survival rate, and reduction of ERβ activity by antiestrogen or gene deletion strategy leads to a protective role against BCa development [35,36]; but epithelial ERα can prevent BCa progression [40]. Meanwhile, we found that 8.2% of stroma in tumors show stronger ERα expression than benign stroma (1.3%). But the expression level of stromal ERβ is not different between cancer and benign groups. In this study, our results demonstrated high expression of ERα in fibroblasts can promote BCa cell invasion.
Cancer associated fibroblasts (CAF) have been demonstrated to promote tumor development and invasion in various cancers [41,42], including prostate [43], breast [44], colon [45] and BCa [46]. Angiogenesis is the major factor to support tumor progression [47] and especially VEGF (Vascular endothelial growth factor) has a promising role in promoting vessel formation [48]. Although VEGF may be produced by difference cells, including cancer cells, CAF and endothelial cells, a previous report has demonstrated that fibroblasts are the principal source of host-derived VEGF [49]. This indicates the importance of CAF in the tumor microenvironment and progression. Other than promoting the capillary formation, CAF also can release HGF and TGF-β to induce tumor growth during tumor initiation [50]. Reported data support that CAF are important to promote cancer metastasis [41]. We observed ERα plays distinct roles in CAFs of prostate and bladder cancers, we demonstrated that ERα in prostate CAF can up-regulate thrombospondin 2 (Thbs2) and down-regulate matrix metalloproteinase 3 (MMP3) to reduce PCa cell invasion [43]. In this study, our results showed that ERα increases in bladder fibroblasts and can promote BCa invasion via manipulating the CCL1 and IL-6 secretion.
Chemokine (C-C motif) ligand 1 (CCL1) has been identified to function against apoptosis and promote angiogenesis [51,52]. In clinical settings, serum concentrations of CCL1 significantly increase in patients with enlarged prostates and may have the potential to be a prognosis marker of benign prostate hyperplasia (BPH) [53]. Increasing data demonstrated the prostate stroma is an etiological factor of BPH and a target for therapy. Elevated CCL1 concentrations may correlate with fibroblast activation and proliferation. Although CCL1’s role in different types of tumor progression remains to be further elucidated, this is the first study to show CCL1 secreted by fibroblasts can consequently promote BCa invasion. Our results showed higher ERα in fibroblasts results in higher expression of CCL1, which provides the first evidence of a positive correlation between stromal ERα and CCL1.
IL-6 has multiple functions in a wide range of biologic activities in different types of cells including tumor cells and has been demonstrated to affect several cancer cells behaviors, including cancer cell migration [54], invasion, proliferation, apoptosis [55], angiogenesis and differentiation [56]. Our results showed co-culturing fibroblasts with BCa cells can promote BCa cell invasion and IL-6 expression. Application of IL-6 neutralizaing antibody can reverse fibroblasts induced BCa cell invasion. An earlier report IL-6 is also an autocrine growth factor in human BCa [57]. Chen et al. demonstrated IL-6 plays a critical role in BCa growth and invasion [58]. They compared IL-6 expression in muscle-invasive and non-muscle invasive BCa samples and thier data revealed that the expression level of IL-6 was significantly correlated with higher clinical stage, higher recurrence rate after treatment, and reduced survival rate. Actually, IL-6 plays dual roles in BCa progression. BCG is a standard treatment for non-muscle invasive BCa by inducing a series of complex systemic humoral and cellular immune responses [59]. Several studies indicated that BCG increased IL-6 production in human and murine BCa [60-62]. Quin et al. demonstrated LPS induced IL-6 expression via activation of p38 and ERK, whereas activation of PI3K/Akt exerts an opposing action [63].
In this study, we demonstrated IL-6 is important in fibroblasts enhanced BCa cell invasion; adding IL-6 neutralizing antibody reversed the fibroblasts enhanced BCa cell invasion. Our results also further indicated BCa fibroblasts secreted CCL1 may affect IL-6 production and invasion capability of BCa. IL-6 has a wide range of functions and is activated by many upstream signaling pathways, including CCL1 [64]. In addition to CCL1, CCL4 was demonstrated to raise IL-6 expression when liver damage occurs [65]. Meanwhile, a previous study showed that in patients who express the syndrome of frailty that the CXCL10 expression level will highly correlate with IL-6 elevation [66]. It will be important and interesting to further exam the potential functional connections of CCL4 and CXCL10 with IL-6 in the fibroblasts of BCa.
Acknowledgements
We thank Dr. Chawnshang Chang and Karen Wolf for help with manuscript preparation.
Disclosure of conflict of interest
The authors confirm that there are no conflicts of interest.
Supporting Information
References
- 1.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 2.Xouri G, Chrisitian S. Origin and function of tumor stroma fibroblasts. Semin Cell Dev Biol. 2010;21:40–46. doi: 10.1016/j.semcdb.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franco OE, Shaw AK, Strand DW, Hayward SW. Cancer associated fibroblasts in cancer pathogenesis. Semin Cell Dev Biol. 2010;21:33–39. doi: 10.1016/j.semcdb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, Onder TT, Wang ZC, Richardson AL, Weinberg RA, Orimo A. Autocrine TGF-β and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A. 2010;107:20009–20014. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abe Y, Horiuchi A, Osuka Y, Kimura S, Granger GA, Gatanaga T. Studies of membrane-associated and soluble (secreted) lymphotoxin in human lymphokine-activated T-killer cells in vitro. Lymphokine Cytokine Res. 1992;11:115–121. [PubMed] [Google Scholar]
- 7.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 8.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 9.Rasanen K, Vaheri A. Activation of fibroblasts in cancer stroma. Exp Cell Res. 2010;316:2713–2722. doi: 10.1016/j.yexcr.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 10.Hayward SW, Wang Y, Cao M, Hom YK, Zhang B, Grossfeld GD, Sudilovsky D, Cunha GR. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Res. 2001;61:8135–8142. [PubMed] [Google Scholar]
- 11.Nakamura T, Matsumoto K, Kiritoshi A, Tano Y, Nakamura T. Induction of hepatocyte growth factor in fibroblasts by tumor-derived factors affects invasive growth of tumor cells: in vitro analysis of tumor-stromal interactions. Cancer Res. 1997;57:3305–3313. [PubMed] [Google Scholar]
- 12.Brauchle M, Angermeyer K, Hubner G, Werner S. Large induction of keratinocyte growth factor expression by serum growth factors and pro-inflammatory cytokines in cultured fibroblasts. Oncogene. 1994;9:3199–3204. [PubMed] [Google Scholar]
- 13.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-ka- ppaB-Dependent Manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 15.Uchida K, Samma S, Momose H, Kashihara N, Rademaker A, Oyasu R. Stimulation of urinary bladder tumorigenesis by carcinogen-exposed stroma. J Urol. 1990;143:618–621. doi: 10.1016/s0022-5347(17)40041-3. [DOI] [PubMed] [Google Scholar]
- 16.Hodges GM, Hicks RM, Spacey GD. Epithelial-stromal interactions in normal and chemical carcinogen-treated adult bladder. Cancer Res. 1977;37:3720–3730. [PubMed] [Google Scholar]
- 17.Blaveri E, Simko JP, Korkola JE, Brewer JL, Baehner F, Mehta K, DeVries S, Koppie T, Pejavar S, Carroll P. Bladder cancer outcome and subtype classification by gene expression. Clin Cancer Res. 2005;11:4044–4055. doi: 10.1158/1078-0432.CCR-04-2409. [DOI] [PubMed] [Google Scholar]
- 18.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 19.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erez N, Truitt M, Olson P, Hanahan D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-κB-dependent manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 21.Torisu H, Ono M, Kiryu H, Furue M, Ohmoto Y, Nakayama J, Nishioka Y, Sone S, Kuwano M. Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanoma: possible involvement of TNFalpha and IL-1alpha. Int J Cancer. 2000;85:182–188. [PubMed] [Google Scholar]
- 22.Chiu HY, Sun KH, Chen SY, Wang HH, Lee MY, Tsou YC, Jwo SC, Sun GH, Tang SJ. Autocrine CCL2 promotes cell migration and invasion via PKC activation and tyrosine phosphorylation of paxillin in bladder cancer cells. Cytokine. 2012;59:423–432. doi: 10.1016/j.cyto.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Kawanishi H, Matsui Y, Ito M, Watanabe J, Takahashi T, Nishizawa K, Nishiyama H, Kamoto T, Mikami Y, Tanaka Y. Secreted CXCL1 is a potential mediator and marker of the tumor invasion of bladder cancer. Clin Cancer Res. 2008;14:2579–2587. doi: 10.1158/1078-0432.CCR-07-1922. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson S, Gustafsson JA. Estrogen receptor transcription and transactivation: Basic aspects of estrogen action. Breast Cancer Res. 2000;2:360–366. doi: 10.1186/bcr81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nimrod A, Ryan K. Aromatization of Androgens by Human Abdominal and Breast Fat Tissue 1. J Clin Endocrinol Metab. 1975;40:367–372. doi: 10.1210/jcem-40-3-367. [DOI] [PubMed] [Google Scholar]
- 26.Callard GV, Petro Z, Ryan KJ. Conversion of androgen to estrogen and other steroids in the vertebrate brain. Endocrinology. 1978;18:511–523. [Google Scholar]
- 27.Frisch RE, Canick JA, Tulchinsky D. Human fatty marrow aromatizes androgen to estrogen. J Clin Endocrinol Metab. 1980;51:394–396. doi: 10.1210/jcem-51-2-394. [DOI] [PubMed] [Google Scholar]
- 28.Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol. 2001;45:S116–S124. doi: 10.1067/mjd.2001.117432. [DOI] [PubMed] [Google Scholar]
- 29.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 30.Pfister C. New trends for optimal management of bladder tumors. J Urol. 2001;165:65–66. doi: 10.1097/00005392-200101000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Noronha RF, Rao BR. Sex hormone receptors in localized and advanced transitional cell carcinoma of urinary tract in humans. Urology. 1986;28:401–403. doi: 10.1016/0090-4295(86)90073-7. [DOI] [PubMed] [Google Scholar]
- 32.Han B, Cui D, Jing Y, Hong Y, Xia S. Estrogen receptor beta (ERbeta) is a novel prognostic marker of recurrence survival in non-muscle-invasive bladder cancer potentially by inhibiting cadherin switch. World J Urol. 2012;30:861–867. doi: 10.1007/s00345-011-0819-4. [DOI] [PubMed] [Google Scholar]
- 33.Mueller SO, Korach KS. Estrogen receptors and endocrine diseases: lessons from estrogen receptor knockout mice. Curr Opin Pharmacol. 2001;1:613–619. doi: 10.1016/s1471-4892(01)00105-9. [DOI] [PubMed] [Google Scholar]
- 34.Cadenas C, Bolt HM. Estrogen receptors in human disease. Arch Toxicol. 2012;86:1489–1490. doi: 10.1007/s00204-012-0928-x. [DOI] [PubMed] [Google Scholar]
- 35.Miyamoto H, Yao JL, Chaux A, Zheng Y, Hsu I, Izumi K, Chang C, Messing EM, Netto GJ, Yeh S. Expression of androgen and oestrogen receptors and its prognostic significance in urothelial neoplasm of the urinary bladder. BJU Int. 2012;109:1716–1726. doi: 10.1111/j.1464-410X.2011.10706.x. [DOI] [PubMed] [Google Scholar]
- 36.Hsu I, Chuang KL, Slavin S, Da J, Lim WX, Pang ST, O’Brien JH, Yeh S. Suppression of ERbeta signaling via ERbeta knockout or antagonist protects against bladder cancer development. Carcinogenesis. 2013;35:651–61. doi: 10.1093/carcin/bgt348. [DOI] [PubMed] [Google Scholar]
- 37.Balkwill F. Cancer and the chemokine network. Nature Reviews Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 38.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 39.Shen SS, Smith CL, Hsieh JT, Yu J, Kim IY, Jian W, Sonpavde G, Ayala GE, Younes M, Lerner SP. Expression of estrogen receptors-alpha and -beta in bladder cancer cell lines and human bladder tumor tissue. Cancer. 2006;106:2610–2616. doi: 10.1002/cncr.21945. [DOI] [PubMed] [Google Scholar]
- 40.Hsu I, Yeh CR, Slavin S, Miyamoto H, Netto GJ, Tsai YC, Muyan M, Wu XR, Messing EM, Guancial EA, Yeh S. Estrogen receptor alpha prevents bladder cancer via INPP4B inhibited akt pathway in vitro and in vivo. Oncotarget. 2014;5:7917–7935. doi: 10.18632/oncotarget.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karagiannis GS, Poutahidis T, Erdman SE, Kirsch R, Riddell RH, Diamandis EP. Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Mol Cancer Res. 2012;10:1403–1418. doi: 10.1158/1541-7786.MCR-12-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slavin S, Yeh CR, Da J, Yu S, Miyamoto H, Messing EM, Guancial E, Yeh S. Estrogen receptor α in cancer-associated fibroblasts suppresses prostate cancer invasion via modulation of thrombospondin 2 and matrix metalloproteinase 3. Carcinogenesis. 2013;35:1301–9. doi: 10.1093/carcin/bgt488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 45.Nakagawa H, Liyanarachchi S, Davuluri RV, Auer H, Martin EW, de la Chapelle A, Frankel WL. Role of cancer-associated stromal fibroblasts in metastatic colon cancer to the liver and their expression profiles. Oncogene. 2004;23:7366–7377. doi: 10.1038/sj.onc.1208013. [DOI] [PubMed] [Google Scholar]
- 46.Enkelmann A, Heinzelmann J, von Eggeling F, Walter M, Berndt A, Wunderlich H, Junker K. Specific protein and miRNA patterns characterise tumour-associated fibroblasts in bladder cancer. J Cancer Res Clin Oncol. 2011;137:751–759. doi: 10.1007/s00432-010-0932-6. [DOI] [PubMed] [Google Scholar]
- 47.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 48.Nör JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154:375–384. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, Seed B. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 50.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-ß signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 51.Homey B, Müller A, Zlotnik A. Chemokines: agents for the immunotherapy of cancer? Nat Rev Immunol. 2002;2:175–184. doi: 10.1038/nri748. [DOI] [PubMed] [Google Scholar]
- 52.Louahed J, Struyf S, Demoulin JB, Parmentier M, Snick JV, Damme JV, Renauld JC. CCR8-dependent activation of the RAS/MAPK pathway mediates anti-apoptotic activity of I-309/CCL1 and Vmip-I. Eur J Immunol. 2003;33:494–501. doi: 10.1002/immu.200310025. [DOI] [PubMed] [Google Scholar]
- 53.Agarwal M, He C, Siddiqui J, Wei JT, Macoska JA. CCL11 (eotaxin-1): a new diagnostic serum marker for prostate cancer. Prostate. 2013;73:573–581. doi: 10.1002/pros.22597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santer FR, Malinowska K, Culig Z, Cavarretta IT. Interleukin-6 trans-signalling differentially regulates proliferation, migration, adhesion and maspin expression in human prostate cancer cells. Endocr Relat Cancer. 2010;17:241–253. doi: 10.1677/ERC-09-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suchi K, Fujiwara H, Okamura S, Okamura H, Umehara S, Todo M, Furutani A, Yoneda M, Shiozaki A, Kubota T, Ichikawa D, Okamoto K, Otsuji E. Overexpression of Interleukin-6 suppresses cisplatin-induced cytotoxicity in esophageal squamous cell carcinoma cells. Anticancer Res. 2011;31:67–75. [PubMed] [Google Scholar]
- 56.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 57.Okamoto M, Hattori K, Oyasu R. Interleukin-6 functions as an autocrine growth factor in human bladder carcinoma cell lines in vitro. Int J Cancer. 1997;72:149–154. doi: 10.1002/(sici)1097-0215(19970703)72:1<149::aid-ijc21>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 58.Chen MF, Lin PY, Wu CF, Chen WC, Wu CT. IL-6 expression regulates tumorigenicity and correlates with prognosis in bladder cancer. PLoS One. 2013;8:e61901. doi: 10.1371/journal.pone.0061901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agarwal A, Agrawal U, Verma S, Mohanty NK, Saxena S. Serum Th1 and Th2 cytokine balance in patients of superficial transitional cell carcinoma of bladder pre-and post-intravesical combination immunotherapy. Immunopharmacol Immunotoxicol. 2010;32:348–356. doi: 10.3109/08923970903300151. [DOI] [PubMed] [Google Scholar]
- 60.Tsui KH, Wang SW, Chung LC, Feng TH, Lee TY, Chang PL, Juang HH. Mechanisms by which interleukin-6 attenuates cell invasion and tumorigenesis in human bladder carcinoma cells. Biomed Res Int. 2013;2013:791212. doi: 10.1155/2013/791212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Boer E, De Jong W, Steerenberg P, Aarden L, Tetteroo E, De Groot E, Van der Meijden A, Vegt P, Debruyne F, Ruitenberg E. Induction of urinary interleukin-1 (IL-1), IL-2, IL-6, and tumour necrosis factor during intravesical immunotherapy with bacillus Calmette-Guerin in superficial bladder cancer. Cancer Immunol Immunother. 1992;34:306–312. doi: 10.1007/BF01741551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Esuvaranathan K, Alexandroff A, McIntyre M, Jackson A, Prescott S, Chisholm G, James K. Interleukin-6 production by bladder tumors is upregulated by BCG immunotherapy. J Urol. 1995;154:572–575. doi: 10.1097/00005392-199508000-00072. [DOI] [PubMed] [Google Scholar]
- 63.Qian Y, Deng J, Xie H, Geng L, Zhou L, Wang Y, Yin S, Feng X, Zheng S. Regulation of TLR4-induced IL-6 response in bladder cancer cells by opposing actions of MAPK and PI3K signaling. J Cancer Res Clin Oncol. 2009;135:379–386. doi: 10.1007/s00432-008-0478-z. [DOI] [PubMed] [Google Scholar]
- 64.Akimoto N, Honda K, Uta D, Beppu K, Ushijima Y, Matsuzaki Y, Nakashima S, Kido MA, Imoto K, Takano Y, Noda M. CCL-1 in the spinal cord contributes to neuropathic pain induced by nerve injury. Cell Death Dis. 2013;4:e679. doi: 10.1038/cddis.2013.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gewiese-Rabsch J, Drucker C, Malchow S, Scheller J, Rose-John S. Role of IL-6 trans-signaling in CCl(4)induced liver damage. Biochim Biophys Acta. 2010;1802:1054–1061. doi: 10.1016/j.bbadis.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 66.Qu T, Yang H, Walston JD, Fedarko NS, Leng SX. Upregulated monocytic expression of CXC chemokine ligand 10 (CXCL-10) and its relationship with serum interleukin-6 levels in the syndrome of frailty. Cytokine. 2009;46:319–324. doi: 10.1016/j.cyto.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


