Abstract
Engagement of CD8 T cells is a crucial aspect of immune responses to pathogens and in tumor surveillance. Nonetheless most vaccination strategies with common adjuvants fail to elicit long-term memory CD8 T cells. Increased knowledge on the cellular and molecular requirements for CD8 T cell activation have unveiled new opportunities to directly modulate CD8 T cells to generate optimal responses. During chronic infections and cancer, immunomodulation strategies to enhance T cell responses may be particularly necessary to overcome the immunosuppressive microenvironment. In this review we will discuss blockade of inhibitory receptors; interleukin-2 administration; regulatory T cell modulation; and targeting of mTOR, as means to enhance CD8 T cell immunity.
1. Introduction
Prophylactic vaccines composed of attenuated or inactivated pathogens usually provide enough signals for the immune system to generate memory cells and antibodies that protect the individual from subsequent encounters. Purified proteins are less immunogenic and need to be combined with adjuvants to enhance T and B cell responses. However, in some situations, optimal immune responses may not be elicited by these vaccination approaches, thus development of novel vaccination strategies is still required.
The word adjuvant is derived from Latin, adiuvare, which means to aid. Adjuvants are compounds that enhance or shape the immune response. Adjuvants preferentially activate the innate immune system to ensure that lymphocytes recognize their cognate antigen in an inflammatory context to generate effective T and B cell responses [1]. However T cell responses elicited by adjuvants approved for use in humans, such as alum or oil-in-water emulsion, could still be improved [2]. With an increased understanding of the immune system and the pathways involved in T cell activation and differentiation, we now know that T cells can be modulated in different ways. In this review we will summarize and discuss some alternative strategies, beyond adjuvants, to improve T cell function, focusing mainly on CD8 T cells. We will address (1) blockade of inhibitory pathways; (2) administration of interleukin (IL)-2; (3) Modulation of Foxp3+ regulatory CD4 T (Treg) cells; (4) Targeting of mechanistic target of rapamycin (mTOR).
Importantly, vaccines can also be used therapeutically: when the immune response has failed to rid the host from an infection (during chronic infection), or to elicit immune responses against a tumor. Therapeutic vaccines have a different risk/benefit profile than prophylactics vaccines that are given to a healthy population. In addition, therapeutic vaccines may require different modulation of the immune system, since both chronic infections and cancer are associated with specific immunosuppression [3]. In those situations strategies that improve T cell function may be particularly necessary to achieve the ideal immunological response [4].
2. Blockade of inhibitory pathways
In order to be activated, T cells need to engage with antigen presenting cells (APCs) presenting cognate peptide-MHC complexes (pMHC). Besides the TCR and agonistic pMHC, the immunological synapse also contains cell adhesion molecules, as well as positive and negative co-receptors. T cells integrate the signals from the immunological synapse, and cell activation only occurs when signals are able to overcome a certain threshold. Hence, to induce an effective immune response, in addition to antigen, T cells need to receive positive signals. CD28 is constitutively expressed on naïve CD4 and CD8 T cells and is the best-studied positive co-stimulatory molecule. CD28 engagement in the immunological synapse decreases the amount of antigen necessary to elicit T cell activation. Importantly, inflammatory signals regulate expression of CD28 binding partners: B7-1 (CD80) and B7-2 (CD86) [5].
To prevent autoimmunity the immune system has evolved intrinsic and extrinsic inhibitory mechanisms that restrain T cell activation. However, inhibitory mechanisms may also dampen desirable immune responses against pathogens and tumors, and to vaccination. In this section we will focus on the intrinsic expression of inhibitory receptors that reduce T cell receptor (TCR) signaling and thereby modulate T cell activation, differentiation and function. In recent years many T cell co-inhibitory receptors have been identified [5,6]. Bellow we discuss cytotoxic T lymphocyte antigen (CTLA)-4 and programmed cell death (PD)-1 co-inhibitory molecules, since manipulation of these two pathways has already reached the clinic.
CTLA-4
CTLA-4 is an inhibitory co-receptor that binds with higher affinity to B7 ligands than CD28. CTLA-4 is induced by TCR signaling, and it competes and physically excludes CD28 from the immunological synapse. In addition, CTLA-4 also recruits phosphatases that dephosphorylate key TCR/CD28 signaling molecules [7]. Accordingly, several reports have shown that preventing CTLA-4 interactions can improve T cell activation. In vivo blockade of CTLA-4 enhances antigen-specific CD4 T cell responses after peptide immunization in complete Freund adjuvant [8]. And administration of anti-CTLA-4 blocking antibodies during Nippostrongylus brasiliensis infection enhances protective Th2 cytokines responses and reduces nematode burden [9]. Transient CTLA-4 blockade increases the number of memory CD8 T cells during low dose Listeria infection in mice; and during recall, CTLA-4 blockade enhanced the response of memory CD8 T cells. In this study, the effects mediated by CTLA-4 blockade were shown to be cell extrinsic, but whether it improved CD4-help or reduced Treg cell function was not fully addressed [10].
Treg cells express high levels of CTLA-4, and it has been shown that CTLA-4 plays a central role in Treg cell suppressive function [11–13]. Mice deficient in CTLA-4 develop fatal autoimmune disease [14,15], in a similar manner to mice lacking functional Treg cells due to mutation in the Foxp3 transcription factor [16]. Accordingly, it has been shown that CTLA-4 expression on Treg cells is necessary to maintain peripheral tolerance in Balb/c mice [17]. CTLA-4 mediates removal of B7 molecules from the surface of APCs by transendocytosis and thus can function in a cell non-autonomous manner to increase the threshold necessary for T cell activation [13]. Antibodies that bind CTLA-4 can impair Treg suppressive activity and may even mediate Treg cell depletion [19–21]. Hence both intrinsic effects on effector T cells and extrinsic effects, through Treg cells, may play a role in the control of T cell responses by administration of anti-CTLA-4 antibodies [12,22]. Importantly, CTLA-4 blockade for unresectable or metastatic melanoma has been approved since 2011 and shows durable anti-tumor responses with increased overall survival rates in up to 49.5% of patients [23]. In summary in vivo studies employing anti-CTLA-4 antibodies demonstrate that blocking this inhibitory pathway can improve T cells responses.
PD-1
PD-1 is an inhibitory receptor that modulates TCR and CD28 signaling through recruitment of the phosphatase SHP2. PD-1 binds PD-L1 (B7-H1/CD274) and PD-L2 (B7-DC/CD273). PD-L2 expression is restricted to antigen presenting cells (dendritic cells, monocytes and some B cell subsets), but PD-L1 expression is widespread. Expression of both PD-1 ligands is modulated by cytokines, such as IFN-γ [24], and PD-L1 expression was recently shown to be induced by hypoxia [25]. An important distinctive feature of the PD-1 pathway, compared to CTLA-4/B7 pathway, is that PD-L1 is expressed on hematopoietic and non-hematopoietic cells, including many tumor cells [24,26]. A differential role of the PD-1 pathway on hematopoietic vs. nonhematopoietic cells was elegantly demonstrated after LCMV infection in bone marrow chimera mice where either hematopoietic cells or nonhematopoietic cells were PD-L1 deficient. When hematopoietic cells lacked PD-L1 expression, both CD4 and CD8 LCMV-specific responses were augmented. However when non-hematopoietic cells lacked PD-L1, even though the magnitude and functionality of virus-specific T responses were similar to WT mice, there was increased viral control [27]. These results show that the PD-1 pathway controls T cell activation, but also operates by inhibiting T cell responses at effector sites. This conclusion is further supported by data with PD-L1 positive tumors, where it was shown that the PD-1 pathway directly inhibits cytotoxic T cell activity [28].
Blocking interactions of PD-1 with PD-L1 can improve primary T cell responses in different settings, as evidenced in acute herpes simplex virus (HSV)-1 infection [29] and lower respiratory viral infection in mice [30], as well as adenovirus vector immunization in non-human primates [31]. Nevertheless, the role of the PD-1 pathway has been best characterized during chronic T cell stimulation.
PD-1 expression is induced and sustained by TCR signaling [32], and as a result, chronically stimulated T cells express high levels of this molecule. Most importantly, sustained PD-1 expression plays a central role in the progressive T cell dysfunction into exhaustion, and blockade of the PD-1 pathway rescues function of exhausted T cells [33,34]. A pivotal role of the PD-1 pathway in T cell exhaustion was first shown in LCMV infection in mice [33] and later demonstrated to occur in many clinically relevant human persistent infections, such as HIV, hepatitis B and hepatitis C virus infections, as well as during cancer [35–41].
Blockade of inhibitory receptors as a strategy to treat chronically infected patients has lagged behind advances in oncology. However one clinical trial with HCV infected patients observed reduction in viral burden in about 10% of patients after a single infusion of anti-PD-1 antibodies, and one patient remained with undetectable HCV RNA for at least one year [42]. In oncology, promising results in different cancer types with blockade of the PD-1 pathway culminated in the recent U.S Food and Drug Administration (FDA) approval in 2014 of anti-PD1 antibodies to treat advanced melanoma patients that failed anti-CTLA-4 therapy [43–45]. Approval for other cancers, such as non-small cell lung cancer is highly anticipated. In patients with metastatic urothelial bladder cancer, positive results with anti-PD-L1 antibodies [46] resulted in “Breakthrough Therapy Designation” by the FDA in 2014.
Combinations with inhibitory receptor blockade
Inhibitory pathways have different mechanisms of suppression, thus combination therapies should increase the frequency of patients responding to immunotherapy. Tumor-specific CD8 T cells express high levels of PD-1, but also co-express CTLA-4 and other inhibitory receptors [47]. Accordingly, a clinical trial combining blockade of PD-1 and CTLA-4 obtained higher response rates than previously reported for either monotherapy in patients with melanoma [48]. Following the example of CTLA-4 and PD-1, several other inhibitory receptors are now targets to improve T cell responses. In animal models it has been shown that combining blockade of Tim-3 or LAG-3 with blockade of the PD-1 pathway can further improve T cell rescue [37,49–51]. Therefore we will probably see an increase in combinatorial interventions, giving opportunity for personalized therapies.
Combining modulation of inhibitory pathways with strategies that boost immune responses is also an area of active investigation [52,53]. During chronic LCMV infection, when blockade of the PD-1 pathway was combined to therapeutic vaccination with recombinant vaccinia virus expressing a LCMV epitope, there was a significant synergistic effect on the rescue of virus-specific CD8 T cells and reduction in viral burden [54]. Similar results have been obtained in tumor animal models. Even though anti-CTLA-4 or tumor-cell vaccine producing granulocyte/macrophage colony-stimulating factor (GM-CSF) as single agents were ineffective for rejection of the poorly immunogenic B16 mouse melanoma, combination of those strategies resulted in therapeutic tumor elimination [55]. Equivalent combination therapy was also necessary to reduce incidence of primary prostate cancer in transgenic mice [56]. Combination of tumor-cell vaccine producing GM-CSF with blockade of the PD-1 pathway also improved immune responses to B16 mouse melanoma and colon carcinoma [57].
Interestingly, in metastatic melanoma patients, when CTLA-4 blockade was combined to a melanosomal protein (glycoprotein 100) peptide vaccine, there was no significant improvement over anti-CTLA-4 monotherapy [58]. One of the challenges in oncology immunotherapy is finding the appropriate antigens that need to be targeted. This challenge partially explains the relative historic failure of therapeutic vaccination approaches in cancer. But we now understand that many immunosuppressive mechanisms in the tumor site reduce or completely ablate immune responses. Thus combining therapeutic vaccination with blockade of inhibitory pathways as an adjuvant strategy is a promising approach, and should further boost the search for tumor antigen candidates.
3. Cytokines to improve T cell responses: IL-2
Besides recognition of pMHC and costimulation, cytokines determine and modulate T cell activation. In many situations, inflammatory cytokines, such as type I IFN or IL-12, are essential for optimal effector and memory CD8 T cell differentiation [59,60]. For CD4 T cells it is well established that cytokines are essential during priming to determine the differentiation program into different helper CD4 T cell subsets [61]. During chronic T cell stimulation, cytokines that share the common gamma chain receptor (IL-2, IL-7, IL-15, IL-21) were shown to be particularly advantageous for T cell stimulation [59,62]. In this review we will describe the potential therapeutic applications of IL-2, highlighting the benefits it could provide when combined to blockade of the PD-1 pathway.
IL-2 was isolated as a T cell growth factor, and it stimulates proliferation and differentiation of T cells. However, since Treg cells constitutively express the high affinity IL-2 receptor (containing CD25) and are highly dependent on this cytokine for survival and function, initial studies on the effects of IL-2 during immune responses generated conflicting observations [63]. Nonetheless, it has been shown that the positive aspects of IL-2 administration are not necessarily hampered by IL-2 mediated Treg cell expansion, for example during chronic LCMV infection [64]. Still, in melanoma patients, it has been suggested that lower frequencies of ICOS+ Treg cells after the first dose of IL-2 are associated with better clinical responses [65].
Several examples have illustrated that the effects of IL-2 on T cells depend very much on the abundance, inflammatory context, as well as the stage of T cell differentiation. Exogenous IL-2 is detrimental during the expansion phase after acute LCMV infection for both CD8 and CD4 virus-specific T cells [66]. Excessive IL-2 during T cell expansion modulates T cell differentiation towards terminal effectors, which increases cell death and hampers memory formation [67]. Nonetheless, IL-2 signaling is important for perforin expression and, consequently, cytolitic function [68]. Hence timing and dosing of IL-2 may need to be carefully considered for each situation.
High dose IL-2 constituted one of the first demonstrations that the immune system could be harnessed to fight cancer [69]. IL-2 is an approved therapy for metastatic melanoma and renal cell carcinoma, eliciting long lasting tumor responses in 5–10% of patients [70]. A recent large clinical trial in advanced melanoma patients observed improved clinical responses when IL-2 therapy was combined with melanosomal protein (glycoprotein 100) peptide vaccine, compared to IL-2 monotherapy [71]. IL-2 is also used to improve survival of expanded tumor infiltrating lymphocytes in adoptive cell transfer strategies. With this approach complete and durable responses were obtained in up to 40% of heavily pre-treated melanoma patients [72]. Thus combining IL-2 to other immunotherapies should further increase the response rate in cancer patients.
During chronic LCMV infection, daily therapeutic low dose IL-2 increased virus-specific T cell responses, resulting in decreased viral burden [66]. Most notably, IL-2 therapy greatly enhanced rescue of exhausted CD8 T cells and viral control when combined to blockade of the PD-1 pathway [64]. IL-2 treatment was associated with increased T-bet expression and reduced expression of inhibitory receptors on exhausted LCMV-specific CD8 T cells [64], consistent with a “less exhausted” phenotype [73]. Remarkably, IL-2 treatment in LCMV chronically infected mice induced CD127 (IL-7Ra) re-expression on virus-specific CD8 T cells [64]. CD127 is expressed on naïve T cells and on memory CD8 T cells, including memory precursors, the small fraction of effector CD8 T cells that survive and differentiate into long-lived memory T cells after acute infection [74]. CD127 expression is functionally important and required for memory differentiation, and for survival of memory CD8 T cells in the absence of antigen. Thus re-expression of CD127 may confer survival advantages to rescued exhausted CD8 T cells.
Interestingly, in metastatic melanoma patients, no additive effects were observed when CTLA-4 blockade was combined to IL-2 therapy [75]. Nevertheless PD-1 and CTLA-4 blockade modulate the immune response by distinct mechanisms and studies combining IL-2 to PD-1 pathway blockade to treat cancer patients are highly anticipated.
4. Treg cell modulation
Treg cells constitute the most important extrinsic inhibitory mechanism that control T cell responses, as demonstrated by their essential role in peripheral tolerance maintenance [76,77]. There are multiple mechanisms whereby Treg cells exert suppressive function and these have been reviewed elsewhere [78–80].
Although in principle, inhibiting Treg cell activity can improve immunological responses to prophylactic vaccination [81,82], Treg cells’ fundamental role in preventing autoimmunity would restrict such strategies in healthy individuals. In addition, it was shown that altered chemokine production after Treg cell depletion promoted priming of low-avidity CD8 T cells, which impaired induction of effective memory CD8 T cells [83]. Similar disadvantage was observed after herpes simplex virus infection in mice, where it was shown that Treg cell depletion increased chemokine production in lymphoid organs leading to decreased migration of effector cells to the infection site [84]. Thus it is not clear whether initiating an immune response in the absence or with inhibition of Treg cells would actually be beneficial to long-term protection.
In contrast, the detrimental role of Treg cells during chronic infection and cancer is well documented [85–89]. Large numbers of Treg cells are present at the tumor site, and increased effector T cell to Treg cell ratio correlates with better prognosis [90–92]. Accordingly, immunotherapies during chronic infection and cancer should greatly benefit from Treg cell manipulation.
Almost three decades ago it was shown that antibody-mediated CD4 depletion in mice lead to lymphoma regression, mediated by CD8 T cells [93]. Later studies demonstrated that a single injection of anti-CD25 antibodies reduced the number of Treg cells and could prevent the growth of several different tumors in mice [94]. Higher efficiency and selective Treg cell depletion can be achieved by diphtheria toxin administration to transgenic mice expressing the human diphtheria toxin receptor on Treg cells [76,95]. Using this system, it has been shown that Treg cell depletion can trigger a CD8 T cell-dependent rejection of established tumors in mice [96]. However, in several tumor models, especially when treatment is given after tumor establishment, combination therapies achieve better tumor control. After inoculation of B16 melanoma expressing ovalbumin (OVA), effects in tumor growth are only observed when Treg depletion is combined to OVA vaccination [97]. In another study, maximal B16 melanoma tumor rejection and CTL responses were obtained when a tumor cell vaccine was combined to anti-CD25 Treg cell depletion and administration of anti-CTLA-4 antibodies [98]. More recently, it was shown that Treg cell ablation was sufficient to deter oncogene-driven breast cancer progression in mice, but combination with local radiation further reduced tumor growth and increased survival [99].
Importantly, local radiation is a standard therapy for cancer management, and Treg cells are more resistant than effector T cells to ionizing radiation [100], thus local radiation may decrease effector/Treg cell ratio at the tumor site. Nonetheless radiation is also thought to have an immunostimulatory effect by reducing tumor burden (and thus reducing the amount of antigenic stimulation on exhausted T cells), and also by releasing tumor antigens to be presented in draining lymph nodes. Hence there is a strong scientific rational to combine Treg cell modulation to radiation therapy [101].
We have recently shown that Treg cells play a pivotal role in maintaining T cell exhaustion during chronic LCMV infection. Treg ablation in chronically infected mice resulted in a striking rescue of anti-viral CD8 T cell responses. After Treg cell depletion exhausted CD8 T cells recognizing several different LCMV epitopes expanded 10–100 fold in multiple organs. More importantly, rescued anti-viral CD8 T cells were functional as assessed by cytokines production, Granzyme B and CD107a/b expression, and ex-vivo cytotoxic activity [102]. In accordance with other reports [76], Treg cell depletion resulted in an increase in B7 molecules on dendritic cells. We showed that rescue of exhausted CD8 T cells after Treg cell depletion was dependent on both B7 co-stimulation and CD4 T cells [102]. We proposed that B7 molecules binding to CD28 on exhausted T cells, overcame the negative effects of PD-1:PD-L1 interactions and led to effective antigen presentation and rescue of LCMV-CD8 specific T cells. Conventional CD4 T cells were also activated by Treg cell depletion and may have aided on the rescue of exhausted CD8 T cells by promoting dendritic cell activation, but also by providing important signals directly to CD8 T cells, such as the cytokines IL-2 and IL-21.
In our recently published work we highlighted that the magnitude and even functionality of LCMV-specific CD8 T cells in LCMV chronically infected mice was greater after Treg cell depletion than after blockade of the PD-1 pathway [102]. Interestingly, viral burden was reduced by blockade of the PD-1 pathway, but not by depletion of Treg cells (Table 1). To reconcile these observations, we proposed that the PD-1 pathway could be hindering viral control after Treg cell depletion in LCMV chronically infected mice. Others have shown, and we also confirmed in our model, that Treg cell depletion leads to a systemic increase in inflammatory cytokines, including IFN-γ, which is known to induce PD-L1 [24,103]. Accordingly we observed that Treg cell ablation triggered an increase in PD-L1 expression, especially on infected cells. Thus we proposed that when rescued LCMV-specific CD8 T cells bind PD-L1 on infected cells, they are unable to exert cytotoxic activity. To corroborate our hypothesis we confirmed that combining Treg cell depletion with anti-PD-L1 antibodies resulted in better viral control than blockade of the PD-1 pathway as a single therapy. In conclusion we demonstrated that (1) Treg cells play a major role in maintaining T cell exhaustion, and (2) the PD-1 pathway distinctively controls T cell activation by APCs and T cell activity at effector sites (Figure 1).
Table 1.
| LCMV-specific CD8 T cell | |||
|---|---|---|---|
| Treatment | Expansion | Cytokine production | Viral control |
| Treg cell depletion | ++++ | +++ | −/+ |
| PD-1 pathway blockade | ++ | +++ | ++ |
| Treg cell depletion + PD-1 pathway blockade | ++++ | ++++ | +++ |
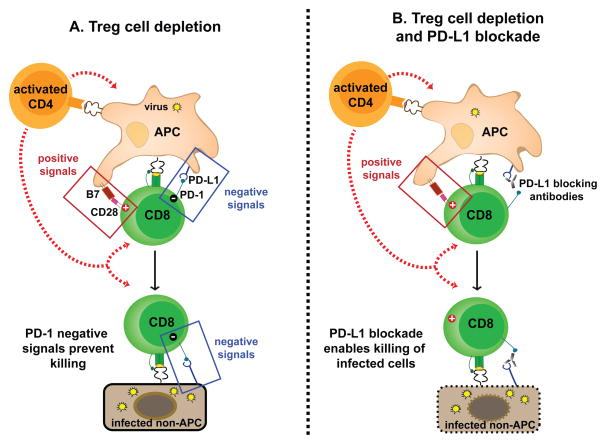
Figure 1. Synergy between Treg cell depletion and PD-1 blockade in the control of chronic viral infection.
(A) Treg cell depletion in LCMV chronically infected mice triggers an increase in B7 and PD-L1 molecules on APCs (partially dependent on activation of auto-reactive CD4 T cells). Increased net positive signals on APCs activate virus-specific CD8 T cells, and cytokines produced by activated CD4 T cells may further contribute for rescue of exhausted CD8 T cells after Treg cell depletion. However, the majority of LCMV infected cells is comprised of non-hematopoietic cells that express PD-L1 but no B7 molecules. Therefore, even though exhausted CD8 T cells are activated, expand and recover function after Treg cell depletion, when they interact with non-hematopoietic target cells, PD-1 negative signals prevail and inhibit cell-mediated cytotoxic activity. Thus Treg cell ablation during chronic LCMV infection rescues virus-specific CD8 T cells but there is no reduction in viral burden. (B) When PD-L1 blocking antibodies are combined with Treg cell depletion, virus-specific CD8 T cells receive positive signals from the APCs without PD-1 negative signaling, resulting in optimal T cell rescue. In addition, blockade of the PD-1 pathway now enables rescued LCMV-specific CD8 T cells to kill PD-L1 expressing infected cells at effector sites. As a result, combining Treg cell ablation to blockade of the PD-1 pathway during chronic LCMV infection results in optimal rescue virus-specific CD8 T cells that can reduce viral burden [102].
In humans, Treg cell modulation could probably be achieved by different strategies, and each would need to be carefully evaluated to achieve the best immunological response, while minimizing adverse effects. Interestingly, some standard therapies such as cyclophosphamide (whose primary goal is to halt tumor cell division) may also preferentially affect Treg cells over conventional T cells, shifting the balance towards effector T cell responses [104]. Strategies that focus on modulation of a population rather than all Treg cells are certainly interesting and promising with regards of safety concerns. For example, it was proposed that Treg cells that infiltrate tumors express high levels of CCR4. Accordingly, administration of anti-CCR4 antibodies to a leukemia-lymphoma patient improved CD8 T cell responses directed against the tumor [105]. Another potential strategy to preferentially target Treg cells in the tumor microenvironment is to block neuropilin-1 (Nrp1), the vascular endothelial growth factor (VEGF) receptor. It has been shown that even though mice in which Treg cells are genetically deficient in Nrp1 have no autoimmune disease, they are better at controlling B16 melanoma tumor growth, which was associated with increased functional intratumoral CD8 T cells [106]. Similar results were also reported in several other mouse tumor models [107]. Nrp1 on Tregs also binds to semaphoring-4a (Sema4a) in other T cells or dendritic cells, promoting functional stability of the Tregs. Therapeutic interference of Nrp1/Sema4a interactions significantly decreased B16 tumor growth in mice [106].
In summary there is great potential to manipulate Treg cells to improve immune responses during chronic infections and cancer, and novel strategies are being developed to decrease adverse autoimmune effects.
5. mTOR modulation
mTOR is an evolutionally conserved serine/threonine kinase that forms two distinct protein complexes: mTORC1 and mTORC2 [108,109]. mTOR complexes are differentially activated and have unique downstream signaling pathways that control multiple cellular activities [108,109]. Rapamycin, a specific inhibitor of mTOR, primarily prevents mTORC1 activity and is currently used as an immunosuppressive drug in transplant recipients.
However, our group found that rapamycin had immunostimulatory effects on the generation of memory CD8 T cells [110]. The drug treatment during acute viral infection or vaccination enhanced the number of antigen specific memory CD8 T cells, and more importantly improved memory T cell quality characterized by high proliferative and protective capacity and increased longevity [110]. We also showed that rapamycin acted intrinsically in antigen specific CD8 T cells and was effective during both the clonal expansion and the contraction phases of T cell responses [110]. During the expansion phase, the drug increased the number of memory precursor cells, which are a subset of effector populations that effectively survive and further differentiate to give rise to long-lived memory CD8 T cells [110]. On the other hand, during the contraction phase (effector to memory transition phase) rapamycin accelerated memory differentiation program [110]. Several independent studies also confirmed improvement of quantity and quality of memory CD8 T cells by rapamycin treatment [111–113].
This immunostimulatory effect of rapamycin on generation of memory CD8 T cells is somehow paradoxical because rapamycin is believed to inhibit T cell responses against allografts in transplant settings. Ferrer et al. showed fundamental differences in the outcome of rapamycin treatment between graft specific versus pathogen specific CD8 T cell responses [114]. Unlike the effect of rapamycin on pathogen specific CD8 T cells described above, the drug failed to enhance graft specific CD8 T cell responses after skin transplantation. Although the exact mechanism underlying the different effects of rapamycin between these two settings remains to be determined, rapamycin seems to specifically improve pathogen specific CD8 T cell immunity [114]. Thus, targeting mTOR by rapamycin is an attractive strategy to enhance generation of memory CD8 T cell immunity after vaccination.
However, since mTOR is expressed in most cells (both immune and non-immune cells) in the body, use of the drug to enhance CD8 T cell immunity in humans will need to be carefully evaluated. In addition, although mTOR is a major regulator of memory CD8 T cell differentiation, this pathway has an essential role in regulating immune responses of other types of immune cells including DCs, effector CD4 T cells, Tregs, and B cells [115,116]. Therefore, in some cases rapamycin treatment could dampen humoral immune responses [117]. To avoid these undesirable issues, specific inhibition of mTOR in activated CD8 T cells may be important for enhancing memory T cell immunity. Berezhnoy et al. demonstrated this proof of concept that targeted inhibition of mTOR could promote superior memory CD8 T cells [118]. They used aptamer-targeted siRNA delivery to inhibit mTOR in T cells and found that this novel approach generated potent memory CD8 T cell immunity and enhanced vaccine-induced protective antitumor immune responses [118]. Thus, developing new technologies that inhibit mTOR only in target immune cells would be key for translating mTOR-related basic findings to humans.
As we described above, inhibiting mTOR in antigen specific CD8 T cells during acute infection or vaccination can enhance generation of memory CD8 T cells. Whether targeting mTOR pathways improve the function of exhausted CD8 T cells arising during chronic infection and cancer is still undetermined. Molecular characterization of exhausted CD8 T cells indicated reduced metabolic fitness, with downregulation of several genes involved in metabolism, as well as in mRNA translation [119]. From these results it seems like exhausted CD8 T cells have a general deficit in energy supply, which would be consistent with their unresponsive state, despite continuous TCR stimulation. Since mTOR regulates both metabolism and mRNA translation [108,109], investigating the role of mTOR in exhausted CD8 T cells is an important subject for future work. Rapamycin and its analogs have been used in clinical trials to test their efficacy for inhibition of tumor growth and some of them are already approved for the treatment of cancer patients [120]. Thus, it will be interesting to examine besides direct effects on cancer cells, whether and/or how mTOR inhibitors affect exhausted CD8 T cells and the immune response to tumor antigens.
6. Conclusion
T cell responses occur in a tightly controlled manner that probably evolved to reduce the incidence of autoimmunity. However, the same mechanisms that prevent excessive autoreactivity, also reduce T cell responses to pathogens or vaccination, and cancer. Increased understanding of the molecules and cells that govern T cell activation has uncovered new pathways that can be modulated to increase T cell immunity. Recent approval of antibodies that block the coinhibitory T cell receptors CTLA-4 and PD-1 for treatment of metastatic melanoma patients has greatly renewed interest in immunotherapy. Similar to the benefits provided by IL-2 therapy, blockade of inhibitory receptors can provide durable clinical responses, which results in increased overall survival on treated cancer patients. While the mechanisms involved in eliciting anti-tumor immune responses have not been completely elucidated, pre-clinical data strongly supports the use of combinatorial immunotherapy. Advances in oncology are now happening in a fast pace and they should encourage the use of immunomodulation to treat chronically infected patients, and possibly even to induce better immunity to prophylactic vaccination.
Highlights.
Inhibitory receptors limit T cell activation, and during chronic infection and cancer, drive T cell dysfunction.
Blockade of the inhibitory receptors PD-1 and CTLA-4 enhance CD8 T cell immunity.
IL-2 synergizes with PD-1 pathway blockade to rescue exhausted T cells.
Treg cell ablation rescues exhausted CD8 T cells during chronic LCMV infection, but blockade of the PD-1 pathway is necessary to control viral load.
mTOR inhibition enhances CD8 T cell memory.
Acknowledgments
Work from our laboratory was supported by grants from the National Institutes of Health R01 AI030048, P01 A1080192 and P01 AI056299. A.O.K was supported by the Irvington Institute Fellowship Program of the Cancer Research Institute.
Abbreviations
- Treg cell
Foxp3+ CD4 regulatory T cell
- mTOR
mechanistic target of rapamycin
- LCMV
lymphocytic choriomeningitis virus
- PD-1
Programmed cell death-1
- CTLA-4
cytotoxic T lymphocyte antigen-4
- APC
antigen presenting cell
- pMHC
peptide-MHC complexes
- FDA
U.S Food and Drug Administration
- IL
interleukin
- Nrp1
neuropilin-1
- VEGF
vascular endothelial growth factor
Footnotes
8. Conflict of interest
R.A. holds patents and receives patent royalties related to the PD-1 inhibitory pathway. The remaining authors declare no conflicting financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19:1597–1608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- 3.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim PS, Ahmed R. Features of responding T cells in cancer and chronic infection. Curr Opin Immunol. 2010;22:223–230. doi: 10.1016/j.coi.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pentcheva-Hoang T, Corse E, Allison JP. Negative regulators of T-cell activation: potential targets for therapeutic intervention in cancer, autoimmune disease, and persistent infections. Immunol Rev. 2009;229:67–87. doi: 10.1111/j.1600-065X.2009.00763.x. [DOI] [PubMed] [Google Scholar]
- 8.Kearney ER, Walunas TL, Karr RW, Morton PA, Loh DY, Bluestone JA, Jenkins MK. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J Immunol. 1995;155:1032–1036. [PubMed] [Google Scholar]
- 9.McCoy K, Camberis M, Gros GL. Protective immunity to nematode infection is induced by CTLA-4 blockade. J Exp Med. 1997;186:183–187. doi: 10.1084/jem.186.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedicord VA, Montalvo W, Leiner IM, Allison JP. Single dose of anti-CTLA-4 enhances CD8+ T-cell memory formation, function, and maintenance. Proc Natl Acad Sci U S A. 2011;108:266–271. doi: 10.1073/pnas.1016791108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 12.Wing K, Yamaguchi T, Sakaguchi S. Cell-autonomous and -non-autonomous roles of CTLA-4 in immune regulation. Trends Immunol. 2011;32:428–433. doi: 10.1016/j.it.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, et al. Trans-Endocytosis of CD80 and CD86: A Molecular Basis for the Cell Extrinsic Function of CTLA-4. Science. 2011 doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 15.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 16.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 17.Friedline RH, Brown DS, Nguyen H, Kornfeld H, Lee J, Zhang Y, Appleby M, Der SD, Kang J, Chambers CA. CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J Exp Med. 2009;206:421–434. doi: 10.1084/jem.20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schubert D, Bode C, Kenefeck R, Hou TZ, Wing JB, Kennedy A, Bulashevska A, Petersen BS, Schaffer AA, Gruning BA, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med. 2014 doi: 10.1038/nm.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulliard Y, Jolicoeur R, Windman M, Rue SM, Ettenberg S, Knee DA, Wilson NS, Dranoff G, Brogdon JL. Activating Fc gamma receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J Exp Med. 2013;210:1685–1693. doi: 10.1084/jem.20130573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, Korman AJ. Anti-CTLA-4 Antibodies of IgG2a Isotype Enhance Antitumor Activity through Reduction of Intratumoral Regulatory T Cells. Cancer Immunology Research. 2013 doi: 10.1158/2326-6066.CIR-13-0013. Published Online First April 7, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolchok JD, Hodi FS, Weber JS, Allison JP, Urba WJ, Robert C, O’Day SJ, Hoos A, Humphrey R, Berman DM, et al. Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Ann N Y Acad Sci. 2013;1291:1–13. doi: 10.1111/nyas.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 27.Mueller SN, Vanguri VK, Ha SJ, West EE, Keir ME, Glickman JN, Sharpe AH, Ahmed R. PD-L1 has distinct functions in hematopoietic and nonhematopoietic cells in regulating T cell responses during chronic infection in mice. J Clin Invest. 2010;120:2508–2515. doi: 10.1172/JCI40040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Channappanavar R, Twardy BS, Suvas S. Blocking of PDL-1 interaction enhances primary and secondary CD8 T cell response to herpes simplex virus-1 infection. PLoS One. 2012;7:e39757. doi: 10.1371/journal.pone.0039757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erickson JJ, Gilchuk P, Hastings AK, Tollefson SJ, Johnson M, Downing MB, Boyd KL, Johnson JE, Kim AS, Joyce S, et al. Viral acute lower respiratory infections impair CD8+ T cells through PD-1. J Clin Invest. 2012;122:2967–2982. doi: 10.1172/JCI62860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finnefrock AC, Tang A, Li F, Freed DC, Feng M, Cox KS, Sykes KJ, Guare JP, Miller MD, Olsen DB, et al. PD-1 blockade in rhesus macaques: impact on chronic infection and prophylactic vaccination. J Immunol. 2009;182:980–987. doi: 10.4049/jimmunol.182.2.980. [DOI] [PubMed] [Google Scholar]
- 32.Blattman JN, Wherry EJ, Ha SJ, van der Most RG, Ahmed R. Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection. J Virol. 2009;83:4386–4394. doi: 10.1128/JVI.02524-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 34.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 35.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Fourcade J, Kudela P, Sun Z, Shen H, Land SR, Lenzner D, Guillaume P, Luescher IF, Sander C, Ferrone S, et al. PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients. J Immunol. 2009;182:5240–5249. doi: 10.4049/jimmunol.0803245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 39.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 41.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gardiner D, Lalezari J, Lawitz E, DiMicco M, Ghalib R, Reddy KR, Chang KM, Sulkowski M, Marro SO, Anderson J, et al. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS One. 2013;8:e63818. doi: 10.1371/journal.pone.0063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas Powles NJV, Fine Gregg Daniel, Eder Joseph Paul, Braiteh Fadi S, Loriot Yohann, Zambrano Cristina Cruz, Bellmunt Joaquim, Burris Howard A, Teng Siew-leng Melinda, Shen Xiaodong, Koeppen Hartmut, Hegde Priti S, Chen Daniel S, Petrylak Daniel Peter. Inhibition of PD-L1 by MPDL3280A and clinical activity in pts with metastatic urothelial bladder cancer (UBC) J Clin Oncol. 2014;32:abstr 5011. [Google Scholar]
- 47.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2012;13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ha SJ, West EE, Araki K, Smith KA, Ahmed R. Manipulating both the inhibitory and stimulatory immune system towards the success of therapeutic vaccination against chronic viral infections. Immunol Rev. 2008;223:317–333. doi: 10.1111/j.1600-065X.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 53.Kamphorst AO, Ahmed R. Manipulating the PD-1 pathway to improve immunity. Current Opinion in Immunology. 2013 doi: 10.1016/j.coi.2013.03.003. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, Freeman GJ, Ahmed R. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008;205:543–555. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, Burg MB, Allison JP. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–2448. [PubMed] [Google Scholar]
- 57.Li B, VanRoey M, Wang C, Chen TH, Korman A, Jooss K. Anti-programmed death-1 synergizes with granulocyte macrophage colony-stimulating factor--secreting tumor cell immunotherapy providing therapeutic benefit to mice with established tumors. Clin Cancer Res. 2009;15:1623–1634. doi: 10.1158/1078-0432.CCR-08-1825. [DOI] [PubMed] [Google Scholar]
- 58.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cox MA, Kahan SM, Zajac AJ. Anti-viral CD8 T cells and the cytokines that they love. Virology. 2013;435:157–169. doi: 10.1016/j.virol.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toe JG, Pellegrini M, Mak TW. Promoting immunity during chronic infection-- the therapeutic potential of common gamma-chain cytokines. Mol Immunol. 2013;56:38–47. doi: 10.1016/j.molimm.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 63.Katzman SD, Hoyer KK, Dooms H, Gratz IK, Rosenblum MD, Paw JS, Isakson SH, Abbas AK. Opposing functions of IL-2 and IL-7 in the regulation of immune responses. Cytokine. 2011;56:116–121. doi: 10.1016/j.cyto.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.West EE, Jin HT, Rasheed AU, Penaloza-Macmaster P, Ha SJ, Tan WG, Youngblood B, Freeman GJ, Smith KA, Ahmed R. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J Clin Invest. 2013;123:2604–2615. doi: 10.1172/JCI67008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sim GC, Martin-Orozco N, Jin L, Yang Y, Wu S, Washington E, Sanders D, Lacey C, Wang Y, Vence L, et al. IL-2 therapy promotes suppressive ICOS+ Treg expansion in melanoma patients. J Clin Invest. 2014;124:99–110. doi: 10.1172/JCI46266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 67.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 68.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosenberg SA, Lotze MT, Muul LM, Chang AE, Avis FP, Leitman S, Linehan WM, Robertson CN, Lee RE, Rubin JT, et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987;316:889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- 70.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192:5451–5458. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, Bikoff EK, Robertson EJ, Lauer GM, Reiner SL, et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012;338:1220–1225. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 75.Maker AV, Phan GQ, Attia P, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Haworth LR, Levy C, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005;12:1005–1016. doi: 10.1245/ASO.2005.03.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 77.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 78.Yamaguchi T, Wing JB, Sakaguchi S. Two modes of immune suppression by Foxp3(+) regulatory T cells under inflammatory or non-inflammatory conditions. Semin Immunol. 2011;23:424–430. doi: 10.1016/j.smim.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 79.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 80.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Toka FN, Suvas S, Rouse BT. CD4+ CD25+ T cells regulate vaccine-generated primary and memory CD8+ T-cell responses against herpes simplex virus type 1. J Virol. 2004;78:13082–13089. doi: 10.1128/JVI.78.23.13082-13089.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moore AC, Gallimore A, Draper SJ, Watkins KR, Gilbert SC, Hill AV. Anti-CD25 antibody enhancement of vaccine-induced immunogenicity: increased durable cellular immunity with reduced immunodominance. J Immunol. 2005;175:7264–7273. doi: 10.4049/jimmunol.175.11.7264. [DOI] [PubMed] [Google Scholar]
- 83.Pace L, Tempez A, Arnold-Schrauf C, Lemaitre F, Bousso P, Fetler L, Sparwasser T, Amigorena S. Regulatory T cells increase the avidity of primary CD8+ T cell responses and promote memory. Science. 2012;338:532–536. doi: 10.1126/science.1227049. [DOI] [PubMed] [Google Scholar]
- 84.Lund JM, Hsing L, Pham TT, Rudensky AY. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 2008;320:1220–1224. doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol. 2014;27:1–7. doi: 10.1016/j.coi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 86.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 87.Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions (*) Annu Rev Immunol. 2009;27:551–589. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 88.Li S, Gowans EJ, Chougnet C, Plebanski M, Dittmer U. Natural regulatory T cells and persistent viral infection. J Virol. 2008;82:21–30. doi: 10.1128/JVI.01768-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72:2162–2171. doi: 10.1158/0008-5472.CAN-11-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 91.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 92.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Awwad M, North RJ. Immunologically mediated regression of a murine lymphoma after treatment with anti-L3T4 antibody. A consequence of removing L3T4+ suppressor T cells from a host generating predominantly Lyt-2+ T cell-mediated immunity. J Exp Med. 1988;168:2193–2206. doi: 10.1084/jem.168.6.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 95.Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, Hamann A, Wagner H, Huehn J, Sparwasser T. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Teng MW, Ngiow SF, von Scheidt B, McLaughlin N, Sparwasser T, Smyth MJ. Conditional regulatory T-cell depletion releases adaptive immunity preventing carcinogenesis and suppressing established tumor growth. Cancer Res. 2010;70:7800–7809. doi: 10.1158/0008-5472.CAN-10-1681. [DOI] [PubMed] [Google Scholar]
- 97.Klages K, Mayer CT, Lahl K, Loddenkemper C, Teng MW, Ngiow SF, Smyth MJ, Hamann A, Huehn J, Sparwasser T. Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res. 2010;70:7788–7799. doi: 10.1158/0008-5472.CAN-10-1736. [DOI] [PubMed] [Google Scholar]
- 98.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bos PD, Plitas G, Rudra D, Lee SY, Rudensky AY. Transient regulatory T cell ablation deters oncogene-driven breast cancer and enhance radiotherapy. J Exp Med. 2013 doi: 10.1084/jem.20130762. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Komatsu N, Hori S. Full restoration of peripheral Foxp3+ regulatory T cell pool by radioresistant host cells in scurfy bone marrow chimeras. Proc Natl Acad Sci U S A. 2007;104:8959–8964. doi: 10.1073/pnas.0702004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Drake CG. Combination immunotherapy approaches. Ann Oncol. 2012;23(Suppl 8):viii41–46. doi: 10.1093/annonc/mds262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Penaloza-MacMaster P, Kamphorst AO, Wieland A, Araki K, Iyer SS, West EE, O’Mara L, Yang S, Konieczny BT, Sharpe AH, et al. Interplay between regulatory T cells and PD-1 in modulating T cell exhaustion and viral control during chronic LCMV infection. J Exp Med. 2014;211:1905–1918. doi: 10.1084/jem.20132577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 104.Le DT, Jaffee EM. Regulatory T-cell modulation using cyclophosphamide in vaccine approaches: a current perspective. Cancer Res. 2012;72:3439–3444. doi: 10.1158/0008-5472.CAN-11-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, Ezoe S, Kanakura Y, Sato E, Fukumori Y, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci U S A. 2013;110:17945–17950. doi: 10.1073/pnas.1316796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J, et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature. 2013;501:252–256. doi: 10.1038/nature12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hansen W, Hutzler M, Abel S, Alter C, Stockmann C, Kliche S, Albert J, Sparwasser T, Sakaguchi S, Westendorf AM, et al. Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J Exp Med. 2012;209:2001–2016. doi: 10.1084/jem.20111497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shimobayashi M, Hall MN. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol. 2014;15:155–162. doi: 10.1038/nrm3757. [DOI] [PubMed] [Google Scholar]
- 110.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bassett JD, Swift SL, VanSeggelen H, Hammill JA, McGray AJ, Evelegh C, Wan Y, Bramson JL. Combined mTOR inhibition and OX40 agonism enhances CD8(+) T cell memory and protective immunity produced by recombinant adenovirus vaccines. Mol Ther. 2012;20:860–869. doi: 10.1038/mt.2011.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ferrer IR, Wagener ME, Robertson JM, Turner AP, Araki K, Ahmed R, Kirk AD, Larsen CP, Ford ML. Cutting edge: Rapamycin augments pathogen-specific but not graft-reactive CD8+ T cell responses. J Immunol. 2010;185:2004–2008. doi: 10.4049/jimmunol.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xu X, Ye L, Araki K, Ahmed R. mTOR, linking metabolism and immunity. Semin Immunol. 2012;24:429–435. doi: 10.1016/j.smim.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Araki K, Ellebedy AH, Ahmed R. TOR in the immune system. Curr Opin Cell Biol. 2011;23:707–715. doi: 10.1016/j.ceb.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang S, Pruitt M, Tran D, Du Bois W, Zhang K, Patel R, Hoover S, Simpson RM, Simmons J, Gary J, et al. B cell-specific deficiencies in mTOR limit humoral immune responses. J Immunol. 2013;191:1692–1703. doi: 10.4049/jimmunol.1201767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Berezhnoy A, Castro I, Levay A, Malek TR, Gilboa E. Aptamer-targeted inhibition of mTOR in T cells enhances antitumor immunity. J Clin Invest. 2014;124:188–197. doi: 10.1172/JCI69856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 120.Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10:868–880. doi: 10.1038/nrd3531. [DOI] [PubMed] [Google Scholar]