Synopsis
While the diagnosis of Autism Spectrum Disorder is based on behavioral signs and symptoms, the evaluation of a child with ASD has become increasingly focused on the identification of the genetic etiology of the disorder. Using chromosomal microarray and whole exome sequencing technology, more than 25% of children with ASD have an identifiable, causative genetic variant or syndrome, and this rate continues to increase with improved methods and widespread use of genetic testing. The identification of genetic variants has been accompanied by a concerted effort to define more homogeneous clinical syndromes that are informed by the underlying genetic etiology of a child’s ASD. In the future, such characterization will facilitate targeted treatments based on mechanisms of disease and common clinical features. In this review we begin with a clinical overview of ASD, highlighting the heterogeneity of the disorder. We then discuss the genetics of ASD and present updated guidelines on genetic testing. We then consider the insights gained from the identification of both single gene disorders and rare variants, with regard to clinical phenomenology and potential treatment targets.
Keywords: Neurodevelopmental disorders, Autism Spectrum Disorders, Genetics, Copy number variants, Chromosomal microarray, Whole exome sequencing
Introduction
Autism Spectrum Disorder (ASD) is a heterogeneous group of disorders defined by impaired social-communication function and the presence of restricted, repetitive patterns of behavior or interests1. While the diagnosis of ASD is based on behavioral signs and symptoms, the evaluation of a child with ASD has become increasingly focused on the identification of the genetic etiology of the disorder. With the advances made in genetic testing over the last decade, more than 25% of children with ASD have an identifiable, causative genetic variant or syndrome, and this rate continues to increase with improved methods in genetic testing. In fact, the term ‘idiopathic autism’ has become increasingly obsolete in this era of genomics, sometimes replaced by the descriptor of “non-syndromic autism” for cases without a defined genetic etiology. The identification of genetic variants has been accompanied by a concerted effort to define more homogeneous clinical syndromes that are informed by the underlying genetic etiology of a child’s ASD. In the future, such characterization will facilitate targeted treatments based on mechanisms of disease and common clinical features. Here we present the clinical phenomenology of ASD, including evaluation and treatment, in the context of our growing appreciation of the genetic basis of this neurodevelopmental disorder.
Diagnosis of ASD is not etiology-based
As with all the neurodevelopmental disorders, the diagnosis of ASD is based on a collection of behavioral and developmental features, not on presumed or known etiology. However, specific clinical characteristics may provide useful clues for the identification of the underlying etiology. Therefore, the diagnostic evaluation of a child with known ASD, as will be outlined in later sections, is motivated by a search for causative or associated genetic variants and syndromes.
ASD is defined by a dyad of impairments in social communication skills and the presence of repetitive patterns of behavior or restricted interests in the early developmental period, with deficits leading to functional impairment in a variety of domains. The diagnosis must be made by an experienced clinician, using a combination of parent report, direct examination of the child and standardized developmental and behavioral testing when needed. The combination of these tools can then be assimilated into a “best clinical estimate” based on diagnostic criteria established in the Diagnostic Statistics Manual (DSM). In May 2013 the revised DSM-5 was published, and in it significant revisions were made to the diagnostic conceptualization of ASD (Figure 1). Two fundamental changes were made. First, the separate categories of social function and communication in DSM-IV were merged into one category of social-communication impairment. This change reflects the fact that deficits in communication, both verbal and non-verbal, are intimately linked to social deficits, particularly early in development. Secondly, the diagnostic categories [Autistic Disorder, Asperger disorder, and Pervasive Developmental Disorder, Not Otherwise Specified (PDD-NOS)] were removed and, instead, one umbrella diagnosis of autism spectrum disorder was created. This change from categories to a continuum better captures the true spectrum of symptom severity of this disorder and reflects the fact that often the separate diagnostic categories were not consistently applied across clinical or research centers.
Figure 1.

Changes from DSM-IV TR to DSM-5 for Autism Spectrum Disorder
The changes in DSM-5 raised concerns that previously diagnosed children would lose services because of changes in nomenclature and a resulting loss of diagnosis. Since then, several studies have compared DSM-IV and DSM-5 diagnoses with structured diagnostic assessments, such as the Autism Diagnostic Observation Schedule (ADOS) with mixed results. Some studies demonstrate very high consistency while others demonstrate more discrepancy, particularly in those previously given a PDD-NOS diagnosis2, 3. Of note, from a clinical perspective, a child diagnosed through DSM-IV need not be re-evaluated for diagnostic purposes simply because of the changes in DSM-5.
Like most neurodevelopmental disorders, ASD has a strong male predominance 4. There are two primary reasons for this uneven gender distribution. First, there exists a diagnostic bias, as boys tend to exhibit more externalizing and disruptive symptoms that facilitate referrals for diagnosis, while girls manifest symptoms such as anxiety and depression that may delay the diagnosis5–7. Secondly, specific genetic factors may protect females from developing ASD (“female protective effect”)8, 9. Support for this theory comes from studies demonstrating a greater ASD-related genetic load in females with ASD compared to males with ASD, and in clinically unaffected female relatives compared to unaffected male relatives of individuals with ASD. Further substantiation of the greater genetic load in females is found by the higher rate of ASD in siblings of females with ASD compared to males with ASD.
Clinical heterogeneity
Variability in clinical presentation is rooted in severity of impairment and co-morbidities. Intellectual disability, ranging from mild to severe, occurs in 70% of children10. Language impairment can range from deficits in pragmatic use of language to complete lack of spoken language, with 30% of children with ASD remaining minimally verbal despite intensive intervention11. Other sources of heterogeneity result from neurological comorbidities (epilepsy, sleep impairment, motor delays and deficits) and psychiatric disorders (depression, anxiety, irritability, Attention Deficit Hyperactivity Disorder). This heterogeneity in clinical presentation requires that treatments, both pharmacological and behavioral, move away from a “one size fits all” approach and, rather, become tailored to a child’s individual clinical profile. As discussed in the following sections, the identification of causative genetic variants can facilitate the characterization of more homogeneous clinical subgroups that, in turn, can guide more targeted therapies.
Heritability of ASD
ASD is one of the most heritable neuropsychiatric disorders, as recognized from the earliest twin studies,12 with concordance rates in monozygotic twins approaching 70%. Recurrence rates in siblings of children with ASD range from 5–20%, with higher rates if the proband is a female. In large prospective cohort studies of infants with older siblings with ASD, the rate of developing ASD has been reported in 18% of infants13. The recurrence rate increases to 33% if a family has two children with ASD. These heritability estimates can be useful when counselling patients about family planning based on family history of ASD14. Considerable research efforts have been dedicated to prospective studies of infant siblings of children with ASD, with the goal of identifying early risk markers and predictors of ASD in this high-risk cohort. Because of the genetic heterogeneity of the sample, no single developmental trajectory or clinical predictor of ASD has been discovered. In fact, these studies have been most successful in identifying overall differences between high and low risk infants, thus reflecting an endophenotype of elevated risk rather than specific predictors of ASD. By 12 months of age, high-risk infants demonstrate more atypical behaviors such as reduced social interest and affect, social smiling, orienting to name, imitation, as well as atypical eye contact. Earlier in infancy, pre-behavioral biomarkers of risk include differences in resting state EEG patterns and face processing15. These studies have been instrumental in reinforcing the fact that atypical patterns of both brain development and behavior can be quantified early in the developmental period, before formal clinical diagnoses can be made which, in turn, has justified continued research in early risk markers for ASD.
Advances in genetic testing
In part due to the well-established heritability of the disorder, genetic testing for children with ASD has been routinely performed for decades. Initially, the standard test in children was comprised of karyotyping alone, which could only identify abnormalities larger than about 3–5 million base pairs, visible under a light microscope. However, recent advances in genetic methods have led to the identification of contributory mutations in up to 30% of children with ASD16, 17. The first breakthrough technology was the chromosomal microarray analysis (CMA)18. Any structural chromosomal duplication or deletion that is larger than 1 kB and causes a deviation from the control copy number is considered a copy number variant (CNV). CNVs can be inherited or sporadic (de novo), with the latter type of mutation considered more likely to be pathogenic. The two types of CMA technologies that are most widely used include the array-based comparative genomic hybridization (aCGH) and the single nucleotide polymorphism (SNP) array, both of which permit high-resolution molecular analysis of chromosome copy number. The SNP array has the advantage of being able to detect specific inheritance patterns, such as uniparental disomy, which cannot be detected by aCGH19. Both aCGH and SNP arrays provided the first opportunity to perform relatively unbiased genome-wide surveys of chromosomal deletions and duplications with much greater resolution.
However, there are limits to the resolution of CMA testing, and point mutations and microdeletions cannot be identified using these methods. More recently, whole exome and whole genome sequencing technology has facilitated investigations at the level of the single base pair, allowing for analysis of single gene defects and for the identification of partial loss of gene function 16, 17, 20, 21. Most large scale exome sequencing studies have been based on data from simplex families, or families with only one affected child (such as the Simons Simplex Collection, a registry of simplex families funded by the Simons Foundation), leading to a growing appreciation of the role of de novo mutations in the pathogenesis of ASD. From these large cohorts of thousands of children, more than 500 candidate genes have been identified, each with 50% chance of being contributory or causative. Network analyses of the functions of the potentially causative genes finds genes implicated in synaptic formation and integrity and in chromatin modulation.22, 23
Guidelines for genetic testing in ASD
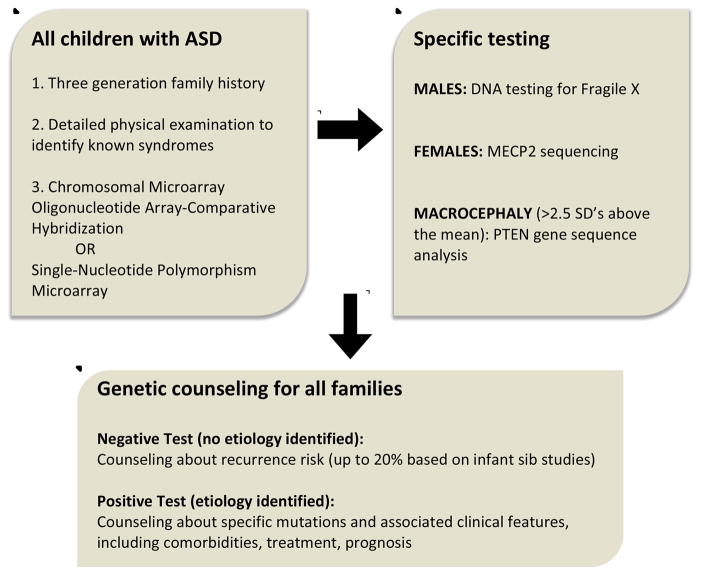
The guidelines for genetic testing for ASD have been revised to reflect the advances in methods, which, in turn, have led to larger populations of individuals with known genetic syndromes and variants associated with ASD. In 2000, the American Academy of Neurology and Child Neurology Society published guidelines on the screening and diagnosis of autism, stating that “high resolution chromosome studies (karyotype) and DNA analysis for fragile X should be performed in the presence of mental retardation…or if dysmorphic features are present24.” Revised guidelines for testing were published by the American College of Medical Genetics (ACMG) in 201325 (figure 2). After a comprehensive 3-generation family history, ACMG recommends a chromosomal microarray analysis for all children. Additionally, Fragile X testing should be performed in boys and MECP2 testing (for Rett syndrome) in girls. Children with macrocephaly (head circumference greater than 2 standard deviations above mean for age) should be tested for PTEN gene mutations. A positive test result should be followed by testing of parents for the determination of heritability of the variant. After testing is complete, genetic counseling should be provided regardless of results, as there are risks to future siblings regardless of genetic etiology, as described above.
Figure 2.
Recommendations for clinical genetic testing in children with ASD (adapted from Schaefer et al, 2013)
Of note, no other neuroimaging or medical testing is routinely recommended for children with ASD. However, certain clinical features may prompt further testing (figure 3). While debate does exist about the implications of the baseline EEG abnormalities found in up to 60% of children with ASD, routine EEG testing is not recommended for all children with an ASD diagnosis. Instead, overnight EEG investigation should be performed in children with a high clinical suspicion for epilepsy or with clear evidence of language regression that would suggest electrical status epilepticus of sleep (ESES). 26, 27 Several genetic syndromes, such as TSC, Rett syndrome, Fragile X and Dup15q syndrome are characterized by a high rate of early onset epilepsy and ASD. In non-syndromic ASD, the risk of epilepsy seems to increase with age. The largest cross sectional study of almost 6,000 children with ASD and epilepsy found that epilepsy in ASD was associated with lower cognitive, adaptive, and language ability as well as greater autism severity, with peak prevalence of epilepsy occurring at age 10.28
Figure 3.

Medical Workup for Autism Spectrum Disorder (ASD)
More than 25% of individuals with ASD have an identifiable genetic cause
With genetic testing now routinely recommended and performed, a growing number of individuals are diagnosed with genetic etiologies for their ASD. Two primary categories of genetic etiologies of ASD exist: single gene disorders and copy number variants. Single gene disorders are detected in 3–5% of children with ASD, and include syndromes such as Fragile X, Tuberous Sclerosis Complex (TSC), Rett syndrome, and Neurofibromatosis. At least 20% of individuals with ASD have identifiable, causative de novo copy number variations and single gene mutations that are identifiable using current genetic testing. No single variation, however, accounts for more than 1% of ASD cases, consistent with the phenotypic heterogeneity of the disorder. 29
Clinical relevance of genetic testing: Moving towards targeted phenotyping and treatment
Parents often voice skepticism about the utility of genetic testing of their child with ASD, highlighting the concern that the knowledge about a causative variant will not actually benefit or inform their child’s management and treatment. In the past, knowledge about an associated genetic syndrome or variant did hold more scientific promise than clinical significance. However, recent research efforts have bolstered the clinical impact of the diagnosis of a genetic syndrome or variant associated with ASD, and these advances in the clinical phenomenology of autism genetics are described in the next sections. First, widespread genetic testing has led to the diagnosis of larger cohorts of children with similar variants, which facilitates the identification of common clinical features that can inform more behavioral intervention targets. Secondly, advances in the identification of causative genes and pathogenic mechanisms associated with these genes have led to molecular treatment targets that, ultimately, may prevent the development of ASD in certain disorders.
Common clinical features: Symptom clusters
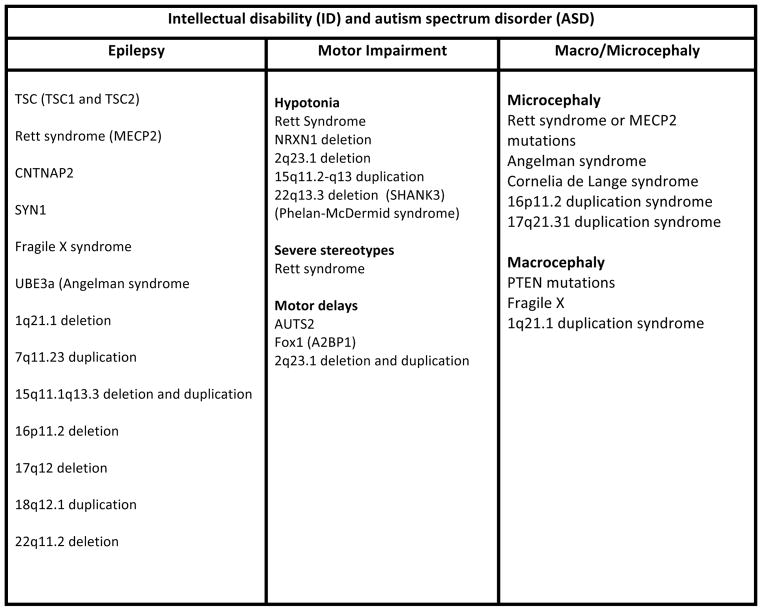
The level of precision in genetic testing still exceeds the precision in clinical phenotyping of the identified genetic syndromes(figure 4). However, definite symptom clusters, or clinical features, have been identified that are highly associated with genetic etiologies of ASD, leading to the commonly used term “syndromic autism30.” These clinical features include intellectual disability (ID), epilepsy, and motor impairment (particularly hypotonia or delay in achieving motor milestones). The presence of macrocephaly or microcephaly (defined by head circumference more than 2.5 standard deviations from the mean) can greatly narrow the differential diagnosis. Of each of these comorbidities, ID certainly is the most prevalent, and its presence can reinforce the need for genetic testing. A recent report from the Simons Simplex Collection found that the mean IQ of affected females with de novo mutations was 78, while the mean IQ of affected males with de novo mutations was 9022. Symptom clusters hold clinical utility in that they may strengthen the argument for genetic testing in children with comorbid ID or epilepsy, and they can guide the need for screening and management of comorbidities, particularly seizures.
Figure 4.
Common clinical features in genetic variants and syndromes associated with ASD
ID and ASD in genetic syndromes
The comorbidity of ID and ASD requires that future studies carefully examine early developmental trajectories and cognitive abilities in these genetic variants and syndromes, in order to confirm the diagnostic specificity of ASD. In DSM-5 it is clearly articulated that, “to make comorbid diagnoses of ASD and ID, social communication should be below that expected for general developmental level.” In other words, clinicians must consider a child’s mental age, not chronological age, when evaluating his/her social, language, and behavioral abilities, as the use of chronological age may lead to an over-diagnosis of ASD. For instance, in a recently published study of developmental trajectories in infants with Tuberous Sclerosis Complex (TSC), cognitive impairment by age 12 months (based on a standardized scale of development: the Mullen Scales of Early Learning) was strongly associated with social communication impairments at age 3, as quantified by the Autism Diagnostic Observation Schedule (ADOS). The confirmation of ASD in these children with elevated ADOS scores required additional evaluation by an experienced clinician in order to determine if the scores were secondary to overall delay or specific to ASD31. Disentangling ID from ASD holds implications for intervention. For instance, social communication impairment secondary to global developmental delay may improve with interventions targeting cognitive and, perhaps, motor skills, while social communication deficits rooted in limited social motivation or attention may respond better to targeted social skills, play based, therapies. As another example, language impairment in ASD can result from deficits in: low level auditory processing, processing of speech sounds, attention to speech cues necessary for language learning, social motivation, or motor impairment that can undermine the production of words. Identification of the specific pathway will facilitate the choice of intervention most effective for the language impairment in subgroups of children.
Overall, future efforts in clinical characterization of children with genetic syndromes may be better served by placing greater emphasis on core deficits, such as social communication skills or language, rather than on categorical clinical diagnoses, in order to then design and direct interventions towards the specific areas of impairment.
Treatment of ASD is not yet etiology-based
Behavioral intervention is the mainstay of treatment for core deficits in ASD, with structured, high-intensity and autism-directed interventions associated with better outcomes32. Under the umbrella term of “ABA” or Applied Behavioral Analysis fall several effective and distinct methods33. The traditional ABA program based on the work of Lovaas et al is intensive and individualized, with the use of discrete trials to teach simple skills that then can build to more complex skills34. Discrete trial therapy is particularly effective for modifying problem behaviors and for teaching specific cognitive and academic skills. More naturalistic and play-based treatments include Pivotal Response Treatment (PRT) and Floortime. The only FDA approved medications for ASD are the atypical antipsychotics Risperidone and Aripiprazole. Both are approved for the treatment of irritability, defined by physical aggression and tantrum behavior. Their primary, sometimes dose-limiting side effects, include weight gain and sedation. Recent guidelines published by Volkmar et al35 emphasize that pharmacologic treatment can, particularly by reducing comorbidities and aberrant behaviors, “increase the ability of persons with ASD to profit from… interventions and to remain in less restrictive environments.” In other words, by improving intrusive or maladaptive behaviors, pharmacotherapy can facilitate a child’s ability to engage in and learn from educational and behavioral interventions for their core ASD symptoms.
With the advances in our knowledge about genetic etiologies of ASD and the identification of molecular pathways that may be aberrant in these disorders, there is hope for pharmacological and behavioral targets that may prevent the development of, or attenuate the impact of, the disease. Two such examples of such treatment targets are provided below.
Targeted treatment example 1: Tuberous Sclerosis Complex
The genes responsible for TSC (TSC 1 and 2) encode for proteins that regulate the mTORC1 protein complex. mTOR is critical for protein synthesis, cell growth and axon formation. Inactivation of the TSC genes causes an upregulation of this mTORC1 pathway, resulting in an increase in protein synthesis, aberrant axon formation and tumor growth. In the last 5 years, based on the known mechanisms of TSC1/2 regulation of the mTOR pathway, mTOR inhibitors have been studied extensively in mouse models of TSC. These studies have revealed that mTOR inhibitors can reverse the cognitive and social impairments found in adult mouse models after surprisingly short courses of treatment36, 37. In turn, these promising findings have inspired the investigation of mTOR inhibitors, such as rapamycin, in patients with TSC. Everolimus, an mTOR inhibitor, is now FDA approved for reduction of Subependymal Giant Cell Astrocytomas (SEGAs) in children with TSC38. Now, with safety profiles established, several international studies are investigating the use of mTOR inhibitors for improving the cognitive delays and behavioral deficits found in children with TSC39.
Additionally, because TSC is often diagnosed inutero due to cardiac rhabdomyomas or SEGAs, these infants can be studied prospectively for the evaluation of early developmental trajectories and risk markers for ASD, providing an opportunity to identify common behavioral and developmental characteristics within TSC that could serve as targets for behavioral intervention. In the first large scale prospective study of development in TSC, infants demonstrated delays in visually mediated behaviors (visual attention, disengagement of attention) in the first year of life. Furthermore, declines in nonverbal cognition in the second year of life predicted symptoms of ASD at 24 and 36 months. This developmental slowing in nonverbal cognition is a trajectory that has not been previously reported in other high-risk groups and, in turn, may represent a TSC-specific developmental trajectory31. Based on this finding, the group is now investigating whether a behavioral intervention that targets nonverbal communication (such as visual attention to social information) in the second year of life can prevent the development of ASD in TSC. Ultimately, for infants with TSC, a combination of targeted molecular and behavioral treatments may attenuate or even prevent the neurodevelopmental disabilities that occur early in development.
Targeted treatment example 2: Dup15q syndrome
Duplication of 15q11.2-q13, or Dup15q syndrome, provides another timely example of the clinical utility of genetic testing for targeted management and, eventually, treatment. Duplications of the 15q11.2-q13 region of maternal origin were first associated with ASD over 15 years ago, and now these duplications are amongst the most common CNV’s associated with ASD and related neurodevelopmental disorders. Duplication of this region leads to the overexpression of several genes, most notably UBE3A (E3 ubiquitin ligase gene) and a cluster of receptor subunits for the neurotransmitter GABAA. There are two major structural versions of this copy number variant: isodicentric chromosome 15 [idic(15)] and interstitial duplication of chromosome 15 [int.dup(15)]. Over the last several years, a national alliance of families affected by this CNV, known as the Dup15q Alliance, has been collecting a registry of patients with the goal of advancing both clinical care and scientific investigation of the disorder. There are now more than 400 patients in the registry with varying duplication types. Through collaborative efforts, studies have identified neurobiological, developmental and behavioral features of Dup15q syndrome.
In addition to ASD, this CNV is characterized by early onset of epilepsy, profound hypotonia in early infancy, moderate to severe intellectual disability, and, in a subgroup of children, excessive beta range activity (15–30 Hz) on clinical EEG, with overall clinical severity greater in the idic(15) cases.40–42. The excessive beta oscillations likely represent an electrophysiological signature of the upregulation of GABAA receptor genes contained in the duplicated chromosomal region.
As a result of data gathered from the national Dup15q syndrome registry, a recent large cohort study of 95 children with Dup15q syndrome sought to identify common characteristics and potential treatments for epilepsy in this population43. Investigators found that epilepsy was much more prevalent in the idic(15) cases than in the int.dup15 cases, multiple seizure types (both generalized and focal) were identified, and that infantile spasms were common, reported in 42% of cases. Both broad spectrum and focal antiepileptic medications (such as carbamazepine) demonstrated efficacy for seizure reduction, suggesting a multifocal etiology to the epilepsy. Importantly, GABAergic medications, such as benzodiazepenes, were relatively ineffective, likely due to abnormalities in GABA transmission in the setting of duplications in GABAB3 receptor genes in the 15q region. This key discovery led to the recommendation that benzodiazepine medications, which are commonly used in the epilepsy population as a whole, be avoided in this genetic subgroup.
In parallel to the efforts in epilepsy, investigators have begun to better characterize the social communication phenotype in Dup15q syndrome. Given the significant hypotonia present in these children, there is particular interest in the effects of motor delays on social communication development, particularly eye contact, nonverbal communication, expressive language, and play. Elucidation of the nature of the core deficits of ASD in Dup15q syndrome will facilitate the design and implementation of targeted behavioral interventions that will specifically benefit this subgroup within the autism spectrum.
Conclusion
Genetic testing for children with ASD is no longer confined to the realm of academia. As cohorts of children with genetic variants and syndromes associated with ASD are identified, common themes across disorders and unique features within disorders can be identified that will ultimately guide targeted interventions rooted in both biological mechanisms and behavior.
Key points.
Like all neurodevelopmental disorders, ASD is a heterogeneous group of disorders characterized by a constellation of symptoms and behaviors that occur in early development.
Genetic testing is the only standard medical workup recommended for all children diagnosed with ASD; more than 25% of children with ASD have an identified genetic cause.
Clinical features, particularly presence of intellectual disability, epilepsy, motor impairment, or certain dysmorphic features support a likely underlying genetic etiology.
The comorbidity of ID and ASD requires that future studies carefully examine early developmental trajectories and cognitive abilities in these genetic variants and syndromes, in order to confirm the diagnostic specificity of ASD.
Common phenotypes and natural history studies within genetic syndromes can help to inform prognosis and treatment targets.
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-5. 5. Washington, D.C: American Psychiatric Association; 2013. [Google Scholar]
- 2.Mazefsky CA, McPartland JC, Gastgeb HZ, Minshew NJ. Brief report: comparability of DSM-IV and DSM-5 ASD research samples. J Autism Dev Disord. 2013;43:1236–1242. doi: 10.1007/s10803-012-1665-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McPartland JC, Reichow B, Volkmar FR. Sensitivity and specificity of proposed DSM-5 diagnostic criteria for autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:368–383. doi: 10.1016/j.jaac.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fombonne E. Epidemiology of pervasive developmental disorders. Pediatr Res. 2009;65:591–598. doi: 10.1203/PDR.0b013e31819e7203. [DOI] [PubMed] [Google Scholar]
- 5.Bolte S, Duketis E, Poustka F, Holtmann M. Sex differences in cognitive domains and their clinical correlates in higher-functioning autism spectrum disorders. Autism. 2011;15:497–511. doi: 10.1177/1362361310391116. [DOI] [PubMed] [Google Scholar]
- 6.Hattier MA, Matson JL, Tureck K, Horovitz M. The effects of gender and age on repetitive and/or restricted behaviors and interests in adults with autism spectrum disorders and intellectual disability. Res Dev Disabil. 2011;32:2346–2351. doi: 10.1016/j.ridd.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 7.Szatmari P, Liu XQ, Goldberg J, et al. Sex differences in repetitive stereotyped behaviors in autism: implications for genetic liability. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:5–12. doi: 10.1002/ajmg.b.31238. [DOI] [PubMed] [Google Scholar]
- 8.Dworzynski K, Ronald A, Bolton P, Happe F. How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? J Am Acad Child Adolesc Psychiatry. 2012;51:788–797. doi: 10.1016/j.jaac.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Solomon M, Miller M, Taylor SL, Hinshaw SP, Carter CS. Autism symptoms and internalizing psychopathology in girls and boys with autism spectrum disorders. J Autism Dev Disord. 2012;42:48–59. doi: 10.1007/s10803-011-1215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baird G, Simonoff E, Pickles A, et al. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the Special Needs and Autism Project (SNAP) Lancet. 2006;368:210–215. doi: 10.1016/S0140-6736(06)69041-7. [DOI] [PubMed] [Google Scholar]
- 11.Kasari C, Brady N, Lord C, Tager-Flusberg H. Assessing the minimally verbal school-aged child with autism spectrum disorder. Autism Res. 2013;6:479–493. doi: 10.1002/aur.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smalley SL, Asarnow RF, Spence MA. Autism and genetics. A decade of research. Arch Gen Psychiatry. 1988;45:953–961. doi: 10.1001/archpsyc.1988.01800340081013. [DOI] [PubMed] [Google Scholar]
- 13.Ozonoff S, Young GS, Carter A, et al. Recurrence risk for autism spectrum disorders: a Baby Siblings Research Consortium study. Pediatrics. 2011;128:e488–495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. JAMA. 2014;311:1770–1777. doi: 10.1001/jama.2014.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zwaigenbaum L. Advances in the early detection of autism. Curr Opin Neurol. 2010;23:97–102. doi: 10.1097/WCO.0b013e3283372430. [DOI] [PubMed] [Google Scholar]
- 16.Neale BM, Kou Y, Liu L, et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485:242–245. doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Roak BJ, Vives L, Girirajan S, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485:246–250. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malhotra D, Sebat J. CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell. 2012;148:1223–1241. doi: 10.1016/j.cell.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heil KM, Schaaf CP. The genetics of Autism Spectrum Disorders--a guide for clinicians. Current psychiatry reports. 2013;15:334. doi: 10.1007/s11920-012-0334-3. [DOI] [PubMed] [Google Scholar]
- 20.Sanders SJ, Murtha MT, Gupta AR, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485:237–241. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu TW, Chahrour MH, Coulter ME, et al. Using whole-exome sequencing to identify inherited causes of autism. Neuron. 2013;77:259–273. doi: 10.1016/j.neuron.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ronemus M, Iossifov I, Levy D, Wigler M. The role of de novo mutations in the genetics of autism spectrum disorders. Nature reviews Genetics. 2014;15:133–141. doi: 10.1038/nrg3585. [DOI] [PubMed] [Google Scholar]
- 23.Pinto D, Delaby E, Merico D, et al. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am J Hum Genet. 2014;94:677–694. doi: 10.1016/j.ajhg.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filipek PA, Accardo PJ, Ashwal S, et al. Practice parameter: screening and diagnosis of autism: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Child Neurology Society. Neurology. 2000;55:468–479. doi: 10.1212/wnl.55.4.468. [DOI] [PubMed] [Google Scholar]
- 25.Schaefer GB, Mendelsohn NJ. Clinical genetics evaluation in identifying the etiology of autism spectrum disorders: 2013 guideline revisions. Genet Med. 2013;15:399–407. doi: 10.1038/gim.2013.32. [DOI] [PubMed] [Google Scholar]
- 26.Ekinci O, Arman AR, Isik U, Bez Y, Berkem M. EEG abnormalities and epilepsy in autistic spectrum disorders: clinical and familial correlates. Epilepsy Behav. 2010;17:178–182. doi: 10.1016/j.yebeh.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Spence SJ, Schneider MT. The role of epilepsy and epileptiform EEGs in autism spectrum disorders. Pediatr Res. 2009;65:599–606. doi: 10.1203/01.pdr.0000352115.41382.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viscidi EW, Triche EW, Pescosolido MF, et al. Clinical characteristics of children with autism spectrum disorder and co-occurring epilepsy. PLoS ONE. 2013;8:e67797. doi: 10.1371/journal.pone.0067797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iossifov I, Ronemus M, Levy D, et al. De novo gene disruptions in children on the autistic spectrum. Neuron. 2012;74:285–299. doi: 10.1016/j.neuron.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosti RO, Sadek AA, Vaux KK, Gleeson JG. The genetic landscape of autism spectrum disorders. Dev Med Child Neurol. 2014;56:12–18. doi: 10.1111/dmcn.12278. [DOI] [PubMed] [Google Scholar]
- 31.Jeste SS, Wu JY, Senturk D, et al. Early developmental trajectories associated with ASD in infants with tuberous sclerosis complex. Neurology. 2014;83:160–168. doi: 10.1212/WNL.0000000000000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Research Council (U.S.). Committee on Educational Interventions for Children with Autism. Educating children with autism. Washington, DC: National Academy Press; 2001. [Google Scholar]
- 33.Vismara LA, Rogers SJ. Behavioral treatments in autism spectrum disorder: what do we know? Annual review of clinical psychology. 2010;6:447–468. doi: 10.1146/annurev.clinpsy.121208.131151. [DOI] [PubMed] [Google Scholar]
- 34.Lovaas OI, Schreibman L, Koegel RL. A behavior modification approach to the treatment of autistic children. J Autism Child Schizophr. 1974;4:111–129. doi: 10.1007/BF02105365. [DOI] [PubMed] [Google Scholar]
- 35.Volkmar F, Siegel M, Woodbury-Smith M, King B, McCracken J, State M. Practice Parameter for the Assessment and Treatment of Children and Adolescents With Autism Spectrum Disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53:237–257. doi: 10.1016/j.jaac.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 36.Tsai PT, Greene-Colozzi E, Goto J, Anderl S, Kwiatkowski DJ, Sahin M. Prenatal rapamycin results in early and late behavioral abnormalities in wildtype C57BL/6 mice. Behav Genet. 2013;43:51–59. doi: 10.1007/s10519-012-9571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ehninger D, Han S, Shilyansky C, et al. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jozwiak J, Sontowska I, Ploski R. Frequency of TSC1 and TSC2 mutations in American, British, Polish and Taiwanese populations. Molecular medicine reports. 2013;8:909–913. doi: 10.3892/mmr.2013.1583. [DOI] [PubMed] [Google Scholar]
- 39.Sahin M. Targeted treatment trials for tuberous sclerosis and autism: no longer a dream. Curr Opin Neurobiol. 2012;22:895–901. doi: 10.1016/j.conb.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Urraca N, Cleary J, Brewer V, et al. The Interstitial Duplication 15q11.2-q13 Syndrome Includes Autism, Mild Facial Anomalies and a Characteristic EEG Signature. Autism Res. 2013 doi: 10.1002/aur.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Battaglia A. The inv dup (15) or idic (15) syndrome (Tetrasomy 15q) Orphanet journal of rare diseases. 2008;3:30. doi: 10.1186/1750-1172-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bolton PF, Park RJ, Higgins JN, Griffiths PD, Pickles A. Neuro-epileptic determinants of autism spectrum disorders in tuberous sclerosis complex. Brain. 2002;125:1247–1255. doi: 10.1093/brain/awf124. [DOI] [PubMed] [Google Scholar]
- 43.Conant KD, Finucane B, Cleary N, et al. A survey of seizures and current treatments in 15q duplication syndrome. Epilepsia. 2014;55:396–402. doi: 10.1111/epi.12530. [DOI] [PubMed] [Google Scholar]