Abstract
OBJECTIVES:
In Brazil, imatinib mesylate is supplied as the first-line therapy for chronic myeloid leukemia in the chronic phase through the public universal healthcare program, Sistema Único de Saúde (SUS). We studied the socio-demographic factors that influenced therapy success in a population in the northeast region of Brazil.
METHODS:
Patients with chronic myeloid leukemia from the state of Piauí were treated in only one reference center. Diagnosis was based on WHO 2008 criteria. Risk was assessed by Sokal, Hasford and EUTOS scores. Patients received 400 mg imatinib daily. We studied the influence of the following factors on the achievement of complete cytogenetic response within one year of treatment: age, clinical risk category, time interval between diagnosis and the start of imatinib treatment, geographic distance from the patient's home to the hospital, years of formal education and monthly income.
RESULTS:
Among 103 patients studied, the median age was 42 years; 65% of the patients had 2-9 years of formal education, and the median monthly income was approximately 100 US$. Imatinib was started in the first year after diagnosis (early chronic phase) in 69 patients. After 12 months of treatment, 68 patients had a complete cytogenetic response. The Hasford score, delay to start imatinib and years of formal education influenced the attainment of a complete cytogenetic response, whereas income and the distance from the home to the healthcare facility did not.
CONCLUSION:
Patients require additional healthcare information to better understand the importance of long-term oral anticancer treatment and to improve their compliance with the treatment.
Keywords: Chronic myeloid leukemia, TKIs, Education level, Socioeconomic status, Treatment
INTRODUCTION
The approval of imatinib (IM) as a targeted therapy for chronic myeloid leukemia (CML) by the Federal Drug Agency (FDA) in 2001 has opened a new era in the treatment of this disease and has considerably improved patients' survival ,1-5. In Brazil, the Ministry of Health initially approved IM for CML in the chronic phase as a second-line treatment and in 2008 as front-line therapy ,6,7. The drug is provided for free to all Brazilian residents through the public health program, Sistema Único de Saúde (SUS). Additionally, recently published national guidelines regulate the cytogenetic and molecular monitoring of patients 7.
In the first Brazilian publication on the results of using IM, presented by a center from São Paulo participating in international cooperative studies 8, 68.8% of the patients starting treatment in the early chronic phase achieved a complete cytogenetic response (CCyR) within 12 months. However, it might be expected that the large social and educational inequalities of the Brazilian population could influence the treatment outcome. Thus, we evaluated socioeconomic factors influencing the treatment success of patients with CML treated in the state of Piauí, located in the northeast region of Brazil.
PATIENTS AND METHODS
All consecutive patients diagnosed with CML in the chronic phase treated at the São Marcos Hospital in Teresina, Piauí, between February 2004 and February 2013 were included in the study. This hospital is the only reference center for CML treatment in the state of Piauí and also treats some patients from the neighboring state of Maranhão. Diagnosis was based on clinical data, peripheral blood counts and cytogenetics or on the detection of the BCR-ABL1 rearrangement by multiplex PCR. Cytogenetics and molecular tests were performed at the central laboratory of the National Institute of Cancer (INCA) in Rio de Janeiro. Patients’ risk categories were assessed by the Sokal, Hasford and EUTOS scores ,9-11.
Patients diagnosed before 2008 and who were treated first with hydroxyurea or interferon but were intolerant to these medications and were thus switched to imatinib were also included in the study. The diagnosis of chronic or accelerated phase disease and blast crisis was performed according to the WHO 2008 criteria 12. Patients were considered to be in the early chronic phase when imatinib therapy was started before 12 months following diagnosis.
For each patient, we collected the geographic distance from the patient's home to the hospital (in kilometers), the years spent in school, the average monthly income of each family member and the time interval between diagnosis and the start of imatinib treatment. The per capita income was also grouped into the income classes established by the Brazilian government (Secretariat of Strategic Affairs).
All patients received 400 mg imatinib daily. Previous treatments included hydroxyurea or interferon. All patents provided informed consent for their participation in the study, which was approved by the local Ethics Committee (proc. no. 045 - 2010 - Federal University of Piauí).
Laboratory monitoring comprised monthly peripheral blood counts performed by local laboratories. Karyotyping was scheduled to be performed at 3, 6 and 12 months; however, in some cases, karyotyping was only possible with some delay. After achieving a complete cytogenetic response, molecular monitoring (quantitative PCR) was performed every 3 months. The response criteria were evaluated according to the 2009 European LeukemiaNet (ELN) guidelines 13.
All time-to-event analyses were made with the log-rank test or Cox regressions. All time-to-event calculations were based on the interval between the beginning of imatinib treatment and the complete cytogenetic response. Cases were censored when there was no response after the third karyotyping (approximately 12 months) or when the patients changed therapy or dropped out of the study before the end of the study. Non-normally distributed variables were normalized by logarithmic transformation. All variables presenting p<0.15 were included in the multivariate analysis. A bootstrap resampling procedure was performed to test the stability of the model, as previously described ,14-17. Winstat 3.1 and SPSS 15.0 software were used.
RESULTS
A total of 103 patients with CML in the chronic phase entered this study. Their descriptive data are summarized in Table 1. Among the patients, 101 were Ph+ (had a Philadelphia chromosome) in the cytogenetic examination; the remaining two had normal karyotypes and were BCR-ABL1 positive.
Table 1. Features of the patients at diagnosis.
| Variable | Value |
|---|---|
| Gender (for all 103 cases) | |
| Male | 56 |
| Female | 47 |
| Age in years (median, range) | 42 (8-81) |
| Sokal score (100 cases) | |
| Low risk | 30 |
| Intermediate risk | 42 |
| High risk | 28 |
| Hasford score (100 cases) | |
| Low risk | 67 |
| Intermediate risk | 27 |
| High risk | 6 |
| EUTOS score (99 cases) | |
| Low risk | 92 |
| Intermediate risk | 7 |
| Treatment before imatinib (no. Patients) | |
| Hydroxyurea | 103 |
| Interferon-α | 34 |
| Median interval diagnosis - start imatinib (days) | 99 (27-1780) |
| For patients in early chronic phase (days) | 60 |
| For patients in late chronic phase (days) | 963 |
| Years in school | 4 (0-17) |
| Median income per family member and month (in R$)* | 226.00 (9.00-3,000.00) |
| Distance from the home to the hospital (in km) | 150 (1-794) |
These values correspond to a mean of 1 Real=0.5 US$ during the study period
All patients had received hydroxyurea for cytoreduction until the result of karyotyping was received, and 35 had also received interferon. Regarding the delay before imatinib treatment, 69 patients were in the early chronic phase, and the remaining 34 started imatinib more than one year following diagnosis. The median time of imatinib treatment in the patients was 45 months (range 7-141). At the end of the observation period, 70 patients (68%) remained on this tyrosine kinase inhibitor (TKI). Imatinib was discontinued due to a loss of response in 28 cases and disease progression in 5 cases. One patient received an allogeneic bone marrow transplantation, and two were lost to follow-up. Among the 68 patients who had achieved a CCR within approximately one year, only 8 later became resistant or progressed.
According to the income classes, 12 patients earned between 0 and 81 reais, 17 between 82-162 reais, 38 between 163-441 reais, 18 between 292-441 reais, 9 between 442-641 and 9 had an income >642 reais.
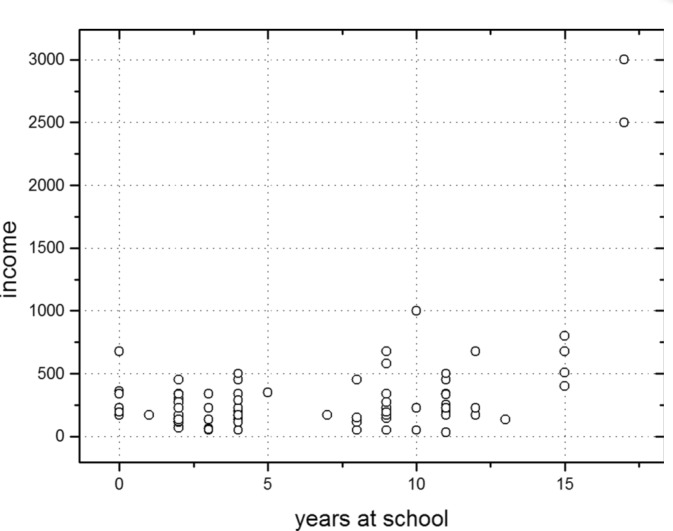
The median time of formal education of the patients was 4 years (Table 1). Eleven patients had never gone to school or had attended school for only one year, 67 patients had spent 2-9 years in school, 17 had spent between 10-12 years in school, and only 8 had some university education (>13 years of education). There was a positive correlation (r=0.20; p=0.012) between the patients' income and the years they had spent in school (Figure 1). However, the distribution of the patients according to the risk groups of any of the scores assessed was not affected by their income classes or years of formal education. Most patients lived far from the healthcare facilities (Table 1).
Figure 1. Patient distribution according to their income and years of formal education. Spearman’s rank order correlation was significant between the two variables: r = 0.20; p = 0.012.
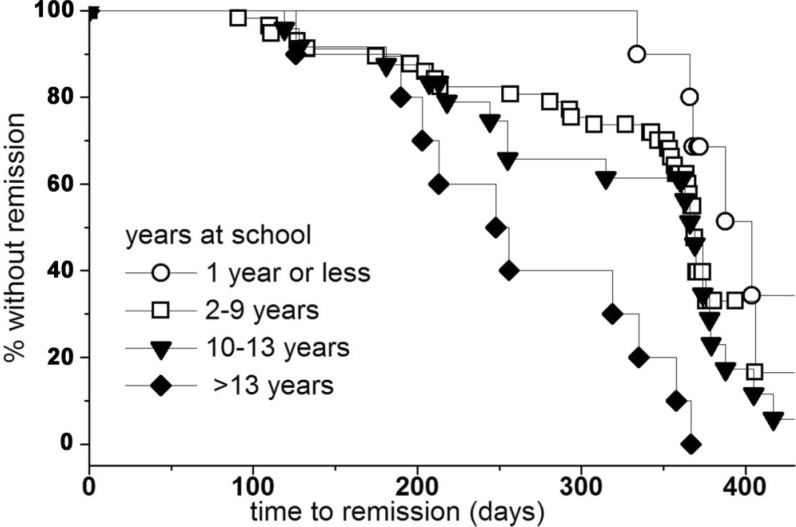
In the Kaplan-Meier plot, the median time to remission for patients with 1 year or less spent in school was 404 days. The median time to remission was 369 days for both the groups with 2 to 9 years of education (basic level) and with 10 to 12 years of education (secondary level) and was 252 days for patients with 13 or more years of education (university level) (log-rank test p=0.006) (Figure 2).
Figure 2. Kaplan-Meier plot analyzing the median time to complete cytogenetic remission according to the patients' educational levels: 0-1 year spent in school (median 404 days), 2-9 years (basic level), 10-12 years (secondary level) (median 369 days) and ≥13 years (university level) (median 252 days). Log-rank test p = 0.006.
In the Cox regression analysis, the Sokal score was of no prognostic relevance, in contrast with the Hasford (p=0.03) and EUTOS (p=0.08) scores. The delay between diagnosis and start of the imatinib therapy was an unfavorable predictive factor for complete cytogenetic remission (B=-0.001; p=0.02), and age was a weak risk factor (B=-0.013; p=0.117). Concerning the demographic and socioeconomic variables, only the educational level had a significant influence on the time to acquire a complete cytogenetic response (B=0.065; p=0.008). Gender, income and the distance from the home to the health facility did not affect the cytogenetic response.
In the multivariate Cox regression analysis, considering age, Hasford and EUTOS scores, years spent in school and the delay to begin IM treatment, only the Hasford score (B=-0.531; p=0.038) and years spent in school remained as independent predictive factors (B=0.374; p=0.019). In the bootstrap stability test, “Hasford score” was present in 71% of the new sets, “years in school” was present in 62%, “delay to begin imatinib” appeared in 32%, and “EUTOS score” appeared in 18%.
DISCUSSION
The introduction of targeted therapy with TKIs has substantially improved the life expectancy and quality of life of patients with CML. However, it has repeatedly been observed that treatment results in daily praxis do not reproduce those of clinical trials ,2,3. The reasons for this observation have been a matter of debate, but more recent data have shown that the results in population-based patient cohorts may be equally good, provided that drug access is unrestricted 3. Since 2008, the Brazilian public healthcare system has provided IM as the first-line treatment for CML for every resident in the country. The main question of our study was to investigate whether the significant social inequalities in the population could be of major clinical importance for the success of long-term TKI treatment for CML. Notably, in our study, the socioeconomic or educational level was not related to the stage of disease at diagnosis.
Among our patients, the only demographic factor influencing the optimal cytogenetic response according to the ELN criteria was the education level, whereas personal income and the distance between home and the health care facility did not. In a population with unrestricted access to medication, patients with a better education level may better understand the disease mechanism and the importance of long-term treatment, as has been previously noted ,4,5,19. Social and educational inequalities have also been shown to influence breast cancer mortality 20 as well as the normal development of low birth weight children 21.
The fact that a family's earnings do not affect therapy is surprising at first glance. The literature is contradictory in this regard ,3,5,22-24. On the one hand, familial resources have been reported to have a significant influence on the outcome of acute lymphoid leukemia (ALL) in both developing and wealthier countries ,22,24. Patient malnourishment and lack of family support have also been implicated in a patient’s incapacity to maintain long-term treatment. On the other hand, family earnings did not affect the outcomes of children with ALL in Norway 23 and the United Kingdom 24, where the public health system provides state of the art treatment for all patients. Nevertheless, several publications have called attention to the fact that better educated patients and those with good family support have much better outcomes after cancer treatment, especially with oral anti-cancer agents, in European countries ,5,23. Highly educated patients read more, better understand the nature of their disease, and are more perseverant and more motivated to adhere to long-lasting treatments, regularly using the prescribed drugs and even arriving at the hospital on the appropriate day ,4,5,19,23. This was fully confirmed in the present study, in which the years spent in school was a strong predictive factor for an optimal cytogenetic response. According to the Kaplan-Meier curve, it was evident that many patients with only one year of formal education delayed the third cytogenetic control. However, even after eliminating these patients from the survival curve, the log rank test still indicated significant differences. As the Kaplan-Meier curve suggests, patients with at least some university education show the best therapy success. A similar finding was reported from Norway, where the outcomes in children with cancer were better when the mothers were highly educated 23. Additionally, in a former work, performed in a more affluent region of our country 19, the time of TKI treatment, participation in clinical trial, higher quality of life scores and socioeconomic statuses, measured by the patients’ income and education levels, respectively, influenced IM treatment compliance.
Although we have found a significant correlation between income and education level, only the years of formal education influenced achievement of CCyR within one year of IM treatment. This finding is in agreement with the hypothesis that in a setting where all patients have equal access to oral anticancer therapy, the patients’ capacity to understand the importance of their treatment is essential for good compliance. It is even more important for the long-term maintenance of therapy. Thus, to optimize the invested public resources in drugs and laboratory support, broad additional healthcare information for patients is necessary.
AUTHOR CONTRIBUTIONS
Rego MN was the postgraduate student who performed her work (her doctor thesis) in the context of the Interinstitutional Postgraduating program from CAPES. She was in charge of patient care, developed the Care Unit, performed the data collection and analysis and participated in the manuscript writing. Metze K participated in the study design, performed the statistics and critically reviewed the manuscript. Lorand-Metze I was the supervising doctor who participated in the study design and reviewed the data collection, data analysis and manuscript.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Hughes TP, Hochhaus A, Branford S, Müller MC, Kaeda JS, Foroni L, Druker BJ, et al. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS) Blood. 2010;116((19)):3758–65. doi: 10.1182/blood-2010-03-273979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pulte D, Barnes B, Jansen L, Eisemann N, Emrich K, Gondos A, et al. GEKID Cancer Survival Working Group. Population level survival of patients with chronic myelocytic leukemia in Germany compared to the US in the early 21st century. J Hematol Oncol. 2013;6((1)):70. doi: 10.1186/1756-8722-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith AG, Painter D, Howell DA, Evans P, Smith G, Patmore R, et al. Determinants of survival in patients with chronic myeloid leukaemia treated in the new era of oral therapy: findings from a UK population-based patient cohort. BMJ Open. 2014;4:e004266. doi: 10.1136/bmjopen-2013-004266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavalli-Björkman N. Implications of patients’ socioeconomic status - what oncologists should be aware of. Acta Oncol. 2014;53((2)):161–3. doi: 10.3109/0284186X.2013.865079. [DOI] [PubMed] [Google Scholar]
- 5.Timmers L, Boons CCLM, Kropff F, Van De Ven PM, Swart EL, Smit EF, et al. Adherence and patients’ experiences with the use of oral anticancer agents. Acta Oncol. 2014;53((2)):259–67. doi: 10.3109/0284186X.2013.844353. [DOI] [PubMed] [Google Scholar]
- 6.Brasil . Brasília: 2008. Ministério da Saúde. Portaria SAS n° 347 de 23 de junho de 2008, Protocolo e Diretrizes Terapêuticas para o tratamento da Leucemia Mieloide Crônica. Diário Oficial da União, n° 120, de 25 de julho de 2008, seção 1, página 54. [Google Scholar]
- 7.Brasil . Brasília: 2013. Ministério da Saúde. Portaria SAS n° 1219 de 14 de novembro de 2013, Protocolo e Diretrizes Terapêuticas para o tratamento da Leucemia Mieloide Crônica. Diário Oficial da União, de 15 de março de 2013, seção 1, página 45-52. [Google Scholar]
- 8.Bendit I, Sanabani SS, Conchon M, Serpa M, Novaes MMY, Nardinelli L, et al. Evaluation of long-term outcomes, cytogenetic and molecular responses with imatinib mesylate in early and late chronic-phase chronic myeloid leukemia: a report from a single institute. Acta Haematol. 2012;128((4)):223–32. doi: 10.1159/000339696. [DOI] [PubMed] [Google Scholar]
- 9.Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, et al. Prognostic discrimination in good-risk chronic granulocytic leukemia. Blood. 1984;63((4)):789–99. [PubMed] [Google Scholar]
- 10.Hasford J, Pfirrmann M, Hehlmann R, Allan NC, Baccarani M, Kluin-Nelemans, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group. J Natl Cancer Inst. 1998;90((11)):850–8. doi: 10.1093/jnci/90.11.850. [DOI] [PubMed] [Google Scholar]
- 11.Hasford J, Baccarani M, Hoffmann V, Guilhot J, Saussele S, Rosti G, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: EUTOS score. Blood. 2011;118((3)):686–92. doi: 10.1182/blood-2010-12-319038. [DOI] [PubMed] [Google Scholar]
- 12.Vardiman JW, Thiele J, Arber DA, Brunning R, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114((5)):937–51. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 13.Baccarani M, Dreyling M, Cottone F, Castagnetti F, Breccia M, et al. ESMO Guidelines Working Group. Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21:165–67. doi: 10.1093/annonc/mdq201. [DOI] [PubMed] [Google Scholar]
- 14.Bedin V, Adam RL, Sá BCS, Landman G, Metze K. Fractal dimension is an independent prognostic factor for survival in melanoma. BMC Cancer. 2010;10:260. doi: 10.1186/1471-2407-10-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lourenço GJ, Delamain MT, Kameo R, Lorand-Metze I, Lima CSP, et al. Polymorphisms of glutathione S-transferase mu 1, theta 1 and pi 1 genes and prognosis in Hodgkin lymphoma. Leuk & Lymph. 2010;50((6)):2215–21. doi: 10.3109/10428194.2010.527402. [DOI] [PubMed] [Google Scholar]
- 16.Ferro DP, Falconi MA, Adam RL, Ortega M, Lima CSP, De Souza CA, et al. Fractal Characteristics of May-Grünwald-Giemsa stained Chromatin are Independent Prognostic Factors for Survival in Multiple Myeloma. PLoS One. 2011;6((6)):e20706. doi: 10.1371/journal.pone.0020706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reis-Alves SC, Traina F, Harada G, Campos PM, Saad ST, Metze K, et al. Immunophenotyping in myelodysplastic syndromes can add prognostic information to well-established and new clinical scores. PLoS One. 2014;8((12)):e81048. doi: 10.1371/journal.pone.0081048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silveira CAP, Ferrari I. First-line treatment with imatinib mesylate in patients with chronic phase chronic myeloid leukemia: experience of a public hospital in a developing country of South America. Leuk & Lymphoma. 2012;53((7)):1417–9. doi: 10.3109/10428194.2011.653641. [DOI] [PubMed] [Google Scholar]
- 19.de Almeida MH, Pagnano KB, Vigorito A C, Lorand-Metze I, Souza CA. Adherence to tyrosine kinase inhibitor therapy for chronic myeloid leukemia: A Brazilian single-center cohort. Acta Haematol. 2013;130((1)):16–22. doi: 10.1159/000345722. [DOI] [PubMed] [Google Scholar]
- 20.Freitas-Junior R, Gonzaga CMR, Freitas NMA, Martins E, Dardes RCM. Disparities in female breast cancer mortality rates in Brazil between 1980 and 2009. Clinics. 2012;67((7)):731–7. doi: 10.6061/clinics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan RG, Portuguez MW, Nunes ML. Cognition, behavior and social competence of preterm low birth weight children at school age. Clinics. 2013;68((7)):915–21. doi: 10.6061/clinics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacDougall LG, Wilson TD, Cohn R, Shuenyane EN, McElligott SE. Compliance with chemotherapy in childhood leukaemia in Africa. S Afr Med J. 1989;75((10)):481–4. [PubMed] [Google Scholar]
- 23.Syse A, Lyngstad TH, Kravdal O. Mortality after childhood cancer dependent on social or economic resources of parents? A population-based study. Int J Cancer. 2012;130((8)):1870–8. doi: 10.1002/ijc.v130.8. [DOI] [PubMed] [Google Scholar]
- 24.Lightfoot TJ, Johnston WT, Simpson J, Smith AG, Ansell P, Crouch S, et al. Survival from childhood acute lymphoblastic leukaemia: the impact of social inequality in the United Kingdom. Eur J Cancer. 2012;48((2)):263–9. doi: 10.1016/j.ejca.2011.10.007. [DOI] [PubMed] [Google Scholar]