Abstract
OBJECTIVE:
This study was performed to determine the effect of N-acetyl-L-cysteine, a modified sulfur-containing amino acid that acts as a strong cellular antioxidant, on the response to environmental stressors and on aging in C. elegans.
METHOD:
The survival of worms under oxidative stress conditions induced by paraquat was evaluated with and without in vivo N-acetyl-L-cysteine treatment. The effect of N-acetyl-L-cysteine on the response to other environmental stressors, including heat stress and ultraviolet irradiation (UV), was also monitored. To investigate the effect on aging, we examined changes in lifespan, fertility, and expression of age-related biomarkers in C. elegans after N-acetyl-L-cysteine treatment.
RESULTS:
Dietary N-acetyl-L-cysteine supplementation significantly increased resistance to oxidative stress, heat stress, and UV irradiation in C. elegans. In addition, N-acetyl-L-cysteine supplementation significantly extended both the mean and maximum lifespan of C. elegans. The mean lifespan was extended by up to 30.5% with 5 mM N-acetyl-L-cysteine treatment, and the maximum lifespan was increased by 8 days. N-acetyl-L-cysteine supplementation also increased the total number of progeny produced and extended the gravid period of C. elegans. The green fluorescent protein reporter assay revealed that expression of the stress-responsive genes, sod-3 and hsp-16.2, increased significantly following N-acetyl-L-cysteine treatment.
CONCLUSION:
N-acetyl-L-cysteine supplementation confers a longevity phenotype in C. elegans, possibly through increased resistance to environmental stressors.
Keywords: N-acetyl-L-cysteine, C. elegans, Stress response, Lifespan, Fertility
INTRODUCTION
The free radical theory of aging suggests that normal aging is due to the accumulation of intracellular damage with time mainly caused by cellular free radicals 1. Free radicals are byproducts of cellular metabolism, and the most abundant free radicals in cells are reactive oxygen species (ROS). Cells use both anti-oxidant enzymes, such as catalase and superoxide dismutase, and anti-oxidant molecules, including glutathione, vitamin E, and vitamin C, to eliminate harmful ROS. However, ROS that escape these cellular defense systems react with other molecules and cause oxidative damage to critical cellular macromolecules, such as DNA, protein, and lipids 2. A positive correlation has been observed between chronological age and the level of cellular oxidative damage in many experimental organisms 3. In addition, 8-hydroxy-2-deoxy-guanosine (a product of DNA oxidative damage) and lipid peroxidation (oxidative damage to lipids) are widely used as molecular markers of aging in model organisms. However, the role of anti-oxidant enzymes in the normal aging process and in the determination of lifespan remains controversial 4.
Nutritional antioxidants act through different mechanisms, including neutralization of free radicals, reduction of peroxide concentrations, repair of oxidized membranes, and decreased ROS production 5. The effect of dietary anti-oxidant molecules on aging has been studied in various species. Resveratrol, a polyphenol found in red wine, reduces the incidence of many age-related diseases, including neurodegeneration, carcinogenesis, and atherosclerosis ,6,7. Dietary supplementation with resveratrol extends the lifespan of Sacchromyces cerevisiae, C. elegans and Drosophila melanogaster ,8,9. The lifespan-extending effect of resveratrol has also been observed in the vertebrate Nothobranchius furzeri 10. Vitamin E supplementation in mice delays age-related transcriptional changes in the heart and brain and prolongs both the mean and maximum lifespan ,11,12. Dietary supplementation of anti-oxidant polyphenols found in green tea and curcumin also extend the lifespan of supplemented mice 13. An Acanthopanax sessiliflorus leaf extract increases resistance to oxidative stress and extends the lifespan of C. elegans 14. The anti-oxidant tempol partially prevents age-related transcriptional changes and significantly reduces mortality in mice ,15,16. In contrast, some anti-oxidants fail to show a lifespan-extending effect. For example, middle age-onset dietary supplementation with coenzyme Q10 or α-lipoic acid significantly mitigates tumor incidence but does not change the lifespan of mice 17.
N-acetyl-L-cysteine (NAC) is a sulfur-containing amino acid derivative with an acetyl group attached to the L-cysteine nitrogen. NAC is a strong anti-oxidant and has liver-protecting activity 18. Supplementation with NAC increases resistance to oxidative stress caused by ROS by elevating intracellular levels of glutathione 19. NAC is also involved in the cellular detoxification of heavy metal ions, such as lead, mercury, and arsenic 20. Lymphocyte and phagocyte membrane function is also enhanced by NAC 21. NAC also has anti-cancer activity, as demonstrated by the inhibition of angiogenesis by endothelial cells during cancer invasion or metastasis 22.
In this study, we examined the effect of NAC supplementation on resistance to various environmental stressors, including oxidative stress, heat shock, and ultraviolet (UV) irradiation in vivo. We also determined whether NAC can extend lifespan and affect fertility, which is closely related to aging. Finally, the expression of genes believed to be predictive molecular markers for longevity in C. elegans was monitored.
MATERIALS AND METHODS
Worm strains and maintenance
The N2 CGCb strain (C. elegans Genetics Center, Minneapolis/St. Paul, MN, USA) was used as the wild-type in all experiments. The green fluorescent protein (GFP)-expressing strains, CL2070 (dvIs70 [Phsp-16.2::GFP, rol-6]) and CF1553 (muIs84 [Psod-3::GFP, rol-6]) were purchased from the C. elegans Genetics Center. The worms were maintained on NGM (25 mM NaCl, 1.7% agar, 2.5 mg/mL peptone, 5 μg/mL cholesterol, 1 mM CaCl2, 1 mM MgSO4, and 50 mM KH2PO4 pH 6.0) plates at 20°C. Escherichia coli OP50 was used as a food source.
Resistance to oxidative stress
Five L4/young adult worms were transferred to a fresh NGM plate and permitted to lay eggs for 5 h. After removing the five adult worms, the eggs were maintained at 20°C for 3 days. Sixty age-synchronized worms were transferred to a fresh NGM plate containing different concentrations of NAC (0, 5, 10, 50, and 100 mM). 5-fluoro-2’-deoxyuridine (12.5 mg/L; Sigma-Aldrich, St. Louis, MO, USA) was also added to prevent internal hatching. After 24 h, 20 mM paraquat (methyl viologen dichloride hydrate, Sigma-Aldrich) was added to the NGM plates to induce oxidative stress in the worms. Thereafter, dead worms were counted three times per day until all of the worms were dead. We used the log-rank test for statistical analysis 23.
Lifespan assay
Sixty age-synchronized worms were transferred to NGM plates containing different concentrations of NAC (0, 1, 2, and 5 mM) and 12.5 mg/L 5-fluoro-2’-deoxyuridine. Thereafter, live worms were transferred to a fresh NGM plate every 2-3 days and counted every day until all of the worms were dead. The lifespan of the worms exposed to NAC was compared to those of untreated control worms using the log-rank test 23.
Heat shock stress resistance
Age-synchronized 3-day-old worms were picked from a NGM plate and transferred to a fresh NGM plate containing 5 mM NAC. After 24 h, the worms were transferred to a 35°C incubator for 10 h to induce heat shock stress. Then, the worms were transferred back to 20°C. Survival rates after 24 h at 20°C were compared between the control and NAC-treated worms. We used the standard two-tailed Student’s t-test for statistical analysis.
UV resistance
Sixty age-synchronized worms were treated with 5 mM NAC for 24 h and then exposed to UV (20 J/cm2/min) for 1 min in a 254 nm-UV crosslinker (BLX-254; Vilber Lourmat, France). Thereafter, dead worms were counted every day until all of the worms were dead. Resistance to UV was compared between the control and NAC-treated worms using the log-rank test 23.
Fertility assay
Five L4/young adult stage worms were transferred to a fresh NGM plate containing 5 mM NAC and permitted to lay eggs for 5 h. The eggs were maintained at 20°C for 2 days. A single worm was transferred to a fresh NGM plate containing 5 mM NAC every day until it laid no eggs. Eggs spawned by a single worm were incubated at 20°C for 48 h, and the number of progeny produced was recorded on each day. The average number of progeny produced by 10 worms treated with 5 mM NAC was compared to those produced from the control worms.
Expression of age-related reporters
Age-synchronized CL2070 and CF 1553 worms containing the hsp-16.2 and sod-3 GFP reporters, respectively, were exposed to 5 mM NAC at 20°C for 7 days. Then, the worms were mounted on a glass slide coated with 2% agarose and anaesthetized with 1 M sodium azide. After covering the slide with a coverslip, expression of each reporter was observed using a confocal microscope (Olympus FV10i, Olympus, Tokyo, Japan). The total fluorescence intensity of a randomly selected single worm was quantified with a fluorescence multi-reader (Infinite F200, Tecan, Grodig, Austria).
RESULTS
Increased resistance to induced oxidative stress by NAC in C. elegans
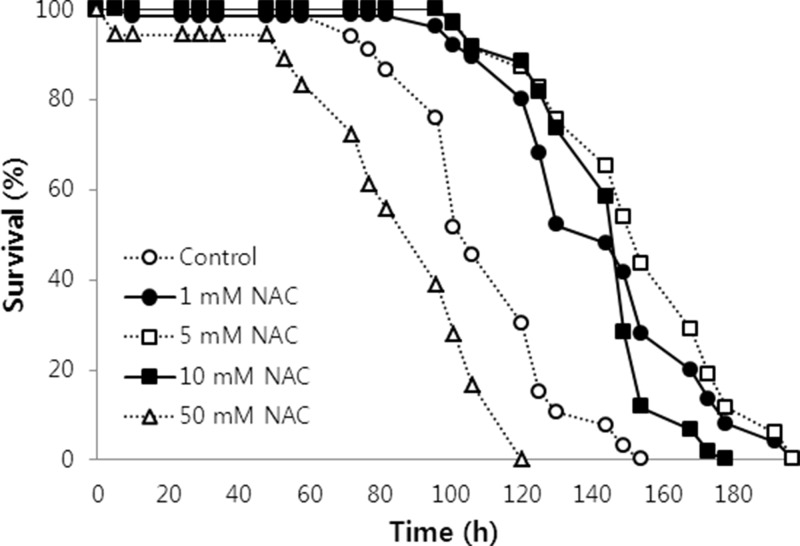
To examine the effect of NAC on the response to oxidative stress, we monitored the survival of worms under oxidative stress conditions with or without NAC. Exposure to a NAC concentration lower than 10 mM conferred increased survival under oxidative stress conditions compared to that of the untreated control (Figure 1). The mean survival time increased from 101.4 h in untreated control worms to 135.3, 146.5, and 136.7 h in 1, 5, and 10 mM NAC-treated worms, respectively. The largest effect was observed in worms exposed to 5 mM NAC (44.5% increase in mean survival time, p<0.001). However, 50 mM NAC had a negative effect on the resistance to oxidative stress in C. elegans, as the mean survival time decreased to 77.6 h. We observed the same dose-dependent anti-oxidative stress effect of NAC in a repeated experiment. The mean survival time increased significantly with 5 and 10 mM NAC treatment, whereas 50 mM NAC reduced resistance to oxidative stress. The most effective concentration of NAC was 5 mM in a replicative experiment, as observed in the first experiment (data not shown).
Figure 1. Effect of NAC on resistance to oxidative stress in C. elegans. Age-synchronized young adult worms were supplemented with different NAC concentrations for 24 h (n=60). Then, the worms were exposed to 20 mM paraquat, an oxidative stress inducer, and the survival of the worms was monitored three times per day until all worms were dead. Oxidatively stressed worms not treated with NAC were used as a control.
Lifespan extension with NAC treatment
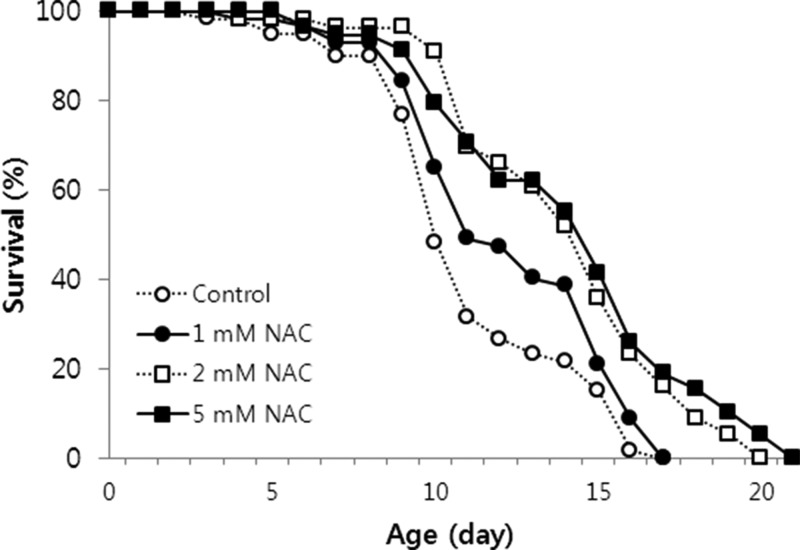
We examined whether NAC could affect C. elegans lifespan. NAC supplementation significantly extended both the mean and the maximum lifespans of C. elegans (Figure 2). Among three different NAC concentrations tested, the lifespan-extending effects of 5 mM NAC was greater than that of 1 and 2 mM NAC. The mean and the maximum lifespan values of untreated control worms were 11.1 and 17 days, respectively. The mean lifespan was extended up to 18.3 days with 5 mM NAC treatment, and maximum lifespan increased by 8 days (Table 1). The effect of NAC on the longevity calculated using the mean lifespan of untreated and 5 mM NAC-treated worms was 30.5% (p<0.001, Table 1). An independent repeat experiment also showed a significant lifespan-extending effect following supplementation with 5 mM NAC (Table 1). These findings indicate that the most effective NAC concentration for resistance to oxidative stress and longevity in C. elegans is 5 mM.
Figure 2. Lifespan of C. elegans treated with different concentrations of NAC. Age-synchronized 3-day-old worms were treated with different NAC concentrations throughout their lifespan (n=60). Both the mean and the maximum lifespan increased significantly in the 2 and 5 mM NAC treated groups (p<0.05 by the log-rank test). Untreated worms were used as a control.
Table 1. Lifespan-extending effect of N-acetyl-L-cysteine in C. elegans.
| Conc. (mM) | Mean lifespan(day)1 | Maximum lifespan(day)2 | p-value3 | % increase4 | ||
|---|---|---|---|---|---|---|
| 1st Experiment | Control | 0 | 11.1 | 17 | ||
| NAC | 1 | 14.2 | 21 | <0.001 | 28.1 | |
| 2 | 14.1 | 20 | <0.001 | 27.1 | ||
| 5 | 18.3 | 25 | <0.001 | 30.5 | ||
| 2nd Experiment | Control | 0 | 14.2 | 21 | ||
| NAC | 1 | 14.9 | 23 | 0.215 | 5.2 | |
| 2 | 15.0 | 23 | 0.211 | 6.1 | ||
| 5 | 16.2 | 25 | 0.003 | 14.6 |
1Mean lifespan is the day when 50% of worms survived.
2Maximum lifespan is the greatest age reached by the last surviving worm.
3p-value was calculated using the log-rank test by comparing the survival of control and NAC-treated groups.
4% effects were calculated by (A-C)/C*100, where A is the mean lifespan of C. elegans treated with each concentration of NAC and C is the mean lifespan of control.
Effect of NAC on response to environmental stressors
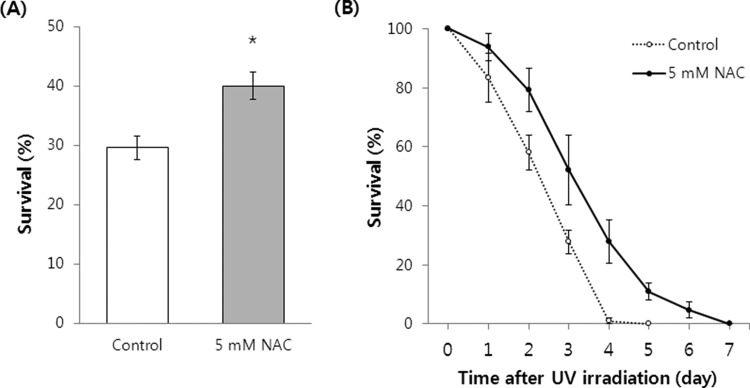
We also measured the change in the response of C. elegans to heat shock and UV irradiation following NAC supplementation. We used a NAC concentration of 5 mM for these experiments because it was the most effective in altering the resistance to induced oxidative stress and extending the lifespan of C. elegans. NAC-treated worms showed increased survival after 10 h of 35°C heat stress compared to the untreated control worms (p=0.009). The mean survival was 40.0±2.30% (mean±standard error of the mean (SEM) of five independent experiments) in NAC-treated worms, whereas only 29.6±2.01% of the untreated worms survived after 10 h of 35°C heat stress (Figure 3A). Resistance to UV irradiation also increased significantly with NAC treatment. Adult worms exposed to 5 mM NAC survived longer than the untreated control worms after UV irradiation (Figure 3B). The mean survival time was 2.4±0.15 h (mean±SEM of three independent experiments) in the untreated worms and 3.4±0.27 h in NAC-treated worms. NAC supplementation increased mean survival time to 54.1, 52.1, and 24.1% in repeat experiments.
Figure 3. Response to environmental stressors with or without NAC treatment. (A) Survival after 10 h of heat stress was compared between untreated control and 5 mM NAC-treated worms. Data show the average of five independent experiments. (B) Time-course survival of worms after UV irradiation was monitored every hour. Values are means of three independent experiments. Asterisk indicates a significant difference (p<0.05 by Student’s t test). Error bars indicate SEM.
Effect of NAC on reproduction
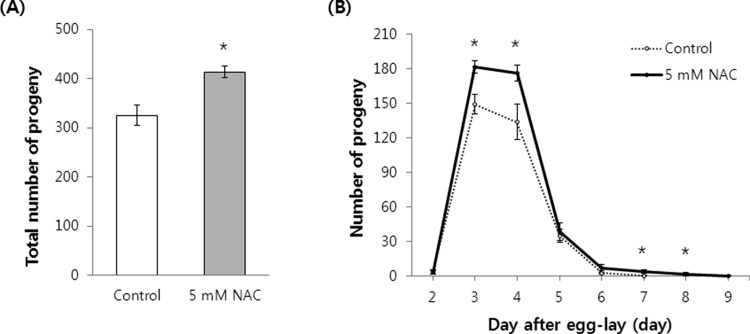
Previous studies have reported that many long-lived C. elegans mutants exhibit a reduced reproductive capacity phenotype ,24-26. Next, we determined whether the NAC lifespan-extending effect also affected reproduction. To our surprise, the fertility of worms supplemented with 5 mM NAC was higher than that of the untreated control worms. The total number of progeny produced increased significantly following exposure to NAC (Figure 4A). Untreated control worms produced 325.6±20.67 (mean±SEM of 10 worms) progeny, whereas 414.2±12.22 progeny were produced by the NAC-treated worms (p=0.002). We also measured the time-course distribution of the number of progeny produced throughout the C. elegans gravid period. The worms exposed to NAC produced more progeny than that of the untreated control worms (Figure 4B). The number of progeny produced by NAC-treated worms increased from 149.4±8.59 to 181.9±5.39 on day 3. Control worms produced 134.0±15.08 progeny and NAC-treated worms produced 176.5±6.69 progeny on day 4. Interestingly, the gravid period was extended by the NAC treatment (Figure 4B). Our fertility assay showed that worms under normal conditions produced progeny for 6 days. However, worms supplemented with NAC produced progeny for 8 days (days 2-9 after egg-lay).
Figure 4. Effect of NAC on C. elegans fertility. The number of progeny produced by gravid worms treated with or without NAC was monitored (n=10). (A) Total number of progeny. Worms exposed to NAC produced significantly more progeny during their gravid period than that of untreated worms (B) Time-course distribution of progeny. NAC-treated worms produced more progeny on days 3 and 4 after egg-laying compared to that by untreated controls and extended their gravid period by 2 days. Asterisk indicates a significant difference (p<0.05 by Student’s t test). Error bars indicate SEM.
Induction of age-related genes by NAC
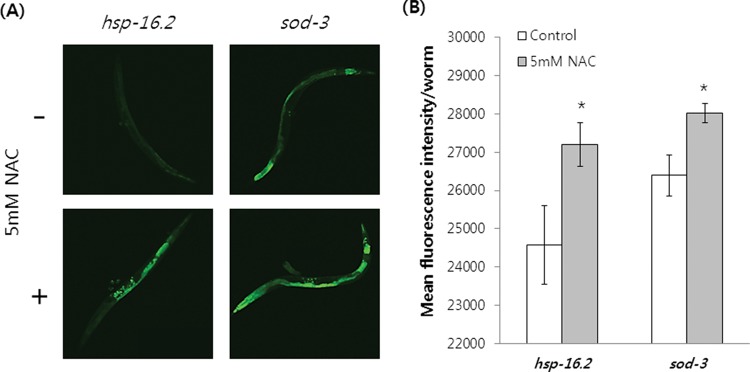
Expression of a GFP reporter coupled to the hsp-16.2 promoter is positively correlated with an organism’s lifespan 27. In addition, fluorescence conferred by sod-3::GFP declines with normal aging and is associated with the remaining lifespan 28. These findings suggest that both hsp-16.2 and sod-3 can be used as predictive molecular markers of C. elegans longevity. In this study, we examined whether hsp-16.2 and sod-3 expression was up-regulated in the long-lived NAC-treated worms. Expression of these longevity fluorescent markers increased significantly in response to NAC treatment. The hsp-16.2::GFP gene was markedly induced by 5 mM NAC treatment (Figure 5A). GFP fluorescence intensity increased from 24,576±1,016.2 (mean±SEM of 20 individual worms) in the control to 27,196±575.8 in NAC-treated worms (p=0.031) (Figure 5B). Supplementation with 5 mM NAC also induced sod-3 expression (Figure 5A). The fluorescence intensities of sod-3::GFP were 26,398±533.5 and 28,023±253.9 in the control and NAC-treated worms, respectively (p=0.009) (Figure 5B).
Figure 5. hsp-16.2 and sod-3 expression in C. elegans treated with 5 mM NAC. Age-synchronized 3-day-old young adult worms were transferred to NGM plates containing 5 mM NAC and cultured for 7 days. (A) Total GFP fluorescence of each whole worm was compared between control and NAC-treated worms for each gene. (B) GFP fluorescence intensity was quantified with a fluorescence multi-reader. Data are mean fluorescence intensity per worm for each gene (n=20) treated with or without 5 mM NAC. Asterisk indicates significant difference (p<0.05 by Student’s t test). Error bars indicate SEM.
DISCUSSION
The free radical theory of aging suggests that the age-related accumulation of oxidative damage caused by free radicals is a major causal factor in aging ,1,29. Many studies have revealed that lifespan-extending interventions are also effective for resisting oxidative stress. Long-lived C. elegans genetic mutants, such as daf-2 and age-1, show increased survival under oxidative-stress conditions 30. Dietary supplementation with anti-oxidants also confers both increased resistance to oxidative stress and longevity. Chicoric acid, a strong ROS scavenger, decreases cellular ROS levels and significantly extends lifespan in C. elegans 31. Treatment with metformin, an anti-diabetic drug, increases both resistance to oxidative stress and lifespan 32. Recent studies using plant extracts also indicate a positive correlation between oxidative stress and longevity. An Alpinia zerumbet leaf extract induces expression of the superoxide dismutase 3, an anti-oxidant gene, and exhibits a lifespan-extending effect 33. Protection against oxidative stress and an increased lifespan is also observed in C. elegans following treatment with extracts from Acanthopanax sessiliflorus leaves 14. The commercially available herb mixture KPG-7 reduces the cellular levels of oxidized proteins and increases lifespan 34. Previous studies have shown that dietary uptake of NAC increased the lifespan of Drosophila melanogaster in a dose-dependent manner 35. In C. elegans, ingestion of liposome with NAC also extended lifespan 36. In our study, resistance to oxidative stress increased markedly following NAC dietary supplementation, and both the mean and maximum lifespan were significantly extended by NAC. These findings suggest that the lifespan-extending effect of NAC could be due to its oxidative stress protecting activity in C. elegans and provide supportive evidence for the free radical theory of aging. In contrast, the elevated superoxide and extended lifespan conferred by mutations in genes involved in mitochondrial electron transport chain are abolished by NAC, suggesting a hormesis effect of superoxide in C. elegans 37. Our results showed that a high concentration of NAC, 50 mM, decreased resistance to oxidative stress, whereas 5 mM NAC increased resistance to oxidative stress and the lifespan. Interestingly, the lifespan of wild-type N2 was not affected by a high concentration of NAC (500 mM) 37. Therefore, the hormesis effect generated by a low concentration of NAC could be another possible mechanism of lifespan extension by NAC.
Many lifespan-extending genetic or nutritional interventions in C. elegans also induce increased resistance to environmental stressors. The age-1 mutation shows resistance to oxidative stress, heat stress, UV irradiation, and heavy metal exposure ,38-41. Daf-2 mutants also have an extended lifespan as well as increased thermotolerance ,24,42. Interestingly, worms whose germ-line precursor cells are ablated are long-lived and resistant to thermal stress 43. A recent study showed that an Acanthopanax sessiliflorus extract reduces susceptibility to heat stress and UV irradiation 14. Worms grown in media prepared with electrolyzed-reduced water live longer than worms cultured in distilled water and also have increased resistance to heat shock and UV irradiation ,44,45. We found that 5 mM NAC was the most effective concentration for resistance to oxidative stress and longevity in C. elegans. NAC (5 mM) also increased survival after heat shock and UV irradiation. Our findings indicate a positive correlation between lifespan extension and increased resistance to environmental stressors, including oxidative stress, heat shock, and UV irradiation, in C. elegans. NAC is a well-known precursor to an anti-oxidant, glutathione. Therefore, additional data regarding the change in cellular level of glutathione and ROS can provide a critical clue for the anti-stress responsive role of NAC.
It has been suggested that there is a trade-off between survival and fitness in lifespan-extending interventions. Resveratrol increases both the mean and maximum lifespan in C. elegans but causes a significant reduction in fecundity during the early gravid period 46. Treatment with blueberry polyphenols confers increased resistance to heat stress and extends lifespan, and the age-related decline in pharyngeal pumping rate is delayed 47. The disposable soma theory of aging suggests that the distribution of cellular resources between cellular fitness and reproductive behavior plays a pivotal role in normal aging 48. Ablation of germ-line precursor cells results in increased lifespan in C. elegans 49. In contrast, we did not observe a reduced reproductive rate as a trade-off for lifespan extension caused by NAC, as the total number of progeny increased. Long-lived worms cultured in medium prepared with electrolyzed-reduced water also produce more progeny compared to that of a control 44. Therefore, it seems that NAC increases C. elegans survival without reducing fertility. However, there could be other trade-offs that were not examined in this study that are associated with increased lifespan induced by NAC.
Previous studies have found that differential expression of hsp-16.2::GFP affects lifespan: worms expressing a high level of hsp-16.2::GFP live longer than worms expressing a low level of hsp-16.2::GFP, and the ability to express different levels of hsp-16.2::GFP is inherited ,27,50. Another well-known transcriptional marker of physiological age is sod-3: sod-3::GFP expression is positively correlated with remaining lifespan in C. elegans 28. Expression of both hsp-16.2::GFP and sod-3::GFP increased significantly in long-lived worms treated with 5 mM NAC, indicating the ability of NAC to induce longevity-assuring transcriptional alterations.
Taken together, our results suggest that dietary supplementation with NAC can modulate an organism’s response to environmental stressors and aging, potentially through the induction of stress-responsive transcriptional markers and genes associated with longevity. Further studies should identify the cellular signaling pathways involved in NAC-induced longevity and reveal the underlying cellular mechanisms of aging in C. elegans.
AUTHOR CONTRIBUTIONS
Park SK conceived and designed the study and wrote the manuscript. Oh SI performed all experiments, analyzed the data, and reviewed the manuscript. Park JK was involved in oxidative stress assay and provided a critical review of the manuscript.
Acknowledgments
This work was supported by the Soonchunhyang University Research Fund (No. 20130593) and Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2010-0022429).
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Herman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11((3)):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 2.Gemma C, Vila J, Bachstetter A, Bickford PC.Oxidative Stress and the Aging Brain: From Theory to PreventionIn:Riddle DR.editorBrain Aging: Models, Methods, and Mechanisms Frontiers in Neuroscience; Boca Raton, (FL)2007 [PubMed] [Google Scholar]
- 3.Johnson TE. Increased life-span of age-1 mutants in Caenorhabditis elegans and lower Gompertz rate of aging. Science. 1990;249((4971)):908–12. doi: 10.1126/science.2392681. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Zhang L, Zhang L, Bai J, Ge H, Liu P. Expression changes in DNA repair enzymes and mitochondrial DNA damage in aging rat lens. Mol Vis. 2010;16:1754–63. [PMC free article] [PubMed] [Google Scholar]
- 5.Berger MM. Can oxidative damage be treated nutritionally? Clin Nutri. 2005;24((2)):172–83. doi: 10.1016/j.clnu.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson LR. Role of plant polyphenols in genomic stability. Mutat Res. 2001;475((1-2)):89–111. doi: 10.1016/S0027-5107(01)00073-2. [DOI] [PubMed] [Google Scholar]
- 7.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275((5297)):218–20. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 8.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425((6954)):191–6. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 9.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430((7000)):686–9. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 10.Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16((3)):296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 11.Navarro A, Gomez C, Sanchez-Pino MJ, Gonzalez H, Bandez MJ, Boveris AD, et al. Vitamin E at high doses improves survival, neurological performance, and brain mitochondrial function in aging male mice. Am J Physiol Regul Integr Comp Physiol. 2005;289((5)):R1392–9. doi: 10.1152/ajpregu.00834.2004. [DOI] [PubMed] [Google Scholar]
- 12.Park SK, Page GP, Kim K, Allison DB, Meydani M, Weindruch R, et al. alpha- and gamma-Tocopherol prevent age-related transcriptional alterations in the heart and brain of mice. J Nutr. 2008;138((6)):1010–8. doi: 10.1093/jn/138.6.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitani K, Yokozawa T, Osawa T. Interventions in aging and age-associated pathologies by means of nutritional approaches. Ann N Y Acad Sci. 2004;1019((1)):424–6. doi: 10.1196/annals.1297.075. [DOI] [PubMed] [Google Scholar]
- 14.Kim CK, Park SK. Effect of Acanthopanax sessiliflorus Extracts on Stress Response and Aging in Caenorhabditis elegans. Food Sci Technol Res. 2013;19((3)):439–44. doi: 10.3136/fstr.19.439. [DOI] [Google Scholar]
- 15.Park SK, Kim K, Page GP, Allison DB, Weindruch R, Prolla TA. Gene expression profiling of aging in multiple mouse strains: identification of aging biomarkers and impact of dietary antioxidants. Aging Cell. 2009;8((4)):484–95. doi: 10.1111/ace.2009.8.issue-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamato M, Ishimatsu A, Yamanaka Y, Mine T, Yamada K. Tempol intake improves inflammatory status in aged mice. J Clin Biochem Nutr. 2014;55((1)):11–4. doi: 10.3164/jcbn.14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee CK, Pugh TD, Klopp RG, Edwards J, Allison DB, Weindruch R, et al. The impact of alpha-lipoic acid, coenzyme Q10 and caloric restriction on life span and gene expression patterns in mice. Free Radic Biol Med. 2004;36((8)):1043–57. doi: 10.1016/j.freeradbiomed.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Yedjou CG, Tchounwou PB. N-acetyl-l-cysteine affords protection against lead-induced cytotoxicity and oxidative stress in human liver carcinoma (HepG2) cells. Int J Environ Res Public Health. 2007;4((2)):132–7. doi: 10.3390/ijerph2007040007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daraie B, Pourahmad J, Hamidi-Pour N, Hosseini MJ, Shaki F, Soleimani M. Uranyl acetate induces oxidative stress and mitochondrial membrane potential collapse in the human dermal fibroblast primary cells. Iran J Pharm Res. 2012;11((2)):495–501. [PMC free article] [PubMed] [Google Scholar]
- 20.Takagi M, Satofuka H, Amano S, Mizuno H, Eguchi Y, Hirata K, et al. Cellular toxicity of cadmium ions and their detoxification by heavy metal-specific plant peptides, phytochelatins, expressed in Mammalian cells. J Biochem. 2002;131((2)):233–9. doi: 10.1093/oxfordjournals.jbchem.a003093. [DOI] [PubMed] [Google Scholar]
- 21.Malorni W, D'Ambrosio A, Rainaldi G, Rivabene R, Viora M. Thiol supplier N-acetylcysteine enhances conjugate formation between natural killer cells and K562 or U937 targets but increases the lytic function only against the latter. Immunol Lett. 1994;43((3)):209–14. doi: 10.1016/0165-2478(94)90225-9. [DOI] [PubMed] [Google Scholar]
- 22.Cai T, Fassina G, Morini M, Aluigi MG, Masiello L, Fontanini G, et al. N-acetylcysteine inhibits endothelial cell invasion and angiogenesis. Lab Invest. 1999;79((9)):1151–9. [PubMed] [Google Scholar]
- 23.Peto R, Peto J. Asymptotically efficient rank invarient test procedures. J Royal Stac Soc Ser A. 1972;135((2)):185–207. doi: 10.2307/2344317. [DOI] [Google Scholar]
- 24.Gems D, Sutton AJ, Sundermeyer ML, Albert PS, King KV, Edgley ML, et al. Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics. 1998;150((1)):129–55. doi: 10.1093/genetics/150.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes SE, Evason K, Xiong C, Kornfeld K. Genetic and pharmacological factors that influence reproductive aging in nematodes. PLoS Genet. 2007;3((2)):e25. doi: 10.1371/journal.pgen.0030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsen PL, Albert PS, Riddle DL. Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics. 1995;139((4)):1567–83. doi: 10.1093/genetics/139.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rea SL, Wu D, Cypser JR, Vaupel JW, Johnson TE. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat Genet. 2005;37((8)):894–8. doi: 10.1038/ng1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanchez-Blanco A, Kim SK. Variable pathogenicity determines individual lifespan in Caenorhabditis elegans. PLoS Genet. 2011;7((4)):e1002047. doi: 10.1371/journal.pgen.1002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78((2)):547–81. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 30.Khare S, Linster CL, Clarke SG. The interplay between protein L-isoaspartyl methyltransferase activity and insulin-like signaling to extend lifespan in Caenorhabditis elegans. PloS One. 2011;6((6)):e20850. doi: 10.1371/journal.pone.0020850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlernitzauer A, Oiry C, Hamad R, Galas S, Cortade F, Chabi B, et al. Chicoric acid is an antioxidant molecule that stimulates AMP kinase pathway in L6 myotubes and extends lifespan in Caenorhabditis elegans. PloS One. 2013;8((11)):e78788. doi: 10.1371/journal.pone.0078788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PloS One. 2010;5((1)):e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Upadhyay A, Chompoo J, Taira N, Fukuta M, Tawata S. Significant longevity-extending effects of Alpinia zerumbet leaf extract on the life span of Caenorhabditis elegans. Biosci Biotechnol Biochem. 2013;77((2)):217–23. doi: 10.1271/bbb.120351. [DOI] [PubMed] [Google Scholar]
- 34.Moriwaki T, Kato S, Kato Y, Hosoki A, Zhang-Akiyama QM. Extension of lifespan and protection against oxidative stress by an antioxidant herb mixture complex (KPG-7) in Caenorhabditis elegans. J Clin Biochem Nutr. 2013;53((2)):81–8. doi: 10.3164/jcbn.13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brack C, Bechter-Thuring E, Labuhn M. N-acetylcysteine slows down ageing and increases the life span of Drosophila melanogaster. Cell Mol Life Sci. 1997;53((11-12)):960–6. doi: 10.1007/PL00013199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shibamura A, Ikeda T, Nishikawa Y. A method for oral administration of hydrophilic substances to Caenorhabditis elegans: Effects of oral supplementation with antioxidants on the nematode lifespan. Mech Ageing Dev. 2009;130((9)):652–5. doi: 10.1016/j.mad.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Yang W, Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. 2010;8((12)):e1000556. doi: 10.1371/journal.pbio.1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barsyte D, Lovejoy DA, Lithgow GJ. Longevity and heavy metal resistance in daf-2 and age-1 long-lived mutants of Caenorhabditis elegans. FASEB J. 2001;15((3)):627–34. doi: 10.1096/fj.99-0966com. [DOI] [PubMed] [Google Scholar]
- 39.Honda Y, Honda S. Life span extensions associated with upregulation of gene expression of antioxidant enzymes in Caenorhabditis elegans; studies of mutation in the age-1, PI3 kinase homologue and short-term exposure to hyperoxia. J Am Aging Assoc. 2002;25((1)):21–8. doi: 10.1007/s11357-002-0003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lithgow GJ, White TM, Hinerfeld DA, Johnson TE. Thermotolerance of a long-lived mutant of Caenorhabditis elegans. J Gerontol. 1994;49((6)):B270–6. doi: 10.1093/geronj/49.6.B270. [DOI] [PubMed] [Google Scholar]
- 41.Murakami S, Johnson TE. A genetic pathway conferring life extension and resistance to UV stress in Caenorhabditis elegans. Genetics. 1996;143((3)):1207–18. doi: 10.1093/genetics/143.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc Natl Acad Sci U S A. 1995;92((16)):7540–4. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science. 2002;295((5554)):502–5. doi: 10.1126/science.1065768. [DOI] [PubMed] [Google Scholar]
- 44.Park SK, Park SK. Electrolyzed-reduced water increases resistance to oxidative stress, fertility, and lifespan via insulin/IGF-1-like signal in C. elegans. Biol Res. 2013;46((2)):147–52. doi: 10.4067/S0716-97602013000200005. [DOI] [PubMed] [Google Scholar]
- 45.Park SK, Kim JJ, Yu AR, Lee MY, Park SK. Electrolyzed-reduced water confers increased resistance to environmental stresses. Mol Cell Toxicol. 2012;8((3)):241–7. doi: 10.1007/s13273-012-0029-1. [DOI] [Google Scholar]
- 46.Gruber J, Tang SY, Halliwell B. Evidence for a trade-off between survival and fitness caused by resveratrol treatment of Caenorhabditis elegans. Ann N Y Acad Sci. 2007;1100((1)):530–42. doi: 10.1196/annals.1395.059. [DOI] [PubMed] [Google Scholar]
- 47.Wilson MA, Shukitt-Hale B, Kalt W, Ingram DK, Joseph JA, Wolkow CA. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging cell. 2006;5((1)):59–68. doi: 10.1111/ace.2006.5.issue-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kirkwood TB. Evolution of ageing. Nature. 1977;270((5635)):301–4. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- 49.Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399((6734)):362–6. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]
- 50.Cypser JR, Wu D, Park SK, Ishii T, Tedesco PM, Mendenhall AR, et al. Predicting longevity in C. elegans: fertility, mobility and gene expression. Mech Ageing Dev. 2013;134((7-8)):291–7. doi: 10.1016/j.mad.2013.02.003. [DOI] [PubMed] [Google Scholar]