Abstract
OBJECTIVE:
Studies suggest an association between vitamin D deficiency and morbidity/mortality in critically ill patients. Several issues remain unexplained, including which vitamin D levels are related to morbidity and mortality and the relevance of vitamin D kinetics to clinical outcomes. We conducted this study to address the association of baseline vitamin D levels and vitamin D kinetics with morbidity and mortality in critically ill patients.
METHOD:
In 135 intensive care unit (ICU) patients, vitamin D was prospectively measured on admission and weekly until discharge from the ICU. The following outcomes of interest were analyzed: 28-day mortality, mechanical ventilation, length of stay, infection rate, and culture positivity.
RESULTS:
Mortality rates were higher among patients with vitamin D levels <12 ng/mL (versus vitamin D levels >12 ng/mL) (32.2% vs. 13.2%), with an adjusted relative risk of 2.2 (95% CI 1.07-4.54; p< 0.05). There were no differences in the length of stay, ventilation requirements, infection rate, or culture positivity.
CONCLUSIONS:
This study suggests that low vitamin D levels on ICU admission are an independent risk factor for mortality in critically ill patients. Low vitamin D levels at ICU admission may have a causal relationship with mortality and may serve as an indicator for vitamin D replacement among critically ill patients.
Keywords: Vitamin D, Septic shock, Critical care
INTRODUCTION
Vitamin D is a fat-soluble vitamin that is synthesized in the skin in response to sunlight exposure (as provitamin D3 and previtamin D3) and is also obtained, to a limited extent, from dietary intake (vitamins D2 and D3). Vitamin D is stored in adipose cells or is converted in the liver to its circulating form, 25-hydroxyvitamin D3 [25(OH)D3]. Mainly in the kidneys, 25(OH)D is converted to its active metabolite, 1,25-dihydroxyvitamin D [1,25(OH)2D], also known as calcitriol. Calcitriol synthesis is enhanced by parathyroid hormone (PTH) and hypophosphatemia. The endocrine action of calcitriol on bone mineral metabolism has been known for decades, and vitamin D supplementation has been advocated in patients with chronic conditions, such as osteoporosis. For many years, vitamin D deficiency was recognized as a chronic condition associated with bone pain, weakness and fractures in the general population. Serum vitamin D levels below 10 ng/mL (generally described as deficiency or severe deficiency) are associated with osteomalacia and rickets, whereas levels in the 20-30 ng/mL range (vitamin D insufficiency or subclinical deficiency) are associated with osteoporosis and elevated PTH and have the potential to cause long-term harmful effects on bone metabolism. Children with rickets are more susceptible to infections, and vitamin D has been implicated in the pathogenesis of tuberculosis ,1-4. The paracrine and autocrine effects of vitamin D have been increasingly reported in the literature. In recent years, it has become clear that several cell lines, including macrophages and cardiac muscle cells, express vitamin D receptors and the enzyme 25(OH)D3-1-alpha-hydroxylase, which allows the cells to convert 25(OH)D to its active form, 1,25(OH)2D 5.
Studies have shown correlations between low vitamin D levels and certain cancers, immune system dysfunction, diabetes, cardiovascular disease, hypertension and metabolic syndrome ,6-8. Thus, investigators in several fields, including critical care medicine, have turned their attention to the nonskeletal effects of vitamin D ,9-15. Some observational studies have demonstrated a high prevalence of vitamin D deficiency in critically ill patients, who are generally deprived of sunlight, confined to bed and fed diets with low vitamin D content.
Data regarding the association between vitamin D deficiency and poor ICU outcomes, such as mortality, increased length of stay, increased length of mechanical ventilation, and infections, are conflicting. After an initial report by Lee et al. 16 suggesting a high prevalence of vitamin D deficiency in critically ill patients, a retrospective study involving 2,399 patients 14 showed an association between vitamin D deficiency, mortality and positive blood cultures. Subsequently, smaller prospective studies did not find this association. Differences in the definition of vitamin D deficiency, the studied populations (surgical ICUs versus medical ICUs), the methodology of vitamin D measurement, the interventions not measured (such as the volume and type of solutions infused) and the geographical location may account for the different findings in these studies ,17,18-24. The relevance of vitamin D measurement and kinetics at ICU admission is open to debate. Furthermore, the 25(OH)D levels required for proper maintenance of the pleiotropic effects of vitamin D remain unknown.
The purpose of this study was to assess the relationship between serum vitamin D levels at admission, vitamin D kinetics during ICU stay, and morbidity and mortality in critically ill patients.
MATERIALS AND METHODS
Study design and setting
This is a prospective study of patients admitted to the medical-surgical ICU of a university hospital (Hospital de Clínicas de Porto Alegre, state of Rio Grande do Sul, Brazil) between March and November 2012. The city of Porto Alegre is located in the South of Brazil at 30°05'S. The study protocol was approved by the institutional research ethics committee, and written informed consent was provided by all patients or their surrogates.
Methods
We included adult patients (age 18 years and older) with a predicted length of ICU stay greater than 3 days. We excluded patients with a length of pre-ICU hospital stay >3 days, chronic renal failure requiring dialysis or pre admission creatinine >2 mg/dL, a life expectancy of less than 24 hours, pregnancy, admission for cardiac or elective surgery, hyper- or hypoparathyroidism, and granulomatous diseases, such as tuberculosis or sarcoidosis.
Patient demographic and clinical data were recorded on ICU admission, including age, sex, comorbidities, current medications, ethnicity, reason for admission, season, APACHE II score, and body mass index (BMI) (Kg/m2). The SOFA score was calculated at ICU admission and at ICU discharge or death. Infections and cultures (blood, sputum, urine, ascites or pleural fluid) were prospectively assessed throughout the ICU stay for each patient. Patients were considered infected when the attending physicians, who were not involved with the study and had no access to serum 25(OH)D levels, diagnosed ICU-acquired infections and prescribed antimicrobials to their patients. In addition to the total number of positive cultures and clinically overt infections, the incidence rates of culture positivity and infection over time were also assessed. Patients were also assessed for the duration of mechanical ventilation, length of ICU stay, length of hospital stay, and 28-day mortality.
Biochemical analysis
Blood samples were collected within 24 hours of ICU admission for the measurement of capillary blood glucose, PTH, ionized calcium, albumin, creatinine, phosphorus, magnesium, C-reactive protein, and lactate. The estimated glomerular filtration rate was calculated using the MDRD equation 10. All tests were performed by routine methods employed at the biochemistry laboratory of Hospital de Clínicas de Porto Alegre. 25(OH)D was measured once weekly for 4 weeks or until ICU discharge. Samples were protected from sunlight exposure, centrifuged and stored under refrigeration until processing, which was performed once weekly on Fridays. 25(OH)D levels were measured using the chemiluminescence method (Liaison; Diasorin, Stillmater, Minnesota; inter- and intra-assay coefficients of variation, 10%) and reported in ng/mL 11. (To convert the 25(OH)D concentration from ng/mL to nmol/L, multiply by 2.5.) PTH levels were measured by a chemiluminescent immunoassay (Advia Centaur XP; Siemens Healthcare Diagnostics Inc.).
Statistical analysis
Normally distributed quantitative variables are expressed as the mean ± standard deviation. Asymmetrically distributed variables are expressed as the median [interquartile range]. The chi-square test was used for the assessment of categorical variables, and Student’s t-test was used for continuous variables. Normally distributed and asymmetric variables were compared using the Mann-Whitney U test. Receiver-operating characteristic (ROC) curves were plotted to illustrate 25(OH)D cutoff values. Relative risks (along with 95% confidence intervals) were estimated using Poisson regression adjusted for 6 covariates: lactate, albumin, SOFA and APACHE II scores, sepsis as the reason for ICU admission and BMI. Survival analysis was performed by means of Kaplan-Meier curves. Log-rank statistics were used to determine significant differences over time. For the comparative analysis of changes in the 25(OH)D levels over time during ICU admission between survivors and non-survivors, we used a generalized estimating equation model for longitudinal data analysis 12. Infection and culture positivity rates were compared by means of incidence density ratio analysis. The significance level was set at p<0.05.
RESULTS
Cohort profile
Overall, 135 patients were included. The 28-day mortality rate was 21.5%. Slightly less than half of all patients (47%) were women, and 78% were Caucasian. The median vitamin D level at admission was 13.3 [8.1-20] ng/mL. Only 16 patients (11.8%) were vitamin D sufficient (25(OH)D>30 ng/mL); 101 (74.8%) were vitamin D insufficient (25(OH)D<20 ng/mL). Five patients were on bisphosphonate, calcium, and vitamin D therapy prior to admission. These patients’ 25(OH)D levels ranged from 4.7 ng/mL to 18 ng/mL. No patient received supplemental vitamin D during his or her ICU stays. The sample profile is shown in Table 1. Non-survivors had higher APACHE II and SOFA scores and PTH and lactate levels on admission and had more difficult clinical courses with more organ dysfunction (lower ΔSOFA). In addition, fewer progressed to enteral feeding; five patients were never fed due to death secondary to refractory shock within the first few days of ICU admission. Electrolytes and renal function were assessed in 117 patients who were not on dialysis at the time of 25(OH)D measurement. Non-survivors had lower ionized calcium levels and lower glomerular filtration rates (Table 2).
Table 1. Sample profile.
| Survivors (n=106) | Non-survivors (n=29) | p-value | |
|---|---|---|---|
| Age, years | 55.4±16.1 | 60.4±16.6 | 0.141 |
| Gender, n (%) | |||
| Female | 48 (45%) | 16 (55%) | 0.462 |
| Male | 58 (55%) | 13 (45%) | |
| Comorbidities, n (%) | |||
| Coronary artery disease | 12 (11.3%) | 3 (10%) | 0.882 |
| Hypertension | 54 (51%) | 14 (48%) | 0.964 |
| Heart failure | 13 (12%) | 3 (10%) | 1.000 |
| Diabetes | 27 (25%) | 6 (21%) | 0.774 |
| Hypothyroidism | 3 (2.8%) | 3 (10%) | 0.113 |
| Chronic renal failure | 3 (2.8%) | 1 (3.4%) | 1.000 |
| COPD | 19 (18%) | 5 (17%) | 1.000 |
| Asthma | 4 (3.8%) | 3 (10%) | 0.169 |
| AIDS | 8 (7.5%) | 0 | 0.201 |
| Cirrhosis | 9 (8.5%) | 3 (10%) | 0.720 |
| Stroke | 7 (6.6%) | 4 (14%) | 0.249 |
| Depression | 8 (7.5%) | 0 | 0.201 |
| Neoplasia | 14 (13%) | 5 (17.2%) | 0.557 |
| Other | 20 (19%) | 7 (24%) | 0.714 |
| Chronic medications, n (%) | |||
| Diuretic | 28 (26.4%) | 11 (38%) | 0.326 |
| Corticosteroid | 8 (7.5%) | 5 (17%) | 0.125 |
| Ca/VITD | 3 (2.8%) | 2 (7%) | 0.292 |
| Bisphosphonate | 1 (1%) | 1 (3.4%) | 0.385 |
| Antiepileptic | 4 (3.8%) | 3 (10.3%) | 0.169 |
| Proton pump inhibitor | 10 (9.4%) | 1 (3.4%) | 0.456 |
| Ethnicity, n (%) | 0.660 | ||
| Caucasian | 83 (78.3%) | 22 (75.9%) | |
| African | 14 (13.2%) | 3 (10.3%) | |
| Other | 9 (8.5%) | 4 (13.8%) | |
| Reason for ICU admission, n (%) | 0.009 | ||
| Sepsis | 34 (32%) | 14 (48%) | |
| Acute respiratory failure | 17 (16%) | 3 (10%) | |
| Post-surgery | 5 (4.7%) | 1 (3.4%) | |
| Post-cardiac arrest | 4 (3.8%) | 1 (3.4%) | |
| Coma | 4 (3.8%) | 5 (17%)* | |
| Non-septic shock | 4 (3.8%) | 3 (10%) | |
| Acute renal failure | 4 (3.8%) | 0 | |
| Other | 34 (32%)* | 2 (7%) | |
| Provenance, n (%) | 0.839 | ||
| Emergency department | 73 (69%) | 21 (72%) | |
| Ward | 2 (1.9%) | 0 | |
| Operating theater | 11 (10%) | 2 (7%) | |
| Other | 20 (30%) | 6 (20.7%) | |
| Admission service, n (%) | 0.924 | ||
| Medical | 80 (75%) | 21 (72%) | |
| Surgical | 26 (25%) | 8 (28%) | |
| Season of admission, n (%) | 0.577 | ||
| Summer | 23 (22%) | 4 (14%) | |
| Autumn | 36 (34%) | 8 (27.5%) | |
| Winter | 28 (26%) | 10 (34.5%) | |
| Spring | 19 (18%) | 7 (24%) | |
| APACHE II score | 17 [12 to 23] | 23 [18 to 29] | 0.001 |
| SOFA score | 4.5 [2 to 8] | 7 [4.5 to 12] | 0.001 |
| ΔSOFA | 3.0 [1 to 6] | −2 [−5.5 to 1.5] | 0.001 |
| BMI (Kg/m2) | 26.7 [23.3 to 30.5] | 26.5 [23.5 to 31.6] | 0.823 |
| Capillary blood glucose (mg/dL) | 146 [113 to 193] | 141 [99 to 189] | 0.485 |
| Albumin (g/dL) | 3.1±0.6 | 2.7±0.8 | 0.004 |
| CRP (mg/L) | 83 [28 to 231] | 168 [23 to 264] | 0.316 |
| Lactate (mmol/L) | 1.4 [1 to 2.1] | 3.1 [1.4 to 5.6] | 0.001 |
| PTH (pg/mL) | 97.9 [56.8 to 198.6] | 200.8 [92.6 to 331] | 0.023 |
COPD, chronic obstructive pulmonary disease; Ca/VITD: calcium/vitamin D ratio; ΔSOFA, SOFA on admission - final SOFA (at ICU discharge or death); BMI, body mass index (Kg/m2); CRP, C-reactive protein; PTH, parathyroid hormone. * denotes a statistically significant change. Summer: December 21 to March 21. Autumn: March 21 to June 21. Winter: June 21 to September 23. Spring: September 23 to December 21.
Table 2. Electrolyte levels and renal function of patients not on acute dialysis at the time of laboratory testing.
| Survivors (n=95) | Non-survivors (n=22) | p-value | |
|---|---|---|---|
| Ca++ (mg/dL) | 4.7 [4.6-4.9] | 4.4 [3.8-4.7] | 0.001 |
| GFR (mL/min/1.73 m2) | 60 [48-60] | 59 [26-60] | 0.042 |
| Phosphorus (mg/dL) | 2.9 [2.3-4.2] | 3.2 [2.7-4.3] | 0.334 |
| Magnesium (mg/dL) | 2.0±0.4 | 1.9±0.36 | 0.345 |
GFR: glomerular filtration rate calculated by the Modification of Diet in Renal Disease Study Group (MDRD). Values are expressed as the total mean ± 95% confidence interval OR the median [interquartile range].
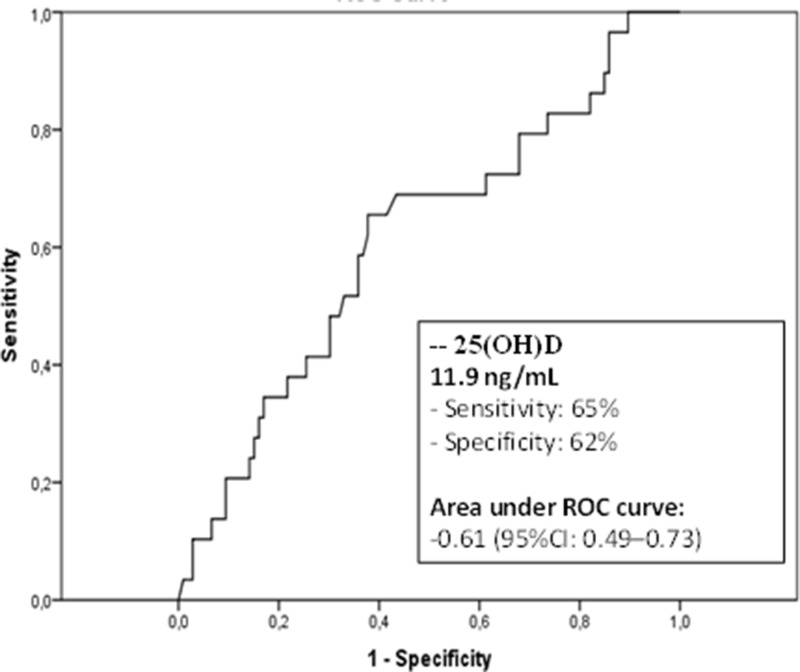
The area under the ROC curve for 25(OH)D levels at admission in relation to mortality was 0.61 (95%CI 0.49-0.73), similar to those for APACHE II (0.7, 95% CI 0.59-0.8) and SOFA scores (0.7, 95% CI 0.6-0.8). The best cutoff was 11.9 ng/mL, with a sensitivity of 65%, a specificity of 62%, a negative predictive value of 86.8%, and a positive predictive value of 32.2% (Figure 1).
Figure 1. Receiver-operating characteristic curve for various cutoff levels of vitamin D [25(OH)D].
Compared with patients with 25(OH)D levels >12 ng/mL, the 59 patients with 25(OH)D≤12 ng/mL (43.7% of the sample) had higher prevalence rates of prior corticosteroid therapy (18% vs. 2.6%; p=0.005) and of acute respiratory failure as a reason for ICU admission (25% vs. 6.6%; p=0.013). Although there is no difference between the median vitamin D levels among seasons, a seasonal association was found for the prevalence of vitamin D deficiency (p=0.041), which was more common in the winter (37.3% vs. 21%) and less common in the summer (10.2% vs. 27.6%). Vitamin D-deficient patients had higher BMIs (27.4 Kg/m2 [24.2-35.2] vs. 26 Kg/m2 [22.7-29.3]; p=0.015). There were no differences in APACHE II scores (19.8±9 vs. 18.7±8.3; p=0.482), SOFA scores at admission (5 [2-8] vs. 5 [3-8]; p=0.170), albumin (3.08±0.78 g/dL vs. 3.06±0.6 g/dL; p=0.87), ionized calcium (4.7 [4.4-5.0] mg/dL vs. 4.7 [4.5-4.8] mg/dL; p=0.770), or PTH (146 [79-260] pg/mL vs. 107 [53-200]; p=0.70), which was elevated in both groups. Patients with 25(OH)D levels ≤12 ng/mL developed greater organ system dysfunction during their ICU stays (ΔSOFA: 0.9±4.7 vs. 3.4±3.9; p=0.001).
Mortality
Over the course of the 28-day observation period, 19 of the 59 patients with 25(OH)D levels ≤12 ng/mL died (32.2%), compared with 10 of the 76 patients with 25(OH)D levels >12 ng/mL (13.2%); this difference was statistically significant (p=0.014). The crude relative risk of death was 2.11 (95% CI 1.08-4.11). After multivariable adjustment, the relative risk remained significantly higher, at 2.20 (95% CI 1.07-4.54) (Table 3).
Table 3. Crude and adjusted mortality by Poisson regression.
| Crude relative risk | 95% CI | Adjusted relative risk | 95% CI | |
|---|---|---|---|---|
| Vitamin D (25[OH]D3 ≤ 12 ng/mL) | 2.11 | 1.08-4.11 | 2.20 | 1.07-4.54 |
| APACHE II | 1.06 | 1.03-1.09 | 1.03 | 0.99-1.8 |
| SOFA | 1.11 | 1.06-1.17 | 1.04 | 0.97-1.11 |
| Lactate | 1.17 | 1.07-1.17 | 1.04 | 0.99-1.10 |
| Albumin | 0.58 | 0.38-0,88 | 0.75 | 0.47-1,19 |
| BMI | 1.01 | 0.96-1.05 | 0.99 | 0.95-1.03 |
| Sepsis | 1.00 | 0.63-1.58 | 1.06 | 0.54-2.07 |
APACHE II: Acute Physiology and Chronic Health Disease Classification System II; SOFA: Sequential Organ Failure Assessment; BMI: body mass index (Kg/m2); CI: confidence interval.
There were no significant differences in mortality (23.5% vs. 15.2%; p=0.275) when the 25(OH)D cutoff was set at 20 ng/mL.
Length of stay
25(OH)D levels ≤12 ng/mL were not associated with any significant differences in the overall length of hospital stay (20 [9-28] vs. 23.5 [14-28], p=0.869) or length of ICU stay (9 [5-21] vs. 9 [5-21], p=0.092), in days.
Mechanical ventilation
Invasive mechanical ventilation was used in 44 (74.6%) patients with 25(OH)D levels ≤12 ng/mL and in 59 (77.7%) patients with 25(OH)D levels >12 ng/mL (p=0.834).
Infection and culture positivity
Overall, 78 patients (57.8%) had positive cultures at some point during their ICU stays. Among the 59 patients with 25(OH)D≤12 ng/mL, a total of 73 infections and 101 positive cultures (including 28 positive blood cultures) were detected. Among the 76 patients with 25(OH)D>12 ng/mL, 88 infections and 121 positive cultures (including 32 positive blood cultures) were detected. The rates of infection and culture positivity per 100 patient-days in the ICU are shown in Table 4. There were no significant differences between groups for either indicator.
Table 4. Mortality, mechanical ventilation and culture positivity according to vitamin D (25[OH]D3) status.
| 25[OH]D3 ≤ 12 ng/mL | 25[OH]D3 > 12 ng/mL | p-value | |
|---|---|---|---|
| 28-day mortality, % | 32.2 | 13.2 | 0.014 |
| Mechanical ventilation,% | 74.6 | 77.4 | 0.834 |
| Infections* | 10.08 | 8.98 | 0.468 |
| Positive cultures* | 13.95 | 12.35 | 0.369 |
| Positive blood cultures* | 3.86 | 3.26 | 0.516 |
ICU: Intensive Care Unit
*Rate of infections per 100 patient-days in ICU
Vitamin D kinetics
A slight decrease in 25(OH)D concentrations occurred over time, reaching statistical significance only on day 21 among all patients. Among non-survivors, a non-significant decline in 25(OH)D levels occurred at the 14th day of ICU stay. The decline in vitamin 25(OH)D levels through ICU stay were not related to mortality or other clinical outcomes.
DISCUSSION
Our findings show that severe vitamin D deficiency is an independent predictor of mortality in critically ill patients. In this sample, a 25(OH)D level of 12 ng/mL was the best cutoff for the identification of critically ill patients at a higher risk of death.
The relationship between mortality and serum vitamin D levels in critically ill patients is a topic of debate in the current literature 13. While some studies have reported such an association ,14-21, others have not ,22-27. The higher mortality rate among severely vitamin D-deficient subjects found in this study is in agreement with the retrospective studies of Braun et al ,14-15. Our study confirms these findings with a prospective design and also demonstrates that the increased mortality attributed to vitamin D deficiency persists even after adjusting the risk for variables associated with mortality. Notably, APACHE II and SOFA scores on admission were similar in 25(OH)D-deficient and non-deficient patients. Nevertheless, the mortality rate was higher in the 25(OH)D-deficient group. In 2012, Higgins et al. 24 conducted a prospective study and found no significant differences in mortality among critically ill vitamin D-deficient patients, although deficient patients had longer ICU lengths of stay. The authors note that their study was not powered to detect differences in mortality. Heterogeneity between studies may explain the different results. The Higgins et al sample was older, and data collection occurred between 2002 and 2003 in Canada, while our data collection occurred in 2012 in Southern Brazil. In our study, we excluded patients with a total length of hospital stay >3 days before ICU transfer, whereas Higgins et al had no such exclusion criterion. We thus excluded patients with normal pre-admission vitamin D levels whose decrease in serum 25(OH)D levels was attributable to hospitalization. Our sample had a higher prevalence of sepsis as the reason for ICU admission. Some studies have suggested that this etiology may be associated with higher mortality related to vitamin D deficiency 6.
Vitamin D may be no more than a marker of severity in critically ill patients. However, the literature reports several vitamin D-dependent physiological mechanisms, suggesting that 25(OH)D deficiency may be implicated in the pathogenesis of organ dysfunction and mortality in critically ill patients, perhaps mediated by its effects on immunity and on the cardiovascular system ,6,7,27-29. As ours was an observational study, we cannot ascertain whether the association between vitamin D deficiency and mortality is causal or attributable to chance. However, even after logistic regression, 25(OH)D levels <12 ng/mL remained an independent predictor of mortality.
The current literature provides no information on the 25(OH)D level that can adequately support the pleiotropic effects of vitamin D. In our population, ROC curve analysis identified 12 ng/mL as the best cutoff for mortality. This finding is similar to that reported in a retrospective study 18. Even if 25(OH)D is merely a marker of severity, these studies suggest that vitamin D levels <10-12 ng/mL can be used to identify patients at a higher risk of mortality and can prompt more intensive monitoring of these patients. In non-critically ill patients, 25(OH)D levels this low are incapable of maintaining serum concentrations of 1,25(OH)2D, leading to clinical repercussions such as rickets and osteomalacia. This study suggests that, similarly to outpatients, critically ill patients with severe vitamin D deficiency suffer the most clinical consequences from the lack of vitamin D; from this finding, we infer that these patients are most likely to benefit from vitamin D replacement during the ICU stay. This hypothesis must still be confirmed by clinical trials. Dietary vitamin D intake is reduced in critically ill patients. Enteral formulas provide 200 to 300 IU/1000 mL, as do multivitamins. The current recommended daily intake of 200 IU/day is not enough to restore vitamin D to a sufficient level 30. Two recent studies showed that higher (yet safe) doses are required for vitamin D replacement in critically ill patients ,31,32. Our results also align with those of a recently published clinical trial 33. In this trial, patients randomized to the intervention group received a loading dose of 540,000 IU of vitamin D followed by 90,000 IU monthly for 5 months. The analysis of the subgroup of patients with baseline levels of vitamin D <10 ng/mL showed a decrease in mortality. This result requires confirmation in future multicenter randomized clinical trials before a therapeutic recommendation can be generated.
As in other prospective studies ,24,34, we found no significant differences in infection and culture positivity rates between vitamin D-deficient and non-deficient patients. Previous studies have shown a trend toward a greater incidence of infections but have lacked the statistical power to conclusively establish this association. Higher rates of infection and culture positivity have been observed among vitamin D-deficient patients in retrospective studies 14, which, despite design bias, are able to include larger samples.
Although the literature suggests an association between 25(OH)D deficiency and morbidity in critically ill patients, our study did not find a longer duration of mechanical ventilation among patients with severe vitamin D deficiency. These results are consistent with other recently published prospective studies conducted in critically ill adult ,17,18,21 and pediatric 26 populations.
In this study, we observed a modest decrease in 25(OH)D levels in critically ill patients, with a tendency toward lower levels among non-survivors. Only two other studies have prospectively measured vitamin D levels during the course of an ICU stay ,24,28. In both studies, data collection was restricted to 7 and 10 days of hospitalization. In our study, we measured 25(OH)D levels once weekly until the 28th day in the ICU and found that non-survivors may have experienced a greater decrease in 25(OH)D concentrations after the second week in the ICU. As the half-life of 25(OH)D is approximately 15 days, these findings partly reflect the lack of vitamin D intake during the ICU stay due to a lack of sunlight exposure and low vitamin D concentrations in the diet provided to critically ill patients. Prior studies have shown a decrease in vitamin D-binding protein (VDBP) concentrations during an ICU stay 29, corroborating the decline in 25(OH)D levels during admission, particularly in non-survivors. This behavior is analogous to that observed with other constitutional proteins, the synthesis of which is inhibited as metabolic pathways shift to the production of acute phase proteins in the setting of systemic inflammatory response syndrome (SIRS). Therefore, the criticality of the illness may also be reflected by serum vitamin D levels.
The high prevalence of vitamin D deficiency and insufficiency observed in our sample is consistent with other studies carried out at other centers, as well as with the high prevalence found in the non-critically ill population in our center 35. We are unaware of other reports of the prevalence and incidence of vitamin D deficiency in critically ill patients in South and Central America.
This study has some limitations. It was conducted at a tertiary care center with a largely Caucasian population and a relatively small sample size. The infection rate was solely calculated based on clinical diagnoses made by the assisting physicians. We cannot completely rule out the potential influence of unassessed variables—such as the volume infused in the first hours after admission or the cause of death—on mortality 36. Vitamin D measurements were not performed in duplicate. VDBP and biologically active 25(OH)D metabolites were not measured. Notably, albumin levels did not differ according to the vitamin D status. Importantly, the pleiotropic effects of vitamin D are dependent on local vitamin D concentrations; it is biologically plausible that these effects would be independent of serum 25(OH)D levels. Furthermore, the association between mortality and severe vitamin D deficiency found in this observational study is not enough to recommend the measurement of vitamin D levels in critically ill patients or supplementation in those with vitamin D deficiency; such changes in practice would depend on the results of clinical trials, which are ongoing.
The strengths of this study include its assessment of a hard endpoint (mortality) that is not prone to measurement bias. In addition to 25(OH)D levels, we measured other components that affect the vitamin D axis such as PTH, ionized calcium, magnesium, and phosphorus. The method employed for 25(OH)D measurement was the DiaSorin radioimmunoassay, which is currently considered the standard for this purpose in view of its excellent agreement with isotope dilution liquid chromatography-tandem mass spectroscopy (LC-MS) ,13,37.
In our study, PTH levels were higher among non-survivors. The elevation of PTH among critically ill patients is due to factors such as peripheral resistance to the action of PTH, especially in the acute phase, in hypovitaminosis D and in hypocalcemia, and may be related to morbidity in critically ill patients 38. Thus, PTH itself appears to be a marker of severity, as observed with TSH or cortisol; however, PTH does not appear to be a potential therapeutic target, unlike vitamin D.
This study found that low vitamin D levels on admission are an independent risk factor for mortality in critically ill patients. The best cutoff of 25(OH)D levels on admission for stratifying the risk of death among critically ill patients was 12 ng/mL. As well as being markers of disease severity, low vitamin D levels and vitamin D kinetics during ICU admission may be causally associated with mortality and may serve as indicators for vitamin D supplementation in this patient population. Nevertheless, randomized trials are required to confirm this hypothesis.
AUTHOR CONTRIBUTIONS
Moraes RB performed the study conception and design, sample collection, data analysis and manuscript preparation. Czepielewski MA participated in the study conception, design and supervision and manuscript preparation and revision. Lisboa TC participated in the sample collection and performed the statistical analysis. Wawrzeniak IC, Marques LS and Nagel FM were involved in the study design and sample collection and storage. Friedman G participated in the study design, data analysis and manuscript preparation and revision. All authors read and approved the final manuscript.
Acknowledgments
This work was supported by grants from Brazilian public agencies of research: Fundo de Incentivo è Pesquisa e Eventos do Hospital de Clínicas de Porto Alegre (FIPE-HCPA) and Grupo de Pesquisa e Pós Graduação - Endocrinologia da Universidade Federal do Rio Grande do Sul (GPPG-Endocrinologia-HCPA).
The authors would like to thank Martha Bergman Senger, Maria Clara Medina Correa, and Luiz Werres Junior at the Hospital de Clínicas de Porto Alegre Biochemistry and Radioimmunoassay Unit for their invaluable assistance with sample processing, Dr. Laisa Bonzanini and undergraduate research fellows Manuela M. Marimon, Luiza Burin, Helena T. Schroeder, and Mauricio Vieira Rodrigues for their assistance with data collection and entry.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Pramyothin P, Holick MF. Vitamin D supplementation: guidelines and evidence for subclinical deficiency. Curr Opin Gastroenterol. 2009;28((2)):139–50. doi: 10.1097/MOG.0b013e32835004dc. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96((7)):1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 3.Biesalski HK. Vitamin D recommendations: beyond deficiency. Ann Nutr Metab. 2011;59((1)):10–6. doi: 10.1159/000332066. [DOI] [PubMed] [Google Scholar]
- 4.Mastala Y, Nyangulu P, Banda RV, Mhemedi B, White SA, Allain TJ. Vitamin D Deficiency in Medical Patients at a Central Hospital in Malawi: A Comparison with TB Patients from a Previous Study. PLoS One. 2013;8((3)):e59017. doi: 10.1371/journal.pone.0059017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94((1)):26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeng L, Yamshchikov AV, Judd SE, Blumberg HM, Martin GS, Ziegler TR, et al. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med. 2009;7:28. doi: 10.1186/1479-5876-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carthy EP, Yamashita W, Hsu A, Ooi BS. 1,25-Dihydroxyvitamin D3 and rat vascular smooth muscle cell growth. Hypertension. 1989;13((6)):954–9. doi: 10.1161/01.HYP.13.6.954. [DOI] [PubMed] [Google Scholar]
- 8.Gysemans CA, Cardozo AK, Callewaert H, Giulietti A, Hulshagen L, Bouillon R, et al. 1,25-Dihydroxyvitamin D3 modulates expression of chemokines and cytokines in pancreatic islets: Implications for prevention of diabetes in nonobese diabetic mice. Endocrinology. 2005;146((4)):1956–64. doi: 10.1210/en.2004-1322. [DOI] [PubMed] [Google Scholar]
- 9.Lee P, Nair P, Eisman JA, Center JR. Vitamin D deficiency in the intensive care unit: an invisible accomplice to morbidity and mortality? Intensive Care Med. 2009;35((12)):2028–32. doi: 10.1007/s00134-009-1642-x. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145((4)):247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 11.Ersfeld DL, Rao DS, Body JJ, Sackrison JL, Jr, Miller AB, Parikh N, et al. Analytical and clinical validation of the 25 OH vitamin D assay for the liaison automated analyzer. Clin Biochem. 2004;37((10)):867–74. doi: 10.1016/j.clinbiochem.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. doi: 10.1093/biomet/73.1.13. [DOI] [Google Scholar]
- 13.Krishnan A, Nair P, Venkatesh B.Vitamin D and the Ctitically ill Patient: An Update for the IntensivistIn: Vincent JL.(Ed) Annual Update in Intensive Care and Emergency Medicine Springer; Verlag Berlin Heidelberg: 2013183–95. [Google Scholar]
- 14.Braun A, Chang D, Mahadevappa K, Gibbons FK, Liu Y, Giovannucci E, et al. Association of low serum 25-hydroxyvitamin D levels and mortality in the critically ill. Crit Care Med. 2011;39((4)):671–7. doi: 10.1097/CCM.0b013e318206ccdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braun AB, Gibbons FK, Litonjua AA, Giovannucci E, Christopher KB. Low serum 25-hydroxyvitamin D at critical care initiation is associated with increased mortality. Crit Care Med. 2012;40((1)):63–72. doi: 10.1097/CCM.0b013e31822d74f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee P, Eisman JA, Center JR. Vitamin D deficiency in critically ill patients. N Engl J Med. 2009;360((19)):1912–4. doi: 10.1056/NEJMc0809996. [DOI] [PubMed] [Google Scholar]
- 17.McKinney JD, Bailey BA, Garrett LH, Peiris P, Manning T, Peiris NA. Relationship between vitamin D status and ICU outcomes in veterans. J Am Med Directors Assoc. 2011;12((3)):208–11. doi: 10.1016/j.jamda.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Venkatram S, Chilimuri S, Adrish M, Salako A, Patel M, Diaz-Fuentes G. Vitamin D deficiency is associated with mortality in the medical intensive care unit. Crit Care. 2011;15((6)):R292. doi: 10.1186/cc10585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews LR, Ahmed Y, Wilson KL, Griggs DD, Danner OK. Worsening severity of vitamin D deficiency is associated with increased length of stay, surgical intensive care unit cost, and mortality rate in surgical intensive care unit patients. Am J Surg. 2012;204((1)):37–43. doi: 10.1016/j.amjsurg.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnson Y, Gringauz I, Itzhaky D, Amital H. Vitamin D deficiency is associated with poor outcomes and increased mortality in severely ill patients. QJM. 2012;105((7)):633–9. doi: 10.1093/qjmed/hcs014. [DOI] [PubMed] [Google Scholar]
- 21.Flynn L, Zimmerman LH, McNorton K, Dolman M, Tyburski J, Baylor A, et al. Effects of vitamin D deficiency in critically ill surgical patients. Am J Surg. 2012;203((3)):379–82. doi: 10.1016/j.amjsurg.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Lucidarme O, Messai E, Mazzoni T, Arcade M, du Cheyron D. Incidence and risk factors of vitamin D deficiency in critically ill patients: results from a prospective observational study. Intensive Care Med. 2010;36((9)):1609–11. doi: 10.1007/s00134-010-1875-8. [DOI] [PubMed] [Google Scholar]
- 23.Cecchi A, Bonizzoli M, Douar S, Mangini M, Paladini S, Gazzini B, et al. Vitamin D deficiency in septic patients at ICU admission is not a mortality predictor. Minerva Anestesiol. 2011;77((12)):1184–9. [PubMed] [Google Scholar]
- 24.Higgins DM, Wischmeyer PE, Queensland KM, Sillau SH, Sufit AJ, Heyland DK. Relationship of vitamin D deficiency to clinical outcomes in critically ill patients. JPEN J Parenter Enteral Nutr. 2012;36((6)):713–20. doi: 10.1177/0148607112444449. [DOI] [PubMed] [Google Scholar]
- 25.McNally JD, Menon K, Chakraborty P, Fisher L, Williams KA, Al-Dirbashi, et al. The association of vitamin D status with pediatric critical illness. Pediatrics. 2012;130((3)):429–36. doi: 10.1542/peds.2011-3059. [DOI] [PubMed] [Google Scholar]
- 26.Rippel C, South M, Butt WW, Shekerdemian LS. Vitamin D status in critically ill children. Intensive Care Med. 2012;38((12)):2055–62. doi: 10.1007/s00134-012-2718-6. [DOI] [PubMed] [Google Scholar]
- 27.Pilz S, Marz W, Wellnitz B, Seelhorst U, Fahrleitner-Pammer A, Dimai HP, et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab. 2008;93((10)):3927–35.0. doi: 10.1210/jc.2008-0784. [DOI] [PubMed] [Google Scholar]
- 28.Nair P, Lee P, Reynolds C, Nguyen ND, Myburgh J, Eisman JA, et al. Significant perturbation of vitamin D-parathyroid-calcium axis and adverse clinical outcomes in critically ill patients. Intensive Care Med. 2013;39((2)):267–74. doi: 10.1007/s00134-012-2713-y. [DOI] [PubMed] [Google Scholar]
- 29.Dahl B, Schiodt FV, Nielsen M, Kiaer T, Williams JG, Ott P. Admission level of Gc-globulin predicts outcome after multiple trauma. Injury. 1999;30((4)):275–81. doi: 10.1016/S0020-1383(99)00080-7. [DOI] [PubMed] [Google Scholar]
- 30.ASPEN Board of Directors and the Clinical Guidelines Task Force Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J Parenter Enteral Nutr. 2002;26((1suppl)):1SA–138SA. [PubMed] [Google Scholar]
- 31.Mata-Granados JM, Vargas-Vasserot J, Ferreiro-Vera C, Luque de Castro MD, Pavon RG, Quesada Gomez JM. Evaluation of vitamin D endocrine system (VDES) status and response to treatment of patients in intensive care units (ICUs) using an on-line SPE-LC-MS/MS method. J Steroid Biochem Mol Biol. 2010;121((1-2)):452–5. doi: 10.1016/j.jsbmb.2010.03.078. [DOI] [PubMed] [Google Scholar]
- 32.Amrein K, Sourij H, Wagner G, Holl A, Pieber TR, Smolle KH, et al. Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: a randomized, double-blind, placebo-controlled pilot study. Crit Care. 2011;15((2)):R104. doi: 10.1186/cc10120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amrein K, Schnedl C, Berghold A, Pieber TR, Dobnig H. Correction of vitamin D deficiency in critically ill patients - VITdAL@ICU study protocol of a double-blind, placebo-controlled randomized clinical trial. BMC Endocr Disord. 2012;12:27. doi: 10.1186/1472-6823-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madden K, Feldman HA, Smith EM, Gordon CM, Keisling SM, Sullivan RM, et al. Vitamin D deficiency in critically ill children. Pediatrics. 2012;130((3)):421–8. doi: 10.1542/peds.2011-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scalco R, Premaor MO, Froehlich PE, Furlanetto TW. High prevalence of hypovitaminosis D and secondary hyperparathyroidism in elders living in nonprofit homes in South Brazil. Endocr. 2008;33((1)):95–100. doi: 10.1007/s12020-008-9061-2. [DOI] [PubMed] [Google Scholar]
- 36.Krishnan A, Ochola J, Mundy J, Jones M, Kruger P, Duncan E, et al. Acute fluid shifts influence the assessment of serum vitamin D status in critically ill patients. Crit Care. 2010;14((6)):R216. doi: 10.1186/cc9341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farrell CJ, Martin S, McWhinney B, Straub I, Williams P, Herrmann M. State-of-the-art vitamin D assays: a comparison of automated immunoassays with liquid chromatography-tandem mass spectrometry methods. Clin Chem. 2012;58((3)):531–42. doi: 10.1373/clinchem.2011.172155. [DOI] [PubMed] [Google Scholar]
- 38.Nair P, Lee P, Reynolds C, Nguyen ND, Myburgh J, Eisman JA, et al. Significant perturbation of vitamin D-parathyroid-calcium axis and adverse clinical outcomes in critically ill patients. Intensive Care Med. 2013;39((2)):267–74. doi: 10.1007/s00134-012-2713-y. [DOI] [PubMed] [Google Scholar]