Abstract
OBJECTIVES:
Fibrolamellar hepatocellular carcinoma is a rare primary malignant liver tumor that differs from conventional hepatocellular carcinoma in several aspects. The aim of this study was to describe the clinical, surgical and histopathological features of fibrolamellar hepatocellular carcinoma and to analyze the factors associated with survival.
METHODS:
We identified 21 patients with histopathologically diagnosed fibrolamellar hepatocellular carcinoma over a 22-year period. Clinical information was collected from medical records and biopsies, and surgical specimens were reviewed.
RESULTS:
The median age at diagnosis was 20 years. Most patients were female (67%) and did not have associated chronic liver disease. Most patients had a single nodule, and the median tumor size was 120 mm. Vascular invasion was present in 31% of patients, and extra-hepatic metastases were present in 53%. Fourteen patients underwent surgery as the first-line therapy, three received chemotherapy, and four received palliative care. Eighteen patients had “pure fibrolamellar hepatocellular carcinoma,” whereas three had a distinct area of conventional hepatocellular carcinoma and were classified as having “mixed fibrolamellar hepatocellular carcinoma.” The median overall survival was 36 months. The presence of “mixed fibrolamellar hepatocellular carcinoma” and macrovascular invasion were predictors of poor survival. Vascular invasion was associated with an increased risk of recurrence in patients who underwent surgery.
CONCLUSION:
Fibrolamellar hepatocellular carcinoma was more common in young female patients without chronic liver disease. Surgery was the first therapeutic option to achieve disease control, even in advanced cases. Vascular invasion was a risk factor for tumor recurrence. The presence of macrovascular invasion and areas of conventional hepatocellular carcinoma were directly related to poor survival.
Keywords: Fibrolamellar hepatocellular carcinoma, Liver cancer, Liver neoplasms, Hepatocellular carcinoma (fibrolamellar variant)
INTRODUCTION
Fibrolamellar hepatocellular carcinoma (FLHCC) was first described by Edmondson in 1956 as “a rare, distinct form of primary hepatocellular carcinoma” 1. It is histologically characterized by large polygonal hepatocytes with eosinophilic and granular cytoplasm surrounded by abundant, thick fibrous bands, which are arranged in a parallel or lamellar distribution 2,3. FLHCC represents 1 - 2% of all primary liver tumors, which is a small percentage compared with that of conventional HCC, which represents 60-80% of liver cancers 4,5. To the best of our knowledge, there are only four reports of FLHCC from Latin American populations ,6-8 and no previous reports from Brazil. The actual incidence of FLHCC in Latin America remains unknown.
FLHCC differs from conventional HCC in several aspects, such as demographics, risk factors and tumor markers 4,5. FLHCC typically occurs in younger patients, with a median age at diagnosis of 25 years old, and has a controversial gender predilection ,9-11. In contrast to conventional HCC, the vast majority of cases occur in patients without chronic liver disease or cirrhosis 4,9,12. Conventional tumor markers for HCC, such as alpha-fetoprotein (AFP), are normal in the majority of patients 4.
Controversy exists regarding whether FLHCC has a better prognosis than conventional HCC. Several studies had formerly described FLHCC as more indolent and associated with a better prognosis 10,, but subsequent studies found that survival after resection was similar in patients with FLHCC and conventional HCC without cirrhosis ,16-18. It is conceivable that a better outcome for FLHCC would be derived from the absence of cirrhosis, high resectability rates, and a younger age at presentation rather than from the distinct biological and clinicopathological features of the tumor itself 4,9,16. A recent multicenter French study has suggested that the presence of some tumor heterogeneity, with classical FLHCC intermingled with conventional HCC, would be responsible for a worse prognosis in a subgroup they called “mixed FLHCC” 19.
The aim of this study was to describe the clinical, surgical, and histopathological features from a series of 21 cases of fibrolamellar hepatocellular carcinoma, with an emphasis on prognostic factors for survival.
MATERIALS AND METHODS
Medical records from 21 patients with histopathologically diagnosed FLHCC from 1990 to 2012 were reviewed and searched for patient demographics, medical histories, the results of imaging studies, laboratory tests, surgical procedures, and outcomes. An experienced liver pathologist (VAFA) reviewed all biopsies and surgical specimens. Patients were staged according to the 7th edition of the American Joint Committee on Cancer (AJCC) staging criteria 20. The study was approved by the Hospital das Clínicas from the University of São Paulo School of Medicine Ethics Committee and was conducted in accordance with the Declaration of Helsinki.
The pathological diagnosis of FLHCC was based on the presence of the following criteria: 1) large tumor cells with deeply eosinophilic cytoplasm; 2) the presence of central macronucleoli; and 3) abundant, fibrous stroma arranged in thin, parallel lamellae around the tumor cells 4.
Similarly to the proposal by Malouf et al. 19, the cases were divided into two groups: pure FLHCC, in which the diagnostic criteria described above were present throughout all samples available from the tumor, and mixed FLHCC, in which at least one focus of conventional HCC was found in a tumor with extensive areas of typical FLHCC. The presence of micro- or macrovascular invasion, satellite nodules, and extra-hepatic disease was also evaluated.
Immunohistochemical staining for the markers Hep-Par, Arginase-1, Muc-1, CK19, CK7, and chromogranin was performed in all specimens. To avoid endogenous avidin-biotin interactions, which might yield undesirable background staining, signal amplification was achieved by the use of a short-polymer peroxidase complex (Novolink, Novocastra, U.K.). The immunoprofile was used to confirm the diagnosis: classical FLHCC was expected to be positive for Hep-Par, Arginase-1, and CK-7 and to present only focal staining for Muc-1 and CK19, whereas the neuroendocrine marker chromogranin was expected to be negative.
Overall survival (OS) was defined as the interval between the diagnosis and the last follow up or death from any cause. A survival analysis was conducted using the Kaplan-Meier method 21 and compared by simple and multiple Cox Regression analyses using R version 2.15.2. Differences were considered significant at p < 0.05. Whenever it was not possible to use the Cox regression, differences were evaluated by the Log-Rank test using R version 2.15.2 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria, 2011)22. We analyzed the impact of the following factors on OS: treatment (surgical vs. nonsurgical), AJCC staging (I/II vs. III/IV), recurrence, and radiological and pathological features (macro- and microvascular invasion, extra-hepatic disease, and pathologic classification - pure FLHCC vs. mixed FLHCC).
RESULTS
The clinicopathologic features and tumor staging, based on imaging studies and histopathological examination, of 21 patients with FLHCC are summarized in Table 1.
Table 1.
Clinicopathological features of 21 patients with fibrolamellar hepatocellular carcinoma.
| Characteristics | All patients |
|---|---|
| Age (years, median, range) | 20 (13 – 49) |
| Gender (male/female, %) | 33/67 |
| Tumor staging (TNM, n, %) | |
| I or II | 6 (28%) |
| III or IV | 15 (72%) |
| Tumor size (cm, median, range) | 12 (6 – 19) |
| Tumor size>10 cm (n, %)* | 13 (68%) |
| Vascular invasion (n, %)* | 8 (42%) |
| Microvascular invasion | 3 (16%) |
| Macrovascular invasion | 5 (26%) |
| Extra-hepatic metastasis (n, %) | 13 (62%) |
| Treatment (n, %) | |
| Surgery | 9 (43%) |
| Surgery + systemic therapy | 5 (24%) |
| QT Chemotherapy | 3 (14%) |
| Palliative care | 4 (19%) |
| Recurrence (n, %)** | 8 (80%) |
| Site of recurrence (n, %) | |
| Liver | 3 (38%) |
| Lymph node | 3 (38%) |
| Liver and lymph node | 1 (12%) |
| Peritoneal implants | 1 (12%) |
| Histology (n, %) | |
| Pure fibrolamellar hepatocellular carcinoma | 18 (86%) |
| Mixed fibrolamellar hepatocellular carcinoma | 3 (14%) |
*Tumor size and vascular invasion were evaluated in 19 patients; ** Recurrence was evaluated in 10 patients.
The diagnosis was made by percutaneous biopsy in 62% of cases and by surgical biopsy or surgical specimen examination in 38%. The histological review confirmed the diagnosis of FLHCC in all 21 specimens. Using the proposed sub-classification by Malouf et al., 18 patients had “pure FLHCC,” whereas three patients (14%) had at least one distinct area of conventional HCC and were thus classified as having “mixed FLHCC”. Macrovascular invasion was analyzed according to radiological and pathological findings. Additionally, histological assessment was performed to search for microvascular invasion.
None of the patients had tumor-associated chronic liver disease or cirrhosis or a history of hepatic adenoma, and the AFP level was normal in all patients at diagnosis. Two patients had portal hypertension associated with portal vein thrombosis. All patients had symptoms at the time of presentation, and the most common symptoms were abdominal pain (65%), weight loss (60%), abdominal mass (50%) and nausea (35%). Two patients in our series had been pregnant but had delivered shortly before tumor diagnosis.
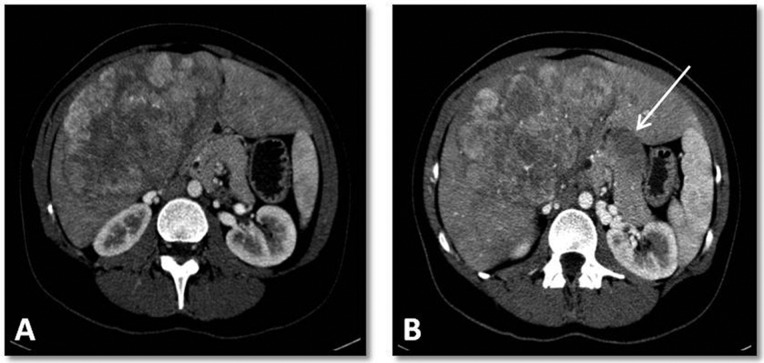
Using imaging studies, tumor staging was evaluated in 20 patients (Figure 1). In radiological studies at diagnosis, most patients had a single nodule (90%), and the median tumor size was 120 mm (6.6-19 cm). Vascular invasion was present in 30% of patients, and extra-hepatic metastases were present in 53%; all cases with extra-hepatic metastases involved metastasis to regional lymph nodes. One patient had also an image suggestive of lung metastasis, whereas 3 patients had mediastinal lymph node metastases.
Figure 1.
Abdominal computerized tomography of a patient with fibrolamellar hepatocellular carcinoma. (A) Contrast-enhanced, arterial-phase computed tomography showing heterogeneous enhancement of a large solid mass with small necrotic foci in segments IV and V of the liver. (B) Contrast-enhanced, arterial-phase computed tomography showing an enlarged lymph node in the hepatic portal (arrow).
Twenty patients underwent surgery. In six cases, the tumor was considered unresectable, due either to extensive liver involvement or to the presence of extra-hepatic disease. Of these, four patients received palliative care, and two received chemotherapy. One patient who did not undergo surgery due to the presence of supraclavicular lymph node metastasis. Fourteen patients underwent resection as the first-line therapy. Nine patients (43%) underwent only surgical resection. Five patients (24%) received surgery plus chemotherapy.
Pathological staging was studied in 13 patients who underwent resection (Figure 2). The median tumor size was 12 cm (6-19 cm) in the surgical specimens. Vascular invasion was present in 5/13 (38%) patients, microvascular invasion was present in 3 patients, and macrovascular invasion was present in 2 patients. One patient had satellite nodules. Extra-hepatic metastases were observed in 6/13 (46%) patients, and the most common sites of metastasis were intra-abdominal lymph nodes (5/6, 83%). A positive surgical margin was present in 5/13 (38%) patients.
Figure 2.
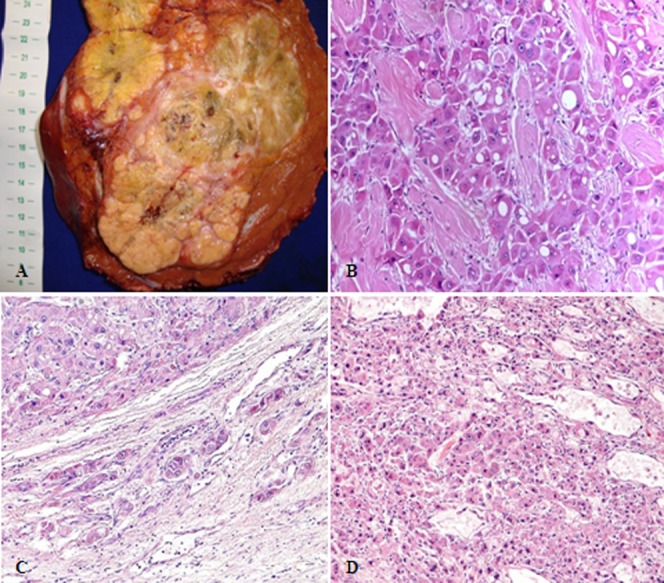
Pathological aspects of fibrolamellar hepatocellular carcinoma. (A) Surgical specimen of a large, well-circumscribed, yellow, heterogeneous mass with areas of necrosis and a central scar. (B) Histopathology of “pure fibrolamellar hepatocellular carcinoma”: large tumor cells with deeply eosinophilic cytoplasm, round, central macronucleoli, and abundant fibrous stroma arranged in thin parallel lamellae around the tumor cells. (C): Fibrolamellar hepatocellular carcinoma showing microvascular invasion; (D): “Mixed fibrolamellar hepatocellular carcinoma”: classical fibrolamellar hepatocellular carcinoma cells are found intermingled with neoplastic cells with features of conventional hepatocellular carcinoma.
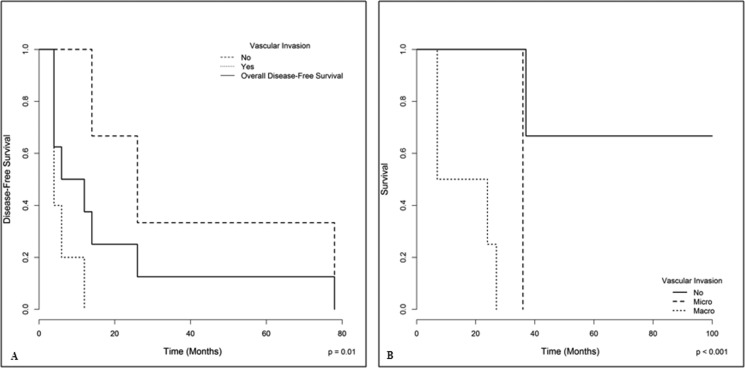
Recurrence after resection was evaluated in 10 patients; eight of them presented FLHCC recurrence. The median time from resection to recurrence was 12 months (Figure 3A). The most common sites of recurrence were the liver (3/8) and abdominal lymph nodes (3/8). One patient had recurrence in both the liver and lymph nodes, and one had peritoneal implants. Two patients underwent surgery for liver recurrences, and both evolved new recurrences. One of them underwent another resection; this patient has now been living disease free for 20 years. The other patient had peritoneal mass recurrence, which evolved to progressive disease and death. Three patients with recurrences received chemotherapy, and the other three received palliative care. As depicted in Figure 3A, the presence of either macro- or microvascular invasion on anatomopathological examination was found to be significantly related to an increased risk of recurrence (p = 0.01). All patients without recurrence were still alive at the end of the study.
Figure 3.
(A) Overall disease-free survival of resected patients with fibrolamellar hepatocellular carcinoma according to vascular invasion. Vascular invasion, either macro- or microscopic, was associated with an increased risk of tumor recurrence (Cox regression, p<0.01). (B) The overall survival of patients with fibrolamellar hepatocellular carcinoma according to the presence of vascular invasion. On the one hand, the presence of macrovascular invasion had a significant impact on survival; on the other hand, although the microvascular invasion data may suggest a trend, the small number of cases (3) did not allow for a significant discrimination with cases without any vascular invasion.
The tumor staging of the 21 patients using the TNM system, according to radiological and pathological findings, was as follows: stage I (TNM) in 5 patients, stage II in one, stage III in three patients and stage IV in 12 patients (60%) (Table 1).
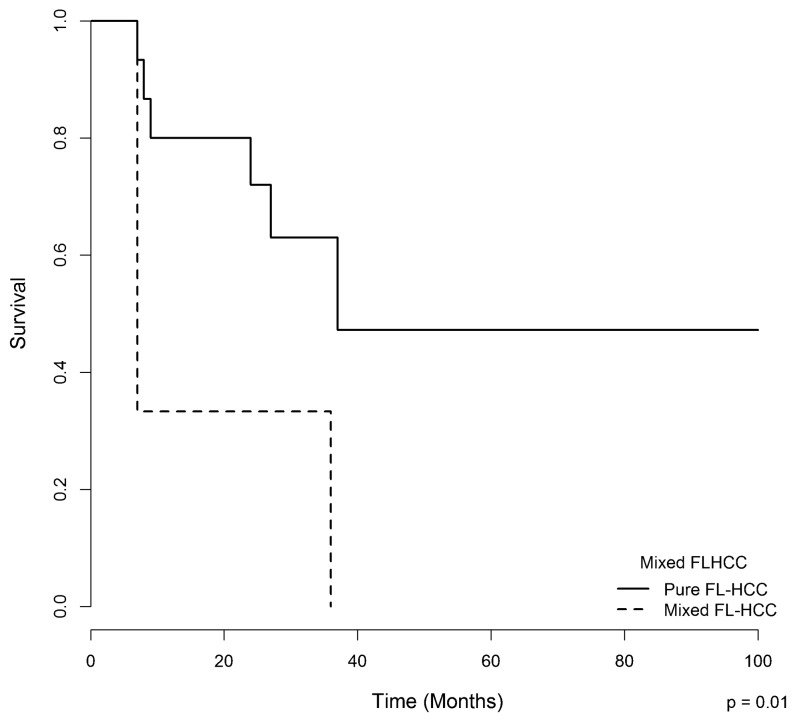
Nineteen patients were followed, with a mean follow-up of 24 months. Fourteen patients died; five patients are still alive, three of whom are disease-free. Two patients are being treated with chemotherapy and are experiencing progressive disease. The median overall survival of the entire series was 36 months (6-228 months). The presence of macrovascular invasion, according to radiological and pathological findings (Figure 3B), and the presence of areas of conventional HCC in “mixed FLHCC” (Figure 4) were associated with a worse survival, as assessed by the Log-Rank test.
Figure 4.
The overall survival of patients with fibrolamellar hepatocellular carcinoma according to the presence of areas of conventional hepatocellular carcinoma (mixed hepatocellular carcinoma vs. pure fibrolamellar hepatocellular carcinoma).
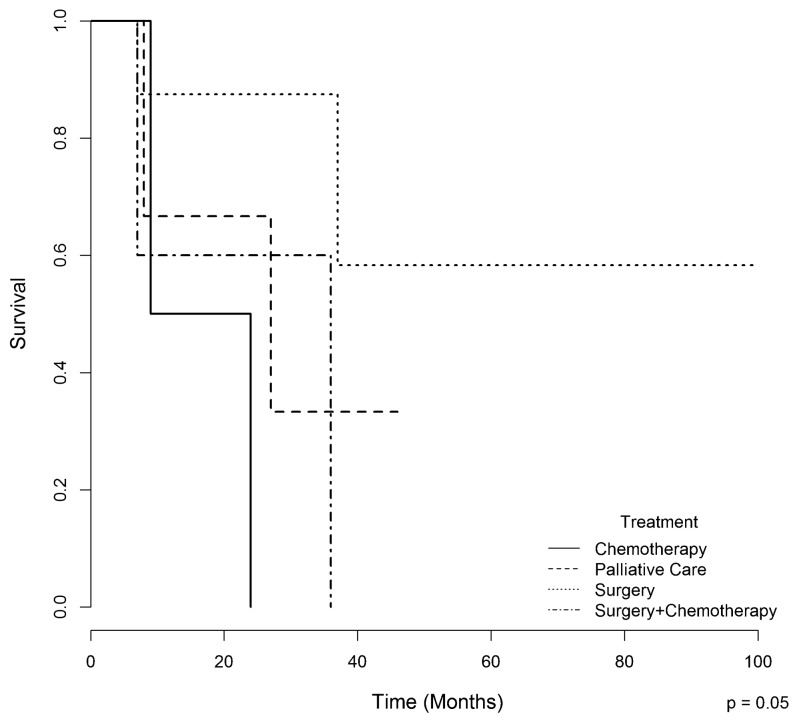
In the univariate and multivariate analyses, the presence of “mixed FLHCC” was a predictor of poor survival (p = 0.04). Patients who underwent surgery had a better one-year survival rate (87.5%) compared with those who underwent chemotherapy, surgery plus chemotherapy, and palliative care (66%, 60%, and 50%, respectively, p = 0.05) (Figure 5).
Figure 5.
The overall survival of patients with fibrolamellar hepatocellular carcinoma according to treatment.
DISCUSSION
FLHCC is an infrequent primary liver tumor presenting significant epidemiological and clinical differences compared with conventional hepatocellular carcinoma. Although the real incidence and clinicopathological features of FLHCC in Latin America remain poorly understood due to scarce available information ,6-8, the present study of 21 cases from a single tertiary hospital suggests either that the incidence of cases at this particular hospital might be increased due to increased referrals or that FLHCC may be less rare than expected. In our series, as observed in other studies, FLHCC occurred in young patients with a median age of 20 years and without associated chronic liver disease ,11-23. Most patients in our study were female (14/21, 67%), similarly to some other studies 8,11; however, other series did not show a gender predilection for FLHCC 9,10,23. Two patients in our series had been pregnant shortly before tumor diagnosis. Some cases of FLHCC have been reported during pregnancy 24,25, and some studies have associated FLHCC with the use of oral contraceptives 4,8. The influence of hormonal stimulation in the development of FLHCC remains unclear. Thus, future studies should address the hypothesis raised herein that estrogens might play a role in the pathophysiology of this tumor.
The data described in the present series highlight relevant epidemiological differences between FLHCC and conventional HCC. In contrast with our study on FLHCC, a multicenter Brazilian study conducted by Carrilho et al. reported that 1,405 patients who were diagnosed with conventional HCC had a median age at diagnosis of 59 years old and were mostly male (78%); additionally, the vast majority of these patients (98%) had liver cirrhosis 27. More clinical and epidemiological differences between FLHCC and HCC have been described previously 10,12.
In the present study, all patients were symptomatic at diagnosis, and the most common symptoms were abdominal pain, weight loss, and nausea, as described in other series 6,8,23, which likely reflects an advanced stage of disease at diagnosis (60% had a pTNM stage IV). Most patients presented a large hepatic mass; the median tumor size was 12 cm. More than half of the patients presented with extra-hepatic disease, including remarkable lymph node involvement. Vascular invasion was also present in 42% of the patients, as evaluated by radiological and pathological findings.
Among the 20 patients who underwent surgery, six tumors were considered unresectable due either to extensive liver involvement or to the presence of unresectable extra-hepatic disease. This was similar to the 13 unresectable cases found by Stipa et al. 12 in a series of 41 patients with FLHCC. Out of the 13 patients resected in our series with pathological staging, the median tumor size was 12 cm, 38% had vascular invasion, and 46% had lymph node metastases. Maniaci et al. 23, reported a series of 10 FLHCC cases with a mean tumor diameter of 13.4 cm, and 7/10 resected patients had lymph node metastases. In another series reported by Pinna et al. 28, 41 patients with FLHCC underwent surgical treatment; these cases had a median tumor size of 13 cm, microvascular invasion (51.2%), and macrovascular invasion (24.4%); 90% were pTNM stage IV.
Diagnosis at an advanced stage of disease may be related to the fact that FLHCC occurs in young patients without chronic liver disease who are thus not subjected to a screening program. In our institution, cirrhotic patients are evaluated through an HCC screening program, which allows for diagnosis in the early and asymptomatic stages of disease 26. In a Brazilian multicenter study, most patients were asymptomatic, and the majority of conventional HCC cases were diagnosed in the early or intermediate stage 27.
Although FLHCC is commonly diagnosed in the advanced stage, resection remains the best option to achieve disease control 23,28,29. In our series, 17/21 (81%) patients were treated. Fourteen patients underwent surgery as the first-line therapy, and liver resection was associated with lymphadenectomy in six. Nine patients (43%) underwent only resection, and five also received adjuvant chemotherapy. The other three patients (14%) received chemotherapy alone. In the present study, patients who underwent surgical treatment alone showed a strong tendency towards a better survival when compared with those who underwent other forms of treatment, such as chemotherapy, surgery plus chemotherapy, or palliative care (p = 0.05). This finding is likely related to an early tumor stage at diagnosis, allowing these patients to undergo surgery without adjuvant chemotherapy. Other studies also reported a better survival in patients who underwent surgical treatment compared with unresectable patients 8,11,12.
The follow-up of the 10 patients who were surgically treated showed tumor recurrence in eight cases. The median time to recurrence was 12 months, and vascular invasion was associated with an increased risk of tumor recurrence. Previous studies have also reported a high recurrence rate, ranging from 36 - 100%, with a median time to relapse between 10 and 33 months 11,28,29. In a series of 28 patients who underwent resection, Stipa et al. 12 reported that the five-year recurrence-free survival rate was 18%, and the only significant negative prognostic factor for recurrence was the presence of lymph node metastases. Pinna et al. 28 reported that the pTNM stage was significantly associated with tumor-free survival. All patients without tumor recurrence were still alive at the end of our study, suggesting that postsurgical tumor recurrence is likely a major prognostic factor in patients with FLHCC.
In contrast to adenocarcinomas from almost all other organs, for which the identification of histological subtypes has led to the search for specific molecular profiles and, in many instances, to the classification of entirely different clinical-pathological entities, most studies report “hepatocellular carcinoma” as a generic tumor type, and thus do not acknowledge the potential for different origins and biological behaviors. In this regard, Malouf et al. 30 have recently reported the relevance of the identification of stromal-rich HCCs, which are subtyped with poor reproducibility even among experts. This fact could at least partially explain the under-reporting of FLHCC even in regions such as Latin America where it does not appear to be so rare. Indeed, this is one of the major motivations for the present report, with an emphasis on updating the clinical and histopathological criteria for the definition of FLHCC as an important subtype of HCC. In the present series, pure FLHCC was associated with a significantly higher overall survival, whereas mixed FLHCC was associated with an increased risk of death. This finding is consistent with recent data from Malouf et al., who reported a longer median overall survival in patients with pure FLHCC compared with patients with mixed FLHCC (9 years vs. 3 years; p<0.02) 19. Mixed FLHCC was reported for 10% to 25% of tumors in some series 12,19.
Other factors that were associated with a worse prognosis in other studies include vascular invasion, lymph node metastasis, positive surgical margins, and old age 4,12. In our study, the presence of macrovascular invasion, according to radiological and pathological findings, was associated with poor survival. The presence of microvascular invasion, however, was not shown to be associated with worse survival; this is likely because only 3 cases presented microvascular invasion. Pinna et al. 28 reported vascular invasion as the most important negative prognostic factor for survival in their series. Lymph node metastasis was the only significant negative prognostic factor in the series reported by Stipa et al. 12. In a series of 15 patients with FLHCC, Moreno-Luna reported that the factors associated with higher survival were age over 23 years, resectability, the absence of major vascular invasion, and free surgical borders 8. In our series, other poor prognostic factors, such as extra-hepatic metastasis and positive margins, were associated with worse survival, but these associations were not statistically significant, likely because of the small number of patients.
An important finding from morphological studies is the growing necessity for extensive histological sampling to search for tumor heterogeneity because the presence of areas of conventional HCC in FLHCC was related to poor survival. Our recommendation is that in addition to the selection of all areas presenting gross features of possible markers of worse prognosis, such as intra-hepatic satellite nodules, vascular invasion, and extra-hepatic invasion, including lymph node and organ metastases, at least one paraffin block should be prepared for each centimeter of the largest extension of the primary tumor.
The results from the multivariate analysis should be interpreted with caution because the small sample size and small number of events by covariate analysis can introduce estimation bias for the hazard ratios 31. However, as FLHCC is considered a rare type of cancer, the number of cases presented herein is among the largest published series from a single institution.
In conclusion, in the present series of 21 cases from a single academic center, fibrolamellar hepatocellular carcinoma was more common in young female patients, none of whom had evidence of chronic liver disease. The majority of patients were diagnosed at an advanced stage and had large tumors; additionally, most already had extra-hepatic metastases, especially to lymph nodes. Whenever feasible, surgery is the best therapeutic option to achieve disease control, even in advanced cases. Vascular invasion was related to a significantly higher risk of recurrence in patients who underwent surgical resection, and the presence of macrovascular invasion in imaging or pathology studies was a predictor of poor survival. Our findings suggest that extensive histological sampling must be performed to search for tumor heterogeneity because the presence of areas of conventional HCC in FLHCC was directly related to poor survival.
ACKNOWLEDGMENTS
The authors would like to thank the Alves de Queiroz Family Fund for Research for the support of our continued work and the Laboratory of Epidemiology and Statistics of Department of Gastroenterology, School of Medicine - University of São Paulo, SP, Brazil for their permanent assistance.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Edmondson HA. Differential diagnosis of tumor and tumor-like lesions of the liver in infancy and childhood. Am J Dis Child. 1956;91(2):168–86. doi: 10.1001/archpedi.1956.02060020170015. [DOI] [PubMed] [Google Scholar]
- 2.Berman MM, Libbey NP, Foster JH. Hepatocellular carcinoma. Polygonal cell type with fibrous stroma – an atypical variant with a favorable prognosis. Cancer. 1980;46(6):1448–55. doi: 10.1002/1097-0142(19800915)46:6<1448::aid-cncr2820460626>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 3.Craig JR, Peters RL, Edmonsdson HA, Omata M. Fibrolamellar carcinoma of the liver: a tumor of adolescents and young adults with distinctive clinico-pathologic features. Cancer. 1980;46(2):372–9. doi: 10.1002/1097-0142(19800715)46:2<372::AID-CNCR2820460227>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 4.Liu S, Chan KW, Wang B, Qiao L. Fibrolamellar hepatocellular carcinoma. Am J Gastroenterol. 2009;104(10):2617–24. doi: 10.1038/ajg.2009.440. [DOI] [PubMed] [Google Scholar]
- 5.McLarney JK, Rucker PT, Bender GN, Goodman ZD, Kashitani N, Ros PR. Fibrolamellar carcinoma of the liver: radiologic/pathologic correlation. Radiographics. 1999;19(2):453–71. doi: 10.1148/radiographics.19.2.g99mr09453. [DOI] [PubMed] [Google Scholar]
- 6.Arista-Nasr J, Gutierrez-Villalobos L, Nuncio J, Maldonaldo H, Bornstein-Quevedo L. Fibrolamellar hepatocellular carcinoma in Mexican patients. Pathol Oncol Res. 2002;8(2):133–7. doi: 10.1007/BF03033723. [DOI] [PubMed] [Google Scholar]
- 7.Silva H, León G, Náquira N. Fibrolamellar hepatocellular carcinoma: report of 4 cases. Rev Med Chil. 1988;116(2):153–6. [PubMed] [Google Scholar]
- 8.Moreno-Luna LE, Arrieta O, García-Leiva J, Martinez B, Torre A, Uribe M, et al. Clinical and pathologic factors associated with survival in young adult patients with fibrolamelar hepatocarcinoma. BMC Cancer. 2005;5:142. doi: 10.1186/1471-2407-5-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toberson M. Review of the clinicopathologic features of fibrolamellar carcinoma. Adv Anat Pathol. 2007;14(3):217–23. doi: 10.1097/PAP.0b013e3180504913. [DOI] [PubMed] [Google Scholar]
- 10.El-Serag HB, Davila JA. Is fibrolamellar carcinoma different from hepatocellular carcinoma. A US population-based study. Hepatology. 2004;39(3):798–803. doi: 10.1002/hep.20096. [DOI] [PubMed] [Google Scholar]
- 11.El-Gazzaz G, Wong W, El-Hadary MK, Gunson BK, Mirza DF, Mayer AD, et al. Outcome of liver resection and transplantation for fibrolamellar hepatocellular carcinoma. Transpl Int. 2000;13(Suppl 1) :S406–9. doi: 10.1007/s001470050372. [DOI] [PubMed] [Google Scholar]
- 12.Stipa F, Yoon SS, Liau KH, Fong Y, Jarnagin WR, D′Angelica M, et al. Outcome of patients with fibrolamellar hepatocellular carcinoma. Cancer. 2006;106(6):1331–8. doi: 10.1002/cncr.21703. [DOI] [PubMed] [Google Scholar]
- 13.Wetzel WJ, Costin JL, Petrino RL. Fibrolamellar carcinoma: distinctive clinical and morphologic variant of hepatoma. South Med J. 1983;76(6):796–8. doi: 10.1097/00007611-198306000-00028. [DOI] [PubMed] [Google Scholar]
- 14.Hemming AW, Langer B, Sheiner P, Greig PD, Taylor BR. Aggressive surgical management of fibrolamellar hepatocellular carcinoma. J Gastroinst Surg. 1997;1(4):342–6. doi: 10.1016/S1091-255X(97)80055-8. [DOI] [PubMed] [Google Scholar]
- 15.Lack EE, Neave C, Vawter GF. Hepatocellular carcinoma. Review of 32 cases in childhood and adolescence. Cancer. 1983;52(8):1510–5. doi: 10.1002/1097-0142(19831015)52:8<1510::aid-cncr2820520830>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 16.Kakar S, Burgart LJ, Batts KP, Garcia J, Jain D, Ferrel LD. Clinicopathologic features and survival in fibrolamellar carcinoma: comparison with conventional hepatocellular carcinoma with and without cirrhosis. Mod Pathol. 2005;18(11):1417–23. doi: 10.1038/modpathol.3800449. [DOI] [PubMed] [Google Scholar]
- 17.Ringe B, Wittekind C, Weimann A, Tusch G, Pichlmayr R. Results of hepatic resection and transplantation for fibrolamellar carcinoma. Surg Gynecol Obstet. 1992;175(4):299–305. [PubMed] [Google Scholar]
- 18.Nagorney DM, Adson MA, Weiland LH, Knight CD, Jr, Smaley SR, Zinsmeister AR. Fibrolamellar hepatoma. Am J Surg. 1985;149(1):113–9. doi: 10.1016/s0002-9610(85)80019-2. [DOI] [PubMed] [Google Scholar]
- 19.Malouf GG, Brugieres L, Le Deley MC, Faivre S, Fabre M, Paradis V, et al. Pure and mixed fibrolamellar hepatocellular carcinoma differ in natural history and prognosis after complete surgical resection. Cancer. 2012;118(20):4981–9. doi: 10.1002/cncr.27520. [DOI] [PubMed] [Google Scholar]
- 20.Sobin LH, Compton CC. TNM seventh edition: what's new, what's changed: communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer. 2010;116(22):5336–9. doi: 10.1002/cncr.25537. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan E, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–81. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 22.R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (2013) URL http://WWW.R-project.org/ [Google Scholar]
- 23.Maniaci V, Davidson BR, Rolles K, Dhillon AP, Hackshaw A, Begent RH, et al. Fibrolamellar hepatocellular carcinoma - prolonged survival with multimodality therapy. Eur J Surg Oncol. 2009;35(7):617–621. doi: 10.1016/j.ejso.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Dahan MH, Kastell P. Fibrolamellar hepatic carcinoma with a presentation similar to that of septic pregnancy. A case report. J Reprod Med. 2002;47(1):47–9. [PubMed] [Google Scholar]
- 25.Gemer O, Segal S, Zohav E. Pregnancy in a patient with fibrolamelar hepatocellular carcinoma. Arch Gynecol Obstret. 1994;255(4):211–2. doi: 10.1007/BF02335087. [DOI] [PubMed] [Google Scholar]
- 26.Paranaguá-Vezozzo DC, Ono SK, Alvarado-Mora MV, Farias AQ, Cunha-Silva M, França JI, et al. Epidemiology of HCC in Brazil: Incidence and risk factors in a ten-year cohort. Ann Hepatol. 2014;13(4):386–93. [PubMed] [Google Scholar]
- 27.Carrilho FJ, Kikuchi L, Branco F, Gonçalves CS, Mattos AA. Clinical and epidemiological aspects of hepatocellular carcinoma in Brazil. Clinics. 2010;65(12):1285–90. doi: 10.1590/S1807-59322010001200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinna AD, Iwatsuki S, Lee RG, Todo S, Madariaga JR, Marsh JW, et al. Treatment of fibrollamelar hepatoma with subtotal hepatectomy or transplantation. Hepatology. 1997;26(4):877–83. doi: 10.1002/hep.510260412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevens WR, Johnson CD, Stephens DH, Nagorney DM. Fibrolamellar hepatocellular carcinoma:stage at presentation and results of agressive surgical management. AJR Am J Roentgenol. 1995;164(5):1153–8. doi: 10.2214/ajr.164.5.7717223. [DOI] [PubMed] [Google Scholar]
- 30.Malouf G, Falissard B, Azoulay D, Callea F, Ferrell LD, Goodman ZD, et al. Is histological diagnosis of primary liver carcinomas with fibrous stroma reproducible among experts. J Clin Pathol. 2009;62(6):519–24. doi: 10.1136/jcp.2008.062620. [DOI] [PubMed] [Google Scholar]
- 31.Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48(12):1503–10. doi: 10.1016/0895-4356(95)00048-8. [DOI] [PubMed] [Google Scholar]