Abstract
Schistosoma japonicum, once endemic all the East Asia, remains as a serious public health problem in certain regions. Ectopic egg embryonation in the liver causes granulomatosis and eventually fatal cirrhosis, so that prevention of this process is one of the keys to reduce its mortality. The embryonation requires cholesteryl ester from HDL of the host blood for egg yolk formation, and this reaction is impaired from the abnormal large HDL in genetic cholesteryl ester transfer protein (CETP) deficiency. When CETP was expressed in mice that otherwise lack this protein, granulomatosis of the liver was shown increased compared to the wild type upon infection of Schistosoma japonicum. The CETP deficiencies accumulated exclusively in East Asia, from Indochina to Siberia, so that Shistosomiasis can be a screening factor for this accumulation. CD36 related protein (CD36RP) was identified as a protein for this reaction, cloned from the cDNA library of Schistosoma japonicum with 1880-bp encoding 506 amino acids. The antibody against the extracellular loop of CD36RP inhibited cholesteryl ester uptake from HDL and suppressed egg embryonation in culture. Therefore, inhibition of CETP is a potential approach to prevent liver granulomatosis and thereby fatal liver cirrhosis in the infection of Schistosoma japonicum.
Keywords: Schistosoma japonicum, liver cirrhosis, granulomatosis, CETP deficiency, HDL, cholesteryl ester
SCHISTOSOMA JAPONICUM IN ASIA
Schistosoma (S.) japonicum, an Asian specific blood fluke parasite, was endemic in East Asia including Indonesia, Philippines, China, and Japan as far as traced in recorded history. Its Mekong strain or S. mekongi is also found around the Mekong basin in Indochina peninsula. The parasite was first identified in Japan early 20th century when a few intensively infected regions were known, which is basically eliminated in these days[1]. It used to infect as many as 12 million people in China until the modern public health effort was initiated[2]. Although the number of the patients has dramatically decreased, it is still active at least in China[2]-[3], Philippines[4]-[5], Cambodia, Laos, Thailand, Malaysia, and Indonesia[6], perhaps accounting for some 2-million patients, and remains as the world second major Shcitosomiasis next to the African blood fluke, S. mansoni.
The life cycle of this parasite includes an intermediate host of specific fresh water snails such as Oncomelenia nosophora. The adults reside in the portal or intestinal veins of the host animals including humans, and lay eggs to be excreted with feces. The eggs embryonate in environmental fresh water and are hatched to release miracidia that penetrate into the snails to infect. They further grow there to sporcytes and then to cercariae, and are released into the water again to swim and penetrate the skin of the terminal hosts. Thus, endemic of S. japonicum is closely associated with intensive contact with natural fresh water reserve in everyday life. The historical geographic distribution of S. japonicum overlaps the regions with the culture of water farming of rice grain. This might have caused intensive endemic of S. japonicum in East Asia.
One of the most life threatening clinical manifestations is liver cirrhosis, which is caused by ectopic egg embryonation. The adult pairs of the parasite locate in the portal and its draining venules and lay eggs to be released to the intestinal tract. However, many of them are flushed back via the portal blood flow to the liver where they embolize and develop into miracidia, a phenomenon that leads to the morbidity and mortality of hepatic granulomatosis and cirrhosis[7]. Thus, egg embryonation is one of targets to prevent fatal development of schistosomiasis. The active mechanism to induce this lesion is unknown. Specific antibodies against various egg antigens have been identified as indicators of the infection, but their relationship with granulomatogenesis is unclear[8]. A potential pathogenesis factor is egg embryonation to the stage of miracidium, as the eggs only after this stage seem to cause the liver lesion when transplanted[9]. L-Selectin of the host binds the eggs only in the stage of miracidium[10]. Vaccination to stabilize the embryonation process has been proposed for an anti-Schistosomiasis therapy[11].
Schistosomas use the host plasma lipoproteins as lipid nutrient sources. S. mansoni express the low density lipoprotein (LDL) receptor-like protein[12]-[13]. The receptors for LDL and very low density lipoprotein (VLDL) were shown to mediate uptake of the lipoproteins in S. mansoni[14]-[15], as well as in S. japonicum[16]-[17]. However, there has not been much information whether and how high density lipoprotein (HDL) interacts with Schistosomas. We investigated a role of HDL in lifecycle of S. japonicum[18]-[20].
HDL FOR EMBRYONATION OF S. JAPONICUM EGGS AND CETP DEFICIENCY
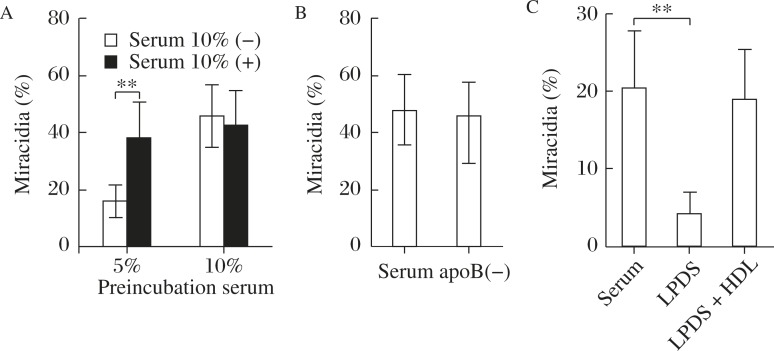
Embryonation of S japonicum eggs to miracidia was investigated in vitro[19]. The paired adult parent parasites removed from the infected mouse portal vein were pre-incubated for laying eggs in the presence of 5% and 10% normal human serum for 2 days. The eggs were isolated and cultured for another 8 days for embryonaiton in the absence and presence of 10 % human serum. When the eggs were laid in the presence of adequate serum source (10%), they did not require serum for their embryonation. When they were laid in the poor nutritional condition (5% serum), the eggs needed the serum for embryonation (Fig. 1A). The finding indicates that the egg yolk is established for embryonation before they are laid but the eggs themselves also uptake nutrients from the serum, to grow. The latter effect was not reduced even when apoB lipoprotein was removed from the serum, indicating that LDL/VLDL are not major nutrient sources for the embryonation. Finally, HDL was shown as a requirement for embryonation (Fig. 1B and C). The results lead us to wonder how alteration of HDL metabolism influences the egg embryonation and accordingly development of the hepatic lesions.
Fig. 1. Embryonation of S. japonicum eggs in culture, monitored as miracidia formation (%), modified from the reference[19].
A: incubation of the eggs with and without human serum (10%) for 8 days after pre-exposure to 5 and 10 % human serum of the parents laying the eggs. B: Normal embryonation after the preincubation with 5 % serum, even with apoB-lipoprotein-deficient human serum (apoB (-)). C: Requirement of HDL for embyonaiton. LPDS; lipoprotein deficient serum. Asterisks ** indicate statistical difference at P<0.01 from serum (-) (A) or serum (C).
Some genetic mutations are known to manifest abnormal HDL metabolism. Deficiencies in HDL is seen in dysfunctional mutations in ATP-binding cassette transporter A1, lecithin: cholesterl acyltransferase and apolipoprotein AI, while deficiency of cholesteryl ester transfer protein (CETP) causes very high HDL cholesterol level as it generates a large abnormal HDL particle. While the former diseases are rare anywhere in the world except for the very limited regions with the founders' effect, CETP deficiency is found with very high prevalence widely in East Asia as discussed later in this text. Therefore, the in vitro experiments for egg embryonation were carried out with the serum from human subjects of genetic CETP deficiency[18].
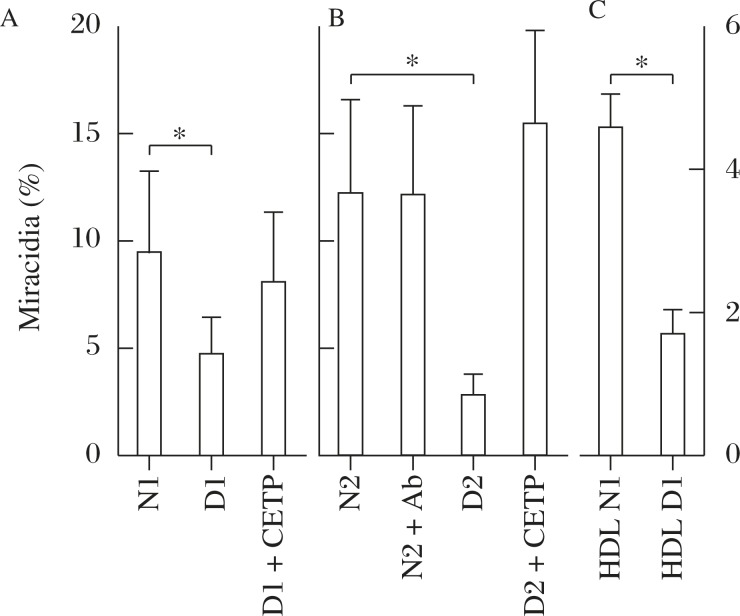
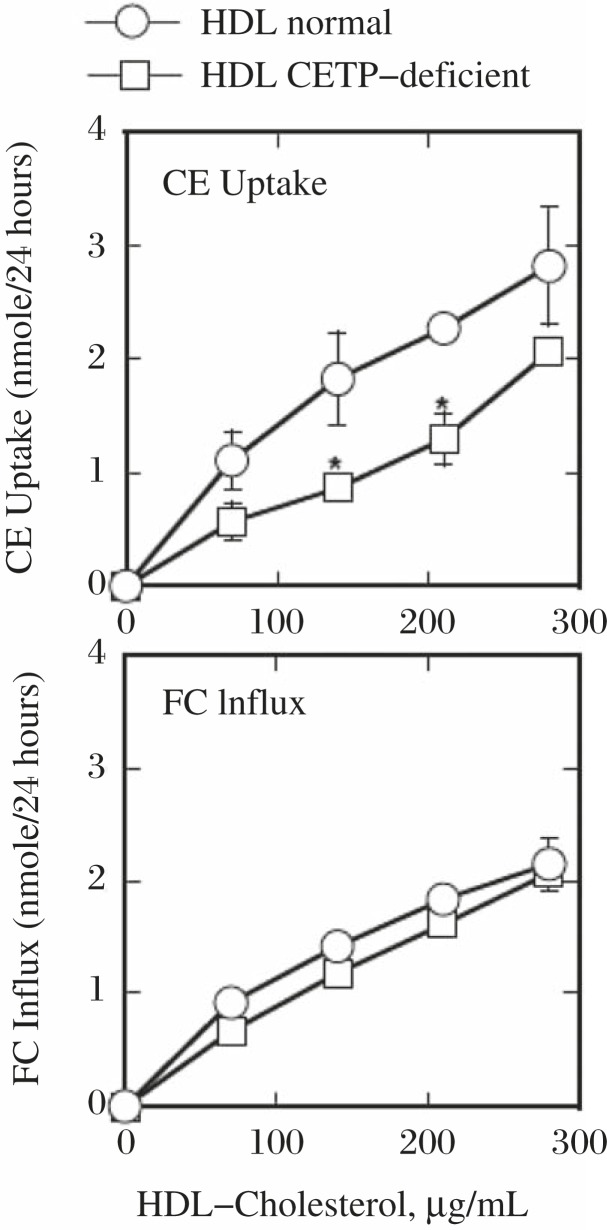
The egg embryonation was significantly low when incubated with the serum of CETP deficient subjects, and this was substantially recovered in the presence of purified human CETP (Fig. 2A). However, the presence of the anti-CETP inhibitory antibody in the normal serum did not attenuate embryonation (Fig. 2B). Finally, normal HDL gave the eggs normal embryonation without other serum components, but HDL of CETP deficient subjects showed inadequate embryonation (Fig. 2C). Selective cholesteryl ester (CE) uptake was slower from HDL from CETP deficient subjects while free cholesterol exchange showed no difference between the normal and CETP-deficiency HDL (Fig. 3)[18]. Wild type mouse serum that lacks CETP activity did not induce adequate embryonation and the serum of the CETP-transgenic mouse enhanced it. Adding purified CETP to the mouse serum partially recovered the rate of embryonation (Fig. 4). Thus the findings with human blood serum were reproduced with mouse serum with and without CETP activity in vitro.
Fig. 2. Embryonation (% Miracidia) of S. japonicum eggs in culture with CETP-deficient human serum in 10% normal human sera (N1 and N2) and that of CETP-deficient subjects (D1 and D2), modified from the reference[18].
Embryonation is retarded in CETP-deficient serum (A) and adding CETP recovers this (B). Normal HDL is adequate for the embryonation but not HDL from CETP-deficiency (C). Asterisk indicates statistical difference at P<0.05 from normal HDL.
Fig. 3. Uptake of cholesteryl ester (CE) and free cholesterol (FC) from HDL by S. japonicum eggs, modified from reference[18].
The eggs were incubated with normal and CETP-deficient HDL with radiolabeled CE or FC.
Fig. 4. The effect of mouse serum on egg embryonation (% miracidia), modified from the reference[18].
Embryonation is poor with wildtype mouse serum that lacks CETP and proceeds with CETP-transgenic mouse serum (CETP-tg). Adding human CETP to the wildtype serum partially restored embryonation. Asterisk indicates statistical difference at P<0.05 from WT.
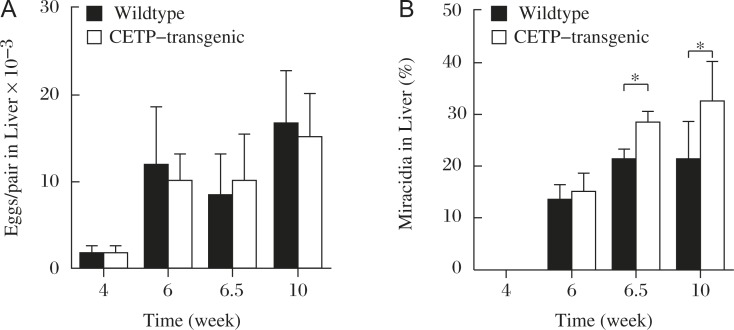
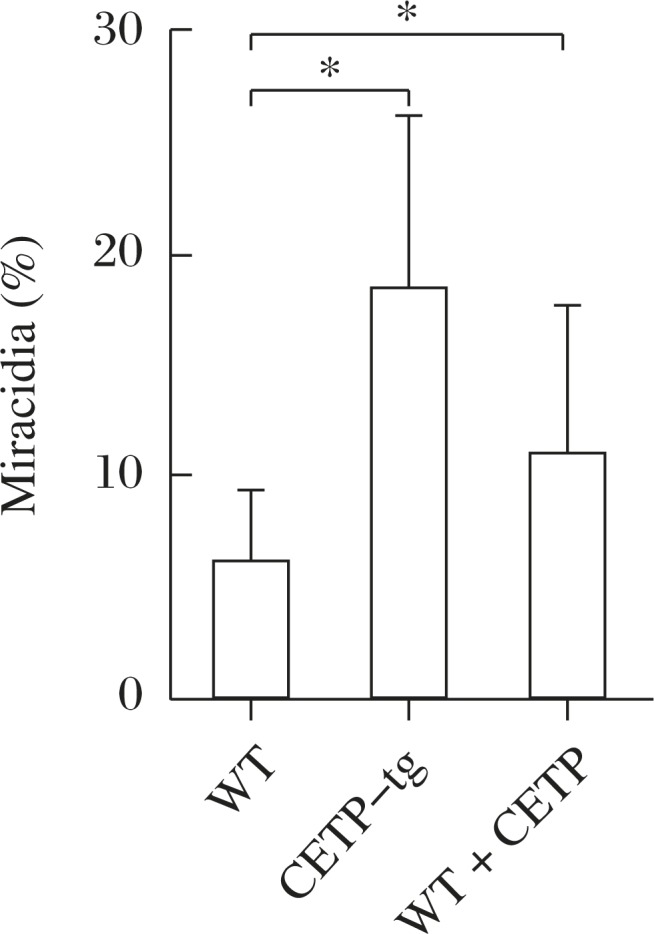
Accordingly, in-vivo studies were carried out in mice, using wildtype mice as a model for CETP-deficiency and the CETP-transgenic mice for normal human subject[18]. Number of the eggs plugged into the liver and the rate of their embryonation were counted microscopically in the liver specimens. While the number of the eggs was similar between the models, their embryonation was significantly higher in the transgenic mouse liver (Fig. 5). The average granulomatous lesion per egg was larger in the transgenic mice (Table 1). Absence of CETP thus seems to reduce ectopic embryonation of S. japonicum eggs in the host liver, making the host resistant to its hepatic complication.
Fig. 5. Number of eggs embolizing in the liver and their ectopic embryonation in the mice infected with S. japonica, modified from the reference[18].
Wildtype and CETP-transgenic mice are infected by S. japonica. The ectopic embryonaiton is accelerated in the transgenic mice. A: Egg count in liver. B: Egg maturation in liver. Asterisk indicates statistical difference at P<0.05 from wildtype.
Table 1. Granulomatous lesion in the liver.
| CETP transgenic | Wildtype | |
| Granuloma area (%) | 10.5±5.5 | 14.4±5.4 |
| Area per egg (µm2) ×10-3 | 86.7±26.5 | 66.1±19.3* |
<?ENTCHAR ast?>: P<0.05. Taken from the reference [8]. The eggs and granulomatous lesion were microscopically identified in the liver specimens of the wild-type and CETP transgenic mice, infected by S. japonicum. The total area of the lesion per egg was calculated for each section. The area per egg was calculated as an mean±SE of the 12 mice, for each of which 12 random liver sections were examined.
ENDEMICS OF CETP DEFICIENCY
CETP is present in the plasma of certain species of mammalians including humans[21]-[24]. Human CETP is composed of 476 amino acid residues[25] with a glycosilated molecular weight of 74,000. It catalyzes non-directional equimolar exchange of CE and triglyceride (TG) among lipoproteins with low substrate specificity[26]-[29]. The reaction equalizes distribution of the core lipids among lipoproteins, and consequently causes the net move of CE from HDL to TG-rich lipoproteins such as VLDL and TG from TG-rich lipoproteins to HDL and LDL[26]. CE is generated in plasma on HDL and plasma TG is present in VLDL and chylomicron originating from the liver and the intestinal cells, so that the CETP reaction results in decrease of CE in HDL and increase of TG both in HDL and LDL. TG is hydrolyzed by hepatic lipase in any lipoprotein subfraction so that the size of HDL and LDL gets smaller by the CETP reaction to produce HDL3 particles and so-to-speak small dense LDL. Increase in plasma TG indeed decreases plasma HDL and increases small dense LDL in the presence of CETP. In turn, HDL-CE increases when CETP reaction decreases[30].
Since generation of CE in HDL is one of the driving force for the removal of cell cholesterol[31], playing an important part of cholesterol transport for catabolism from peripheral somatic cells to the liver as its catabolic cite, the CETP reaction may facilitate this transport by sending HDL-CE to LDL for the pathway of recovery of LDL through the hepatic LDL receptor[32]. On the other hand, the increase in the HDL surface area in low CETP activity may provide more capacity to accept cell cholesterol.
CETP became a popular topic of lipoprotein study since discovery of the genetic defect of CETP. The patients were first described in Japan in 1985 as cases with hyperalphalipoproteinemia with the lack of CE transfer reaction between lipoproteins[33]-[34]. Its genetic background was soon established[35], and many cases were found thereafter in Japan. Two major mutations have been identified as major disorders, intron 14 G(+1)-to-A (Int14A) and exon 15 missense mutation (D442G)[36]-[38]. Prevalence of these two mutants was found very high in Japanese general population, as int14A 1% to 2% and D442G 6% to 7%. In addition, sporadic cases with 10 or more other types of mutations were also identified among Japanese[39]-[41]. Accordingly, the estimated number of the heterozygotes can be around 10 millions in Japan and the homozygotes would be as many as 150,000 to 250,000. CETP deficiency may account for 27.6 % of the people with HDL cholesterol ≥ 60 mg/dL and 31.4%–32.5% of those with HDL ≥ 80 mg/dL in Japan[36],[42]. Above all, Omagari of Akita district in Northern Japan was discovered as the region with high accumulation of the Int14A mutant, with the prevalence of the heterozygote of 27%[38]. Thus, genetic CETP deficiency was found highly common among Japanese.
The first non-Japanese patient was reported from Switzerland as a Chinese descent[43]. Several reports thereafter described CETP deficiency among other Asians. Genetic prevalence of D442G mutant heterozygote case was found to be 3.3%–10.8% in the main-land Chinese population[44]-[46], and 4.5%–7.7% in the population of Taiwan[42],[47]-[49]. It could be estimated up to 12% among Koreans based on its allele frequency of 6%[50]. Vietnamese D442G mutants were estimatedg to be 6.9% in the general population[51]. Nine cases were identified to be D442G heterozygotes out of the 35 individuals with hyperalphalipoproteinemias in Thailand[52], accounting for 26%, similar to the ratio among Japanese[36],[42] strongly indicating CETP mutants common in Thailand. Further detailed information is available in the previous review article[53]. More recent result for elderly Siberian Yakuts indicates the prevalence of D442G mutant 16.2% in the native Yakuts and 5.2% among the non-indigenous[54], mostly Russians and Ukrainians, whose intermarriage rate with Yakuts may be 10 to 20% (Ariev AL, personal communication). Not much reliable information is available for the Int14A mutation, except for two out of the 145 subjects (1.4%) in Hong Kong Chinese[18],[47],[55]-[59] and none of the 346 Vietnamese[51]. In a unique spot, Omagari of Akita district in Northern Japan, accumulation of Int14A mutant was found with the prevalence of the heterozygote 27.0%[38].
In contrast, CETP deficiency is rare in other ethnic groups. The first Caucasian case was reported in 1997[60], and one case of Int14A was reported in 1998 in Canada without ethnic background identification[61]. It was thus concluded that CETP deficiency is rare among North American Caucasians[62]. Nevertheless, a few studies reported sporadic cases of CETP deficiency in The United States[63], Italy[64]-[65], and the Netherlands[62],[66]. There was an estimate of D442G mutant as “less than 1%”[67], but the ground for this estimation, as well as how “less”, is unclear.
Clinical manifestation of CETP deficiency is limited to abnormal plasma lipoprotein profile represented by very high HDL cholesterol and moderately reduced LDL cholesterol. In the lack of CETP activity, CE generated in HDL is detained there, so that the core CE compartment expands and HDL particles become larger in their size, as large as LDL, and rich in apoE[68]-[71]. The patients in general do not exhibit any serious clinical symptoms. It has been wondered whether this large HDL is protective against atherosclerosis. The answer has been controversial for the genetic CETP deficiency[38],[72]-[73] and the attempts of pharmacological inhibition of CETP were also inconclusive so far[74]-[75].
In summary, CETP deficiency is highly prevalent in East Asia, at least among Japanese, Chinese both in mainland and Taiwan, and Koreans, predominantly with the D442G mutant. It is likely similarly high in Thailand. The Int14A mutant may be the second common, but it is not clearly demonstrated except for Japan. Other types of mutation have also been found frequently in Japan. It may also be so in other East Asian regions, but the information is not adequate. In contrast, this disease is rare in other ethnic groups.
For geographic or ethnic accumulation of a genetic abnormality, two potential hypotheses may be considered, “founders' effect” or screening by a regional fatal disease(s). The former cases are normally found in limited communities of the descendants of earlier settlers. Typical examples are accumulation of familial hypercholesterolemia in French Canadians[76], Afrikaners in South Africa[77] and perhaps in Lebanon[78]. In this case, the accumulated mutations may not be highly diverse as originating in a few carrier families. The latter is represented by sickle cell anemia that is resistant to malaria infection, as a believed reason for high prevalence of this genetic anemia among African ethnic groups[79]-[81]. This case may affect large populations historically exposed to such diseases, mostly infectious diseases.
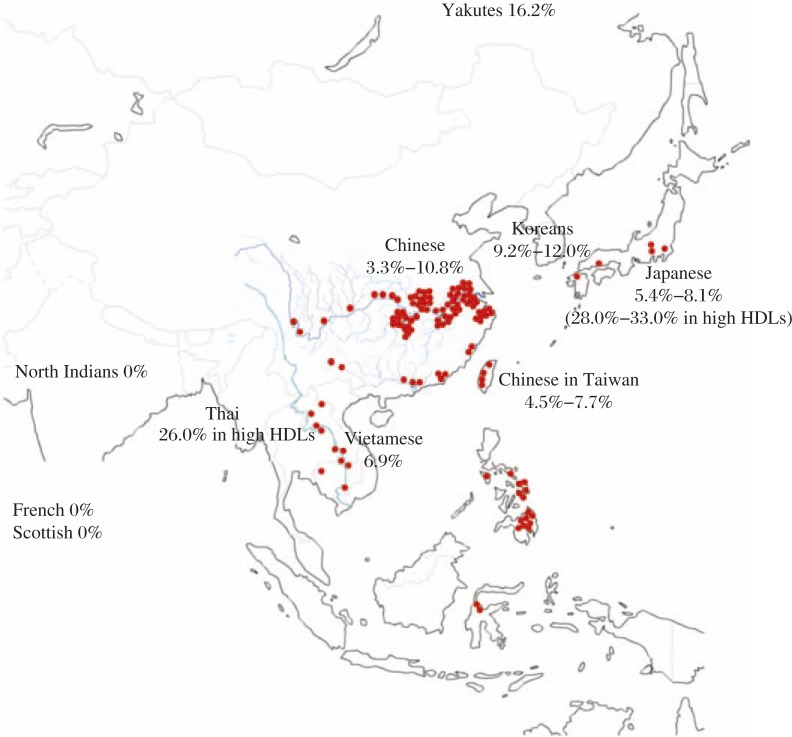
Accumulation of CETP deficiency can also be discussed from such points of view. Majority of the patients of the region may be limited to one or two type(s) of mutations although further diversity of the mutation is also observed in the region, being not inconsistent with founders' effect. However, the region where this disease is found with high prevalence seems a large portion of East Asia, far beyond founder's effect. No specific settler family can be conceivable to account for such large descending population affected. However, extreme accumulation of int14A in Omagari may be the case of local “founders' effect”[38]. It should be noted that its only significant clinical phenotype is abnormal plasma lipoprotein metabolism. Very few infectious diseases are found with any relation to or dependency on plasma lipoproteins. Schistosomas may be one of the few to meet such criteria. The regions of historic endemics of S. japonicum and of high prevalence of CETP deficiency in fact largely overlap (Fig. 6).
Fig. 6. Endemics map of S. japonicum and CETP deficiency (D442G).
Red spots show the regions where the cases were found since the parasite was identified in the early 20th Century. The regions spread over Japan, China including Taiwan, Mekong valleys, Thailand, Philippines, and Indonesia. The endemic was eliminated in many of these regions in the 21st Century. Data of the prevalence of G442G available in literature till today are listed as percentage in each ethnic general population unless otherwise indicated.
IDENTIFICATION AND CHARACTERIZATION OF CD36-RELATED PROTEIN IN S. JAPONICUM
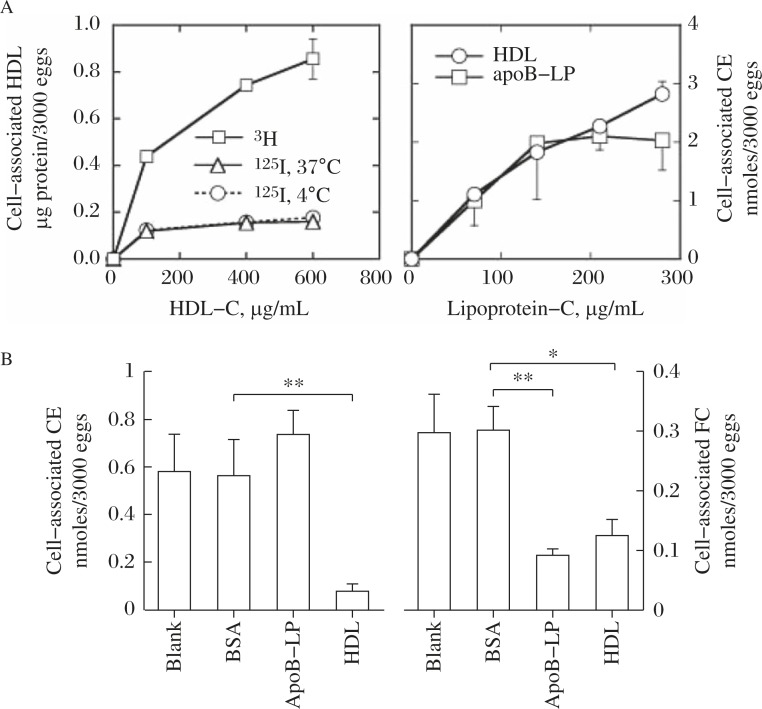
In order to investigate a specific role of HDL for egg embryonation, uptake by the S. japonicum eggs of cholesterol, the most specific nutrient carried by lipoproteins, was observed. Uptake of lipoprotein CE by the eggs is shown in Fig. 7[19]. CE is selectively taken up from HDL. The uptake also seems to occur from apoB-lipoprotein, but it is saturated at much lower level and the pathways are independent of each other. In contrast, free cholesterol (FC) exchange between the eggs and lipoproteins is by a common pathway for HDL and apoB-lipoproteins (Fig. 7).
Fig. 7. Selective uptake of CE from HDL by the eggs of S. japonicum modified from the reference[19].
A: The labeled HDL with 125I for protein and with 3H for CE was incubated with the eggs and the uptake of each radioactivity was measured and standardized for HDL protein (left). The CE uptake was also measured from HDL and LDL (right). B: CE (left) and FC (right) uptake from HDL in the presence of each plasma component indicated (BSA, ApoB lipoprotein, and HDL). Asterisks indicate statistical difference at P<0.05 (*) and P<0.01 (**) from BSA.
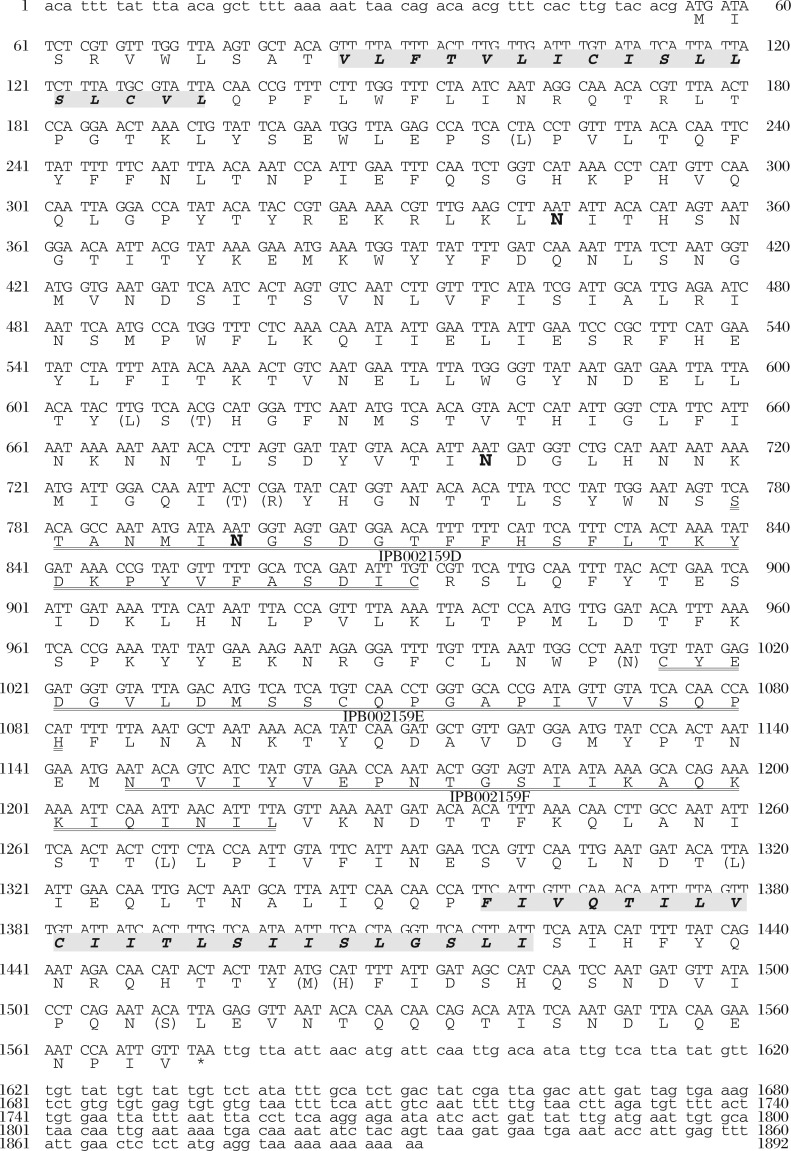
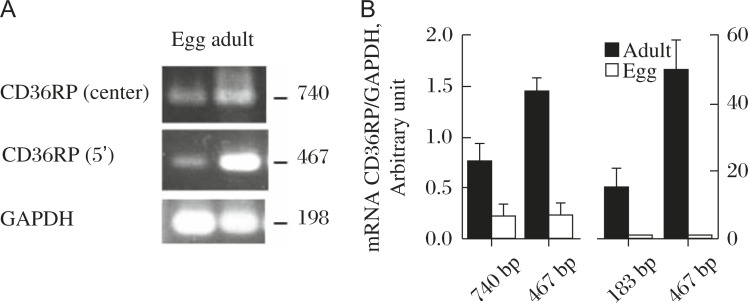
Selective cellular uptake of CE has been shown to be mediated by CD36-like proteins, such as scavenger receptor-B1 (SR-B1) in rodent[82] or CLA1 in human[83]. Assuming a similar protein mediates the reaction, expression of mRNA was searched in S. japonicum by using the 489-bp probe derived from the cDNA of Sj-Ts2 protein that has one of the CD36 domains (671-bp submitted and registered as Genbank AF291715). From the S. japonicum adult cDNA library, Sj-Ts2-containing cDNAs of variable sizes longer than 1-kb were obtained all seemingly derived by single transcription, including the sequence of the reported Sj-Ts2 protein. The sequence of the full length (1880-bp) original mRNA was determined and deduced to 506 amino acid residues (Fig. 8) (GenBank accession no. AY496973)[19]. Though there is an indication of alternative splicing of this mRNA, the size of the PCR product, with the first-strand cDNA derived from total RNA as template, was similar (467-bp) between eggs and adults (Fig. 9). Therefore, the message is likely to be expressed in eggs though the level seems lower.
Fig. 8. Nucleotide sequence and the deduced amino acid residue sequence of CD36RP cloned from S. japonicum, modified from the reference[19].
Shadowed portions are predicted membrane spanning regions. Bald letters of N indicate potential glycosylation sites. The sequences double underline portions are potential CD36-related regions (IPB 002159D, 002159E, and 002159F).
Fig. 9. Expression of CD36RP in S. japonicum, the adults and the eggs, modified from the reference[19].
Two different probes (near the center and 5′ region) were used to yield PCR products of 740 bp and 467 bp, respectively. A: The results of conventional PCR. B: The results of real-time PCR.
The amino acid sequence indicated that it has two transmembrane regions and at least three CD36 domains that are conserved in 32 CD36 proteins from variety of organism according to Conserved Domain search analysis (Fig. 8), having high homology with rat SR-B1, rat CD36 and human CLA-1. The protein was thus thought to be of the CD36 family and termed CD36-related protein (CD36RP)[19]. It had 15 nucleotide polymorphism sites in the coding region identified during screening, resulting in 11 amino acid substitutions. Three N-glycosylation sites (aa97, 205, 248) identified among the 15 candidates asparagine in CD36RP were conserved in mammalian SR-BI and CD36 (Fig. 8). In fact, the size of CD36RP in the S. japonicum adult decreased from 82 kDa to 62 kDa after N-glycanase treatment[19].
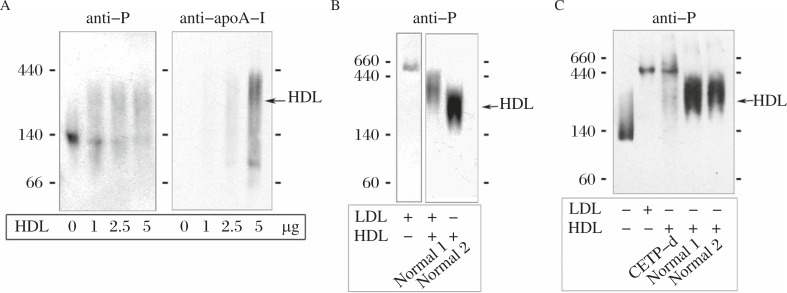
A recombinant peptide representing the extracellular domain containing the conserved Cys and Pro-rich domain of CD36RP was used for binding to lipoproteins[19]. The water-soluble extracellular domain peptide was shown to strongly bind to normal HDL, but neither to LDL nor to the HDL from a CETP deficient subject as poorly as to LDL (Fig. 10)[19]. The antibody against the extracellular domain peptide of CD36RP significantly suppressed the active CE uptake at 37°C, as well as the egg embryonation to miracidia (Fig. 11)[19].
Fig. 10. Interaction of the peptide representing the extracellular domain of CD36RP (residues 249-369) with lipoproteins, modified from the reference[19].
HDL was visualized by immunoblotting with anti-apoA-I antibody and the peptide was visualized by the antibody against the shorter peptide (331–348)(anti-P). A: Binding of the peptide with human HDL. B: Interaction of the peptide with HDL and LDL. C: Binding of the peptide with HDL from the normal and the CETP-deficient subjects.
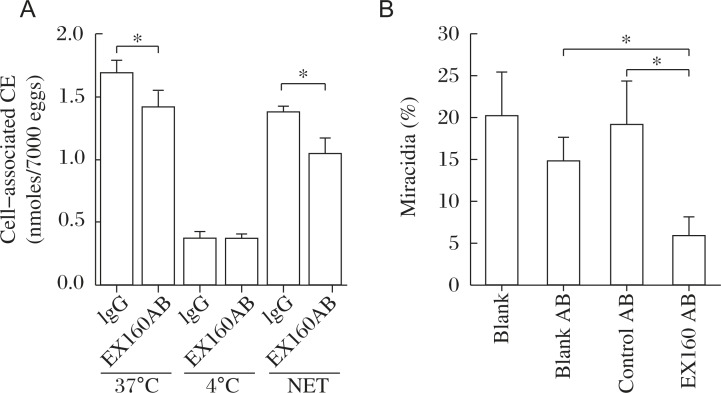
Fig. 11. Suppression of CE uptake by and embryonation of the S. japonicum eggs in culture, by the antibody raised against the extracellular domain peptide of CD36RP representing the residues 249-408, modified from the reference[19].
Blank AB, nonimmunized rabbit antibody, Control AB, antibody against the intracellular domain of CD36RP (residues 331–348). Asterisk indicates statistical difference at P<0.05 from IgG or Blank AB/Control AB.
PROPOSAL OF THE HYPOTHESIS
Available information can be summarized as follows. 1) CETP deficiency is a unique inborn error to manifest very high HDL cholesterol due to extreme enlargement of HDL particles, without other apparent clinical features. CETP deficiency was shown highly prevalent among East Asians, at least shown with those in Japan, mainland China, Taiwan, Vietnam, Korea, Siberia and probably Thailand, while it seems rare among other ethnic groups. 2) One of the regional endemic diseases having any relation to plasma lipoproteins is schistosomiasis where the parasites use plasma lipoproteins as nutrient sources. S japonicum has been known evidently endemic at least in Japan, China, Philippine, Indonesia, and its close strain in the Mekong basin, substantially overlapping with the regions of high prevalence of the CETP deficiency (Fig. 6). 3) The ectopic egg embryonation to miracidia of S japonicum in the liver requires normal plasma HDL, and it is impaired with the HDL of CETP deficiency. 4) CD36RP, a membrane protein of the CD36 family, was identified in S japonicum. The antibodies against the extracellular domain of this protein suppressed selective uptake of CE by the eggs as well as the embryonation of the eggs.
Based on these findings and insights into them, we propose that CD36RP is a candidate for a mediator of selective uptake of CE from HDL by S. japonicum necessary for the egg embryonation to miracidia[19]-[20]. Pre-exposure of the adult schistosomes to wildtype HDL is sufficient for the eggs to mature, perhaps because the yolk of the egg was preformed adequately in such a condition. The eggs laid with inadequate yolk may still mature provided that normal HDL is supplied after they are laid. Absence of normal HDL retards embryonation of the S. japonicum eggs in the host liver and, accordingly, prevents hepatic granulomatosis, in a situation such as CETP deficiency where abnormal large HDL does not efficiently bind CD36RP. Therefore, it is not unreasonable to speculate that CD36RP is a strong candidate for a mediator of HDL-CE uptake by the adults and eggs of S. japonicum as a key molecule for embryonation of the egg to the miracidium, based on structural similarity of CD36RP to CD36 or SR-BI, selective binding of the extracellular domain of CD36RP to HDL, and suppression of the HDL-CE uptake and embryonation of the eggs in culture by the antibody against CD36RP. CD36RP and host plasma HDL are both key elements for hepatic granulomatosis in S. japonicum infection, a fatal pathological process in infected pateints.
If this hypothesis is valid, inhibition of CETP could be useful to prevent hepatic granulomatosis in schistosomiasis. CETP inhibitors have been developed hoping to raise HDL cholesterol to prevent atherosclerotic vascular diseases such as coronary heart disease. The hypothesis has been proven valid in an animal experiment[84], but has not been successful to demonstrate clinical outcome yet[74]-[75]. The inhibitors, however, can be useful to intend the off-target effect on other serious public health problem, prevention of the fatal liver cirrhosis in Schitosomiasis[20].
Acknowledgements
This work was supported in part by MEXT-Supported Program for the Strategic Research Foundation at Private Universities (S1201007) and by Grant-in-aid from MEXT Japan (24614018).
References
- 1.Iida F, Iida R, Kamijo H, et al. Chronic Japanese schistosomiasis and hepatocellular carcinoma: ten years of follow-up in Yamanashi Prefecture, Japan[J] Bull World Health Organ. 1999;77(7):573–581. [PMC free article] [PubMed] [Google Scholar]
- 2.McManus DP, Gray DJ, Li Y, et al. Schistosomiasis in the People's Republic of China: the era of the Three Gorges Dam[J] Clin Microbiol Rev. 2010;23(2):442–466. doi: 10.1128/CMR.00044-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang LD, Chen HG, Guo JG, et al. A strategy to control transmission of Schistosoma japonicum in China[J] N Engl J Med. 2009;360(2):121–128. doi: 10.1056/NEJMoa0800135. [DOI] [PubMed] [Google Scholar]
- 4.Bergquist R, Tanner M. Controlling schistosomiasis in Southeast Asia: a tale of two countries[J] Adv Parasitol. 2010;72:109–144. doi: 10.1016/S0065-308X(10)72005-4. [DOI] [PubMed] [Google Scholar]
- 5.Gordon CA, Acosta LP, Gray DJ, et al. High prevalence of Schistosoma japonicum infection in Carabao from Samar Province, the Philippines: implications for transmission and control[J] PLoS Negl Trop Dis. 2012;6(9):e1778. doi: 10.1371/journal.pntd.0001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harinasuta C. Epidemiology and control of schistosomiasis in Southeast Asia[J] Southeast Asian J Trop Med Public Health. 1984;15(4):431–438. [PubMed] [Google Scholar]
- 7.Ross AGP, Bartley PB, Sleigh AC, et al. Schistosomiasis[J] New Eng J Med. 2002;346(16):1212–1220. doi: 10.1056/NEJMra012396. [DOI] [PubMed] [Google Scholar]
- 8.Mohda J, Redman CA, Thormhill JA, et al. Schstosomes: Unanswered questions on the basic biology of the host-parasite relationship[J] Prasitol Today. 1998;14(10):396–401. doi: 10.1016/s0169-4758(98)01321-0. [DOI] [PubMed] [Google Scholar]
- 9.Hirata M, Takushima M, Kaga M, et al. Induction of experimental murine granuloma formation against Schistosoma japonicum eggs produced by in vitro ova deposition, in vitro tissue extraction, or lyophilization[J] Parasitol Res. 1991;77(4):315–319. doi: 10.1007/BF00930907. [DOI] [PubMed] [Google Scholar]
- 10.El Ridi R, Velupillai P, Harn DA. Regulation of Schistosome egg granuloma formation: Host-soluble l-selectin enters tissue-trapped eggs and binds to carbohydrate antigens on surface membrane of miracidia[J] Infect Immun. 1996;64(11):4700–4705. doi: 10.1128/iai.64.11.4700-4705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell G, Garcia EG, Rivera PT, et al. Evidence for and implications of anti-embryonation immunity in Schistosomiasis[J] Exper Parasitol. 1994;79(4):546–549. doi: 10.1006/expr.1994.1116. [DOI] [PubMed] [Google Scholar]
- 12.Rumjanek FD, Campos EG, Afonso LC. Evidence for the occurrence of LDL receptors in extracts of schistosomula of Schistosoma mansoni[J] Mol Biochem Parasitol. 1988;28(2):145–152. doi: 10.1016/0166-6851(88)90062-x. [DOI] [PubMed] [Google Scholar]
- 13.Rumjanek FD, McLaren DJ, Smithers SR. Serum-induced expression of a surface protein in schistosomula of Schistosoma mansoni: a possible receptor for lipid uptake[J] Mol Biochem Parasitol. 1983;9(4):337–350. doi: 10.1016/0166-6851(83)90090-7. [DOI] [PubMed] [Google Scholar]
- 14.Xu X, Caulfield JP. Characterization of human low density lipoprotein binding proteins on the surface of Schistosomula of Schistosoma mansoni[J] Eur J Cell Biol. 1992;57(2):229–235. [PubMed] [Google Scholar]
- 15.Furlong ST, Thibault KS, Morbelli LM, et al. Uptake and compartmentalization of fluorescent lipid analogs in larval Schistosoma mansoni[J] J Lipid Res. 1995;36(1):1–12. [PubMed] [Google Scholar]
- 16.Rogers MV, Quilici D, Mitchell GF, et al. Purification of a putative lipoprotein receptor from Schistosoma japonicum adult worms[J] Mol Biochem Parasitol. 1990;41(1):93–100. doi: 10.1016/0166-6851(90)90100-z. [DOI] [PubMed] [Google Scholar]
- 17.Fan J, Xiaoxian G, Yang W, et al. A Schistosoma japonicum very low-density lipoprotein-binding protein[J] Int J Biochem Cell Biol. 2003;35(10):1436–1451. doi: 10.1016/s1357-2725(03)00105-5. [DOI] [PubMed] [Google Scholar]
- 18.Okumura-Noji K, Sasai K, Zhan R, et al. Cholesteryl ester transfer protein deficiency causes slow egg embryonation of Schistosoma japonicum[J] Biochem Biophys Res Com. 2001;286(2):305–310. doi: 10.1006/bbrc.2001.5386. [DOI] [PubMed] [Google Scholar]
- 19.Okumura-Noji K, Miura Y, Lu R, et al. CD36-related protein in Schistosoma japonicum: candidate mediator of selective cholesteryl ester uptake from high-density lipoprotein for egg maturation[J] FASEB J. 2013;27(3):1236–1244. doi: 10.1096/fj.12-219816. [DOI] [PubMed] [Google Scholar]
- 20.Yokoyama S. A potential screening factor for accumulation of cholesteyl ester transfer protein deficiency in East Asia: Schistosoma japonicum[J] Biochim Biophys Acta. 2014;1841(4):495–504. doi: 10.1016/j.bbalip.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Bruce C, Davidson WS, Kussie P, et al. Molecular determinants of plasma cholesteryl ester transfer protein binding to high density lipoproteins[J] J Biol Chem. 1995;270(19):11532–11542. doi: 10.1074/jbc.270.19.11532. [DOI] [PubMed] [Google Scholar]
- 22.Jiang XC, Bruce C, Cocke T, et al. Point mutagenesis of positively charged amino acids of cholesteryl ester transfer protein: conserved residues within the lipid transfer/lipopolysaccharide binding protein gene family essential for function[J] Biochemistry. 1995;34(21):7258–7263. doi: 10.1021/bi00021a042. [DOI] [PubMed] [Google Scholar]
- 23.Tall A. Plasma lipid transfer proteins[J] Ann Rev Biochem. 1995;64:235–257. doi: 10.1146/annurev.bi.64.070195.001315. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Kussie P, Deng L, et al. Defective binding of neutral lipids by carboxyl-terminal deletion mutant of cholesteryl ester transfer protein[J] J Biol Chem. 1995;270(2):612–618. doi: 10.1074/jbc.270.2.612. [DOI] [PubMed] [Google Scholar]
- 25.Drayna D, Jarnagin AS, McLean J, et al. Cloning and sequencing of human cholesteryl ester transfer protein cDNA[J] Nature. 1987;327(6123):632–634. doi: 10.1038/327632a0. [DOI] [PubMed] [Google Scholar]
- 26.Ko KW, Ohnishi T, Yokoyama S. Triglyceride transfer is required for net cholesteryl ester transfer between lipoproteins in plasma by lipid transfer protein. Evidence for a hetero-exchange transfer mechanism demonstrated by using novel monoclonal antibodies[J] J Biol Chem. 1994;269(45):28206–28213. [PubMed] [Google Scholar]
- 27.Ohnishi T, Hicks LD, Oikawa K, et al. Properties of human plasma lipid transfer protein in aqueous solution and at interfaces[J] Biochemistry. 1994;33(20):6093–6099. doi: 10.1021/bi00186a008. [DOI] [PubMed] [Google Scholar]
- 28.Ohnishi T, Tan C, Yokoyama S. Selective transfer of cholesteryl ester over triglyceride by human plasma lipid transfer protein between apolipoprotein-activated lipid microemulsion[J] Biochemistry. 1994;33:4533–4542. doi: 10.1021/bi00181a014. [DOI] [PubMed] [Google Scholar]
- 29.Qiu X, Mistry A, Ammirati MJ, et al. Crystal structure of cholesteryl ester transfer protein reveals a long tunnel and four bound lipid molecules[J] Nat Struct Mol Biol. 2007;14(2):106–113. doi: 10.1038/nsmb1197. [DOI] [PubMed] [Google Scholar]
- 30.Foger B, Ritsch A, Doblinger A, et al. Relationship of plasma cholesteryl ester transfer protein to HDL cholesterol. Studies in normotriglyceridemia and moderate hypertriglyceridemia[J] Arterioscler Thromb Vasc Biol. 1996;16(12):1430–1436. doi: 10.1161/01.atv.16.12.1430. [DOI] [PubMed] [Google Scholar]
- 31.Czarnecka H, Yokoyama S. Regulation of lecithin-cholesterol acyltransferase reaction by acyl acceptors and demonstration of its “idling” reaction[J] J Biol Chem. 1993;268(26):19334–19340. [PubMed] [Google Scholar]
- 32.Francis GA, Ko KW, Hara H, et al. Regulation of the uptake of high-density lipoprotein-originated cholesteryl ester by HepG2 cells: role of low-density lipoprotein and plasma lipid transfer protein[J] Biochim Biophys Acta. 1991;1084(2):159–166. doi: 10.1016/0005-2760(91)90215-4. [DOI] [PubMed] [Google Scholar]
- 33.Kurasawa T, Yokoyama S, Miyake Y, et al. Rate of cholesteryl ester transfer between high and low density lipoproteins in human serum and a case with decreased transfer rate in association with hyperalphalipoproteinemia[J] J Biochem. 1985;98(6):1499–1508. doi: 10.1093/oxfordjournals.jbchem.a135418. [DOI] [PubMed] [Google Scholar]
- 34.Koizumi J, Mabuchi H, Yoshimura A, et al. Deficiency of serum cholesteryl-ester transfer activity in patients with familial hyperalphalipoproteinaemia[J] Atheroscler. 1985;58(1-3):175–186. doi: 10.1016/0021-9150(85)90064-4. [DOI] [PubMed] [Google Scholar]
- 35.Brown ML, Inazu A, Hesler CB, et al. Molecular basis of lipid transfer protein deficiency in a family with increased high-density lipoproteins[J] Nature. 1989;342(6248):448–451. doi: 10.1038/342448a0. [DOI] [PubMed] [Google Scholar]
- 36.Inazu A, Jiang XC, Haraki T, et al. Genetic cholesteryl ester transfer protein deficiency caused by two prevalent mutations as a major determinant of increased levels of high density lipoprotein cholesterol[J] J Clin Invest. 1994;94(5):1872–1882. doi: 10.1172/JCI117537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakai N, Yamashita S, Hirano K, et al. Frequency of exon 15 missense mutation (442D:G) in cholesteryl ester transfer protein gene in hyperalphalipoproteinemic Japanese subjects[J] Atherosclerosis. 1995;114(2):139–145. doi: 10.1016/0021-9150(94)05477-z. [DOI] [PubMed] [Google Scholar]
- 38.Hirano K, Yamashita S, Nakajima N, et al. Genetic cholesteryl ester transfer protein deficiency is extremely frequent in the Omagari area of Japan. Marked hyperalphalipoproteinemia caused by CETP gene mutation is not associated with longevity[J] Arterioscler Thromb Vasc Biol. 1997;17(6):1053–1059. doi: 10.1161/01.atv.17.6.1053. [DOI] [PubMed] [Google Scholar]
- 39.Maruyama T, Yamashita S, Matsuzawa Y, et al. Mutations in Japanese subjects with primary hyperlipidemia–results from the Research Committee of the Ministry of Health and Welfare of Japan since 1996[J] J Atheroscler Thromb. 2004;11(3):131–145. doi: 10.5551/jat.11.131. [DOI] [PubMed] [Google Scholar]
- 40.Yamashita S, Hirano K, Sakai N, et al. Molecular biology and pathophysiological aspects of plasma cholesteryl ester transfer protein[J] Biochim Biophys Acta. 2000;1529(1-3):257–275. doi: 10.1016/s1388-1981(00)00164-5. [DOI] [PubMed] [Google Scholar]
- 41.Nagano M, Yamashita S, Hirano K, et al. Molecular mechanisms of cholesteryl ester transfer protein deficiency in Japanese[J] J Atheroscler Thromb. 2004;11(3):110–121. doi: 10.5551/jat.11.110. [DOI] [PubMed] [Google Scholar]
- 42.Nagano M, Yamashita S, Hirano K, et al. Two novel missense mutations in the CETP gene in Japanese hyperalphalipoproteinemic subjects: high-throughput assay by Invader assay[J] J Lipid Res. 2002;43(7):1011–1018. doi: 10.1194/jlr.m200024-jlr200. [DOI] [PubMed] [Google Scholar]
- 43.Ritsch A, Drexel H, Amann FW, et al. Deficiency of cholesteryl ester transfer protein: Description of molecular defect and the dissociation of cholesteryl ester and triglyceride transfer in plasma[J] Arterioscler Thromb Vasc Biol. 1997;17(16):3433–3441. doi: 10.1161/01.atv.17.12.3433. [DOI] [PubMed] [Google Scholar]
- 44.Chen DW, Yang JF, Tang Z, et al. Cholesteryl ester transfer protein polymorphism D442G associated with a potential decreased risk for Alzheimer's disease as a modifier for APOE epsilon4 in Chinese[J] Brain Res. 2008;1187:52–57. doi: 10.1016/j.brainres.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 45.Chiba H, Akita H, Tsuchihashi K, et al. Quantitative and compositional changes in high density lipoprotein subclasses in patients with various genotypes of cholesteryl ester transfer protein deficiency[J] J Lipid Res. 1997;38(6):1204–1216. [PubMed] [Google Scholar]
- 46.Hui S-P. Frequency and effect on plasma lipoprotein metabolism of a mutation in the cholesteryl ester transfer protein gene in the Chinese[J] Hokkaido J Med Sci. 1997;72(3):319–327. [PubMed] [Google Scholar]
- 47.Hsu LA, Ko YL, Hsu KH, et al. Genetic variations in the cholesteryl ester transfer protein gene and high density lipoprotein cholesterol levels in Taiwanese Chinese[J] Hum Genet. 2002;110(1):57–63. doi: 10.1007/s00439-001-0640-z. [DOI] [PubMed] [Google Scholar]
- 48.Jap TS, Wu YC, Tso YC, et al. A novel mutation in the intron 1 splice donor site of the cholesterol ester transfer protein (CETP) gene as a cause of hyperalphalipoproteinemia[J] Metabolism. 2002;51(3):394–397. doi: 10.1053/meta.2002.30527. [DOI] [PubMed] [Google Scholar]
- 49.Yang GJ, Gemperli A, Vounatsou P, et al. A growing degree-days based time-series analysis for prediction of Schistosoma japonicum transmission in Jiangsu province, China[J] Am J Trop Med Hyg. 2006;75(3):549–555. [PubMed] [Google Scholar]
- 50.Song G-J, Han G-H, Chae J-J, et al. The effect of the cholesterol ester transfer protein gene and environmental factors on the plasma high density lipoprotein cholesterol levels in the Korean population[J] Molecules & Cells. 1997;7(5):615–619. [PubMed] [Google Scholar]
- 51.Thu NN, Mai TT, Ohmori R, et al. Effect of the cholesteryl ester transfer protein genotypes on plasma lipid and lipoprotein levels in Vietnamese children[J] Pediatr Res. 2005;58(6):1249–1253. doi: 10.1203/01.pdr.0000183782.57705.fc. [DOI] [PubMed] [Google Scholar]
- 52.Plengpanich W, Siriwong S, Khovidhunkit W. Two novel mutations and functional analyses of the CETP and LIPC genes underlying severe hyperalphalipoproteinemia[J] Metabolism. 2009;58(8):1178–1184. doi: 10.1016/j.metabol.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 53.Thompson JF, Reynolds JM, Williams SP, et al. Frequency and function of CETP variants among individuals of Asian ancestry[J] Atherosclerosis. 2009;202(1):241–247. doi: 10.1016/j.atherosclerosis.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 54.Arkhipova NS, Ar'ev AL, Popova EK, et al. Association of polymorphism of angiotensin-converting enzyme gene I/D and D442G cholesteryl ester transfer protein gene with risk factors for atherosclerosis among elderly and senile patients with coronary heart disease in the Republic Sakha (Yakutia)[J] Adv Gerontol. 2013;3(4):277–281. [PubMed] [Google Scholar]
- 55.Finkelstein JL, Schleinitz MD, Carabin H, et al. Decision-model estimation of the age-specific disability weight for schistosomiasis japonica: a systematic review of the literature[J] PLoS Negl Trop Dis. 2008;2(3):e158. doi: 10.1371/journal.pntd.0000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma YQ, Thomas GN, Critchley JA, et al. Identification of the intron 14 splicing defect of the cholesteryl ester transfer protein gene in Hong Kong Chinese[J] Clin Genet. 2001;59(4):287–289. doi: 10.1034/j.1399-0004.2001.590413.x. [DOI] [PubMed] [Google Scholar]
- 57.Nihei N, Kajihara N, Kirinoki M, et al. Establishment of a GIS monitoring system for schistosomiasis japonica in Kofu, Japan[J] Ann Trop Med Parasitol. 2006;100(2):143–153. doi: 10.1179/136485906X86293. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi H, Takahashi A, Maki M, et al. Effect of CETP on the plasma lipoprotein profile in four strains of transgenic mouse[J] Biochem Biophys Res Commun. 2001;283(1):118–123. doi: 10.1006/bbrc.2001.4743. [DOI] [PubMed] [Google Scholar]
- 59.Zhang R, Yoshida A, Kumagai T, et al. Vaccination with calpain induces a Th1-biased protective immune response against Schistosoma japonicum[J] Infect Immun. 2001;69(1):386–391. doi: 10.1128/IAI.69.1.386-391.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Teh EM, Dolphin PJ, Breckenridge WC, et al. Human plasma CETP deficiency: identification of a novel mutation in exon 9 of the CETP gene in a Caucasian subject from North America[J] J Lipid Res. 1998;39:442–456. [PubMed] [Google Scholar]
- 61.Hill SA, Nazir DJ, Jayaratne P, et al. Mutations in cholesteryl ester transfer protein and hepatic lipase in a North American population[J] Clin Biochem. 1997;30(5):413–418. doi: 10.1016/s0009-9120(97)00009-x. [DOI] [PubMed] [Google Scholar]
- 62.van der Steeg WA, Hovingh GK, Klerkx AH, et al. Cholesteryl ester transfer protein and hyperalphalipoproteinemia in Caucasians[J] J Lipid Res. 2007;48(3):674–682. doi: 10.1194/jlr.M600405-JLR200. [DOI] [PubMed] [Google Scholar]
- 63.Rhyne J, Ryan MJ, White C, et al. The two novel CETP mutations Gln87X and Gln165X in a compound heterozygous state are associated with marked hyperalphalipoproteinemia and absence of significant coronary artery disease[J] J Mol Med (Berl) 2006;84(8):647–650. doi: 10.1007/s00109-006-0070-4. [DOI] [PubMed] [Google Scholar]
- 64.Cefalu AB, Noto D, Magnolo L, et al. Novel mutations of CETP gene in Italian subjects with hyperalphalipoproteinemia[J] Atherosclerosis. 2009;204(1):202–207. doi: 10.1016/j.atherosclerosis.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 65.Calabresi L, Nilsson P, Pinotti E, et al. A novel homozygous mutation in CETP gene as a cause of CETP deficiency in a Caucasian kindred[J] Atherosclerosis. 2009;205(2):506–511. doi: 10.1016/j.atherosclerosis.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 66.Chantepie S, Bochem AE, Chapman MJ, et al. High-density lipoprotein (HDL) particle subpopulations in heterozygous cholesteryl ester transfer protein (CETP) deficiency: maintenance of antioxidative activity[J] PLoS One. 2012;7(11):e49336. doi: 10.1371/journal.pone.0049336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thompson A, Di Angelantonio E, Sarwar N, et al. Association of cholesteryl ester transfer protein genotypes with CETP mass and activity, lipid levels, and coronary risk[J] JAMA. 2008;299(23):2777–2788. doi: 10.1001/jama.299.23.2777. [DOI] [PubMed] [Google Scholar]
- 68.Inazu A, Brown ML, Hesler CB, et al. Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation[J] N Engl J Med. 1990;323(18):1234–1238. doi: 10.1056/NEJM199011013231803. [DOI] [PubMed] [Google Scholar]
- 69.Yamashita S, Sprecher DL, Sakai N, et al. Accumulation of apolipoprotein E-rich high density lipoproteins in hyperalphalipoproteinemic human subjects with plasma cholesteryl ester transfer protein deficiency[J] J Clin Invest. 1990;86(3):688–695. doi: 10.1172/JCI114764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sakai N, Matsuzawa Y, Hirano K, et al. Detection of two species of low density lipoprotein particles in cholesteryl ester transfer protein deficiency[J] Arterioscler Thromb. 1991;11(1):71–79. doi: 10.1161/01.atv.11.1.71. [DOI] [PubMed] [Google Scholar]
- 71.Yamashita S, Hui DY, Wetterau JR, et al. Characterization of plasma lipoproteins in patients heterozygous for human plasma cholesteryl ester transfer protein (CETP) deficeiency: Plasma CETP regulated high-density lipoprotein concentration and composition[J] Metabolism. 1991;40(7):756–763. doi: 10.1016/0026-0495(91)90097-g. [DOI] [PubMed] [Google Scholar]
- 72.Moriyama Y, Okamura T, Inazu A, et al. A low prevalence of coronary heart disease among subjects with increased high-density lipoprotein cholesterol levels, including those with plasma cholesteryl ester transfer protein deficiency[J] Prev Med. 1998;27(5 Pt 1):659–667. doi: 10.1006/pmed.1998.0340. [DOI] [PubMed] [Google Scholar]
- 73.Curb JD, Abbott RD, Rodriguez BL, et al. A prospective study of HDL-C and cholesteryl ester transfer protein gene mutations and the risk of coronary heart disease in the elderly[J] J Lipid Res. 2004;45(5):948–953. doi: 10.1194/jlr.M300520-JLR200. [DOI] [PubMed] [Google Scholar]
- 74.Nissen SE, Tardif JC, Nicholls SJ, et al. Effect of torcetrapib on the progression of coronary atherosclerosis[J] N Engl J Med. 2007;356(13):1304–1316. doi: 10.1056/NEJMoa070635. [DOI] [PubMed] [Google Scholar]
- 75.Schwartz GG, Olsson AG, Abt M, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome[J] N Engl J Med. 2012;367(22):2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 76.Betard C, Kessling AM, Roy M, et al. Molecular genetic evidence for a founder effect in familial hypercholesterolemia among French Canadians[J] Hum Genet. 1992;88(5):529–536. doi: 10.1007/BF00219339. [DOI] [PubMed] [Google Scholar]
- 77.Torrington M, Botha JL, Pilcher GJ, et al. Association between familial hypercholesterolaemia and church affiliation. Is this the result of sociocultural isolation of migrant farmers in 19th-century South Africa?[J] S Afr Med J. 1984;65(19):762–767. [PubMed] [Google Scholar]
- 78.Abifadel M, Rabes JP, Jambart S, et al. The molecular basis of familial hypercholesterolemia in Lebanon: spectrum of LDLR mutations and role of PCSK9 as a modifier gene[J] Hum Mutat. 2009;30(7):E682–691. doi: 10.1002/humu.21002. [DOI] [PubMed] [Google Scholar]
- 79.Allison AC. Protection afforded by sickle-cell trait against subtertian malareal infection[J] Br Med J. 1954;1(4857):290–294. doi: 10.1136/bmj.1.4857.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hedrick PW. Resistance to malaria in humans: the impact of strong, recent selection[J] Malar J. 2012;11:349. doi: 10.1186/1475-2875-11-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taylor SM, Parobek CM, Fairhurst RM. Haemoglobinopathies and the clinical epidemiology of malaria: a systematic review and meta-analysis[J] The Lancet Infectious Diseases. 2012;12(6):457–468. doi: 10.1016/S1473-3099(12)70055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Acton S, Gigotti A, Landschulz T, et al. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor[J] Science. 1996;271(5248):518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 83.Murao K, Terpstra V, Green SR, et al. Characterization of CLA-1, a human homologue of rodent scavenger receptor BI, as a receptor for high density lipoprotein and apoptotic thymocytes[J] J Biol Chem. 1997;272(28):17551–17557. doi: 10.1074/jbc.272.28.17551. [DOI] [PubMed] [Google Scholar]
- 84.Okamoto H, Yonemori F, Wakitani K, et al. A cholesteryl ester transfer protein inhibitor attenuates atherosclerosis in rabbits[J] Nature. 2000;406(6792):203–207. doi: 10.1038/35018119. [DOI] [PubMed] [Google Scholar]