Abstract
Accurate and timely diagnosis of prosthetic joint infection is essential to initiate early treatment and achieve a favorable outcome. In this study, we used a rabbit model to assess the feasibility of technetium-99m-labeled annexin V for detecting prosthetic joint infection. Right knee arthroplasty was performed on 24 New Zealand rabbits. After surgery, methicillin-susceptible Staphylococcus aureus was intra-articularly injected to create a model of prosthetic joint infection (the infected group, n = 12). Rabbits in the control group were injected with sterile saline (n = 12). Seven and 21 days after surgery, technetium-99m-labeled annexin V imaging was performed in 6 rabbits of each group. Images were acquired 1 and 4 hours after injection of technetium-99m-labeled annexin V (150 MBq). The operated-to-normal-knee activity ratios were calculated for quantitative analysis. Seven days after surgery, increased technetium-99m-labeled annexin V uptake was observed in all cases. However, at 21 days a notable decrease was found in the control group, but not in the infected group. The operated-to-normal-knee activity ratios of the infected group were 1.84 ± 0.29 in the early phase and 2.19 ± 0.34 in the delay phase, both of which were significantly higher than those of the control group (P = 0.03 and P = 0.02). The receiver operator characteristic curve analysis showed that the operated-to-normal-knee activity ratios of the delay phase at 21 days was the best indicator, with an accuracy of 80%. In conclusion, technetium-99m-labeled annexin V imaging could effectively distinguish an infected prosthetic joint from an uninfected prosthetic joint in a rabbit model.
Keywords: prosthetic joint infection, annexin V, technetium-99m, diagnosis
INTRODUCTION
Currently, prosthetic joint replacements are the gold standard treatment for patients with advanced arthritis. The procedures are usually highly successful and improve a patient's quality of life. However, they are occasionally associated with complications such as aseptic loosening and prosthetic joint infection (PJI)[1]. Accurate differentiation between early aseptic loosening and PJI is very important because treatments, surgical interventions and outcomes are quite different between them[2]. So far, several studies designed to distinguish the two conditions have shown inadequate sensitivity and specificity3-5.
Nuclear medicine for the evaluation of patients with PJI maintains an advantage over other modalities as it is noninvasive and has high specificity. The most accurate imaging test available is labeled autologous leukocyte/technetium-99m-labeled sulfur colloid imaging[6]. However, this technique requires separating and labeling leukocytes ex vivo, which is complex, time-consuming and increases the chance of iatrogenic errors. In addition, imaging of the delay phase after 24 hours plays an important role in diagnosis, which further adds to the complexity of the procedure,7-8.
PJI induces a complex set of biological responses to pathological stimuli. Polymorphonuclear neutrophils (PMNs) in PJI serve as one of the key envoys from the immune system, whose primary function is bactericidal. After phagocytosing infective agents, PMNs become activated and produce large amounts of reactive oxygen species that kill pathogens and induce massive cell apoptosis[9]. In addition, the pathological consequences of infection are also secondary to the release of proteases of microbial origin, which induce fibrinolytic activities and neighboring tissue injury[10].
It has been demonstrated that technetium-99m-labeled annexin V (99mTc-annexin V) can bind with high affinity to phosphatidylserine, which can be externalized in the early stages of apoptosis. It is widely used for in vivo detection of apoptotic cells in cardiovascular disease and cancer[11]. Other studies using various animal models and human beings have shown that 99mTc-annexin V can also detect acute, subacute, and chronic infection[9],[12-13]. In the present study, we investigated whether 99mTc-annexin V imaging was effective in differentiating early stage PJI from uninfected prosthetic joints in a validated rabbit model[14].
MATERIALS AND METHODS
PJI model
The Institutional Animal Experiment Committee of Nanjing Medical University approved the study protocol. Twenty-four New Zealand white male rabbits weighing 3.0 kg − 3.5 kg were housed in individual cages with a natural light–dark cycle. The protocol for establishing the rabbit model of PJI due to methicillin-sensitive Staphylococcus Aureus (MSA) was previously reported[14]. Briefly, an orthopedic surgeon replaced the rabbit's right knee joint with a silicone elastomer implant (Silastic, great toe implant HP, Swanson Design; Dow Corning, Valbonne, France). The operation was performed under general anesthesia induced by ketamine and xylazine hydrochloride (ratio 3.5:1.5). An arthrotomy was made through a midline longitudinal incision and a medial parapatellar approach with the patella dislocated laterally was used to expose the knee joint. This was followed by removal of the epiphysial plates and reaming of the medullary cavity of the proximal tibia and distal femora. A silicone implant (stem: 14 mm, head: 15 mm×5 mm) was inserted in the joint. The deep fascia and skin were then closed.
After wound closure, rabbits were divided at random into two groups (n = 12 for each group). In the infected group, 0.5 mL inoculum containing 106 CFU of MSSA (ATCC 29213) was intra-articularly injected with a 1-mL syringe at the level of the lower patellar border. In the control group, 0.5 mL sterile saline solution was injected at the same location. A postoperative radiograph was obtained for examining the location of the prosthesis.
99mTc-annexin V preparation and imaging
His10-annexin V was synthesized at the Department of Biochemistry of Nanjing University[15]. For radiolabeling, SnCl2·2H2O in 0.05 mol/L HCl was diluted with citrate buffer solution to the concentration of 1.0 mg/mL and mixed vigorously. His10-annexin V (5 μg), 99mTcO4− (600 MBq) and vitamin C (100 μL of 10 mg/mL) were successively added to the reaction mixture (100 μL), mixed thoroughly and left for 30 minutes at room temperature. The radiochemical purity of 99mTc-annexin V was assayed in accordance with the standard procedure using high performance liquid chromatography and the stability of the radiopharmaceutical was tested via metabolite analysis of rabbit urine.
99mTc-annexin V imaging was performed in 6 rabbits from each group at 7 days after surgery. The same procedure was performed on the remaining rabbits of the two groups at 21 days after surgery. After the preparation of 99mTc-annexin V, the rabbits in both groups were injected with approximately 150 MBq 99mTc-annexin V via the ear vein. Before imaging, the rabbits were anesthetized the same as above, tied to a board, and imaged at 1 hour (early phase) and 4 hours (delay phase) after injection. Ten-minute planar anterior and posterior viewings of the legs (matrix 256 × 256) were obtained using a dual-head γ-camera (Millennium VG, Hawkeye; General Electric Medical Systems) equipped with high-resolution parallel collimators.
Data was analyzed by two readers (F.W., Y.J.H) with 12 and 5 years of experience of nuclear medicine, respectively, blinded to the 99mTc-annexin V examination, other diagnosis information, and final clinical diagnosis. Regions of interest were drawn over the operated and contralateral knee on each anterior scintigram. Mean activity per pixel was determined in each region of interest. Final decisions were made by the consensus of the two readers and used for analysis. Inter-rater agreement was measured by using κ statistics. The operated-to-normal knee activity ratios (ONKRs) were calculated (n = 6).
Biodistribution of 99mTc-annexin V
All rabbits were euthanized immediately after the delay phase of 99mTc-annexin V imaging at 21 days after surgery. Tissue radioactive uptake of 99mTc-annexin V (i.e., activity per gram of tissue/injected dose/gram body weight) was determined for the thigh muscle, patellar tendon, femoral cartilage, upper tibial bone, and pus/articular fluid of both the operated and contralateral knees of rabbits in each group. The operated-to-normal knee tissue radioactive uptake ratios were calculated for quantitative analysis (n = 6).
Infection assessment
Clinical manifestation
The operated knee joints were evaluated for clinical manifestations of inflammation, including local warmth, tenderness, drainage and effusion, before harvest by two investigators blinded to the two groups. The soft tissue and intramedullary cavity of the adjacent knee joint were assessed for joint effusion, pus, abscess formation and cortical lysis.
MRI imaging
Seven and 21 days after surgery, 3 rabbits were randomly selected from each group and subjected to MRI, using a 3.0-T magnetic resonance system (Magnetom Trio, Siemens Medical Systems, Germany). The MRI sequences consisted of T1-weighted imaging (T1WI): repetition time (TR)/echo time (TE) = 765/19 ms, T2-weighted imaging (T2WI): TR/TE = 4590/42 ms, and proton density-weighted imaging (PDWI): TR/TE = 3600/20 ms. Other parameters were the same, as follows: field of view 20 cm×20 cm, matrix 320×320, and section thickness 1.5 mm.
Microbiological analyses
After harvest, the exudate surrounding the prosthetic joint of each rabbit was carefully removed under sterile conditions, spread over a blood-agar plate, and incubated for 72 hours at 37 °C. Infection was confirmed by the formation of MSSA colonies on the plates.
Histological analyses
The operated knees of all rabbits were fixed with 4% formalin for at least 24 hours, then decalcified in 5% nitric acid and embedded in paraffin. The samples were sectioned to 4 μm along the longitudinal line of medullary cavity and stained with hematoxylin-eosin (H&E) for microscopic analysis.
Statistical analyses
Data was expressed as the mean ± standard deviation (SD) and analyzed by using SPSS 13.0 (SPSS, Chicago, USA). Differences in the ONKRs (early phase and delay phase) and tissue radioactive uptake ratio between the control group and infected group were compared by Student's t-test. The ONKR cutoff value that could predict acute PJI with the highest sensitivity and specificity was defined by constructing a receiver operator characteristic (ROC) curve for the prosthetic model. The P value was two-sided and values of less than 0.05 were considered statistically significant.
RESULTS
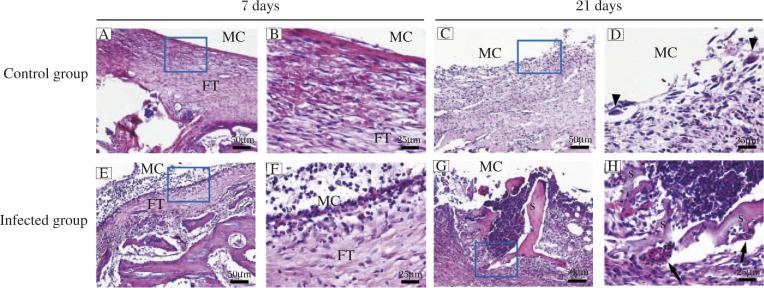
All rabbits in the infected group showed obvious clinical signs of purulent infection, whereas those of the control group were free of infection signs. Most infected rabbits (10 of 12 rabbits) demonstrated obvious joint swelling, effusion and intramedullary pus formation. The culture results indicated that the exudate surrounding the prosthesis of each rabbit in the control group was sterile, while MSSA colonies were detected in all rabbits of the infected group. There was a notable difference in histology between the control and infected groups. In the control group, H&E staining (Fig. 1A, B, C and D) revealed a fibrous tissue with multiple hemorrhages at 7 days after surgery. Local fibrous membrane formation indicated rejection of the prosthetic implant. Moreover, at 21 days after surgery, many macrophages, monocytes and multinucleated giant cells accumulated within fibrous tissue and were visible in the medullary cavity. However, in the infected group (Fig. 1E, F, G and H), typical inflammatory response was observed around the prosthetic joint at 7 days after surgery, including fibrous tissue formation accompanied by neutrophil infiltration, vascular dilation, and bacterial colonies. Twenty-one days after surgery, abscess formation, bone and bone marrow necrosis, cortical lysis, or sequestra were found in the medullary cavity.
Fig. 1. H&E staining of operated knees from the control group (A–D) and infected group (E–H).
Magnification: 100× (A, C, E and G) and 400× (B, D, F and H). Black arrowheads indicate multinucleated giant cells. MC, medullary cavity. FT, fibrous tissue. Black arrows reveal neutrophil infiltration. S, sequestra formation.
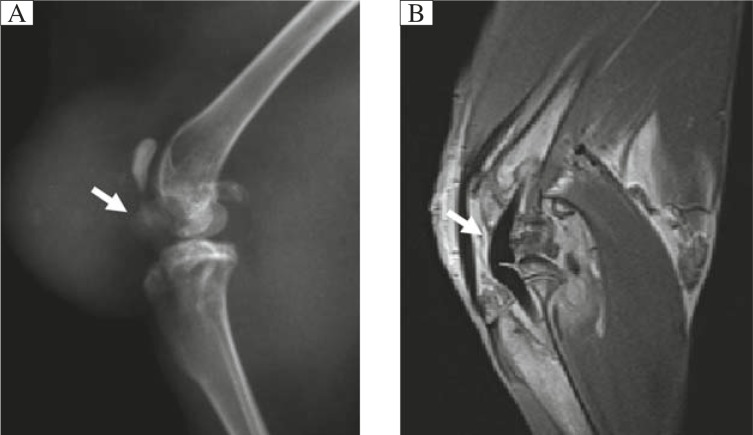
The location of the prosthesis and tissue changes around the prosthetic joint in the operated knee were clearly seen on both radiographs (Fig. 2A) and MRI (PDWI, Fig. 2B).
Fig. 2. Focal X-ray (A) and MRI (B; PD sequence) clearly show the prosthesis and tissue changes in the operated knee after arthroplasty.
Characterization of 99mTc-annexin V
Intense renal and bladder activity was observed in whole-body imaging as 99mTc-annexin V was excreted through the urinary system. Negligible accumulation was observed in the liver, spleen, bowel and thyroid, while little99mTc-annexin V was accumulated in the femoral and tibial epiphysis, normal bone, muscle, and bone marrow. The radiochemical purity of 99mTc-annexin V was confirmed > 95%. After injection of 99mTc-annexin V for 120 minutes, the high percentage of radiolabeled metabolites in rabbit urine confirmed a high radiolabeled metabolite fraction in the bladder.
Visual and quantitative analysis of 9mTc-annexin V imaging
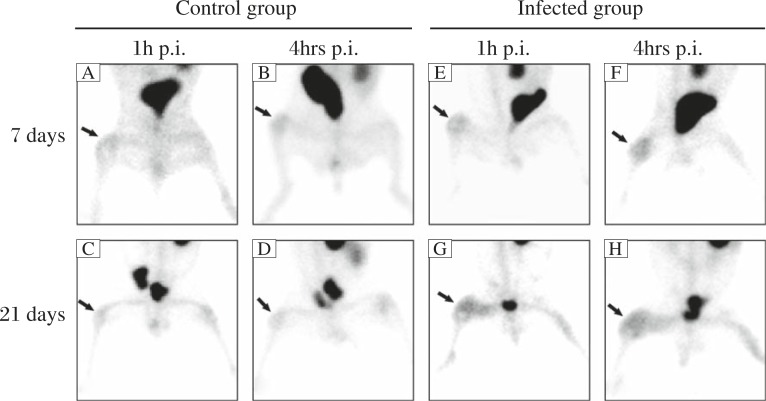
Visual analysis of images in the early and delay phase showed significantly increased radioactivity uptake of 99mTc-annexin V in the operated knees, compared with the contralateral knees, in all cases at 7 days after surgery (Fig. 3A, B, E and F). Significantly decreased uptake in both the early and delay phase was seen in the control group at 21 days after surgery, but similar decreased activity was not noted in the infected group.
Fig. 3. 99mTc-annexin V images in the control (A, B, C and D) and infected groups (E, F, G, and H) at 7 and 21 days after surgery.
Compared with the contralateral knees, notable uptakes of 99mTc-annexin V in the operated knees (arrows) were found in all rabbits of both groups at 7 days after surgery. However, no obvious uptake was noted in the control group at 21 days, while high activities were seen in the infected group.
Seven days after surgery, the ONKRs of both the control group and infected group were 1.98±0.19 and 2.09±0.23 in the early phase (P = 0.42), 1.79±0.15 and 1.99±0.43 in the delay phase (P = 0.31) (Table 1). However, at 21 days after surgery, the ONKRs of the infected group were 1.84±0.29 in the early phase and 2.19±0.34 in the delay phase, which were significantly higher than those of the control group (P = 0.03 and P = 0.02). The weighted kappa value for inter-rater agreement was 0.802.
Table 1. ONKR values and ROC curve analysis of 99mTc-annexin V in the control group and the infected group.
| ONKR values | ROC curve analysis | ||||||||
| Control group | Infected group | P | AUC | Cutoff | Se(%) | Sp(%) | Ac(%) | ||
| 7 days after surgery | 1 hour p.i. | 1.98 ± 0.19 | 2.09 ± 0.23 | 0.42 | 0.653 | 1.89 | 83.3 | 50 | 37.5 |
| 4 hours p.i. | 1.79 ± 0.15 | 1.99 ± 0.43 | 0.31 | 0.639 | 1.9 | 66.7 | 83.7 | 80 | |
| 21 days after surgery | 1 hour p.i. | 1.42 ± 0.29 | 1.84 ± 0.29 | 0.03 | 0.833 | 1.44 | 100 | 50 | 66.7 |
| 4 hours p.i. | 1.53 ± 0.48 | 2.19 ± 0.34 | 0.02 | 0.917 | 1.86 | 100 | 83.3 | 85.7 | |
ONKR: Operated to Normal Knee activity Ratio; p.i.: post 99mTc-annexin V injection; In the calculation of sensitivity (Se), specificity (Sp) and accuracy (Ac), the scan was considered positive for infection when ONKR value was > cutoff; The ONKRs of the infected group were significantly higher than those of the control group at 21 days after surgery, especially in delay phase.
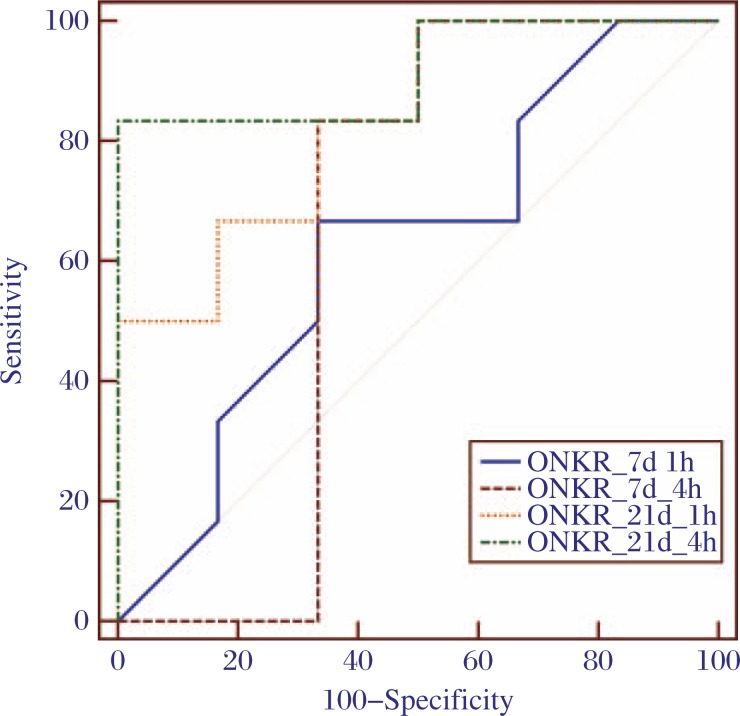
Taking into account of all supporting evidence for infection, the ROC curve analysis (Fig. 4) identified an ONKR threshold of 1.86, in the delay phase at 21 days after surgery, to have the highest sensitivity (100%) and specificity (83.3%) for the diagnosis of PJI, with an accuracy of 80%.
Fig. 4. The ROC curves of ONKR in the control and infected groups at 7 and 21 days after surgery.
The ROC curve analysis showed that the ONKR of delay phase at 21 days was the best indicator for differentiating the infected group from the control group.
Biodistributions of 99mTc-annexin V
The operated-to-normal knee tissue radioactive uptake ratio (Table 2) of the thigh muscle in the infected group (1.65±0.61) was significantly higher than that of the control group (0.67±0.18) (P = 0.01). There were no significant differences, between the infected group and control group in the patellar tendon, femoral cartilage, upper tibial bone and pus/articular fluid.
Table 2. Biodistribution of 99mTc-annexin V in the control group and the infected group.
| Tissue | Control group | Infected group | P |
| Thigh muscle | 0.67 ± 0.18 | 1.65 ± 0.61 | 0.01 |
| Patellar tendon | 1.8 ± 0.74 | 1.9 ± 0.49 | 0.78 |
| Femoral cartilage | 1.81 ± 0.34 | 1.63 ± 0.42 | 0.43 |
| Upper tibial bone | 1.67 ± 0.12 | 1.74 ± 0.41 | 0.71 |
| Pus/articular fluid | 1.64 ± 0.25 | 1.99 ± 0.46 | 0.14 |
Data were presented as tissue radioactive uptake ratio between the operated and the normal contralateral knee. There was no significant difference in the control group and the infected group, except in thigh muscle.
MRI imaging
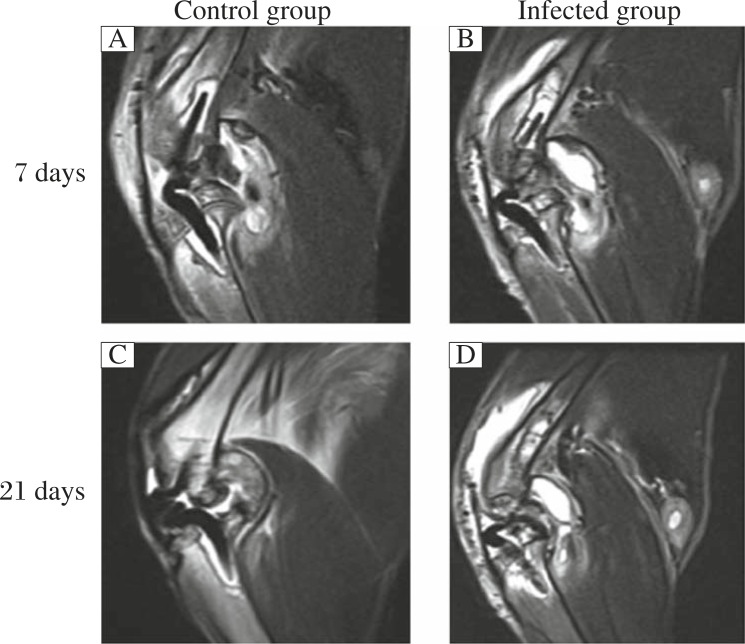
Seven days after surgery, intra-articular swelling and effusion were seen on T2WI in both groups (Fig. 5A and B). However, 21 days after surgery, swelling and effusion were obviously decreased in the control group (Fig. 5C) and became aggravated in the infected group (Fig. 5D).
Fig. 5. MRI (T2WI) imaging was used for detecting intra-articular changes in the control (A and C) and infected (B and D) groups.
Seven days after surgery, intra-articular swelling and effusion could be observed in both groups (A and B). However, 21 days after surgery, the swelling and effusion obviously decreased in the control group (C), but was aggravated in the infected group (D).
DISCUSSION
We hypothesized that 99mTc-annexin V imaging could detect the metabolic changes of local infection, thereby assisting the diagnosis of PJI. The results from this study support that hypothesis. Twenty-one days after surgery, there was a significantly increased uptake of 99mTc-annexin V in the infected group compared with the control group. This increased uptake persisted even in the delay phase. In addition, our results show good reproducibility in both groups and high diagnostic accuracy.
Recently, Mordechai et al.[13] reported that in a clinical trial 99mTc-recombinant human annexin V imaging showed high sensitivity (100%) and low specificity (67%) for diagnosing PJI. The results of our present study improved on this specificity (83.3%). The difference might be attributed to low-grade, cross-stage infection and the limitations of the clinical trial. Clinical trials are difficult to design due to individual differences in patient course and recovery. In particular, the time from onset to detection of PJI is variable[14],[16]. Therefore, experiments using animal models should be a prerequisite to further human trials.
It is important to note that although many factors (e.g., hematogenous dissemination) can cause PJI contamination in total joint replacement, it is mainly due to wound infection from the air in the operating room[14],[17-19]. The rabbit model used in the present study simulates this source by immediate injection of the bacteria after wound closure. Animal model enabled us to perform 99mTc-annexin V imaging at different time points, especially in the delay phase, which helped to ascertain the temporal changes of infection and reach a definite diagnosis. In comparison, it is difficult to confirm and identify infections in humans even by aspiration or tissue biopsy.
Increased uptake of 99mTc-annexin V in the operated knee of both groups was found at 7 days after surgery. According to Rouzet et al.[10], the peak of uptake reflects tissue remodeling, fibrosis, and the formation of granulation tissue in the early stage of inflammation. A similar rise was also noted in 18F-FDG imaging in a study by Yamada[20], in which the zone of high uptake at autoradiography was directly correlated with the marginal zone of the young fibroblast, new vessels, and phagocytes. The peak uptake of 18F-FDG was associated with histological changes of early chronic inflammation. From the observations and inferences of the present study, we hypothesize that this may also be secondary to local hydrodynamics changes caused by the biological process of wound healing and maturation. These changes increase vascular permeability and blood flow[21]. However, in the present study we found that 99mTc-annexin V uptake in the control group unexpectedly decreased within 21 days, while that of the infected group slightly increased, even in delay phase. This suggested that the reaction of local tissues to both surgery and prosthesis in uninfected joints gradually subsided by 21 days after surgery. The accumulation of 99mTc-annexin V in this stage was specifically due to the inherent pathological changes of infection[22-23].
From histology, we easily observed cellular necrosis in the tissues surrounding the prosthetic joint, accompanied by massive acute inflammatory cell infiltration. As Penn et al. has reported[9], PMNs are recruited in microbial infection as a part of systemic and local process in a continuous manner. As the number of apoptotic PMNs increase, the concentration of radiolabeled annexin V increases in the area. It is possible that infection leads to lysis of several types of acute inflammatory cells, including neutrophils, lymphocytes, and macrophages. As their physical integrity is compromised, the externalization of phosphatidylserine increases[24-25]. In addition, proteases released by staphylococcus aureus strains induce detachment and death of neighboring tissue. The staphylokinase conveyed by bacteria could lead to the generation of plasmin, which in turn may activate host proteases[26]. The bacteria may secrete cell toxin and various proteases, which promote neighboring tissue injury and intensify cell stress, apoptosis, and necrosis[27]. In the present study, the operated-to-normal knee tissue radioactive uptake ratio of the thigh muscle in the infected group was significantly higher than that of the control group. We consider that local tissue vasodilation, increased vascular permeability and blood flow, as the results of inflammation, may lead to 99mTc-annexin V accumulation.
Since His10-annexin V (molecular weight 35.8 kDa) can be directly labeled to 99mTc, our results showed a faster clearance rate via the renal system and negligible abdominal accumulation because there is no chelated compound formation. Considering all these advantages and the improved target-to-background ratios, 99mTc-annexin V imaging is feasible for clinical application.
There are a number of limitations in this preliminary study. First, intrinsic response of PJI in animal models usually differs from that of humans. However, the rabbit model is well-accepted and has been used extensively for diagnosis and treatment studies[28-30]. Second, the silicone-elastomer implants used are different from those used in clinical practice.
In conclusion, our results showed 99mTc-annexin V accumulation in PJI, but not in uninfected prosthetic joint, at 21 days after surgery. Radioactive accumulation seemed to be related to the inherent pathological changes of inflammation and injury tissues. The findings of this study indicate that 99mTc-annexin V imaging may be a potential tool for clinical diagnosis of PJI. Further study is warranted.
Acknowledgements
This research was supported by the Chinese National Nature Sciences Foundation (31070861, 81171745).
References
- 1.Ong KL, Kurtz SM, Lau E, et al. Prosthetic joint infection risk after total hip arthroplasty in the Medicare population[J]. J Arthroplasty 200924((6 Suppl)), 105–109. [DOI] [PubMed] [Google Scholar]
- 2.El Helou OC, Berbari EF, Marculescu CE, et al. Outcome of enterococcal prosthetic joint infection: is combination systemic therapy superior to monotherapy?[J] Clin Infect Dis 200847((7)), 903–909. [DOI] [PubMed] [Google Scholar]
- 3.Bottner F, Wegner A, Winkelmann W, et al. Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement[J]. J Bone Joint Surg Br 200789((1)), 94–99. [DOI] [PubMed] [Google Scholar]
- 4.Fuerst M, Fink B, Ruther W.The value of preoperative knee aspiration and arthroscopic biopsy in revision total knee arthroplasty[J]. Z Orthop Ihre Grenzgeb 2005143((1)), 36–41. [DOI] [PubMed] [Google Scholar]
- 5.Rahman L, Hall-Craggs M, Muirhead-Allwood SK.Radiology of the resurfaced hip[J]. Skeletal Radiol 201140((7)), 819–830. [DOI] [PubMed] [Google Scholar]
- 6.Palestro CJ, Love C, Tronco GG, et al. Combined labeled leukocyte and technetium 99m sulfur colloid bone marrow imaging for diagnosing musculoskeletal infection[J]. Radiographics 200626((6)), 859–870. [DOI] [PubMed] [Google Scholar]
- 7.Magnuson JE, Brown ML, Hauser MF, et al. In-111-labeled leukocyte scintigraphy in suspected orthopedic prosthesis infection: comparison with other imaging modalities[J]. Radiology 1988168((1)), 235–239. [DOI] [PubMed] [Google Scholar]
- 8.Love C, Marwin SE, Tomas MB, et al. Diagnosing infection in the failed joint replacement: a comparison of coincidence detection 18F-FDG and 111In-labeled leukocyte/99mTc-sulfur colloid marrow imaging[J]. J Nucl Med 200445((11)), 1864–1871. [PubMed] [Google Scholar]
- 9.Penn DL, Kim C, Zhang K, et al. Apoptotic abscess imaging with 99mTc-HYNIC-rh-Annexin-V[J]. Nucl Med Biol 201037((1)), 29–34. [DOI] [PubMed] [Google Scholar]
- 10.Rouzet F, Dominguez Hernandez M, Hervatin F, et al. Technetium 99m-labeled annexin V scintigraphy of platelet activation in vegetations of experimental endocarditis[J]. Circulation 2008117((6)), 781–789. [DOI] [PubMed] [Google Scholar]
- 11.Stadelmann C, Lassmann H.Detection of apoptosis in tissue sections[J]. Cell Tissue Res 2000301((1)), 19–31. [DOI] [PubMed] [Google Scholar]
- 12.Ye F, Fang W, Wang F, et al. Evaluation of adenosine preconditioning with 99mTc-His10-annexin V in a porcine model of myocardium ischemia and reperfusion injury: preliminary study[J]. Nucl Med Biol 201138((4)), 567–574. [DOI] [PubMed] [Google Scholar]
- 13.Lorberboym M, Feldbrin Z, Hendel D, et al. The use of 99mTc-recombinant human annexin V imaging for differential diagnosis of aseptic loosening and low-grade infection in hip and knee prostheses[J]. J Nucl Med 200950((4)), 534–537. [DOI] [PubMed] [Google Scholar]
- 14.Belmatoug N, Cremieux AC, Bleton R, et al. A new model of experimental prosthetic joint infection due to methicillin-resistant Staphylococcus aureus: a microbiologic, histopathologic, and magnetic resonance imaging characterization[J]. J Infect Dis 1996174((2)), 414–417. [DOI] [PubMed] [Google Scholar]
- 15.Zhang LN, Yang X, Hua ZC.Expression and purification of recombinant human annexin V in Escherichia coli[J]. Prep Biochem Biotechnol 200030((4)), 305–312. [DOI] [PubMed] [Google Scholar]
- 16.Cremieux AC, Carbon C.Experimental models of bone and prosthetic joint infections[J]. Clin Infect Dis 199725((7)), 1295–1302. [DOI] [PubMed] [Google Scholar]
- 17.Love C, Marwin SE, Palestro CJ.Nuclear medicine and the infected joint replacement[J]. Semin Nucl Med 200939((1)), 66–78. [DOI] [PubMed] [Google Scholar]
- 18.Garvin KL, Konigsberg BS.Infection following total knee arthroplasty: prevention and management[J]. J Bone Joint Surg Am 201193((12)), 1167–1175. [DOI] [PubMed] [Google Scholar]
- 19.Cataldo MA, Petrosillo N, Cipriani M, et al. Prosthetic joint infection: recent developments in diagnosis and management[J]. J Infect 201061((6)), 443–448. [DOI] [PubMed] [Google Scholar]
- 20.Yamada S, Kubota K, Kubota R, et al. High accumulation of fluorine-18-fluorodeoxyglucose in turpentine-induced inflammatory tissue[J]. J Nucl Med 199536((7)), 1301–1306. [PubMed] [Google Scholar]
- 21.Cho KA, Joo SY, Han HS, et al. Osteoclast activation by receptor activator of NF-kappaB ligand enhances the mobilization of hematopoietic progenitor cells from the bone marrow in acute injury[J]. Int J Mol Med 201026((4)), 557–563. [DOI] [PubMed] [Google Scholar]
- 22.Morawietz L, Classen RA, Schröder JH, et al. Proposal for a histopathological consensus classification of the periprosthetic interface membrane[J]. J Clin Pathol 200659((6)), 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del BV, Graci C, Spinelli MS, et al. Histological and ultrastructural reaction to different materials for orthopaedic application[J]. Int J Immunopathol Pharmacol 201124((1 Suppl 2)), 91–94. [DOI] [PubMed] [Google Scholar]
- 24.Scheel-Toellner D, Wang KQ, Webb PR, et al. Early events in spontaneous neutrophil apoptosis[J]. Biochem Soc Trans 200432((Pt3)), 461–464. [DOI] [PubMed] [Google Scholar]
- 25.Blankenberg FG.In vivo detection of apoptosis[J]. J Nucl Med 200849((Suppl 2)), 81S–95S. [DOI] [PubMed] [Google Scholar]
- 26.Widmer E, Que YA, Entenza JM, et al. New concepts in the pathophysiology of infective endocarditis[J]. Curr Infect Dis Rep 20068((4)), 271–279. [DOI] [PubMed] [Google Scholar]
- 27.Shaw L, Golonka E, Potempa J, et al. The role and regulation of the extracellular proteases of Staphylococcus aureus[J]. Microbiology 2004150((Pt 1)), 217–228. [DOI] [PubMed] [Google Scholar]
- 28.Saleh-Mghir A, Muller-Serieys C, Dinh A, et al. Adjunctive rifampin is crucial to optimizing daptomycin efficacy against rabbit prosthetic joint infection due to methicillin-resistant Staphylococcus aureus[J]. Antimicrob Agents Ch 201155((10)), 4589–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alt V, Bitschnau A, Böhner F, et al. Effects of gentamicin and gentamicin-RGD coatings on bone ingrowth and biocompatibility of cementless joint prostheses: an experimental study in rabbits[J]. Acta Biomater 20117((3)), 1274–1280. [DOI] [PubMed] [Google Scholar]
- 30.Sarda-Mantel L, Saleh-Mghir A, Welling MM, et al. Evaluation of 99mTc-UBI 29-41 scintigraphy for specific detection of experimental Staphylococcus aureus prosthetic joint infections[J]. Eur J Nucl Med Mol Imaging 200734((8)), 1302–1309. [DOI] [PubMed] [Google Scholar]