Abstract
We sought to investigate the expression of Fas and FasL on T cell surface and caspase 8 involvement in T cell apoptosis promoted by serum IL-10 in systemic lupus erythematosus (SLE) patients. Cells and sera were obtained from 35 SLE patients. Apoptosis of T cells in patients with SLE was increased and associated with the SLE disease activity index (SLEDAI). Elevated expression of Fas and FasL on T cell surface contributed to increased apoptosis of T cells. Increased IL-10 in the sera of SLE patients was capable of inducing Fas and FasL expression on CD4+T cell surface, promoting apoptosis of this cell subset. Decreased IL-10 serum levels and low expression of Fas were found in 5 patients of the first follow-up group after 2-month treatment. In another group with one-year treatment, the SLEDAI declined to inactive scores. Serum IL-10 was decreased significantly, and expression of Fas and FasL on T cells was also reduced. Declined apoptosis was predominant only in CD4+T cell subset. When sera with high level of IL-10 were used to culture PBMCs from healthy controls, activated caspase 8 was elevated in CD3+T, CD4+T and CD8+T cells. The study showed that serum IL-10 induced apoptosis of T cell subsets via the caspase 8 pathway initiated by Fas signaling. Increased apoptosis of T cells contributes to autoantigen burden, which is pathogenic in the development of SLE.
Keywords: apoptosis, T cell, lupus erythematosus, systemic
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by the production of pathogenic autoantibodies to a spectrum of nuclear antigens. The pathogenesis of the disease remains poorly understood. The process of apoptosis may be relevant to lupus pathogenesis in three key ways. First, impairment of death pathways may lead to defective elimination of autoreactive T or B lymphocytes and loss of self-tolerance. Second, self-antigens may be displayed on cell surfaces during cell death and under certain conditions, which may lead to immune responses against autoantigens. Finally, induction of apoptotic death may be an important effector mechanism causing cell damage in individuals with SLE[1].
Many studies have presented evidence for impaired immune cell apoptosis in SLE models[2]-[4]. Despite apoptosis abnormalities were found in animal lupus models, apoptosis is generally reported to be normal or even increased in lupus patients[5]. Our previous study reported that T cells from SLE patients exhibited increased apoptosis and IL-10 could induce the apoptosis of T cells by promoting sFas and sFasL production[6]. However, the impact of serum IL-10 from SLE patients on the membrane expressions of Fas and FasL was unclear. Membrane death receptor Fas ligated by FasL initiates intracellular signal transduction of death domain and thus results in apoptosis. An increased expression of intracellular activated caspase 3 in CD4+ and CD8+ T cells from patients with SLE has been proven[7]; however, the key initiator, caspase 8, needs to be elucidated. Here, we investigated the effect of serum IL-10 from patients with SLE on the expression of membrane Fas and FasL, and on caspase 8 involvement in the apoptosis of T cell subsets.
Patients and methods
Patients and controls
Thirty-five adult patients (31 females and 4 males with a mean age of 36.7 years ±9.8 years) with a diagnosis of SLE based on the American College of Rheumatology criteria[8]-[9] were enrolled in the study after giving informed consent. The patients with SLE were recruited from the authors' affiliated institution and were divided into 2 groups based on their SLE disease activity index (SLEDAI) score[10]. One group included 25 patients with active SLE (SLEDAI≥6; 23 females and 2 males with a mean age of 36.2 years±9.4 years), and the second group included 10 patients with inactive SLE (SLEDAI<6; 8 females and 2 males with a mean age of 38.4 years±11.6 years). Twenty healthy blood donors were enrolled in our study after giving informed consent.
There were 2 follow-up groups. Five patients with initial diagnosis complained fever, skin rash, joint pain, together with proteinuria. They had received 2 months of therapy at the time of analysis. Another follow-up group consisting of 5 active SLE patients who had received 12 months of therapy was also examined.
Cell preparation
After venipuncture, 10 mL blood was collected in heparinized and plain tubes for isolation and serum separation of peripheral blood mononuclear cells (PBMCs), respectively. Serum was stored at −80 °C until analysis. PBMCs were isolated from heparinized blood within 1–2 hours after collection by density gradient centrifugation by using Ficoll-histopaque 1077 (Sigma Chemical Co., Dorset, England).
Cell culture
PBMCs from SLE patients or healthy controls were cultured in complete 1640 medium for 48 hours. PHA, IL-10 or FasL antibodies were added into the culture. When factors in SLE sera were studied, we chose sera from SLE patients with higher IL-10 levels, with comparison to sera from healthy controls with no detectable IL-10.
Flow cytometry
In isolated PBMCs, 100 μL cell suspension was performed in 3 flow cytometry tubes, and 2 μL each of fluorescein isothiocyanate labeled annexin V (FITC-annexin V), propidium iodide, PE labeled anti-CD4 or CD8 and PE-Cy5 labeled anti-CD3 or isotype control (BD Biosciences, Mountain View, CA, USA) was added and kept at 4 °C for 20 minutes. After staining, cells were washed twice and resuspended in phosphate buffered saline, and analyzed in a FACSCalibur system (BD Biosciences), and analyzed by using FlowJo software (Tree Star, San Carlos, CA). The frequencies of CD3+, CD4+ and CD8+ T cells were determined by measuring positive population on histograms in the PBMC gate. After gating for T cells, dot plot of annexin V/ propidium iodide was made and early apoptotic cells were identified as annexin V+ propidium iodide- events. The data for every T cell subset were represented as % of cells in the PBMC population, whereas the percentage of apoptotic cells was out of each T cell subset. Compensation was performed using non-stained and single stained controls.
Flow cytometry
FITC-conjugated anti-Fas, PE-conjugated anti-Fas, anti-FasL and isotype control (BD Biosciences, Mountain View, CA, USA) were used to analyze the expression of Fas and FasL on different T cell subsets according to the manufacturer's instructions. Activated caspase 8 was determined by flow cytometry using a commercially available activated caspase 8 detection kit (eBioscience) according to the manufacturer's instructions.
Enzyme-linked immunosorbent assay (ELISA) Concentrations of sFas and IL-10 in the serum were measured by enzyme-linked immunosorbent assay (ELISA) using commercial kits (R&D Systems, MN, USA).
Statistical analysis
Data were analyzed on SPSS10.0 using non-parametric tests. Mann Whitney test was used for inter-group comparison, Wilcoxon test for paired samples and Kruskal-Wallis test for comparisons of more than 2 groups. Correlation was performed using Spearman's coefficient of correlation.
Results
Apoptosis of T cell subsets increased in patients with SLE and positively correlated with disease activity
The clinical features of the study subjects are shown in Table 1. Compared with the healthy controls, apoptosis of T cells in PBMCs from patients with SLE was significantly increased and positively correlated with SLEDAI (r = 0.45, P<0.05). Among T cells, apoptosis of CD4+ T cell subset was significantly greater than that of CD8+ T cell subset. Apoptosis of CD4+ T cell subset, rather than CD8+ T cell subset, was obviously elevated in active SLE patients (Table 1). The unbalanced apoptosis between CD4+ and CD8+ T cell subsets led to a reduction in the percentage of CD4+ T cells in CD3+ T cells and decreased the ratio of CD4 to CD8. The decrease of CD4/CD8 ratio was more significant in active patients than that in inactive patients (Table 1).
Table 1. Clinical findings, apoptosis of T subsets and their percentages in 35 patients with SLE and 20 healthy controls.
| Groups | Healthy controls (n = 20) | SLE patients (n = 35) | Active SLE (n = 25) | Inactive SLE (n = 10) |
|---|---|---|---|---|
| Clinical findings | ||||
| SLEDAI | N | 12.28±1.97 | 6.10±1.91 | |
| Anti-dsDNA (Farr RIA,%) | N | 37.27±20.71* | 18.39±16.42 | |
| ESR (mm/hour) | UN | 47.60±17.49** | 28.80±10.35 | |
| WBC (109/L) | UN | 3.83±0.91** | 4.96±0.76 | |
| IgG (g/L) | UN | 18.66±4.87** | 12.20±2.58 | |
| C3 (g/L) | UN | 0.61±0.30** | 1.08±0.43 | |
| C4 (g/L) | UN | 0.13±0.08** | 0.25±0.10 | |
| Apoptosis of T subsets (x±s,%) | ||||
| CD3+AV+PI− | 15.73±8.02 | 23.44±11.49* | 28.52±14.11** | 20.87±9.79 |
| CD4+AV+PI− | 11.25±5.13 | 16.88±8.77* | 21.89±12.64**Δ | 12.08±5.91 |
| CD8+AV+PI− | 13.24±7.11 | 16.69±9.00* | 20.27±13.91* | 13.98±7.89 |
| Percentages of peripheral T lymphocytes (x±s,%) | ||||
| CD3+ | 67.28±3.84 | 70.08±6.96 | 69.26±7.70 | 70.20±4.55 |
| CD4+ | 37.34±5.04 | 27.78±6.32* | 26.04±4.66*Δ | 30.26±8.40* |
| CD8+ | 28.19±2.86 | 42.15±8.09* | 42.90±8.35* | 39.97±7.37* |
| CD4/CD8 | 1.36±0.31 | 0.69±0.25* | 0.63±0.21*Δ | 0.83±0.29* |
Apoptosis of T cells was determined by flow cytometry and early apoptotic cells were identified as annexin V+ PI–. CD3+T, CD4+T, and CD8+T cell frequencies were determined by measuring positive population on histograms in the PBMCs gate.
Compared with healthy controls, *: P<0.05; **: P<0.01; compared with inactive group, Δ: P<0.05. UN: unknown; N: negative.
Expressions of Fas and FasL on T cell surface were elevated in SLE patients, paralleled by an increased apoptosis of T cells and correlated with disease activity
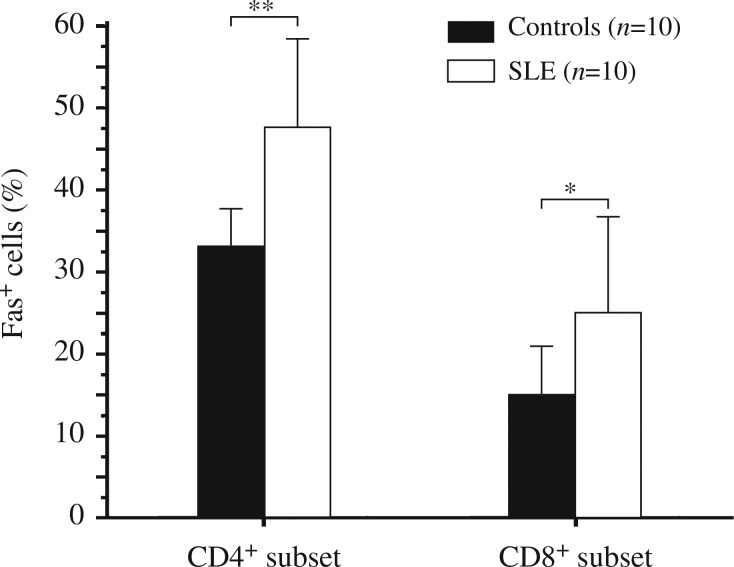
Compared with the healthy controls, expression of Fas on the membrane of CD3+ T cells in fresh isolated PBMCs was significantly increased in patients with both active and inactive SLE, though there was less significant increase of FasL expression on T cell surface in inactive patients (Fig. 1). There was only a slight increase of FasL in inactive patients without significance. Fas and FasL expression was correlated with SLEDAI (r = 0.46, P<0.05 and r = 0.49, P<0.05, respectively). Among fresh isolated T cells, increased Fas and FasL expressions were consistent both on CD4+ and CD8+ T cell subsets in SLE patients. Moreover, patients with active SLE showed a significant increase in Fas and FasL expression (Fig. 2). Furthermore, these Fas and FasL expressions were associated with the apoptosis of T cell subsets. Fas and FasL expressions in CD4+ T cells were associated with the apoptosis of CD4+ T cell, (r = 0.45 and 0.43, respectively). However, FasL expression in CD8+ T cells was correlated with apoptosis of both CD4+ and CD8+ T cells (r = 0.42 and 0.49, respectively).
Fig. 1. Expression of Fas (A) and FasL (B) on CD3+ T cell surface.
CD3+ T cells were isolated freshly from peripheral blood mononuclear cells (PBMCs) and labeled by fluorescein antibodies for flow cytometry. PBMCs were obtained from patients with active and inactive SLE and healthy blood donors. ** P<0.01.
Fig. 2. Increased expression of Fas (A) and FasL (B) on CD4+ and CD8+T cell subsets in patients with SLE.
PBMCs were obtained from patients with active and inactive SLE and healthy blood donors. CD3+ T cells were isolated freshly from PBMCs. Gating for T cells, expression of Fas and FasL on CD4+ and CD8+T cells is shown according to the labeled fluoresceins. * P<0.05; ** P<0.01.
Additionally, the level of sFas in serum from SLE patients was obviously higher and associated with several clinical features, including disease activity, antinuclear antibody titer, anti-dsDNA antibody occurrence, reduced C3 and lupus nephritis (Table 2). When PBMCs from patients were cultured with PHA for 48 hours, Fas expression on T cell was increased (Fig. 3). FasL blocking antibody reduced the apoptosis of cultured T cells, especially in CD4+ T cell subset (Fig. 4).
Table 2. Level of sFas in serum of patients with SLE (x±s,ug/L).
| Groups | n | sFas |
|---|---|---|
| Healthy controls | 20 | 2.27±1.04 |
| SLE patients | 36 | 4.18±2.08** |
| Active | 18 | 5.14±1.78** |
| Inactive | 18 | 3.22±1.23 |
| ANA ≥ 1:1,000 | 19 | 4.90±2.29** |
| ANA < 1:1,000 | 17 | 3.41±1.45 |
| Anti-dsDNA (+) | 10 | 5.59±2.42* |
| Anti-dsDNA (-) | 26 | 3.66±1.67 |
| C3 < 0.9 g/L | 17 | 4.57±2.30 |
| C3 ≥ 0.9 g/L | 19 | 3.82±1.78 |
| Lupus nephritis (+) | 11 | 5.83±2.03** |
| Lupus nephritis (-) | 25 | 3.40±1.54 |
Concentration of sFas in serum was measured by enzyme-linked immunosorbent assay (ELISA) using commercial kits. Compared with the control group, * P<0.05; ** P<0.01.
Fig. 3. Expression of Fas on CD4+ or CD8+ T cell surface with PBMCs from SLE patients cultured with PHA for 48 hours.
PBMCs were obtained from SLE patients and healthy blood donors. PHA stimulation was performed for 48 hours. Expression of Fas on CD4+ or CD8+ T cell surface was determined by flow cytometry analysis. * P<0.05; ** P<0.01.
Fig. 4. T cell apoptosis declined in the presence of FasL antibody.
T cells were cultured with or without anti-FasL antibody. A and B: Flow cytometric dot plots of annexin V versus propidium iodide (PI) in CD4 gate. A: culture without FasL antibody; B: culture with FasL antibody. C: * P<0.05.
Increased serum IL-10 induced Fas and FasL expression on T cells and promoted T cell apoptosis
An increased IL-10 level was found in the serum from SLE patients. Compared with healthy controls (12.80 pg/mL±4.24 pg/mL), patients with SLE had increased IL-10 level (68.90 pg/mL±34.72 pg/mL). Among SLE patients, IL-10 level was much higher in active patients (81.26 pg/mL±31.42 pg/mL) than that found in inactive ones (33.38 pg/mL± 11.84 pg/mL). Serum IL-10 level was correlated with SLEDAI (r = 0.43, P<0.05), as well as with anti-dsDNA antibody (r = 0.83, P<0.01) and serum IgG level (r = 0.41, P<0.05).
Increased IL-10 level correlated with the percentage of FasL-expressing-cells in CD3+, CD4+ and CD8+ cells (r = 0.59, P<0.05; r = 0.52, P<0.05; r = 0.63, P<0.01; respectively). In active SLE patients, increased IL-10 level was correlated with the apoptosis of the CD4+ T cell subset (r = 0.54, P<0.05) and negatively with the ratio of CD4 to CD8 (r = −0.43, P<0.05).
When neutralizing IL-10 antibody was employed in the culture of PBMCs isolated from SLE patients, Fas and FasL expressions on CD3+, CD4+ and CD8+ T cell subsets were reduced (Table 3). Furthermore, IL-10 antibody could inhibit the apoptosis of T cells, and improve the ratio of CD4/CD8 (Table 4).
Table 3. Effect of IL-10 antibody neutralization on expression of Fas and FasL on T cell surface in cultured PBMCs from SLE patients (x±s, %, n = 10).
| CD3+ T cell | CD4+ T subset | CD8+ T subset | ||||
|---|---|---|---|---|---|---|
| Culture system | Fas+ | FasL+ | Fas+ | FasL+ | Fas+ | FasL+ |
| Without IL-10 Ab | 35.61±11.76 | 5.10±2.50 | 17.25±7.13 | 1.68±1.42 | 21.69±10.81 | 2.97±1.69 |
| With IL-10 Ab | 32.72±12.96* | 4.20±2.30* | 15.04±7.82* | 1.56±1.21* | 20.14±10.30* | 2.33±1.57* |
| Reduced by | 10.65±0.14 | 12.10±0.25 | 13.92±0.14 | 14.12±0.21 | 7.06±0.08 | 6.96±0.48 |
PBMCs isolated from SLE patients were cultured and neutralizing IL-10 antibody was added into the culture. Fas and FasL expressions on CD3+ T cells, CD4+ and CD8+ T cell subsets were determined by by flow cytometry. Compared with no IL-10 antibody system, *: P<0.05. Ab: antibody.
Table 4. Effects of IL-10 antibody neutralization on apoptosis of T cell subsets and ratio of CD4/CD8 in SLE PBMCs culture (x±s, %, n = 10).
| Percentages of apoptosis (AV+PI–) | ||||||
|---|---|---|---|---|---|---|
| Culture system | CD3+ T cell | CD4+ T subset | CD8+ T subset | Ratio of CD4/CD8 | ||
| Without IL-10 Ab | 21.08±8.14 | 17.80±6.35 | 18.01±7.01 | 0.79±0.26 | ||
| With IL-10 Ab | 19.02±6.55* | 16.25±5.94* | 16.53±6.47* | 0.92±0.23* | ||
| Changes (%) | −(8.35±0.13) | −(8.45±0.11) | −(8.14±0.09) | 16.46±0.25 | ||
Neutralizing IL-10 antibody was employed in the SLE PBMCs cultures system, apoptosis of T cell subsets were determined by flow cytometry. Compared with no IL-10 antibody system, *: P<0.05. Ab: antibody.
In a follow-up study with 5 cases at first visit, after 2-month treatment, patients showed normal temperature, relieved skin and joint symptoms, decreased protein concentration in the urine and SLEDAI declined by 6 points. Then, we examined their serum IL-10 level and found that after 2-month treatment, there was a significant decline of IL-10 in the sera of these 5 patients. We also found that Fas expression on CD4+ T cell surface and apoptosis of the CD4+ T cell subset was decreased as well (Fig. 5).
Fig. 5. A follow-up study of 5 patients with active SLE after 2 months of treatment.
A: serum IL-10 level was detected by ELISA; B: expression of Fas on T cell susbets was determined by flow cytometry; C: expression of FasL on T cell susbets was determined by flow cytometry. D: apoptosis of T cell population displayed by flow cytometric analysis of annexin V versus propidium iodide. * P<0.05.
Another group of 5 patients with active SLE were followed up for 12 months. The SLEDAI score decreased from more than 10 to lower than 4 with relieved symptoms. Their sera IL-10 level declined after 12 months of treatment. The expression of Fas and FasL and the apoptosis of T cells also decreased (Table 5).
Table 5. Changes happened from active to inactive stage in 5 follow-up SLE patients (x±s).
| Active stage | Inactive stage | P value | |
|---|---|---|---|
| Serum IL-10 (pg/mL) | 101.00±37.32 | 34.20±12.34 | <0.01 |
| Fas+ cell percentage (%) | |||
| in CD3+ T cell | 42.23±9.08 | 30.96±8.49 | <0.05 |
| in CD4+ T subset | 22.30±6.48 | 12.29±4.74 | <0.01 |
| in CD8+ T subset | 21.24±5.42 | 15.18±6.69 | <0.05 |
| FasL+ cell percentage (%) | |||
| in CD3+ T cell | 2.39±0.58 | 1.98±0.45 | >0.05 |
| in CD4+ T subset | 1.17±0.47 | 0.87±0.45 | <0.05 |
| in CD8+ T subset | 1.13±0.51 | 0.70±0.49 | <0.05 |
| Apoptosis (%) | |||
| in CD3+ T cell | 22.79±10.03 | 20.95±7.61 | >0.05 |
| in CD4+ T cell | 18.75±8.47 | 14.10±6.27 | <0.05 |
| in CD8+ T cell | 15.40±8.51 | 14.17±7.19 | >0.05 |
Five patients with SLE were visited after 12-month treatment. FITC-conjugated anti-Fas, PE-conjugated anti-Fas, anti-FasL, and isotype control were used in the analysis of expression of Fas and FasL on different T cell subsets according to the manufacturer's instructions. Apoptosis of T cells was determined by flow cytometry.
IL-10 in SLE serum promoted active caspase 8 production by T cells in PBMCs from healthy individuals
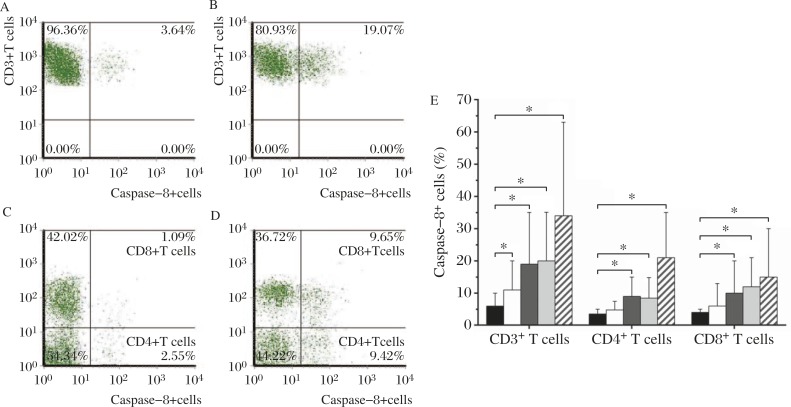
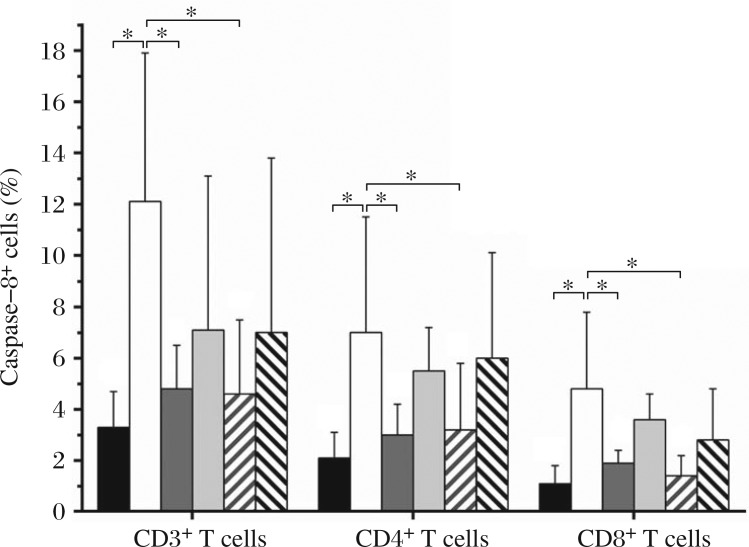
The serum from SLE patients with high level of IL-10 was added into the culture of PBMCs from healthy individuals for 48-hour incubation. The percentage of active caspase 8 positive cells in CD3+, CD4+ and CD8+ T cell subsets cultured with SLE serum were elevated compared with the controls and showed a dose-dependent increase (Fig. 6). CD3+ T cells were isolated freshly from PBMCs and different cell populations were labeled by fluorescein antibodies for flow cytometry analysis. Expression of caspase 8 on CD3+, CD4+ or CD8+ T cell surface was determined by flow cytometry analysis. Neutralizing IL-10 antibody inhibited the elevation of active caspase 8 production. Moreover, when FasL neutralizing antibody was employed in the culture system, the percentage of caspase 8-expressing cells were also decreased significantly (Fig. 7).
Fig. 6. Active caspase 8-producing cells in CD3+, CD4+ and CD8+ T cells with PBMCs from healthy individuals cultured with sera from SLE patients or controls.
A,B,C, and D: flow cytometric dot plots of positive cells. A and C: PBMCs cultured with sera from controls, a: the percentage of active caspase 8 positive CD3+ T cells, c: the percentage of active caspase 8 positive CD4+ and CD8+ T cells. B and D: PBMCs cultured with sera from SLE patients, B: the percentage of active caspase 8 positive CD3+T cells, D: the percentage of active caspase 8 positive CD4+ and CD8+ T cells. E: different concentrations of sera from SLE patients were designed to study their effects on active caspase 8 production. Healthy individuals' sera with undetectable IL-10 were used in the control culture system. * P<0.05.  sera from SLE patients 0% +sera from healthy controls 20%,
sera from SLE patients 0% +sera from healthy controls 20%,  sera from SLE patients 5% +sera from healthy controls 15%,
sera from SLE patients 5% +sera from healthy controls 15%,  sera from SLE patients 10% +sera from healthy controls 10%,
sera from SLE patients 10% +sera from healthy controls 10%,  sera from SLE patients 15% +sera from healthy controls 5%,
sera from SLE patients 15% +sera from healthy controls 5%,  sera from SLE patients 20% +sera from healthy controls 0%.
sera from SLE patients 20% +sera from healthy controls 0%.
Fig. 7. Impact of IL-10 neutralizing antibody or FasL antibody on active caspase 8 expression in CD3+, CD4+ and CD8+ T cell subsets.
CD3+ T cells were isolated freshly from PBMCs and different cell subpopulations were labeled by fluorescein antibodies for flow cytometry. Expression of caspase 8 on CD3+, CD4+ or CD8+ T cell surface was determined by flow cytometry. The results of 6 separate experiments are reported as mean ± SD. *: P < 0.05.  sera from healthy controls,
sera from healthy controls,  sera from SLE patients,
sera from SLE patients,  SLE sera with IL-10 antibody,
SLE sera with IL-10 antibody,  SLE sera with IL-10 antibody isotype controls,
SLE sera with IL-10 antibody isotype controls,  SLE sera with FasL antibody,
SLE sera with FasL antibody,  SLE sera with FasL antibody isotype controls.
SLE sera with FasL antibody isotype controls.
Discussion
Aberrant apoptosis is implicated in the development of SLE and is a major contributing factor in disease susceptibility[1]. Increased spontaneous apoptosis of lymphocytes has been linked to increased IL-10 production, the release of Fas ligand and the overexpression of Fas receptor in SLE[11]. A significant number of investigations have shown different results of apoptosis in human SLE from lupus animal models in which immune cell apoptosis is impaired[2]-[4]. MRL/lpr mice express very low levels of Fas, produce lupus-like autoantibodies to DNA and chromatin and cryoglobulins, and exhibit immune-mediated renal disease and vasculitis. Gld, a null mutation of Fas ligand, exerts its effect on autoimmunity by a similar mechanism[12]. However, these mutations were not found in patients with lupus. To the contrary, Emlen et al. found evidence of increased apoptosis of peripheral blood lymphocytes[5], which was confirmed by subsequent studies[13]-[15].
Recently, the study of V Dhir et al. have reported increased T cell apoptosis, which correlated with disease activity[16]. In the present study, our data demonstrated similar results of T cell apoptosis and correlation with SLEDAI. Furthermore, the molecular basis of T cell apoptosis and its upstream factor were elucidated. First, in patients with SLE, T cell apoptosis was increased in CD4 and CD8 cell subsets, especially in CD4+ T cell subset in active SLE patients. The unbalanced apoptosis between CD4+ and CD8+ T cell subsets led to a decreased ratio of CD4 to CD8. Second, on the basis of previous studies, Fas and FasL expressions on T cell surface were observed to explain whether this death receptor/ligand is responsible for T cell apoptosis in lupus. Fas and FasL expressions were increased in CD3+, CD4+ and CD8+ T cell subsets in active SLE patients. Fas and FasL expression was correlated with increased apoptosis of T cell subsets. Function blocking antibody against FasL inhibited the apoptosis of CD4+ T cell subset. In addition, sFas in the serum from SLE patients was increased and associated with disease activity, production of autoantibodies and organ damages. Third, a considerable amount of work offered evidence that IL-10 was pathogenic and could be a potential target for lupus therapy[16]-[20]. In the present study, higher IL-10 level in the serum of SLE patients was confirmed and correlated with SLEDAI. Fas and FasL expressions and the apoptosis of CD4+ T cell subset correlated with IL-10 levels. Anti-IL-10 antibodies inhibited Fas and FasL expression and the apoptosis of T cell subsets.
Follow-up studies demonstrated that after treatment, when disease activity was relieved, IL-10 secretion decreased with Fas expression on CD4+ T cell surface and apoptosis of this cell subset were down-regulated. Fas triggers the extrinsic apoptosis signaling pathway by ligand engagement. Activation of caspase-8 is the key step in death signal transduction. An increased expression of intracellular activated caspase3 in CD4+ and CD8+ T cells from patients with SLE has been reported[7]. Our study demonstrated that IL-10 in the serum of SLE patients promoted active caspase 8 production by T cells.
In conclusion, the current study has shown that serum IL-10 induced apoptosis of T cell subsets via the caspase 8 pathway initiated by Fas signaling. Increased apoptosis of T cells, especially the CD4+T cell subset, not only contributes to the autoantigen burden, but also upsets immune defense as manifested by decreased CD4/CD8 ratio. Our findings suggest that aberrant apoptosis of CD4+T cells induced by IL-10, at least partly, is involved in the development and relapse of SLE as well as in the pathogenesis of the disease.
References
- 1.Cohen PL. Apoptotic cell death and lupus[J] Springer Semin Immun. 2006;28(2):145–152. doi: 10.1007/s00281-006-0038-z. [DOI] [PubMed] [Google Scholar]
- 2.Bouillet P, Metcalf D, Huang DC, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity[J] Science. 1999;286(5445):1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 3.Mehrad B, Park SJ, Akangire G, et al. The lupus-susceptibility locus, Sle3, mediates enhanced resistance to bacterial infections[J] J Immunol. 2006;176(5):3233–3239. doi: 10.4049/jimmunol.176.5.3233. [DOI] [PubMed] [Google Scholar]
- 4.Roy V, Chang NH, Cai Y, et al. Aberrant IgM signaling promotes survival of transitional T/B cells and prevents tolerance induction in lupus-prone NewZealand black mice[J] J Immunol. 2005;175(11):7363–7371. doi: 10.4049/jimmunol.175.11.7363. [DOI] [PubMed] [Google Scholar]
- 5.Emlen W, Niebur J, Kadera R. Accelerated in vitro apoptosis of lymphocytes form patients with systemic lupus erythematosus[J] J Immunol. 1994;152(7):3685–3692. [PubMed] [Google Scholar]
- 6.Wang H, Xu J, Ji X, et al. The abnormal apoptosis of T cell subsets and possible involvement of IL-10 in systemic lupus erythematosus[J] Cell Immunol. 2005;235(2):117–121. doi: 10.1016/j.cellimm.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 7.Xue C, Lan-Lan W, Bei C, et al. Abnormal Fas/FasL and caspase-3-mediated apoptotic signaling pathways of T lymphocyte subset in patients with systemic lupus erythematosus[J] Cell Immunol. 2006;239(2):121–128. doi: 10.1016/j.cellimm.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus[J] Arthritis Rheum. 1982;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 9.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus[J] Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 10.Bombardier C, Gladman DD, Urowitz MB, et al. Derivation of the SLEDAI: a disease activity index for lupus patients[J] Arthritis Rheum. 1992;35(6):630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 11.Georgescu L, Vakkalanka RK, Elkon KB, et al. Interleukin 10 promotes activation-induced cell death of SLE lymphocytes mediated by Fas ligand[J] J Clin Invest. 1997;100(10):2622–2633. doi: 10.1172/JCI119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease[J] Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 13.Perniok A, Wedekind F, Herrmann M, et al. High levels of circulating early apoptic peripheral blood mononuclear cells in systemic lupus erythematosus[J] Lupus. 1998;7(2):113–118. doi: 10.1191/096120398678919804. [DOI] [PubMed] [Google Scholar]
- 14.Gröndal G, Traustadottir KH, Kristjansdottir H, et al. Increased T-lymphocyte apoptosis/necrosis and IL-10 producing cells in patients and their spouses in Icelandic systemic lupus erythematosus multicase families[J] Lupus. 2002;11(7):435–442. doi: 10.1191/0961203302lu223oa. [DOI] [PubMed] [Google Scholar]
- 15.Funauchi M, Sugiyama M, SukYoo B, et al. A possible role of apoptosis for regulating autoreactive responses in systemic lupus erythematosus[J] Lupus. 2001;10(4):284–288. doi: 10.1191/096120301680416977. [DOI] [PubMed] [Google Scholar]
- 16.Dhir V, Singh AP, Aggarwal A, et al. Increased T-lymphocyte apoptosis in lupus correlates with disease activity and may be responsible for reduced T-cell frequency: a cross-sectional and longitudinal study[J] Lupus. 2009;18(9):785–791. doi: 10.1177/0961203309103152. [DOI] [PubMed] [Google Scholar]
- 17.Kalechman Y, Gafter U, Da JP, et al. Delay in the onset of systemic lupus erythematosus following treatment with the immunomodulator AS101: association with IL-10 inhibition and increase in TNF-alpha levels[J] J Immunol. 1997;159(6):2658–2667. [PubMed] [Google Scholar]
- 18.López P, Gómez J, Prado C, et al. Influence of functional interleukin 10/tumor necrosis factor-α polymorphisms on interferon-α, IL-10, and regulatory T Cell population in patients with systemic lupus erythematosus receiving antimalarial treatment[J] J Rheumatol. 2008;35(8):1559–1566. [PubMed] [Google Scholar]
- 19.Schotte H, Gaubitz M, Willeke P, et al. Interleukin-10 promoter microsatellite polymorphisms in systemic lupus erythematosus: association with the anti-Sm immune response[J] Rheumatology. 2004;43(11):1357–1363. doi: 10.1093/rheumatology/keh353. [DOI] [PubMed] [Google Scholar]
- 20.Csiszár A, Nagy G, Gergely P, et al. Increased interferon-gamma (IFN-gamma), IL-10 and decreased IL-4 mRNA expression in peripheral blood mononuclear cells (PBMC) from patients with systemic lupus erythematosus (SLE)[J] Clin Exp Immunol. 2000;122(3):464–470. doi: 10.1046/j.1365-2249.2000.01369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viallard JF, Pellegrin JL, Ranchin V, et al. Th1 (IL-2, interferon-gamma (IFN-gamma)) and Th2 (IL-10, IL-4) cytokine production by peripheral blood mononuclear cells (PBMC) from patients with systemic lupus erythematosus (SLE)[J] Clin Exp Immunol. 1999;115(1):189–195. doi: 10.1046/j.1365-2249.1999.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]