Abstract
Accumulating studies have proved that perinatal exposure to environmental dose causes long-term potentiation in anxiety/depression-related behaviors in rats. Hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis is one of the most consistent biological findings in anxiety- and depression-related disorders. The HPA axis is reported to be susceptible to developmental reprogramming. The present study focused on HPA reactivity in postnatal day (PND) 80 male rats exposed perinatally to environmental-dose BPA. When female breeders were orally administered 2 μg/(kg.day) BPA from gestation day 10 to lactation day 7, their offspring (PND 80 BPA-exposed rats) showed obvious anxiety/depression-like behaviors. Notably, significant increase in serum corticosterone and adrenocorticotropin, and corticotropin-releasing hormone mRNA were detected in BPA-exposed rats before or after the mild stressor. Additionally, the level of glucocorticoid receptor mRNA in the hippocampus, but not the hypothalamus, was decreased in BPA-exposed rats. The levels of hippocampal mineralocorticoid receptor mRNA, neuronal nitric oxide synthase and phosphorylated cAMP response element binding protein were increased in BPA-exposed rats. In addition, the testosterone level was in BPA-exposed rats. The results indicate that reprogramming-induced hyperactivity of the HPA axis is an important link between perinatal BPA exposure and persistent potentiation in anxiety and depression.
Keywords: BPA, affective behaviors, HPA axis, glucocorticoid receptor, testosterone
INTRODUCTION
Early development of the central nervous system (CNS) is a subtle and intricate process which is greatly sensitive to environmental adverse conditions such as toxic compounds, stress, and drug treatments[1]-[3]. A large body of evidence has indicated that the disturbance of brain development produces persistent influence on either the function or structure of the brain. Among the potentially neurotoxic agents, bisphenol A (BPA), which possesses estrogenic properties, is a major source for human exposure as an important raw material for manufacturing epoxy resins and polycarbonate plastics[4]. In recent years, increasing studies have confirmed that perinatal exposure of BPA at doses less than the referred safe dose (50 μg/(kg.day), considered as ‘low-dose’ BPA) can interfere with brain development, resulting in CNS dysfunctions, including mental abnormality, hyperactivity, and deficits in language and motor learning. Among these, affective abnormality including anxiety and depression disorders is a common consequence induced by the negative action of low-dose BPA on brain development[5]-[7].
Although the mechanisms underlying the relationship between developmental BPA exposure and affective abnormality are unclear, epigenetic reprogramming of the hypothalamic-pituitary-adrenal (HPA) axis or neuronal circuits involved in regulating the HPA axis is seemingly of plausible explanation. HPA axis activity plays a critical role in regulating affective behaviors. HPA-axis dysfunction is regarded as the neurobiological basis of affective disorders characterized by anxiety disorders, major depressive disorder, psychotic depression, and bipolar disorder[8]. Increased corticosteroid levels representing a ‘hyperactive’ HPA axis is detected in the majority of patients with depression or anxiety disorder[9]. It has been established that the hippocampus is an important region involved in HPA-axis regulated affective behaviors[10]. Alterations in the hippocampus are widely reported to be accompanied with hyperactivity of the HPA axis in patients and animal models with affective disorders. The hippocampus participates in corticosteroid-mediated negative feedback to the HPA axis through expressing low affinity glucocorticoid receptors (GR) and high affinity mineralocorticoid receptors (MR). An imbalance between hippocampal MR and GR is implicated in patients with affective disorders[11]. Hippocampal GR downregulation impairing the corticosteroid negative feedback is a crucial factor for the hyperactivity of the HPA axis in depression[12]. During early development, impaired feedback control of the HPA-axis may persist into adulthood to acquire GR resistance in some specific feedback areas[13] and GR hypersensitivity in other brain regions[14]. Some literatures have confirmed that the hippocampus and HPA axis are the critical brain regions for estrogen-mediated organizational effects[15]. Previous studies have indicated that perinatal BPA exposure results in increase of estrogen receptors in the preoptic area, the pituitary and the dorsal raphe nucleus[16]-[18]. In addition, increased level of the hippocampus steroid receptor co-activator 1 was also detected in 1-week old male rats exposed perinatally to BPA[19]. Based on the above background, it is conceivable that the perturbance of the hippocampus HPA pathway may underlie BPA-induced anxiety and depression-like behaviors.
In this study, female breeders were subcutaneously injected with BPA (2 μg/kg) from gestation day 10 to lactation day 7. Anxiety- and depression-like behaviors were evaluated through the time spent in center-area of the open field test (OFT), in light-box of dark-light transfer (DLT) and the immobility time in forced swim test (FST) in BPA-exposed male offspring when they were mature. To explore the molecular mechanisms underlying BPA-altered affective behaviors, we further examined basal HPA-axis activity, GR-mediated HPA-axis responses to the stressor, and the hippocampal MR-induced neuronal nitric oxide synthase (nNOS)-cAMP response element binding protein (CREB) signaling.
MATERIALS AND METHODS
Animals
The study was performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by Animal Care and Ethical Committee of Nanjing Medical University. All measures were taken to minimize the number used as well as the pain and suffering of the animals. Sprague-Dawley female and male rats (Oriental Bio Service Inc., Nanjing, Jiangsu, China), weighing approximately 250–300 g, were used in the study. Animal rooms were maintained on a 12:12 light-dark cycle starting at 07:00 am and kept at a temperature of 22 °C–23 °C. All animals were allowed ad libitum access to clean water or food.
Preparation of animal models
Adult female and male rats were housed in separate cages for over 1 week. The estrous cycles in females were examined daily by vaginal cytology. Female rats with two regular estrous cycles were used to mate with males. Male rats were placed in the cages housing female rats. Vaginal plug was observed in the next morning, which was counted as day 1 of pregnancy. The dams of the experimental group were orally administered 2 μg/(kg.day) BPA (Sigma, St. Louis, MO, USA) from day 10 of pregnancy to day 7 of lactation. The dose was chosen according to the reported environmental exposure dose of BPA (2.4 μg/(kg.day)[20]. BPA was dissolved in ethanol, and then further diluted in water with the final concentration of ethanol less than 1%. Control dams received the same volume of vehicle. All offspring were weaned on postnatal day (PND) 22, and then were transferred to other cages by litter and sex. During treatment with BPA, dams or their offspring (BPA-exposed rats) did not exhibit obvious alteration of body weight gain (data not shown). One male offspring was randomly selected from each litter for study. Female rats were not taken into experiments to avoid fluctuations in mnemonic capacity related to the estrus cycle and changing estrogen levels.
Experimental paradigms
PND 80 control and BPA-exposed rats were randomly divided into three sets for the following experimental paradigms: (1) One set (n = 12 rats per group) was used to examine affective behaviors in the morning, following OFT→DLT→FST sequence. (2) The second set (n = 10 rats per group) was used to examine gonadal hormones, GR expression in the hippocampus and paraventricular nucleus (PVN), hippocampal MR and nNOS expression, and CREB phosphorylation. Brains and trunk blood were collected in the morning by rapid decapitation. (3) The third set (n = 10 rats per group) was used to investigate the activity of the HPA axis pre- and post-stress stimulation. Baseline blood samples were collected at circadian nadir in the morning via the lateral tail veins. The stress stimulation was administered by forcing rats to swim in a container filled with water (35 cm depth) for 5 minutes. Post-stress trunk blood was obtained immediately following the end of the stressor by decapitation.
Behavioral analysis
OFT: All rats were familiarized with the test environment for 60 minutes and then placed into a 75 cm×75 cm white Plexiglas arena. The time spent in the center field was recorded for 5 minutes as a parameter of anxiety.
DLT: A 43 cm ×43 cm×30 cm Plexiglas chamber was equally divided in two compartments, one consisting of white Plexiglas illuminated brightly and the other of black Plexiglas. Two compartments were separated by an appropriate insert. The total time in the illuminated compartment was continuously recorded for 5 minutes reflecting anxiety level.
FST: The swimming tank is a cylindrical acrylic container (50 cm tall ×30 cm in diameter) with water (24 °C ± 1 °C) filled to a depth of 35 cm. Animals were placed in the water for 15-minutes pretest swimming (data not shown) and were subjected to a 5-minutes test swimming 24 hours later. Increased immobile time is regarded as a typical sign of depressive-like phenotype.
Hormones assay
Serum was separated and stored at −20 °C for hormones assay. Serum corticosterone was assayed by rat corticosterone ELISA kit (Assay Designs, Inc. Ann Arbor, MI, USA). Serum adrenocorticotropin (ACTH), estrogen and testosterone were determined by a standard radioimmunoassay (MP Biomedicals, Irvine, CA, USA) according to the instructions of the manufacturer.
Real-time RT-PCR
The hippocampus and PVN were collected from frozen brain slices (2 mm thick), and then stored at −80°C until assay. Total RNA was extracted from the PVN and hippocampus using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. One μg RNA was used for reverse transcription (TaKaRa, Shiga, Japan). The primer sequences were: rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH; NM_017008.4) sense: 5′-TCCCTCAAGATTGTCAGCAA-3′, and antisense: 5′-AGATCCACAACGGATACATT-3′; rat corticotropin-releasing hormone (CRH; NM_031019.1) sense: 5′-CAGAACAACAGTGCGGGCTCA-3′, and antisense: 5′-AAGGCAGACAGGGCGACAGAG-3′; rat MR (NM_013131.1) sense: 5′-TGTCTCAGACCTTGGAGCGTT-3′, and antisense: 5′-TGTTCGGAATAGCACCGGAA-3′; rat GR (AB115420.1) sense: 5′-ACAGCTCACCCCTACCTTGGT-3′, and antisense: 5′-CTTGACGCCCACCTAACATGT-3′. RT-PCR was performed using a Light Cycler Fast Start DNA Master SYBR Green I kit and ABI Prism Step One Plus Sequence Detection System (Applied Biosystems, Foster City, CA, USA) and relative expression of genes was determined using the 2-ΔΔCt method by normalization against GAPDH expression. The relative levels of CRH, MR and GR mRNA were expressed as percent of control value.
Western blot analysis
The hippocampal region was carefully dissected on ice, frozen immediately and stored in liquid nitrogen (−80°C) until analysis. The samples were homogenized on ice in a lysis buffer containing 1 mM phenylmethylsulfonyl fluoride and protease inhibitor cocktail (Complete; Roche, Mannheim, Baden-Wuerttemberg, Germany), 150 mmol/L NaCl, 50 mmol/L Tris- HCl (pH 7.5), 10 mmol/L NaF, 5 mmol/L EDTA, 1 mmol/Lsodium orthovanadate, 0.5% sodium deoxycholate, and 1% Triton X-100. Total proteins (20 μg) were electrophoretically separated on SDS-polyacrylamide gel. The resolved proteins were electrophoretically transferred to a polyphorylated difluoride membrane. After blocking with 5% nonfat dried milk, the membranes were probed with rabbit anti-p-CREB-ser133 (1:1000; Cell Signaling, Beverley, CA, USA), or rabbit anti-CREB (1:1,000; Cell Signaling, Beverley, CA, USA), or mouse anti-nNOS antibody(1:5,000, BD Transduction Laboratories, San Jose, CA, USA) at 4 °C overnight. β-actin antibody (1:1,000, Sigma) was used as the internal control. After incubating with an HRP-labeled secondary antibody (1:500; Cell Signaling), the membranes were developed using the ECL detection Kit (Amersham Biosciences, Piscataway, NJ, USA). Quantitative expression of the proteins was analyzed with the image analysis software package ((Bio-Rad, Hercules, CA, USA).
Data analysis/statistics
The software Microcal Origin 6.1 (Micro-Cal Software Inc., Northampton, MA, USA) was employed to process and summarize the experimental data. The group data were expressed as mean ±SEM. Two-way ANOVA followed by Bonferroni post hoc test was introduced to analyze the effect of BPA treatment and 5-min forced swimming stress on serum ACTH and corticosterone. Student′s t test was used for comparisons between control and BPA-exposed groups. State7 software (STATA Corporation, College Station, TX, USA) was used to perform statistical analysis. P<0.05 was required for statistical significance.
RESULTS
Perinatal BPA exposure increases anxiety- and depression-like behaviors in rats
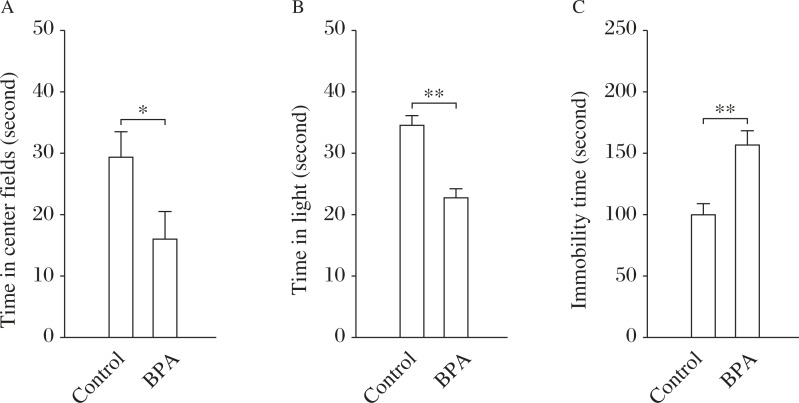
The time in the central area of the OFT and in the light-box of the DLT has been shown to inversely correlate with the anxiety level[21]. As shown in Fig. 1A and Fig. 1B, BPA-exposed rats (n = 12) spent less time in the central area of the OFT (t(1,22) = 2.33, P = 0.014) and in the light-box of the DLT (t(1,22) = 3.71, P = 0.001) than the control rats (n = 12), indicating signs of anxiety[22]. The immobility time of the FST reflects the depression-like state[23]. BPA-exposed rats showed a significant increase in the immobility time of the FST in comparison with control rats (t(1,22) = 3.25, P = 0.004; Fig. 1C). The results suggest that rats perinatally exposed to BPA exhibited typical signs of affective disorders. Hence, BPA-exposed rats were used as the animal model with BPA-induced affective disorders to investigate the alteration of the hippocampus-HPA axis circuits associated with the abnormality of affective behaviors.
Fig. 1. Perinatal BPA exposure leads to anxiety- and depression-like behaviors.
A&B: Effects of perinatal BPA exposure on anxiety-like behaviors. Bar graphs show mean time in center fields of open field test (OFT) (A) and light box of dark-light transfer (DLT) (B) in control (n = 12) and BPA-exposed rats (n = 12). C: Effects of perinatal BPA exposure on depression-like behaviors (FST). Bar graph shows mean immobility time in forced swim test (FST). *P<0.05 and **P<0.01 (n = 12).
Perinatal BPA exposure leads to the hyper-activity of HPA axis in rats
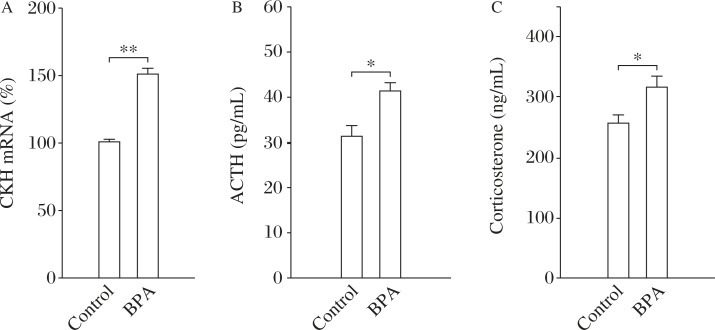
The pre-stress CRH mRNA, or serum ACTH and corticosterone were firstly examined to determine the basal HPA-axis activity. As shown in Fig. 2A-C, BPA-exposed rats (n = 10) showed a significant increase in the levels of CRH mRNA (t(1,18) = 4.46, P<0.001), ACTH (t(1,18) = 2.41, P = 0.027) and corticosterone (t(1,18) = 2.78, P = 0.012) compared to the control rats (n = 10).
Fig. 2. Perinatal BPA exposure enhances basal activity of HPA-axis in rats.
The comparison of CRH mRNA in the paraventricular nucleus (PVN) (A), ACTH (B) and corticosterone (C) in control (n = 10) and BPA-exposed rats (n = 10). *P<0.05 and **P<0.01 (n = 10).
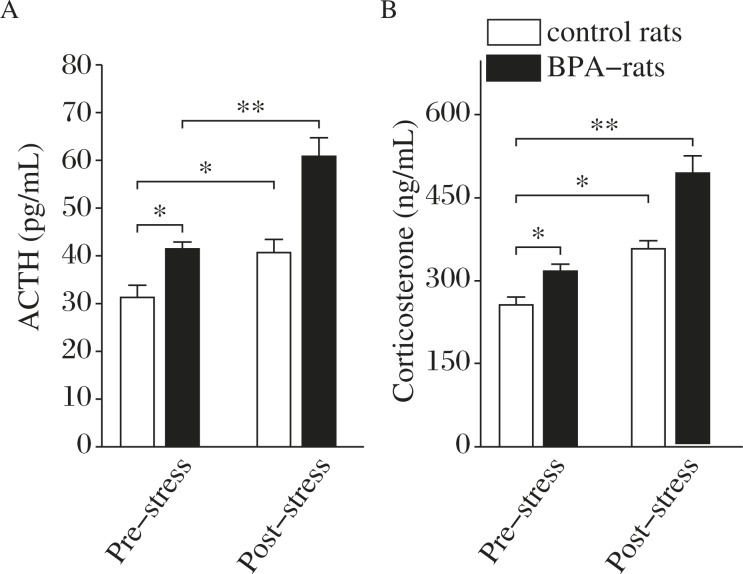
Two-way ANOVA analysis indicated that either BPA treatment or 5-min forced swimming stress significantly affected the level of corticosterone (BPA treatment: F(1,36) = 77.496, P<0.001; stress: F(1,36) = 136.656, P<0.001; interaction: F(1,36) = 10.626, P = 0.002) and ACTH (BPA treatment: F(1,36) = 41.614, P<0.001; stress: F(1,36) = 61.840, P<0.001; interaction: F(1,36) = 7.384, P = 0.010). Bonferroni post hoc test further revealed that following the stressor of 5-min forced swimming, the levels of ACTH and corticosterone were increased approximately 30–40% in the control rats (ACTH: P = 0.039, corticosterone: P = 0.013; Fig. 3A&B), whereas a larger increase in the level of ACTH (P<0.001) or corticosterone (P<0.001) was induced by the stressor in BPA-exposed rats than those in the control rats (Fig. 3A&B). These results showed that HPA-axis function in either basal or stress condition was significantly enhanced in BPA-exposed rats.
Fig. 3. The perinatal BPA exposure enhances the pre- or post-stress reactivity of HPA-axis.
A&B: The influence of BPA treatment and stress on ACTH (A) and corticosterone (B) levels. *P<0.05 and **P<0.01 (n = 10).
Perinatal BPA exposure decreases hippocampal GR expression in rats
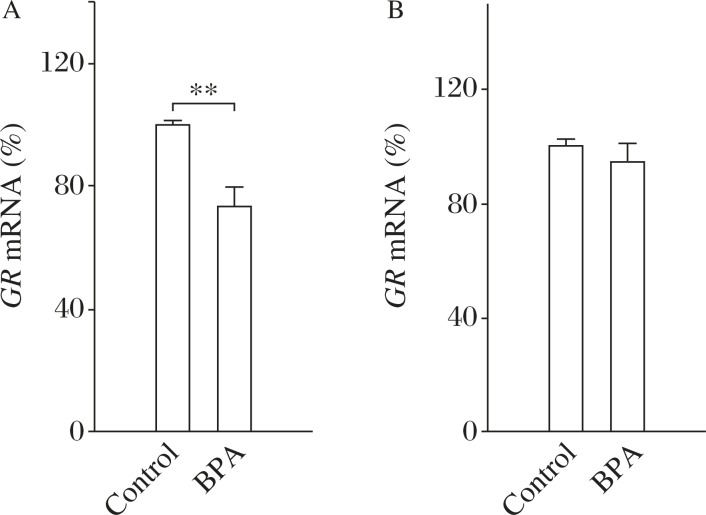
Considering the importance of the GR system for negative-feedback regulation of HPA activity[24], GR mRNA expression in the PVN or hippocampus was then examined. In comparison with the control rats (n = 10), BPA-exposed rats (n = 10) showed a significant decrease in the expression of hippocampal GR mRNA (t(1,18) = 3.35, P = 0.006; Fig. 4A). However, there was no difference in the expression of GR mRNA in the PVN region between the control and BPA-exposed rats (t(1,18) = 1.89, P = 0.08; Fig. 4B). The results indicate that the GR-mediated negative feedback on HPA-axis was depressed in BPA-exposed rats.
Fig. 4. GR-mediated-negative feedback on HPA-axis is de-pressed in BPA-exposed rats.
The comparison of GR mRNA in the hippocampus (A) or PVN (B) of control and BPA-exposed rats. **P<0.01 (n = 10).
The expression of hippocampal MR, nNOS and CREB is enhanced in BPA-exposed rats
Hippocampal MR-mediated nNOS-p-CREB signaling pathway is reported to affect the HPA axis and affective behaviors via negatively regulating GR expression[12]. We designed further experiments to examine MR-induced nNOS and p-CREB signaling in the hippocampus. As shown in Fig. 5A-C, BPA-exposed rats (n = 10) showed increased expression of MR mRNA (t(1,18) = 3.73, P = 0.002), nNOS (t(1,18) = 3.79, P = 0.001) and p-CREB proteins (t(1,18) = 5.43, P<0.001) in relative to those in the hippocampus of the control rats (n = 10). The results indicate that the MR-mediated nNOS-CREB signaling pathway was potentiated in BPA-exposed rats.
Fig. 5. Hippocampal MR-nNOS-CREB pathway is potentiated in BPA-exposed rats.
A: The comparison of hippocampal MR mRNA level in control and BPA-exposed rats. B&C: Upper: Representative Western blots are shown for nNOS and β-actin in (B), and p-CREB and CREB in (C). Lower panels: The comparison of hippocampal nNOS (B) and p-CREB (C) in control and BPA-exposed rats. **P<0.01.
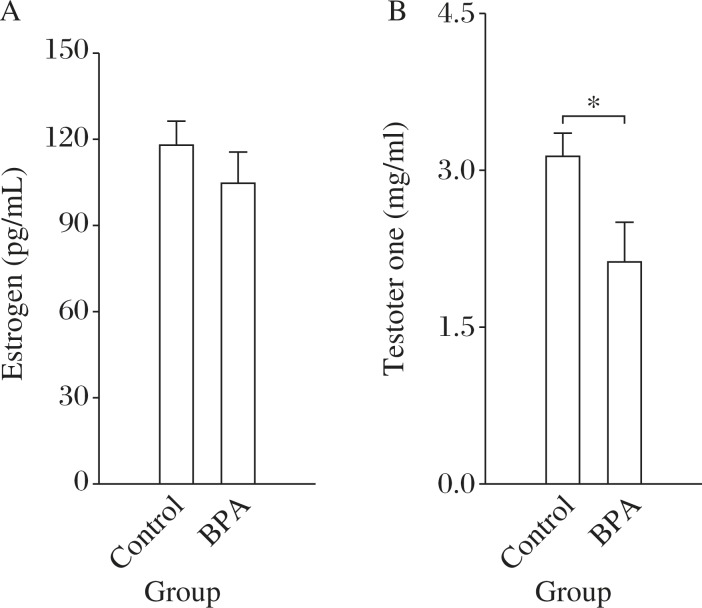
Perinatal BPA exposure inhibits peripheral production of testosterone in rats
Because gonadal hormones are involved in regulating the response of the HPA-axis[25]-[27], serum estrogen and testosterone levels were measured. There was no difference in the serum level of estrogen (t(1,18) = 0.57, P = 0.41; Fig. 6A) between control (n = 10) and BPA-exposed rats (n = 10), but the serum level of testosterone in BPA-exposed rats was significantly lower than that of control rats (t(1,18) = 2.19, P = 0.04; Fig. 6B).
Fig. 6. Perinatal BPA exposure decreases peripheral testos-terone level in rats.
A&B: The comparison of estrogen (A) and testosterone (B) levels in control and BPA-exposed rats. *P<0.05 (n = 10).
DISCUSSION
Our study reveals that perinatal exposure to low dose of BPA results in the hyperactivity of the HPA axis, which is associated with anxiety/depression-like behaviors, through weakening the inhibition on the HPA axis by hippocampal GR-mediated feedback and peripheral testosterone. BPA-exposed rats spent less time in the center area of the OFT and in the light box of the DLT and showed more immobility in the FST than control rats. The hyperactivity of the pre- or post-stress HPA-axis was accompanied with decreased hippocampal GR expression and serum testosterone level in BPA-exposed rats. In comparison with controls, the levels of hippocampal MR mRNA, nNOS and p-CREB were increased in BPA-exposed rats.
Affective behaviors in PND 80 BPA-exposed rats were studied using three separate behavioral paradigms. BPA-exposed rats were more inactive, spent less time in the center area of the OFT or in the light box of the DLT and showed more immobility in the FST than control rats. These results indicate that BPA-exposed rats are prone to exhibit an increase in anxiety- and depression-related behaviors, which is consistent with the previous studies[28]-[30]. The HPA axis is widely reported to be highly susceptible to reprogramming during fetal and neonatal development, leading to persistent abnormality in the physiology and behaviors of the offspring[31],[32]. Here, we found that perinatal BPA exposure produced long-term potentiated effect on the HPA axis in offspring as reflected by the persistent increase in the level of serum corticosterone and ACTH and CRH mRNA either in basal condition or after stress. The results suggest that HPA hyperactivity underlies affective disorders associated with developmental BPA exposure.
The hyperactivity of the HPA axis is attributed, at least in part, to the impaired negative feedback of endogenous corticosterone. Through binding to GR and MR in the limbic-HPA axis stress system, corticosterone serves as a potent negative regulator of HPA axis activity, in particular the synthesis and release of CRH from the PVN region of hypothalamus and ACTH from the pituitary. MR via binding corticosterone mainly participates in stress appraisal and response onset, whereas GR is implicated in promoting adaptation to and recovery from stress and establishing struggle strategies. GR thus is thought to play a critical role in the corticosterone-mediated negative feedback inhibition of the HPA axis and stress adaptation[24]. It has been found that hippocampal GR deficiency is correlated with the hyperactivity of the HPA axis, and contributes to the etiology of depression[33]. In the present study, the decease of GR mRNA levels was observed in the hippocampus of BPA-exposed rat offspring. This change might lead to the decrease of GR-mediated feedback inhibition on the HPA axis, equally producing HPA-axis hyperactivity and the ensued anxiety/depression-like behaviors. Accumulating evidence indicates that hippocampal MR negatively regulates GR expression and activity via activating nNOS mediated CREB signal pathway[12],[34]. In comparison with control rats, the expression of hippocampal MR was increased in BPA-exposed rats. Furthermore, the levels of nNOS and p-CREB were higher than those in control rats. Thus, the findings indicate that the upregulation of hippocampal MR-induced nNOS-CREB signaling is likely implicated in BPA-induced hyperactivity of HPA-axis and high levels of anxiety and depression-related behaviors.
Findings from clinical and animal studies have proved that testosterone after sexual maturation plays an important role in anti-anxiety and anti-depressant behaviors,3536. The decease or depletion of testosterone by various measures in adults results in increase of anxiety and depression,3738. Hence, the reduced serum testosterone level is explained to be an important peripheral mechanisms underlying the affective abnormality in BPA-exposed offspring. A growing body of evidence has indicated that testosterone affects affective behaviors at multiple levels of the HPA axis. Testosterone has been shown to reduce adrenal sensitivity to ACTH[26] and suppress ACTH release from the anterior pituitary and CRH release from the PVN[27],[39]. Thus, it is conceivable that the low level of testosterone may be another factor causing HPA-axis hyperactivity associated with anxiety and depression of BPA-exposed rats. Although estrogen has been reported to have a potentiating effect on the HPA axis[25], the observed effects in BPA-exposed offspring seems to be disconnected with peripheral estrogen secretion due to its unchanged serum level.
The present study suggests that perinatal BPA exposure results in obvious anxiety- and depression-like behaviors probably via long-term potentiatiation of the HPA axis in offspring. However, the present data cannot explain the underlying mechanisms of the relationship between developmental experiences and life-long phenotypic consequences. The development period is crucial for establishing and maintaining epigenetic marks[40]. Epigenetic alterations were consequently regarded as the most plausible explanation that environmental toxicants including BPA could exert long-lasting effects. Indeed, there is accumulating evidence for BPA-induced epigenetic alterations,4142. For example, Dolinoy et al.[42] found that maternal BPA exposure shifted the coat color distribution of offspring toward yellow by decreasing methylation at specific CpG sites in yellow agouti allele. A study by Ho et al.[41] indicated that developmental BPA exposure increases adult susceptibility to prostate cancer via hypomethylating specific CpG sites of phosphodiesterase type 4 variant 4 gene in the prostate. One report of our laboratory showed that the decrease of glutamic acid decarboxylase (GAD) 67 expression in the amygdala of rat perinatally exposed to BPA is due to the long-lasting epigenetic changes of GAD67 promoter[43]. It has been confirmed that the HPA axis and hippocampus are always the targets of epigenetic reprogramming by development experience. For example, low maternal care methylates and hypoacetylates the hippocampal GR promoter in offspring leading to the HPA hyperactivity,4445. Hence, it is necessary to confirm the epigenetic mechanisms undying the effects of perinatal BPA exposure on HPA axis associated with affective behaviors.
Accumulating studies have indicated the negative effects of prenatal stress or early-life adversity on the development and maturation of corticosteroid-mediated stress system[32],[46]. However, to date, there are few studies paying attention to the influence of environmental toxins on this stress-related system. The present study shows convincingly that perinatal BPA exposure brings the long-term potentiation of HPA response to stress accompanied with obvious anxiety- and depression-like behaviors. Future research should put emphasis on investigating the underlying mechanisms of the link between perinatal BPA exposure and HPA axis hyperactivity, such as epigenetic alterations of relevant genes.
Acknowledgments
This work was supported by China Postdoctoral Science Foundation (2013M540456), Jiangsu Planned Projects for Postdoctoral Research Funds (1301065B) and Grants for 973 (2014CB943303), NSFC (81071027; 31171440; 81000482).
References
- 1.Ansorge MS, Hen R, Gingrich JA. Neurodevelopmental origins of depressive disorders[J] Curr Opin Pharmacol, 2007;7(1):8–17. doi: 10.1016/j.coph.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS. Stress and hippocampal plasticity[J] Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 3.Mendola P, Selevan SG, Gutter S, et al. Environmental factors associated with a spectrum of neurodevelopmental deficits[J] Ment Retard Dev Disabil Res Rev. 2002;8(3):188–197. doi: 10.1002/mrdd.10033. [DOI] [PubMed] [Google Scholar]
- 4.Kang JH, Kito K, Kondo F. Factors influencing the migration of bisphenol A from cans[J] J Food Prot. 2003;66(8):1444–1447. doi: 10.4315/0362-028x-66.8.1444. [DOI] [PubMed] [Google Scholar]
- 5.Jasarevic E, Sieli PT, Twellman EE, et al. Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A[J] Proc Natl Acad Sci U S A. 2011;108(28):11715–11720. doi: 10.1073/pnas.1107958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu X, Tian D, Hong X, et al. Sex-specific influence of exposure to bisphenol-A between adolescence and young adulthood on mouse behaviors[J] Neuropharmacology. 2011;61(4):565–573. doi: 10.1016/j.neuropharm.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Patisaul HB, Bateman HL. Neonatal exposure to endocrine active compounds or an ERbeta agonist increases adult anxiety and aggression in gonadally intact male rats[J] Horm Behav. 2008;53(4):580–588. doi: 10.1016/j.yhbeh.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis[J] JAMA. 1992;267(9):1244–1252. [PubMed] [Google Scholar]
- 9.Holsboer F, Ising M.Stress hormone regulation: biological role and translation into therapy[J] Annu Rev Psychol 20106181–109. C101–111 [DOI] [PubMed] [Google Scholar]
- 10.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease[J] Nat Rev Neurosci. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 11.Young EA, Lopez JF, Murphy-Weinberg V, et al. Mineralocorticoid receptor function in major depression[J] Arch Gen Psychiatry. 2003;60(1):24–28. doi: 10.1001/archpsyc.60.1.24. [DOI] [PubMed] [Google Scholar]
- 12.Zhou QG, Zhu LJ, Chen C, et al. Hippocampal neuronal nitric oxide synthase mediates the stress-related depressive behaviors of glucocorticoids by downregulating glucocorticoid receptor[J] J Neurosci. 2011;31(21):7579–7590. doi: 10.1523/JNEUROSCI.0004-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Bellis MD, Baum AS, Birmaher B, et al. A.E. Bennett Research Award. Developmental traumatology. Part I: Biological stress systems[J] Biol Psychiatry. 1999;45(10):1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- 14.Nemeroff CB. The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions[J] Mol Psychiatry. 1996;1(4):336–342. [PubMed] [Google Scholar]
- 15.O'Keefe JA, Handa RJ. Transient elevation of estrogen receptors in the neonatal rat hippocampus[J] Brain Res Dev Brain Res. 1990;57(1):119–127. doi: 10.1016/0165-3806(90)90191-z. [DOI] [PubMed] [Google Scholar]
- 16.Khurana S, Ranmal S, Ben-Jonathan N. Exposure of newborn male and female rats to environmental estrogens: delayed and sustained hyperprolactinemia and alterations in estrogen receptor expression[J] Endocrinology. 2000;141(12):4512–4517. doi: 10.1210/endo.141.12.7823. [DOI] [PubMed] [Google Scholar]
- 17.Ramos JG, Varayoud J, Kass L, et al. Bisphenol a induces both transient and permanent histofunctional alterations of the hypothalamic-pituitary-gonadal axis in prenatally exposed male rats[J] Endocrinology. 2003;144(7):3206–3215. doi: 10.1210/en.2002-0198. [DOI] [PubMed] [Google Scholar]
- 18.Kawai K, Murakami S, Senba E, et al. Changes in estrogen receptors alpha and beta expression in the brain of mice exposed prenatally to bisphenol A[J] Regul Toxicol Pharmacol. 2007;47(2):166–170. doi: 10.1016/j.yrtph.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Xu X, Liu Y, Sadamatsu M, et al. Perinatal bisphenol A affects the behavior and SRC-1 expression of male pups but does not influence on the thyroid hormone receptors and its responsive gene[J] Neurosci Res. 2007;58(2):149–155. doi: 10.1016/j.neures.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Akingbemi BT, Sottas CM, Koulova AI, et al. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells[J] Endocrinology. 2004;145(2):592–603. doi: 10.1210/en.2003-1174. [DOI] [PubMed] [Google Scholar]
- 21.Xu X, Hong X, Xie L, et al. Gestational and lactational exposure to bisphenol-A affects anxiety- and depression-like behaviors in mice[J] Horm Behav. 2012;62(4):480–490. doi: 10.1016/j.yhbeh.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Cao J, Belluzzi JD, Loughlin SE, et al. Acetaldehyde, a major constituent of tobacco smoke, enhances behavioral, endocrine, and neuronal responses to nicotine in adolescent and adult rats[J] Neuropsychopharmacology. 2007;32(9):2025–2035. doi: 10.1038/sj.npp.1301327. [DOI] [PubMed] [Google Scholar]
- 23.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review[J] Eur J Pharmacol. 2003;463(1–3):3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 24.Dzirasa K, Covington HE. 3rd. Increasing the validity of experimental models for depression[J] Ann N Y Acad Sci. 2012;1265:36–45. doi: 10.1111/j.1749-6632.2012.06669.x. [DOI] [PubMed] [Google Scholar]
- 25.Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment[J] Biol Psychiatry. 2001;49(5):391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 26.Kirschbaum C, Schommer N, Federenko I, et al. Short-term estradiol treatment enhances pituitary-adrenal axis and sympathetic responses to psychosocial stress in healthy young men[J] J Clin Endocrinol Metab. 1996;81(10):3639–3643. doi: 10.1210/jcem.81.10.8855815. [DOI] [PubMed] [Google Scholar]
- 27.Rubinow DR, Roca CA, Schmidt PJ, et al. Testosterone suppression of CRH-stimulated cortisol in men[J] Neuropsychopharmacology. 2005;30(10):1906–1912. doi: 10.1038/sj.npp.1300742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes[J] J Neuroendocrinol. 2002;14(6):506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- 29.Matsuda S, Matsuzawa D, Ishii D, et al. Effects of perinatal exposure to low dose of bisphenol A on anxiety like behavior and dopamine metabolites in brain[J] Prog Neuropsychopharmacol Biol Psychiatry. 2012;39(2):273–279. doi: 10.1016/j.pnpbp.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Fujimoto T, Kubo K, Aou S. Prenatal exposure to bisphenol A impairs sexual differentiation of exploratory behavior and increases depression-like behavior in rats[J] Brain Res. 2006;1068(1):49–55. doi: 10.1016/j.brainres.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 31.DeRijk R, de Kloet ER. Corticosteroid receptor genetic polymorphisms and stress responsivity[J] Endocrine. 2005;28(3):263–270. doi: 10.1385/ENDO:28:3:263. [DOI] [PubMed] [Google Scholar]
- 32.de Kloet ER, Sibug RM, Helmerhorst FM, et al. Stress, genes and the mechanism of programming the brain for later life[J] Neurosci Biobehav Rev. 2005;29(2):271–281. doi: 10.1016/j.neubiorev.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Boyle MP, Brewer JA, Funatsu M, et al. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior[J] Proc Natl Acad Sci U S A. 2005;102(2):473–478. doi: 10.1073/pnas.0406458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou QG, Hu Y, Hua Y, et al. Neuronal nitric oxide synthase contributes to chronic stress-induced depression by suppressing hippocampal neurogenesis[J] J Neurochem. 2007;103(5):1843–1854. doi: 10.1111/j.1471-4159.2007.04914.x. [DOI] [PubMed] [Google Scholar]
- 35.Carnahan RM, Perry PJ. Depression in aging men: the role of testosterone[J] Drugs Aging. 2004;21(6):361–376. doi: 10.2165/00002512-200421060-00002. [DOI] [PubMed] [Google Scholar]
- 36.Frye CA, Seliga AM. Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats[J] Cogn Affect Behav Neurosci. 2001;1(4):371–381. doi: 10.3758/cabn.1.4.371. [DOI] [PubMed] [Google Scholar]
- 37.Enayati M, Solati J, Hosseini MH, et al. Maternal infection during late pregnancy increases anxiety- and depression-like behaviors with increasing age in male offspring[J] Brain Res Bull. 2012;87(2–3):295–302. doi: 10.1016/j.brainresbull.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Egashira N, Koushi E, Okuno R, et al. Depression-like behavior and reduced plasma testosterone levels in the senescence-accelerated mouse[J] Behav Brain Res. 2010;209(1):142–147. doi: 10.1016/j.bbr.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 39.Bao AM, Fischer DF, Wu YH, et al. A direct androgenic involvement in the expression of human corticotropin-releasing hormone[J] Mol Psychiatry. 2006;11(6):567–576. doi: 10.1038/sj.mp.4001800. [DOI] [PubMed] [Google Scholar]
- 40.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development[J] Science. 2001;293(5532):1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 41.Ho SM, Tang WY, Belmonte de Frausto J, et al. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4[J] Cancer Res. 2006;66(11):5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development[J] Proc Natl Acad Sci U S A. 2007;104(32):13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou R, Chen F, Chang F, et al. Persistent overexpression of DNA methyltransferase 1 attenuating GABAergic inhibition in basolateral amygdala accounts for anxiety in rat offspring exposed perinatally to low-dose bisphenol A[J] J Psychiatr Res. 2013 doi: 10.1016/j.jpsychires.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 44.Weaver IC. Epigenetic programming by maternal behavior and pharmacological intervention. Nature versus nurture: let's call the whole thing off[J] Epigenetics. 2007;2(1):22–28. doi: 10.4161/epi.2.1.3881. [DOI] [PubMed] [Google Scholar]
- 45.Weaver IC, Cervoni N, Champagne FA, et al. Epigenetic programming by maternal behavior[J] Nat Neurosci. 2004;(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 46.Szyf M, Weaver IC, Champagne FA, et al. Maternal programming of steroid receptor expression and phenotype through DNA methylation in the rat[J] Front Neuroendocrinol. 2005;26(3–4):139–162. doi: 10.1016/j.yfrne.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Haenisch B, Bonisch H. Depression and antidepressants: insights from knockout of dopamine, serotonin or noradrenaline re-uptake transporters[J] Pharmacol Ther. 2011;129(3):352–368. doi: 10.1016/j.pharmthera.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Zhou R, Zhang Z, Zhu Y, et al. Deficits in development of synaptic plasticity in rat dorsal striatum following prenatal and neonatal exposure to low-dose bisphenol A[J] Neuroscience. 2009;159(1):161–171. doi: 10.1016/j.neuroscience.2008.12.028. [DOI] [PubMed] [Google Scholar]