Abstract
Background
This study aimed to evaluate freeze-dried sera as an alternative to non-freeze dried for detection of anti-Leishmania infantum antibodies over the course of 11 months using the direct agglutination test (DAT).
Methods
Altogether, 60 serum samples (30 from humans and 30 from dogs) were collected from various geographical locations in Iran. All the collected sera were pooled and each pooled serum sample contained 10 different sera. In the beginning, the human and dog pooled sera were categorized as positive (weak and strong) and negative based on anti-L. infantum antibodies using the DAT. All the freeze-dried and non-freeze-dried sera were stored at −70°C, −20°C, 4°C, 22−28°C and 56°C for 11 months. The positive and negative human and dog pooled sera were separately tested using the DAT each month and the results were compared to non-freeze-dried sera kept under the same conditions.
Results
We found strong agreement (100%) between the results obtained from freeze-dried human and dog in strong DAT positive sera kept at -70°C, -20°C, 4°C and 22-28°C during this study. The human and dog pooled sera stored at 56°C were corrupted after 2 weeks. The DAT results were highly reproducible using freeze-dried human pooled sera in the beginning and month 11 of this study (CV = 0.036).
Conclusion
Freeze-dried human and dog strong DAT positive sera are highly stable under different temperature conditions, are easy to transport and are safe for use as positive and negative serum controls in laboratories.
Keywords: Leishmania infantum, Freeze-dried sera, Preservation
Introduction
Visceral leishmaniasis (VL) is a potentially fatal protozoan infection distributes in some parts of Iran (1). Early diagnosis of VL in the human and domestic animal reservoirs have significantly been enhanced by the introduction of the direct agglutination test (DAT) (2). Despite the fact that the DAT is a simple, cost-effective and field applicable technique, but the high ambient temperatures and the lack of cold chain facilities in most of the VL endemic areas have limited application of the technique (2, 3). Now, some improvements were established to DAT antigen processing in order to preserve solidity under rural conditions (4).
A freeze-dried antigen is capable of stable for long periods at temperature levels up to 45 °C (2, 5, 6). The major limitations of this method are that preservation by freeze-dried needs highly equipment such as sophisticated equipment, a continuous electrical supplies and a high level of expertise. Furthermore, the require of expensive equipped laboratories for producing the DAT antigen greatly limits application of this reliable diagnostic tool to economically privileged VL endemic areas (7–10). Preservation of non-freezed dried sera has a few problems such as simple transportation, blood infections to health care staffs and laboratory technicians and decrease of the antibody titres due to trnsportation. While, the freeze-dried sera to be highly stable at fluctuating laboratory temperatures, easy to transport and can be used for the serodiagnosis human and canine VL without the maintenance of a cold chain.
In the current study, we produced freeze-drying of sera containing anti-L.infantum antibodies. The freeze-dried sera were stored at various temperatures, and its ability to detect anti-L. infantum antibodies in the human and animal sera was compared with the non- freeze-dried sera.
Materials and Methods
Study design
The investigation was based on evaluation of freeze-dried human and dog sera for long-term preservation and detection of anti-Leishmania infantum antibodies using the direct agglutination test (DAT) over 330 days. Initially, all freeze-dried and non-freeze-dried sera were divided into two groups based on positive (weak and strong) and negative DAT results. Subsequently, freeze-dried and non-freeze-dried sera were stored at temperature levels of at −70 °C, −20 °C, 4 °C, 22−28 °C and 56 °C for 11 months (Fig.1). All the freeze-dried and non-freeze-dried sera were evaluated by the DAT for detection of anti-L.infantum antibodies two times monthly for 11 months.
Fig. 1.
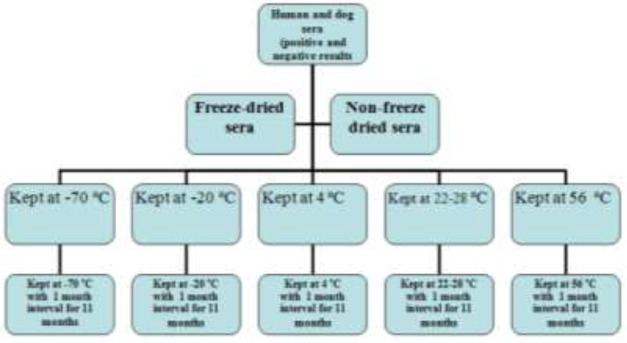
Flow chart, which outlines the major parts of the study
Subjects
The current study was carried out in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Tehran University of Medical Sciences, Tehran, Iran.
Altogether, 60 appropriate sera (30 human sera and 30 dog sera) collected from different geographical locations of Iran were used to produce pooled sera. Each pooled serum sample contained 10 sera. All prepared sera were separated from the blood samples immediately and freeze-dried until testing. The study was carried out at the School of Public Health, Tehran University of Medical Sciences from 2012 to 2013.
Preparetion of freeze-drying of sera
The Lyophilizator used in this study was a model Lyotrap Plus (SLS, UK). This instrument consisted of two sections: 1) vacuum pumps and 2) a freezer with temperature levels ranging from −35 °C to −45 °C. Prior to freeze-drying, all the samples were pre-frozen at −20 °C for 24 hours. The sera were freeze-dried for 4 hours and after which we obtained the white pellets of freeze-dried sera and placed them into vials. It should be noted that the physiological sera has been used for recovery of the freeze-dried sera. Sera were further classified according to degrees of agglutination reactions and varied in titres from less than 1:800 (0, negative) to 1:800-1:6400 (weak) and greater than or equal to 1:6400-102800 (strong) in human pooled sera. The dog pooled sera were categorized by titres less than 1:80 (0, negative), 1:80-1:640 (weak) and 1:640-1:20480 (strong).
DAT performance
Monthly DAT sera tests were conducted between from Mar 2012 to Dec 2013. To reconstitute the FD pooled sera, 50 ml of normal saline (0.9 % NaCl) was added to each vial half of an hour before testing.
The pooled human sera into two groups (freeze-dried and non- freeze-dried) were diluted over a range of 1:100 to 1:102400 with normal saline containing 0.2% gelatin and 0.78% ß-mercapthanol (2-ME) (Sigma Lot No. 45H0508). The pooled canine sera (freeze-dried and non- freeze-dried) also were diluted from 1:10to 1:20480 with than same diluents, but 1.56 % 2-ME were added to the V-shape plates. Following the step described above, the dog sera plates were incubated for one hour at 37 °C. Fifty micro liter DAT antigens was added into the sera plates and the plates were manually shaken for one minute. All the sera plates kept 24 hour under ambient temperature and moist conditions, the results also evaluated (6, 9). Following the test control, semi quantitative results achieved with DAT are cleared as an antibody titer, the reciprocal of the highest dilution at which agglutination (large diffuse blue mats) was still detectable after 24 hour incubation at room condition, compared with positive and negative control correctly (8, 10). All pooled sera were tested in duplicate using DAT.
Data analyzes
Analyses were conducted computing descriptive statistics and comparing subgroups using nonparametric statistics, such as the Wilcoxon-Mann-Whitney or Kruskal-Wallis test as appropriate. Analyses were conducted using ANOVA test and SPSS software version 18 with a probability (P) value of < 0.05 as statistically significant. Cohen’s kappa values express the agreement between freeze-dried and non- freeze- dried sera, and a value of 0.21-0.60 represents a fair to moderate agreement, a value of 0.61-0.80 represents a substantial agreement, and a value ≥ 0.81 represents almost perfect agreement.
The coefficient of variation (CV) represents the ratio of the standard deviation to the mean, and it is a useful statistic for comparing the degree of variation from one data series to another, even if the means are drastically different from each other. CV was calculated with the following formula:
Results
The results comparing freeze-dried to non-freeze-dried sera are given in Tables 1 and 2. The ranges of DAT sera titres in the whole series of the human and canine sera were 1:800 to 1:102400 and 1:320 to 1:20480, respectively. The correlation between freeze-dried and non-freeze-dried sera was analysed at different storage temperatures. The correlation between the human sera at −20 °C was very high (93%). In contrast, the canine sera showed a high correlation (93%) at both 4 and −20°C. The results also showed that the sera stored at the 56°C were of unacceptable quality for both human and dog pooled sera. The human freeze-dried sera agreement at 4, −20, −70 and 22−28 °C between the first and month11 was 100%. The human non-freeze-dried sera agreement at −20 and 22−28 °C between the first and month 11 was 100%. The dog freeze-dried sera agreement at −70 °C between the first and month 11 was 100%. The dog non-freeze-dried sera agreement at −20 °C between the first and month 11 was 100%. The coefficient of variation (CV) of anti-Leishmania antibodies titers using DAT on human and dog frozen-dried pooled sera in the beginning and month 11 repetition was shown in Table 3.
Table 1.
The distribution of anti-Leishmania antibodies titre in human pooled sera stored in ambient temperatures with DAT method, in the beginning and month 11
| Kind of human sera pooled | Primary titer (Beginning of study) | Titers(at End of the study after 11 months) | |||||
|---|---|---|---|---|---|---|---|
| 4°C | -20°C | -70°C | 22-28°C | 56°C | |||
| freeze-dried | strong | 1:102400 | 1:102400 | 1:102400 | 1:102400 | 1:51200 | Denature |
| weak | 1:3200 | 0 | 1:800 | 1:800 | 1:800 | Denature | |
| non freeze-dried | strong | 1:102400 | 1:12800 | 1:102400 | 1:102400 | 1:12800 | Denature |
| Weak | 1:3200 | 0 | 1:800 | 1:1600 | 0 | Denature | |
100% correlation were found on strong DAT positive sera using freeze-dried sera before and month 11 while no correlation was observed on week DAT sera.
50% correlation were found on strong DAT positive sera using non freeze-dried sera before and month 11 and no correlation was observed on week DAT sera.
Table 2.
The distribution of anti-Leishmania antibodies titer in human pooled sera stored in ambient temperatures with DAT method, in the beginning and month11
| Kind of dog sera pooled | Primary titer(Beginning of study) | Titer (at End of the study after 11 months) | |||||
|---|---|---|---|---|---|---|---|
| 4°C | -20°C | -70°C | 22-28°C | 56°C | |||
| freeze-dried | strong | 1:20480 | 1:20480 | 1:20480 | 1:20480 | 1:20480 | Denature |
| weak | 1:320 | 1:80 | 1:80 | 1:80 | 1:80 | Denature | |
| non freeze-dried | strong | 1:20480 | 1:5120 | 1:5120 | 1:5120 | 1:320 | Denature |
| weak | 1:320 | 0 | 1:80 | 1:160 | 0 | Denature | |
100% correlation were found on strong DAT positive sera using freeze-dried sera before and month 11 while no correlation was observed on week DAT sera.
No correlation were found on strong as well as weak DAT positive sera using non freeze-dried sera before and month 11 and no correlation was observed on week DAT sera.
In non freeze-dried sera, we found one fold reduction in anti-Leishmania antibodies in strong positive sera while one or two-fold reduction in weak positive sera
Table 3.
The coefficient of variation (CV) of anti-Leishmania antibodies titers using DAT on human and dog frozen-dried pooled sera in the beginning and month 11 repetition
| CV (%) | ||
|---|---|---|
| Primary titer | Repetition titer | |
| Human | 0.037 | 0.035 |
| Dog | 0.34 | 0.35 |
After the CV determinations, it is illustrated that there was no differences between human and canine freez-edried and non- freeze-dried sera in the beginning and month 11repetition.
Discussion
The most remarkable results that have emerged from previous studies were that high ambient temperatures and lack of cold chain facilities in most of the VL-endemic areas have limited application of DAT despite the fact that the technique is a simple, cost-effective and field-applicable technique (8, 10). As noted in the introduction, the FD-DAT antigen was introduced to solve this problem. However, the preservation by FD needs highly specialized tools and techniques such as sophisticated equipment, a continuous electrical supply and a high level of expertise (2, 5, 11).
In 2011, a simple and applicable method for making GP-DAT antigen from an Iranian strain of L. infantum was successfully introduced by Akhoundi and Mohebali (2). High ambient temperatures and a lack of cold chain facilities have limited the application of the preservation of new human and animal sera.
In this study, freeze-dried pooled sera were prepared from humans and domestic dogs and stored at different temperatures in order to detect anti-L. infantum antibodies using DAT. As the results showed, the correlation between the freeze-dried and non-freeze-dried human sera at −20°C was very high (94%) while there was a lower correlation at the laboratory temperature (25°C). The freeze-dried and non-freeze-dried canine sera showed a high correlation (94%) at the 4 and −20°C.
In our practical study, the sera stored at 56°C showed negative reactions in both pooled human and dog sera. Congruent with previous studies, this study confirms that the FD-DAT, freeze-dried sera or GP-DAT at 56°C was of unacceptable quality (2, 6, 9).
Conclusion
The results indicated there were no significant differences between freeze-dried and non-freeze-dried sera over a period of 11 months at certain temperatures. Furthermore, the human freeze-dried sera were shown to be highly stable at - 20°C while the canine sera were shown to be highly stable at both 4 and -20°C.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgments
This study was funded by Tehran University of Medical Sciences (Project No: 91-04-27-20562) and it also was thesis of Miss. Zahra Kakoei for receiving MSc degree in Medical Parasitology. We thank the late Prof. H. Kakooei from the School of Public Health, Tehran University of Medical Sciences for editorial comments. The authors also thank from Dr. H. Hajjaran, Mr. Z. Zarei, Mrs. S. Charehdar and all colleagues from the leishmania-sis laboratory at the School of Public Health at the Tehran University of Medical Sciences. The authors declare that there is no conflict of interests.
References
- Mohebali M (2013). Visceral leishmaniasis in Iran: Review of the Epidemiological and Clinical Features. Iran J Parasitol, 8: 348–358. [PMC free article] [PubMed] [Google Scholar]
- Akhoundi B, Mohebali M, Edrissian GH, Eslami MB, Keshavarz H, Malekafzali H, Rokni MB (2012). Preparation and evaluation of a glycerol-preserved direct agglutination antigen for long-term preservation: a comparative study of the detection of anti-Leishmania infantum antibodies in human and dog. Asian Pac J Trop Med, 5(2): 117–120. [DOI] [PubMed] [Google Scholar]
- Akhoundi B, Mohebali M, Babakhan L, Edrissian GH, Eslami MB, Keshavarz H, Malekafzali H (2010). Rapid detection of human Leishmania infantum infection: A comparative field study using the fast agglutination screening test and the direct agglutination test. Travel Med Inf Dis, 8(5): 305–310. [DOI] [PubMed] [Google Scholar]
- Harith A, Kolk AHJ, Kager PA, Leeuwenburg J, Muigai R, Kiugu S, Laarman JJ(1986). A simple and economical direct agglutination test for serodiagnosis and sero-epidemiological studies of visceral leishmaniasis. Trans R Soc Trop Med Hyg, 80(4): 583–587. [DOI] [PubMed] [Google Scholar]
- Meredith SE, Kroon NC, Sondorp E, Seaman J, Goris MG, Vaningen CW, Oosting H, Schoone GJ, Terpstra WJ, Oskam L(1995). A stable direct agglutination test based on freeze-dried antigen for serodiagnosis of visceral leishmaniasis. J Cin Microbiol, 33(7): 1742–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie SH, Hawkey PM (1995). Medical parasitology: a practical approach series. Oxford: Oxford University Press, 159–160. [Google Scholar]
- World Health Organization (1996). Manual on visceral leishmaniasis control. Geneva: WHO, 60–63. [Google Scholar]
- Mohebali M, Edrissian GH, Shirzadi MR et al. (2011). An observational study for current distribution of visceral leishmaniasis in different geographical zones of Iran for implication of health policy. Travel Med Infect Dis, 9(2): 67–74. [DOI] [PubMed] [Google Scholar]
- Mohebali M, Hajjaran H, Hamzavi Y, Mobedi I, Arshi S, Zarei Z, Akhoundi B, Naeini KM, Avizeh R, Fakhar M (2005). Epidemiological aspects of canine visceral leishmaniosis inthe Islamic Republic of Iran. Vet Parasitai, 129(3–4): 243–251. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2010). Report of a meeting of the WHO expert committee on the control of leishmaniases. Technical report series 949, 22–26, Geneva, Switzerland: WHO. [Google Scholar]
- Harith A, Mutasim M, Mansour D, Mustafa EF, Arvidson H (2003). Use of glycerol as an alternative to freeze-drying for long-term preservation of antigen for the direct agglutination test. Trop Med Inter Health, 8(11): 1025–1102. [DOI] [PubMed] [Google Scholar]


