Highlight
Root bending, tensile strength, and ability to penetrate hard soil are related to anatomical phenes that are subject to selection in crop breeding programs.
Key words: Anatomy, bending strength, phenes, tensile strength.
Abstract
The ability of roots to penetrate hard soil is important for crop productivity but specific root phenes contributing to this ability are poorly understood. Root penetrability and biomechanical properties are likely to vary in the root system dependent on anatomical structure. No information is available to date on the influence of root anatomical phenes on root penetrability and biomechanics. Root penetration ability was evaluated using a wax layer system. Root tensile and bending strength were evaluated in plant roots grown in the greenhouse and in the field. Root anatomical phenes were found to be better predictors of root penetrability than root diameter per se and associated with smaller distal cortical region cell size. Smaller outer cortical region cells play an important role in stabilizing the root against ovalization and reducing the risk of local buckling and collapse during penetration, thereby increasing root penetration of hard layers. The use of stele diameter was found to be a better predictor of root tensile strength than root diameter. Cortical thickness, cortical cell count, cortical cell wall area and distal cortical cell size were stronger predictors of root bend strength than root diameter. Our results indicate that root anatomical phenes are important predictors for root penetrability of high-strength layers and root biomechanical properties.
Introduction
Soil compaction adversely affects crop production in many parts of the world. The formation of strong or compacted soil layers restricts root exploration and access to nutrients and water thereby promoting early onset of stress (Barraclough and Weir, 1988). Soil strength and mechanical impedance to root growth typically increases with decreasing soil moisture (Cairns et al., 2004; Iijima and Kato, 2007; Whitmore and Whalley, 2009). Impedance causes physical limitations to root growth in the rooting zone with a typical soil penetrometer resistance of 2.0MPa, the threshold for root elongation (Bengough et al., 2011). Root growth in drying soil is generally constrained by a combination of mechanical impedance and water stress. However, soil strength increases nonlinearly with decreasing soil moisture, which may result in mechanical impedance limiting root growth to a relatively greater extent than water stress per se (Bengough et al., 2011). Deep rooting is important for drought adaptation by improving utilization of water in deep soil strata (Lynch, 2013) but the presence of strong or compacted soil layers often precludes the exploitation of deep water and nutrients. Thus, deep roots that are capable of penetrating hard soil are important for improving crop productivity in compacted soils.
Soil management approaches to ameliorate subsoil compaction, such as deep ripping and the application of gypsum to improve soil structure and aggregate stability, are often used as a solution to soil compaction. However, in the long-term, these management practices may not be a good solution to alleviate soil compaction because they encourage decomposition of organic matter, breakdown of soil aggregates and weakening of soil structure (Brady and Weil, 2008) and are costly in terms of energy and time. The viable alternative is to find ways of developing root system traits capable of penetrating these hard soils and alleviating compaction with minimum cost, maintaining sustainability.
There is evidence of differences in root penetration ability between, and also within, species. Within-species variation in root penetration has been reported in cotton (May and Kasperbauer, 1999; Klueva et al., 2000), maize and soybean (Bushamuka and Zobel, 1998), rice (Price et al., 2000; Zheng et al., 2000; Clark et al., 2002), and wheat (Acuña and Wade, 2005; Botwright Acuna et al., 2007; Kubo et al., 2006, 2008). Generally the ability of roots to penetrate hard soils is associated with phenes [‘phene’ is to ‘phenotype’ as ‘gene’ is to ‘genotype’ (Lynch, 2011)] that reduce the likelihood of root buckling when penetrating hard soils (Clark et al., 2003; Bengough et al., 2011; Jin et al., 2013).
Mechanically, roots form an essential component in anchoring plants in the soil providing the foundations for plant stability, with most terrestrial plants dependent on their ability to stand upright. Root biomechanical properties have been of interest regarding their implications for the mechanics of plant anchorage and lodging resistance (Ennos et al., 1993; Goodman and Ennos, 1998; Oladokun and Ennos, 2006). The resistance to lodging is a function of the resistance of the compressed leeward roots to bending, the anchorage of the windward roots under tension, the strength of the soil, and the mass of the soil-root plate (Coutts, 1986). Both attributes—tensile strength ‘the maximum force per unit area required to cause a material to break’ and bending strength—are thus key traits in enabling plants to resist lodging. In addition bending strength or stiffness is associated with good root penetration of hard soils (Clark et al., 2008).
The maize root is comprised of the stele and cortex. The stele contains vascular bundles: the phloem and xylem. The cortex consists of a single layer of endodermis with casparian strips forming a barrier to the radial flow of water and nutrients with 8–15 layers of parenchymatous cortical cells. In this study the cortex is divided into three bands: the epidermis plus two or three layers beneath it forming the outer cortical region that can be thought of as the protective layer of the root; the middle 50% of the cortex or mesodermis; and the inner cortical region close to the endodermis.
Anatomical phenes have been associated with efficient soil resource acquisition under abiotic stresses; for example high root cortical aerenchyma (RCA), small living cortical area, reduced cortical cell file number, and large cortical cells (Fan et al., 2003; Zhu et al., 2010; Jaramillo et al., 2013; Lynch, 2013; Lynch 2014; Chimungu et al., 2014a,b ; Lynch et al., 2014, Chimungu et al., 2015). The effects of root anatomical phenes on penetration and root biomechanics are not well understood (Lynch and Wojciechowski 2015). Some phenes such as RCA can overcome the effects of drought by improving soil exploration (Zhu et al., 2010), but may also weaken the root structure (Engelaar et al., 1993; Striker et al., 2006). Reduced cortical cell file number (i.e. a thinner cortex) may reduce metabolic cost for soil exploration (Chimungu et al., 2014a ) but it may also entail significant costs in terms of reduced mechanical strength. On the other hand, tissue made of smaller cells might have a higher tissue density providing rigidity and strength, and thus more resistance to buckling or rupture. However, no attempt has been made to relate variation in root anatomical phenes to root penetration ability and biomechanical properties. In this study we examine if biomechanical properties and anatomical phenes affect root penetrability of hard layers. We hypothesize that stele anatomical phenes influence root tensile strength, assuming that the cortical tissue is weaker compared to the stele; cortical traits influence root bending strength and root penetration, with penetrability driven by bending stiffness. The thicker cortex roots would presumably be less prone to buckling and develop the greater axial pressure necessary to penetrate the harder soil.
Given the potential for root anatomical phenes in improving crop adaptation to abiotic stresses, and the inconclusive nature of existing information on the effects of anatomical phenes on root biomechanical properties and penetrability, the objective of this study was to investigate the effects of anatomical phenes on root penetration ability of hard layers and biomechanical properties. Variation in anatomical phenes, root penetration ability, tensile strength and Young’s modulus of roots were quantified in growth chamber, greenhouse and field-grown plants. To our knowledge, this is the first mechanistic study on the relationship between root anatomical phenes and root penetrability of hard layers linked to biomechanical properties of maize.
Materials and methods
Experiment 1: evaluation of root penetration ability
Twenty-six maize genotypes contrasting in root anatomical traits were used to assess root penetration ability in a temperature-controlled growth chamber (Environmental Growth Chambers, Model GC-36, Chagrin Falls, OH44022, US) using a thin wax layer system (Taylor and Gardner, 1960; Yu et al., 1995). Based on preliminary experiments, wax-petrolatum layers used in this study consisted of 60% wax (Royal Oak Sales, Inc, GA30076, US) and 40% petrolatum (Unilever, CT06611, US) by weight. The mixture was melted at 80°C, poured into molds and allowed to solidify at room temperature. The resulting wax-petrolatum disks were 125mm in diameter and 3mm thick (with equivalent strength of 1.7MPa at 27°C). Plants were grown in a randomized complete block design, with three replicates, in the temperature-controlled growth chamber. The mean minimum and maximum air temperatures during the experimental period were 25±3°C and 30±2°C, respectively with maximum illumination of 800 μmol photons m-2 s-1 and average relative humidity of 40%. Mesocosms were constructed of two stacked PVC pipes sealed together with duct tape, each 130mm long with an internal diameter of 10mm. The bottom pipe was capped, filled with 1279g of media (i.e. bulk density of 1.3g cm-3), and one wax-petrolatum layer placed on top with the top pipe placed onto the wax layer prior to filling with media. Growth medium consisted of (by volume) 50% commercial grade sand (Quikrete Companies Inc. Harrisburg, PA, USA), 25% vermiculite (Whittemore Companies Inc., Lawrence, MA, USA), and 25% topsoil (Hagerstown silt loam top soil, a fine, mixed, mesic Typic Hapludalf). Mineral nutrients were provided by mixing media with 10g per column of OSMOCOTE PLUS fertilizer (5–6 months release) (Scotts-Sierra Horticultural Products Company, Marysville, Ohio, USA) consisting of (%); NO3 (8) NH+ 4 (7), P (9), K (12), S (2.3), B (0.02) Cu (0.05), Fe (0.68), Mn (0.06), Mo (0.02), and Zn (0.05) for each column. Seeds were pre-germinated in a darkened germination chamber at 28±1°C, rolled in germination paper (Anchor Paper Company, St. Paul, MN, USA) and moistened with 0.5mM CaSO4 for 2 d. Two seedlings per mesocosm were transplanted, and thinned to one uniform seedling per mesocosm 3 d after planting. Planting was staggered by 1 d for each replicate. Top and bottom sections were hydraulically separated with the wax layer and moisture levels maintained by irrigating each layer separately. Plants were grown for 25 d and each replicate harvested in 1 d. Number of roots reaching the wax layer, numbers penetrating the wax layer, and total number of roots at the base of the stem were counted. Root penetrability was calculated as a ratio of the number of roots penetrating the wax-petrolatum layer to number of roots reaching the wax layer per plant; the ratio expressed as the root-penetration index (PR) (Materechera et al., 1992). Three penetrated roots were sampled and preserved in 75% ethanol for quantification of anatomical traits.
Experiment 2: evaluation of root tensile strength
Plants were grown in a greenhouse (February–March 2014) at University Park, PA, USA (40°4′N, 77°49′W), using 14/10h day/night: 23/20°C day/night: 40–70% relative humidity with natural light 500–1200 μmol photons m-2 s-1 PAR, and supplemental light 500–600 μmol photons m-2 s-1 PAR was provided with 400-W metal-halide bulbs (Energy Technics, York, PA, USA) for 14h/day. The mesocosms consisted of PVC cylinders 1.5 m in height by 0.15 m in diameter and lined with transparent hi-density polyethylene film to facilitate root sampling. The growth medium consisted of (by volume) 50% commercial grade sand (Quikrete Companies Inc. Harrisburg, PA, USA), 25% vermiculite and 25% topsoil [Hagerstown silt loam top soil (fine, mixed, mesic Typic Hapludalf)]. Mineral nutrients were provided by mixing the media with 70g per column of OSMOCOTE PLUS fertilizer (Scotts-Sierra Horticultural Products Company, Marysville, Ohio, USA) consisting of (%); N (15), P (9), K (12), S (2.3), B (0.02) Cu (0.05), Fe (0.68), Mn (0.06), Mo (0.02), and Zn (0.05) for each column. Seeds were germinated and planted as in Experiment 1 before thinning to one uniform seedling per mesocosm 5 d after planting.
Roots were sampled 40 d after planting with polyethylene liners extracted from mesocosms and laid on a root washing station. Root segments of 10cm were collected 0–20, 20–40, 40–60, 60–80, 80–100, 100–120, 120–140cm from the base of the primary, seminal, and first, second and third whorl crown roots (i.e. to assess the impact of root age on root tensile strength). Root segments were refrigerated at 4°C to preserve them for 24–30h until testing. Tensile measurements were carried out at the Mechanical Testing laboratory at The Pennsylvania State University using a universal testing machine (Instron, model 5866, Norwood, MA, USA). Prior to testing, all roots were inspected with damaged roots discarded. For tensile testing samples were clamped between two grips. Clamping is the most critical issue when measuring root strength. In our tests, the roots were clamped using wedges to avoid slippage and fine sandpaper was also attached to the clamps to increase friction. Each tested segment was 100mm in length allowing 50mm above and below to be clamped, minimizing potential slippage. Force was recorded during tensile testing with extension at a constant rate of 10mm min-1. Tensile load was measured using a 100 N load cell (Instron 2525–807 Series, Norwood, MA, USA) accurate to ±2.5 mN at maximum load. Root tensile strength was calculated as maximum tensile force, at ultimate failure, divided by root cross-sectional area for root tensile strength or by stele cross sectional area for stele tensile strength.
Experiment 3: evaluation of root bending properties
Root bending properties of six field grown maize genotypes was quantified. Plants were grown at the Russell E Larson Agricultural Research Center in Rock Springs, PA, USA (40°42′N, 77°57′W,), during the summer of 2013. The soil is classified as a Hagerstown silt loam (fine, mixed, mesic Typic Hapludalf) with bulk density of 1.6g cm-3. Genotypes were grown in a randomized complete block design with three replications of each genotype. Each plot consisted of three rows, with each row being 2.5 m long, with 25cm spacing between plants and 75cm between rows. Three plants (i.e. flowering stage) were randomly excavated for analysis 60 d after planting. Six to eight root segments 10cm in length were collected 4cm from the base of each plant for bend tests. Before the tests all lateral roots were removed using a razor. Segments were placed between moist germination paper (Anchor Paper Co., St. Paul, MN, USA) and refrigerated at 4°C for 24–30h to preserve them until measurement, minimizing degradation. Three-point bending tests were carried out at The Pennsylvania State University Mechanical Testing laboratory, using a universal testing frame (Instron, model 5866, Norwood, MA, USA). Before testing, roots were inspected and damaged roots removed from the study. Samples were placed between two supports (set apart approximately 15 times the diameter of the sample) and a pushing probe of radius 10mm lowered until contact with the sample. During the test the crosshead was lowered at a rate of 10mm min-1, with peak bending force recorded. The force applied to the root was continuously registered by a 100 N (±2.5) load cell (Instron 2525–807 Series, Norwood, MA, USA).
Root anatomical phene measurement
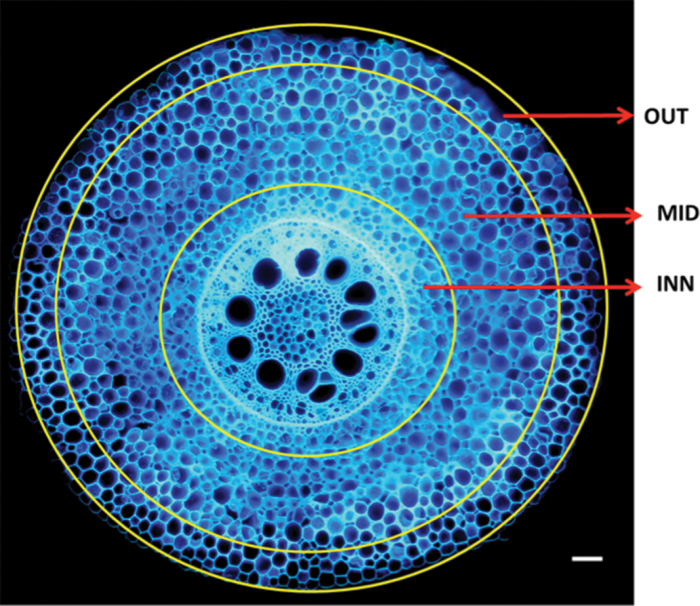
Root segments were ablated using laser ablation tomography (LAT) to obtain images for anatomical analysis. In brief, LAT is a semi-automated system that uses a laser beam (Avia 7000, 355nm pulsed laser) to vaporize or sublimate the root at the camera focal plane ahead of an imaging stage. The sample is incrementally extended into the beam, vaporized or sublimated, and imaged simultaneously. Imaging of root cross-sections was performed using a Canon T3i camera (Canon Inc. Tokyo, Japan) and 5× micro lens (MP-E 65mm) on the laser-illuminated surface. Image analysis was performed using RootScan software, an image analysis tool developed for analysing root anatomy (Burton et al., 2012). Some of the primary anatomical phenes measured or calculated are presented in Table 1. For cell size determination cortex was divided into three bands (Fig. 1).
Table 1.
Anatomical traits measured or derived using RootScan from laser ablated cross-section images
| Trait by tissue region | Abbreviation |
|---|---|
| Root cross-section | |
| Root diameter (mm2) | RD |
| Cortex | |
| Total cortical area (mm2) | TCA |
| Cortical thickness (mm) | CT |
| Cortical cell wall area (mm2) | CCWA |
| Cortical cell file number | CCFN |
| Cortical cell count | CCC |
| Median cell size, inner cortex (μm2) | INN |
| Median cell size, middle cortex (μm2) | MID |
| Median cell size, outer cortex (μm2) | OUT |
| Root cortical aerenchyma (%) | RCA |
| Stele | |
| Stele diameter (mm) | SD |
| Stele cell wall area (mm2) | SCWA |
Fig. 1.
Maize root cross-section showing three cortical bands; outer cortex (OUT), middle cortex (MID), and inner cortex (INN). Bar=100 µm.
Data analysis
The R statistical package (R Development Core Team, 2014) was used for data analysis. Correlation analysis was used to test for relationships between root penetrability and bending strength and root anatomical phenes. To describe correlation patterns among phenes, principal components analyses (PCA) was performed for seven traits, using original data. This PCA helps elucidate the relationships among many traits simultaneously and summarizes them on a single graph. All traits that were significantly correlated to root penetration or bending strength were used as independent variables in a multiple linear regression to identify their contribution to root penetrability and bending strength; phenes with close interrelationships, or derived from each other, were excluded. The anova command was used to compare multiple regression models. Stepwise multiple linear regression procedure was used to identify phenes that correlated with root penetrability and bending strength variation. Final models were selected by using Akaike information criterion (AIC). Power law regressions were carried out to determine the relationship between root tensile strength and root diameter and stele diameter. The analysis of covariance (ANCOVA) was used to evaluate the effect of age on root tensile strength as influenced by diameter.
Results
Experiment 1. Relationship of root penetration and anatomical phenes in maize
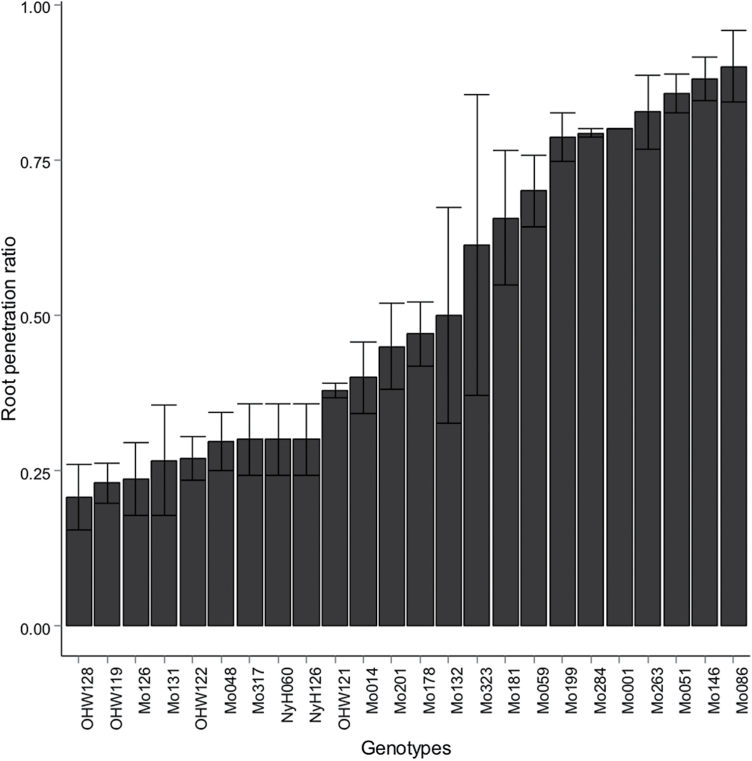
Root penetration (RP) indicates the relative ability of a plant’s root to penetrate a wax layer. An RP value of 1 signifies that all roots penetrated the wax layer, while an RP value of 0 signifies that none of the roots that reached the wax layer were able to penetrate it. Across the 24 genotypes, there was 3-fold variation for RP, ranging from a minimum of 0.22 to a maximum of 0.90 (Fig. 2).
Fig. 2.
Root penetration (RP) of 26 maize genotypes ranked in ascending order. RP is calculated as the ratio of number of roots reaching the wax layer to number of roots that penetrated the wax layer, with an RP of 1 representing that all roots reaching the wax layer penetrated the layer.
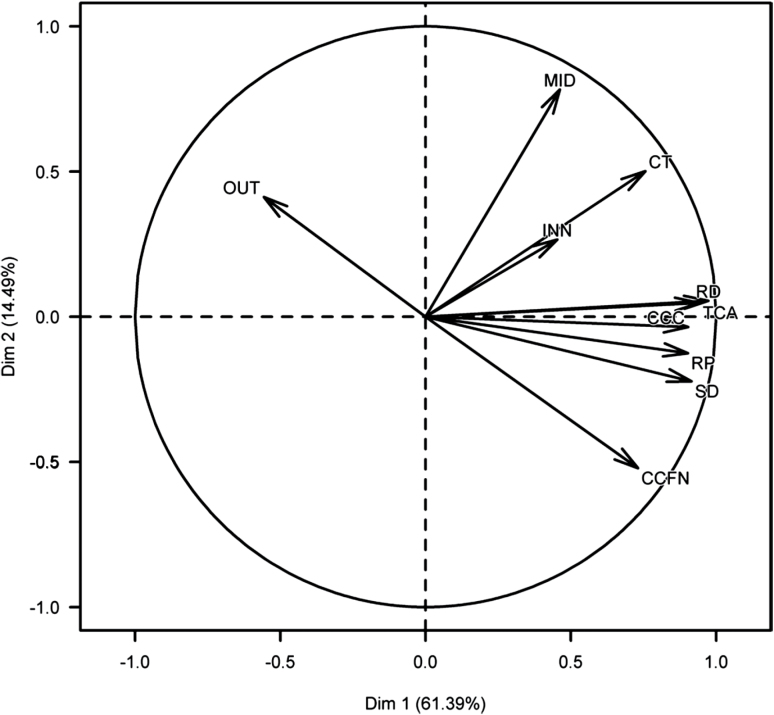
Multiple anatomical phenes were measured (Table 2) with substantial variation found in: total cortical area (TCA), cortex thickness (CT), cortical cell count (CCC), cortical cell file number (CCFN), cell size inner cortical region (INN), cell size mid-cortical region (MID), cell size in outer cortical region (OUT), and stele diameter (SD) (Table 2). Root diameter was correlated with root penetration (Supplementary Fig. S1). Several root phenes were strongly correlated with RD and RP (Fig. 3, Supplementary Table S1). Results of the PCA showed that the first axis explained 61.4% of the variation among the eight phenes and was mostly associated with TCA, CT, CCC, and SD (Fig. 3). The second axis explained 14.5% of the variation and was associated with CCFN, MID and OUT (Fig. 3). Specific Pearson’s correlations showed that both CT and SD were positively correlated with RD. Cortical phenes: TCA, CCC, CCFN, INN and MID were positively correlated with RD (Fig. 3, Supplementary Table S1), while DIS was negatively correlated with RD (r=−0.41, P<0.05) (Supplementary Table S1). The relationship between RD and RCA was not significant (Fig. 3, Supplementary Table S1). In addition, correlation analysis showed a significant and positive relationship between RP and SD, TCA, CCC, CCFN, CT, INN and MID (Fig. 3, Supplementary Table S1). Interestingly DIS was negatively correlated with RP (Supplementary Table S1). Among the anatomical phenes, some were negatively or positively correlated.
Table 2.
Summary statistics with median, minimum (Min.), maximum (Max.) and fold-variation for root diameter (RD) and root anatomical phenes: total cortical area (TCA), cortex thickness (CT), cortical cell count (CCC), cortical cell file number (CCFN), cortical cell size in the outer cortical region (OUT), middle (MID) and inner cortical region of the cortex (INN) (see Fig. 1 for description), and stele diameter (SD) for 26 maize genotypes
| Phene | Median | Min. | Max. | Fold-variation |
|---|---|---|---|---|
| RD | 1.45 | 1 | 2.2 | 1.2 |
| TCA | 1.2 | 0.5 | 2.8 | 4.6 |
| CT | 0.4 | 0.3 | 0.6 | 1.0 |
| CCC | 770.5 | 273 | 2149 | 6.9 |
| CCFN | 11 | 7 | 15 | 1.1 |
| INN | 249.2 | 169 | 383.5 | 1.3 |
| MID | 408.55 | 300.8 | 786.5 | 1.6 |
| OUT | 238.5 | 102.8 | 487 | 3.7 |
| SD | 0.7 | 0.4 | 1.1 | 1.8 |
Fig. 3.
Principal component analyses (PCA) on genotype means for eight anatomical phenes among 24 genotypes. The angle between two arrows represents the correlation of the respective variables. There is no linear dependence if the angle is 90 degrees. RP, root penetration, RD, root diameter; SD, stele diameter; CT, cortex thickness, TCA, total cortical area; CCC, cortical cell count, INN, inner cortical cell size, MID, middle cortical cell size, OUT, outer cortical cell size, CCFN, cortical cell file number.
Root anatomical phenes significantly correlated with RP, i.e. TCA, CT, SD, CCC, TCA, CCFN and DIS were included in multiple regression analysis as independent variables. Three multiple regression models were used to explain variation in RP (Supplementary Table S2). The first model elucidated relationships between RP and RD plus anatomical phenes (Model 1), the second model was for RP and anatomical phenes (Model 2) and the third model was for RP and RD only (Model 3, Supplementary Fig. S1). Analysis of variance shows that removing RD from Model 1 (i.e. Model 2) does not significantly affect the fit of the model (P=0.78). Model 2 was significantly different from Model 3 (P<0.001). In addition Model 2 was a better model than Model 3 with a slightly greater coefficient of determination (0.79 versus 0.66). Anatomical phenes were a much better predictor for RP than root diameter per se. Stepwise multiple linear regression was applied to Model 2 to determine the anatomical phenes accounting for the majority of variability in RP. The lowest AIC stepwise model for RP included CT, OUT and SD, explaining 78% of the variability in PR (Table 3).
Table 3.
Summary of multiple regression model (Model 2) of root penetration as predicted by cortex thickness (CT), cortical cell size of the outer cortical region (OUT) and stele diameter (SD); SE is the standard error of the coefficients, *P≤0.05; ***P≤0.001
| Coefficient | SE | ||
|---|---|---|---|
| (Intercept) | −0.2543 | 0.1196 | * |
| CT | 0.8779 | 0.2220 | *** |
| OUT | −0.0007 | 0.0002 | *** |
| SD | 1.1170 | 0.1595 | *** |
| R2 | 0.788 | ||
| Adjusted R2 | 0.776 |
Experiment 2. Relationship of root tensile strength and anatomical phenes in maize
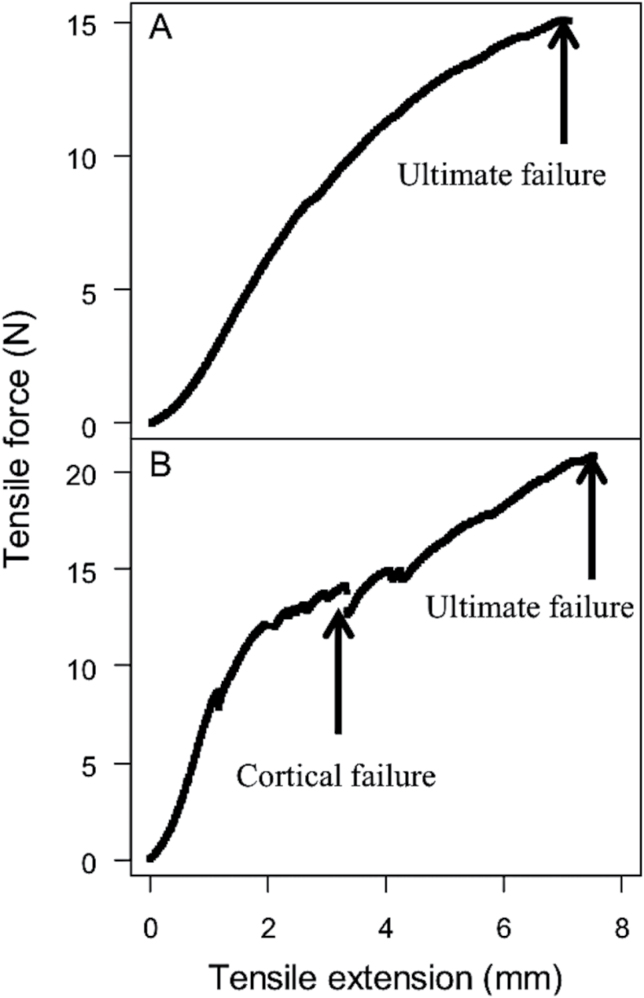
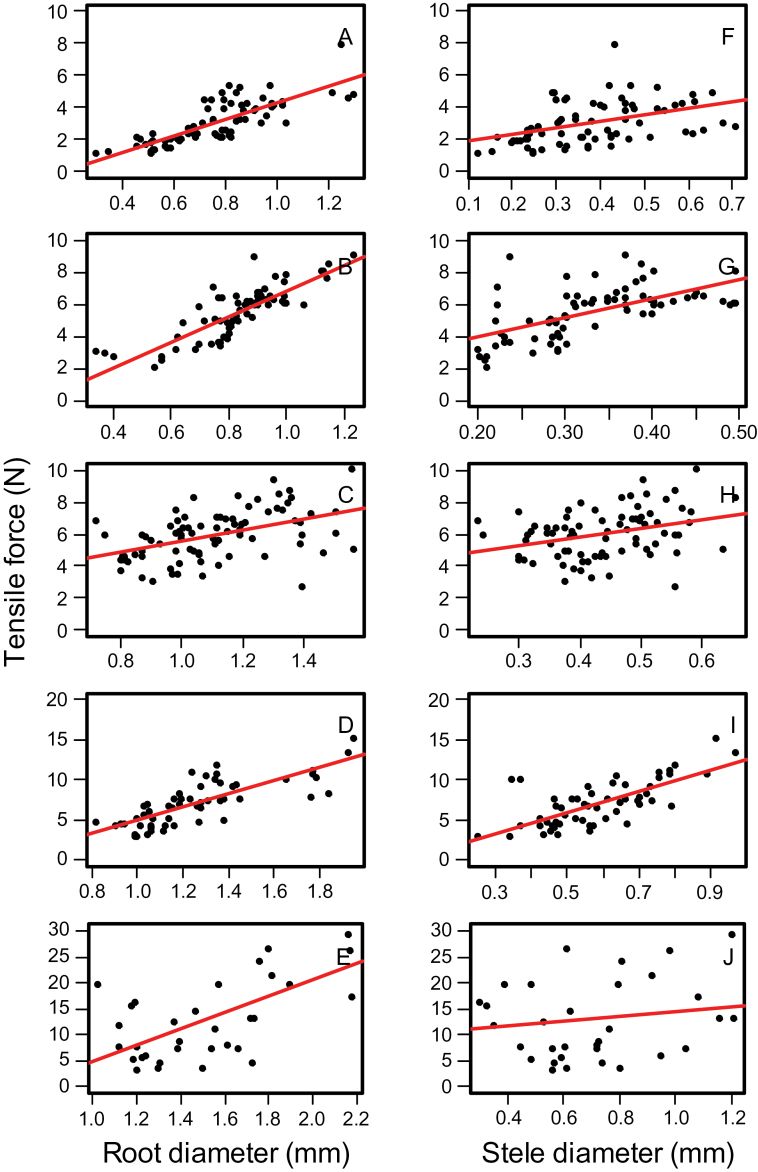
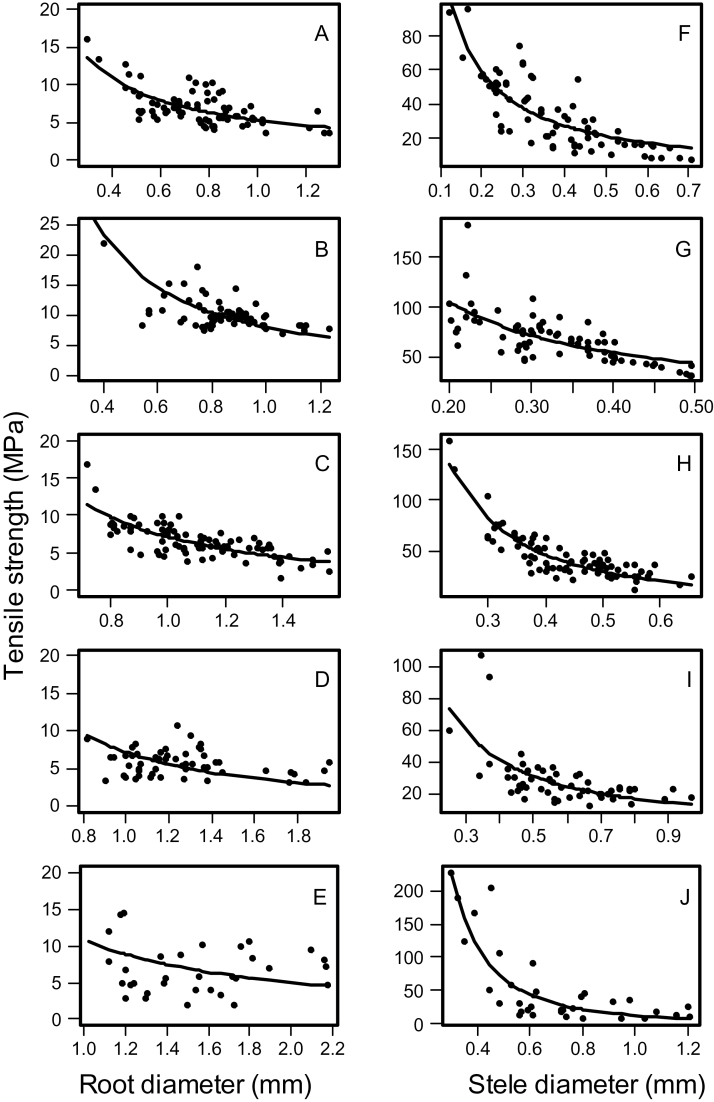
The root diameter of the tested samples ranged from 0.67 to 1.92mm (Supplementary Table S3). During tensile testing roots displayed typical elastic-plastic deformation with initially steep stress-strain curves in the elastic region before plastic deformation beyond the yield point. At the final stages of the test, the irregular sounds of the cortex snapping were heard in some samples prior to ultimate tensile failure. Two distinct peak values were observed in the force-displacement curves (Fig. 4). The first peak value is due to failure of the cortex with the second peak ultimate root failure (i.e. failure of the stele in tension) (Fig. 4). The results showed that the tensile breaking force (TBF) increased with increasing root and stele diameter (Fig. 5). Accordingly, tensile strength was calculated based on root cross-sectional area (root tensile strength) and stele cross-sectional area assuming that tensile force was concentrated within the stele only (stele tensile strength) with no load on the cortex. Relationships between the tensile strength and either root diameter or stele diameter were fitted with power-law relationships (Fig. 6). Table 4 presents the parameters and the coefficient of determination for the fitted models (tensile strength = ad b where d is diameter, a and b are regression coefficients). In general increasing stele or root diameter were both associated with decreased tensile strength (Fig. 6) following a power law equation for all root classes. Stele diameter was a stronger predictor of tensile strength than root diameter with a greater coefficient of determination (Fig. 6, Table 4).
Fig. 4.
Force-displacement curves (A) with a single peak representing root ultimate failure, and (B) with multiple peaks, the first peak value is due to failure of the cortex and the second peak is the ultimate root failure.
Fig. 5.
The relationship between tensile force (N) and root diameter (A–E) and stele diameter (F–J) for different root types: primary (A and F), seminal (B and G), first crown root (C and H), second crown root (D and I), and third crown root (E and J), 45 d after planting. Solid lines are fitted linear regression lines. See Table 5 for regression coefficients and significance levels.
Fig. 6.
The relationship between tensile strength and root diameter (A–E) and stele diameter (F–J) for different root types: primary (A and F), seminal (B and G), first crown root (C and H), second crown root (D and I), and third crown root (E and J), 45 d after planting. Solid lines are fitted curvilinear negative power regression lines. See Table 4 for regression coefficients and significance levels.
Table 4.
Parameters (a and b) and coefficient of determination (R 2) values for the power regression, expressing the decrease in root tensile strength and stele tensile strength with increasing root diameter and stele diameter for different root types in maize; n indicates number of samples
| Root tensile strength | ||||
|---|---|---|---|---|
| Root type | a | b | n | R 2 |
| Primary | 5.33 | −0.77 | 71 | 0.39 |
| Seminal | 8.02 | −1.16 | 70 | 0.52 |
| Nodal1 | 7.11 | −1.42 | 77 | 0.51 |
| Nodal2 | 6.01 | −0.29 | 54 | 0.06 |
| Nodal3 | 10.86 | −1.11 | 32 | 0.08 |
| Stele tensile strength | ||||
| Primary | 9.68 | −1.12 | 71 | 0.58 |
| Seminal | 22.47 | −0.95 | 70 | 0.59 |
| Nodal1 | 7.31 | −2.01 | 77 | 0.55 |
| Nodal2 | 12.97 | −1.26 | 54 | 0.42 |
| Nodal3 | 12.71 | −2.41 | 32 | 0.52 |
Root tensile strength decreased with increasing distance from the stem base (younger tissue) in all root types (Table 5). ANCOVA showed that tensile strength was significantly affected by age (i.e. distance from the stem base) with regard to root diameter in seminal, second and third order nodal roots, while the relationship was not significant in primary and first nodal roots (Table 5).
Table 5.
Summary of analysis of covariance models (F-value and degrees of freedom) of root tensile strength as influenced by age (distance from the stem base), root diameter and stele diameter; *P≤0.05; **P≤0.01; ***P≤0.001
| Tensile strength-Root diameter | |||||
|---|---|---|---|---|---|
| Primary | Seminal | Nodal1 | Nodal2 | Nodal3 | |
| Age | 1.1(1,72) | 9.1(1,69)** | 0.006(1,76) | 5.3(1,51)* | 6.6(1,29)* |
| Root diameter | 23.6(1,72)*** | 0.3(1,69) | 18.2(1,76)*** | 20.7(1,51)*** | 4.3(1,29)* |
| Age*Root diameter | 0.4(1,72) | 10.8(1,69)** | 1.8(1,76) | 1.2(1,51) | 3.1(1,29) |
| R 2 | 0.41 | 0.53 | 0.67 | 0.36 | 0.43 |
| Tensile strength-Stele diameter | |||||
| Age | 7.5(1,72)** | 8.1(1,69)** | 5.8(1,76)* | 1.5(1,51) | 26.6(1,29)*** |
| Root diameter | 43.1(1,72)*** | 43.5(1,69)*** | 31.2(1,76)*** | 24.1(1,51)*** | 24.3(1,29)*** |
| Age*Root diameter | 0.5(1,73) | 2.3(1,69) | 0.9(1,76) | 0.3(1,51) | 12.1(1,29)** |
| R 2 | 0.74 | 0.73 | 0.68 | 0.63 | 0.43 |
Experiment 3. Root bending strength is related to cortical anatomy
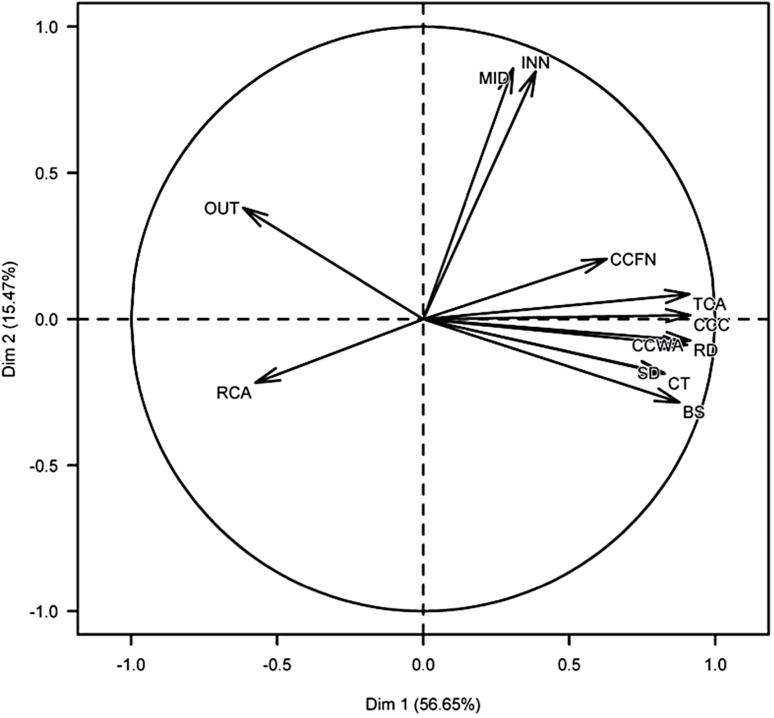
Root bending strength was positively correlated with RD, CCWA, CT, SD, TCA, CCC, and CCFN whereas it was negatively correlated with RCA and OUT (Supplementary Table S4, Supplementary Fig. S2). No significant correlations were found between bending strength and either INN or MID (Supplementary Table S4). PCA showed that the first axis explained 56.6% of the variation among the eight phenes and was mostly associated with TCA, CT, CCC, CCWA and SD (Fig. 7). The second axis explained 15.4% of the variation and was associated with INN and MID (Fig. 7).
Fig. 7.
Principal component analyses (PCA) for eight anatomical phenes. The angle between two arrows represents the correlation of the respective variables. There is no linear dependence if the angle is 90 degrees. BS, root bending strength, RD, root diameter; SD, stele diameter; CT, cortex thickness, TCA, total cortical area; RCA, root cortical aerenchyma, CCC, cortical cell count, INN, inner cortical cell size, MID, middle cortical cell size, OUT, outer cortical cell size, CCFN, cortical cell file number.
Root anatomical phenes significantly correlated with bending strength were included in multiple regression analysis to determine which were most important in predicting bending strength; with CCWA, CT, SD, CCC, TCA, CCFN, RCA and OUT as independent variables. Three multiple regression models were used to explain variation in bending strength (Supplementary Table S5). Model 1 assumes that bending strength can be accurately predicted by RD plus anatomical phenes: Model 1 accounted for 77% of the variation. Model 2 assumes that bending strength can be accurately predicted by root anatomical phenes alone: Model 2 accounted for 71% of the variation. Model 3 assumes that bending strength can be accurately predicted by RD alone: this model accounting for 47% of the variation (Supplementary Fig. S2). Results suggest that anatomical phenes were better predictors of bending strength than RD alone. The parsimonious model, based on Model 2, for root bending strength included CCC, OUT, CT and CCWA, explaining 86% bending strength variation (Table 6).
Table 6.
Summary of multiple regression model of root bending strength as predicted by cortical cell wall area (CCWA), cortical cell count (CCC), cortex thickness (CT), and cortical cell size in the outer cortical region (OUT); P≤0.05
| Coefficient | Std. Error | Pr(>|t|) | |
|---|---|---|---|
| (Intercept) | 0.88 | 19.40 | 0.964 |
| CCWA | 7.19 | 3.22 | 0.0324 * |
| CCC | 0.01 | 0.00 | 0.0106 * |
| CT | 53.88 | 20.63 | 0.0134 * |
| OUT | −0.30 | 0.12 | 0.0130 * |
| R2 | 0.86 | ||
| Adjusted R2 | 0.84 |
Discussion
Root diameter is considered an important trait that strongly influences root biomechanical properties and the ability of roots to penetrate hard soil. We demonstrated that root anatomical phenes are better predictors of RP, tensile strength and bending strength than root diameter per se. Root tensile strength decreases with increasing diameter according to a negative power law (Bischetti et al., 2005; Loades et al., 2013), thicker roots are associated with good penetration of strong soils by relieving stress at the growing root tip (Hettiaratchi, 1990; Kirby and Bengough, 2002). Additionally, thicker roots are more resistant to buckling and deflection when encountering hard soil domains (Whiteley et al., 1982; Clark et al., 2003; Jin et al., 2013). Clark et al. (2008) reported log-log positive relationships between root diameter and bending stiffness.
RP was evaluated using wax-petrolatum layers, which simulate strong soil. The use of a wax layer system proved to be a suitable method for detailed root penetration evaluation in maize. The advantage of the wax-layer system is that physical properties of wax are not affected by changes in moisture content that alter the strength of artificially compacted soil (Yu et al., 1995). This type of system has been successfully used in the identification of rice genotypes capable of penetrating strong soils (Yu et al., 1995; Clark et al., 2000, 2002; Zheng et al., 2000), and wheat (Acuña and Wade, 2005; Botwright Acuna et al., 2007), with results on rice confirmed in field trials (Samson et al., 2002).
A particularly interesting observation in this study was the strong negative relationship between RP and OUT. The presence of smaller cells in this region might play an important role in stabilizing the root against ovalization or compression and thereby reducing the risk of local buckling and collapse during penetration. Root resistance to compression is known to increase with the thickness of the multiseriate epidermal layer (Striker et al., 2007). We observed that the smaller cells in the outer cortical region have thick cell walls and are closely packed, which may make the root stronger and more resistant to compression deformation due to external forces.
Integrating the preceding concepts and taking rhizoeconomics and the distribution of soil resources into account, the ‘steep, cheap and deep’ ideotype has been useful for identifying phenes for improving resource acquisition under edaphic stress (Lynch, 2013; 2014). Many elements of this ideotype are also relevant for improving root penetration ability in hard soils, such as steep growth angles, which can improve root penetration by deploying roots near vertical incidences with compacted soil layers, thereby reducing the probability of buckling, and large diameter, which may also improve root penetration as discussed below (Lynch and Wojciechowski 2015). However, the structural investments and metabolic costs of root systems are substantial and can exceed half of daily photosynthesis (Lambers et al., 2002). Root anatomical phenes influence the metabolic costs of soil exploration (Zhu et al., 2010; Jaramillo et al., 2013; Lynch 2014; Lynch et al., 2014). These phenes include root cortical aerenchyma (RCA), living cortical area (LCA), cortical cell file number, and cortical cells. We suggest that an anatomical root ideotype for greater root penetration in hard soils should include (i) small outer cortical region cells to provide mechanical reinforcement for the root to resist bending or buckling when penetrating hard layers, (ii) large cortical cells in the mesodermis to reduce the metabolic cost of soil exploration (Lynch, 2013; Chimungu et al., 2014b ); (iii) thick axial roots with more aerenchyma to reduce root metabolic cost, in favour of root growth to penetrate hard soils. The presence of aerenchyma will not affect root penetration ability since RCA forms in mature root tissue behind the zone of active root elongation and root hair formation. It is important to note that studies have demonstrated that the penetration of roots through soils with a large amount of fine particles (silt and clay) and densely compacted soils is probably achieved through the possession of large root diameter, which resists buckling, while in coarse-textured sandy or well-structured soils thin roots would penetrate the soil more easily through gaps between soil aggregates and large pores (Scholefied and Hall, 1985; Pietola and Smucker, 1998; Allah et al., 2010).
The utility of phenes is affected by the external environment as well as the plant phenotype in which it is expressed. Knowledge of interactions among phenes is essential in developing an ideotype for optimizing root penetration. These phene interactions can be additive, synergistic or antagonistic (York et al., 2013). For example, root hairs have been linked to good root penetration of hard soils by providing anchorage to the growing root tip (Haling et al., 2013), and steep root angles are also associated with improved root penetration (Dexter and Hewitt, 1978; Whalley et al., 2012). We suggest synergisms or additive interactions between root phenes such as root growth angle, root hairs and anatomical phenes, because a combination of greater anchorage of the root tip, reduced axial stress, and resistance to bending or buckling would work together to improve root penetrability of hard soils.
Root tensile strength is another key factor in understanding and predicting plant anchorage and contributions to soil stabilization. Root strength decreases with increasing root diameter; one explanation may be cellulose content in small diameter roots (Genet et al., 2005), or it may be due to autocorrelation with root diameter as tensile stress is calculated using diameter (Hales et al., 2009). Abiotic stress has also been found to influence root biomechanical properties (Loades et al., 2013) and it is possible that soil physical heterogeneity may also account for differing biomechanical properties.
Karrenberg et al. (2003) argued that other root parameters may be better predictors of tensile strength than root diameter, especially in roots with a thick cortex. The potential implications of cortical failure during tensile testing reduces the area over which an applied force acts, so the actual tensile stress will be greater than that calculated based on root diameter. Indeed, calculated tensile stress based on stele area was generally greater than calculated stress based on root cross-sectional area, as would be expected due to radial diameter reduction. The strength of the stele may be attributable to greater cellulose content, which forms long chain polymers in the cell walls of root xylem tissue (Genet et al., 2005). Although lateral roots were removed from root samples prior to testing they can anchor the cortex to the stele. It is of interest to evaluate the effect of lateral root density on individual root tensile strength and this merits further research.
Root biomechanical properties change with root age (Genet et al., 2005; Loades et al., 2013). In this study we found that root tensile strength decreased with increasing distance from the stem base, showing an age effect (Table 5). Maize, like most monocots, lacks secondary root growth. The change in tensile strength with distance from the stem base is likely attributable to age or root development. These results are consistent with previous studies in other grasses, with strength decreasing with distance from the base of the stem (Ennos et al., 1993; Easson et al., 1995; Loades et al., 2013).
Bending tests revealed potentially interesting variations in terms of root stiffness. By comparing stress/strain curves beyond the yield point, curves were interrupted by one or more small steps indicating localized failures and probable fractures of the cortex. Previous studies showed that bending strength is associated with root diameter (Clark et al., 2008). In this study cortical traits were better predictors for root bending strength than root diameter per se. This is consistent with our observations that the cortex failed first during bending test (Supplementary Fig. S3). Since mechanical stresses are additive, the failure of the peripheral tissue (i.e. the cortex) is expected to decrease root stiffness, so the stele has a minor role in bending strength. The inclusion of DIS in the stepwise model for bending strength is consistent with previous studies that have shown that, in the mature maize root system, roots are strengthened near the base by a heavy lignified exodermis, which makes them rigid in bending (Ennos et al., 1993; Striker et al., 2007). These results provide further evidence that in order to fully understand root biomechanical properties it is necessary to consider the various functional phenes simultaneously and attempt to unravel causal relationships among them.
Root biomechanical properties are influenced by a number of different factors and also by the soil environment. Soil physical conditions such as mechanical impedance affect biomechanical properties of roots. Goodman and Ennos (1999) found that bending strength of maize roots changes with soil bulk density, with roots from stronger soil being less stiff than those from weak soil. In addition Pollen and Simon (2005) reported that change in soil moisture content, soil texture and nutrient status, can affect root tensile strength. Considering those effects, caution must be taken when interpreting such relationships like those reported in this study without taking into consideration the growth conditions. In this study plants were grown in the field and in the greenhouse under high light intensity using uniformly packed mesocosms. Topsoil was added to the growth medium in the greenhouse to mimic soil conditions in the field. We therefore propose that our experimental conditions are not likely to be artifactual.
Conclusion
Root diameter is a function of both stele diameter and cortical thickness. We found that cortical thickness is important for bending, while stele diameter is important for tensile strength. However, many other anatomical phenes were stronger predictors of root penetration and biomechanical properties than root diameter. More work is required to fully understand the effect of root anatomical phenes on root penetrability in field. Researchers of these properties should consider root anatomy. Because anatomical phenes are more elemental than aggregate traits like root diameter they are more likely to be under simpler genetic control, and may be more fruitful selection criteria in crop breeding programmes.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Fig. S1. Correlation between root diameter (mm) and penetration for roots of maize genotypes grown in temperature-controlled growth chamber.
Supplementary Fig. S2. Correlation between root diameter (mm) and bending strength (Nm) for roots of maize genotypes grown in field
Supplementary Fig. S3. Cross-section of root segment following tensilometry.
Supplementary Table S1. Correlation coefficients for root penetration and anatomical traits of 24 maize genotypes
Supplementary Table S2. Summary of a multiple regression models of root penetration ability as predicted by root anatomical phenes and diameter.
Supplementary Table S3. Root diameter with distance from the stem base for different root types: primary, seminal, first crown root (Nodal1), second crown root (Nodal2) and third crown root (Nodal3).
Supplementary Table S4. Correlation coefficients between root bending strength and anatomical phenes.
Supplementary Table S5. Summary of a multiple regression models of root bending strength as predicted by root anatomical phenes and diameter.
Acknowledgements
We thank Larry York for critical reading of the manuscript, David Shelleman for technical support for root biomechanical properties and Bob Snyder for root penetration studies. This research was supported by the National Science Foundation/Basic Research to Enhance Agricultural Development (grant number: 4184–UM–NSF–5380).
References
- Acuña TLB, Wade LJ. 2005. Root penetration ability of wheat through thin wax-layers under drought and well-watered conditions. Australian Journal of Agricultural Research 56, 1235. [Google Scholar]
- Allah AAA, Shimaa A, Zayed BA, Gohary AA El, Badawy SA. 2010. The role of root system traits in the drought tolerance of rice (Oryza sativa L.). International Journal of Agricultural and Biological Sciences 44, 83–87. [Google Scholar]
- Barraclough PB, Weir AH. 1988. Effects of a compacted subsoil layer on root and shoot growth, water use and nutrient uptake of winter wheat. Journal of Agricultural Science 110, 207–216. [Google Scholar]
- Bengough G, McKenzie BM, Hallett PD, Valentine TA. 2011. Root elongation, water stress, and mechanical impedance: a review of limiting stresses and beneficial root tip traits. Journal of Experimental Botany 62, 59–68. [DOI] [PubMed] [Google Scholar]
- Bischetti GB, Chiaradia EA, Simonato T, Speziali B, Vitali B, Vullo P, Zocco A. 2005. Root strength and root area ratio of forest species in Lombardy (Northern Italy). Plant and Soil 278, 11–22. [Google Scholar]
- Botwright Acuna TL, Pasuquin E, Wade LJ. 2007. Genotypic differences in root penetration ability of wheat through thin wax layers in contrasting water regimes and in the field. Plant and Soil 301, 135– 149. [Google Scholar]
- Brady N, Weil RR. 2008. The Nature and Properties of Soils . New Jersey: Prentice Hall. [Google Scholar]
- Burton AL, Williams M, Lynch JP, Brown KM. 2012. RootScan: Software for high-throughput analysis of root anatomical traits. Plant and Soil 357, 189–203. [Google Scholar]
- Bushamuka VN, Zobel RW. 1998. Differential genotypic and root type penetration of compacted soil layers. Crop Science 38, 776–781. [Google Scholar]
- Cairns JE, Audebert A, Townend J, Price AH, Mullins CE. 2004. Effect of soil mechanical impedance on root growth of two rice varieties under field drought stress. Plant and Soil 267, 309–318. [Google Scholar]
- Chimungu JG, Brown KM, Lynch JP. 2014. a Reduced root cortical cell file number improves drought tolerance in maize. Plant Physiology 166: 1943–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimungu JG, Brown KM, Lynch JP. 2014. b . Large root cortical cell size improves drought tolerance in maize (Zea mays L.). Plant Physiology 166:2166–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimungu JG, Maliro MFA, Nalivata PC, Kanyama-Phiri G, Brown KM, Lynch JP. 2015. Utility of root cortical aerenchyma under water limited conditions in tropical maize (Zea mays L.). Field Crops Research http://dx.doi.org/10.1016/j.fcr.2014.10.009 [Google Scholar]
- Clark L, Cope R, Whalley W. 2002. Root penetration of strong soil in rainfed lowland rice: comparison of laboratory screens with field performance. Field Crops Research 76, 189–198. [Google Scholar]
- Clark L, Price A, Steele K, Whalley W. 2008. Evidence from near-isogenic lines that root penetration increases with root diameter and bending stiffness in rice. Functional Plant Biology 35, 1163–1171. [DOI] [PubMed] [Google Scholar]
- Clark LJ, Aphale SL, Barraclough PB. 2000. Screening the ability of rice roots to overcome the mechanical impedance of wax layers : importance of test conditions and measurement criteria. Plant and Soil 219, 187–196. [Google Scholar]
- Clark LJ, Whalley WR, Barraclough PB. 2003. How do roots penetrate strong soil? Plant and Soil 255, 93–104. [Google Scholar]
- Coutts M. 1986. Components of tree stability in sitka spruce on peaty gley soil. Forestry 59, 173–197. [Google Scholar]
- Dexter A, Hewitt J. 1978. The deflection of plant roots. Journal of Agricultural Engineering Research 23, 17–22. [Google Scholar]
- Easson D, Pickles S, White E. 1995. A study of the tensile force required to pull wheat roots from soil. Annals of Applied Biology 127, 363–374. [Google Scholar]
- Engelaar W, Jacobs M, Blom C. 1993. Root growth of Rumex and Plantago species in compacted and waterlogged soils. Acta Botanica Neerlandica 45, 25–35. [Google Scholar]
- Ennos AR, Crook MJ, Grimshaw C. 1993. The anchorage mechanics of maize, Zea mays . Journal of Experimental Botany 44, 147–153. [Google Scholar]
- Fan M, Zhu J, Richards C, Brown KM, Lynch JP. 2003. Physiological roles for aerenchyma in phosphorus-stressed roots. Functional Plant Biology 30, 493–506. [DOI] [PubMed] [Google Scholar]
- Genet M, Stokes A, Salin F, Mickovski S. 2005. The influence of cellulose content on tensile strength in tree roots. Plant and Soil 278, 3–11. [Google Scholar]
- Goodman A, Ennos A. 1998. Responses of the root systems of sunflower and maize to unidirectional stem flexure. Annals of Botany 82, 347–357. [Google Scholar]
- Goodman A, Ennos A. 1999. The effects of soil bulk density on the morphology and anchorage mechanics of the root systems of sunflower and maize. Annals of Botany 83, 293–302. [Google Scholar]
- Hales TC, Ford CR, Hwang T, Vose JM, Band LE. 2009. Topographic and ecologic controls on root reinforcement. Journal of Geophysical Research 114, F03013. [Google Scholar]
- Haling RE, Brown LK, Bengough AG, Young IM, Hallett PD, White PJ, George TS. 2013. Root hairs improve root penetration, root-soil contact, and phosphorus acquisition in soils of different strength. Journal of Experimental Botany 64, 3711–3721. [DOI] [PubMed] [Google Scholar]
- Hettiaratchi D. 1990. Soil compaction and plant root growth. Philosophical Transactions of the Royal Society of London , B329, 343–355. [Google Scholar]
- Iijima M, Kato J. 2007. Combined soil physical stress of soil drying, anaerobiosis and mechanical impedance to seedling root growth of four crop species. Plant Production Science 10, 451–459. [Google Scholar]
- Jaramillo RE, Nord EA, Chimungu JG, Brown KM, Lynch JP. 2013. Root cortical burden influences drought tolerance in maize. Annals of Botany 112, 429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Shen J, Ashton RW, Dodd IC, Parry MaJ, Whalley WR. 2013. How do roots elongate in a structured soil? Journal of Experimental Botany 64, 4761–4777. [DOI] [PubMed] [Google Scholar]
- Karrenberg S, Blaser S, Kollmann J, Speck T, Edwards PJ. 2003. Root anchorage of saplings and cuttings of woody pioneer species in a riparian environment. Functional Ecology 17, 170–177. [Google Scholar]
- Kirby J, Bengough A. 2002. Influence of soil strength on root growth: experiments and analysis using a critical‐state model. European Journal of Soil Science 53, 119–128. [Google Scholar]
- Klueva N, Joshi R, Joshi C, Wester D, Zartman R, Cantrell R, Nguyen H. 2000. Genetic variability and molecular responses of root penetration in cotton. Plant Science 155, 41–47. [DOI] [PubMed] [Google Scholar]
- Kubo K, Iwama K, Yanagisawa A. 2006. Genotypic variation of the ability of root to penetrate hard soil layers among Japanese wheat cultivars. Plant Production Science 9, 47–55. [Google Scholar]
- Kubo K, Uchino H, Jitsuyama Y, Iwama K. 2008. Relationship between deep root distribution and root penetration capacity estimated by pot experiments with a paraffin and vaseline layer for landraces and recent. Plant Production Science 11, 487–497. [Google Scholar]
- Lambers H, Atkin OK, Millenaar FF. 2002. Respiratory patterns in roots in relation to their functioning. In: Waisel Y, Eshel A, Kafkaki K, eds. Plant Roots: The Hidden Half . New York: Marcel Dekker, Inc, 521–552. [Google Scholar]
- Loades K, Bengough A, Bransby M, Hallett P. 2013. Biomechanics of nodal, seminal and lateral roots of barley: effects of diameter, waterlogging and mechanical impedance. Plant and Soil 370, 407–418. [Google Scholar]
- Lynch JP. 2011. Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiology 156, 1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. 2013. Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Annals of Botany 112, 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. 2014. Root phenes that reduce the metabolic costs of soil exploration: opportunities for 21st century agriculture. Plant, Cell and Environment doi 10.1111/pce.12451 [DOI] [PubMed] [Google Scholar]
- Lynch JP, Wojciechowski T. 2015. Opportunities and challenges in the subsoil: pathways to deeper rooted crops. Journal of Experimental Botany 66, 2199–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP, Chimungu JG, Brown KM. 2014. Root anatomical phenes associated with water acquisition from drying soil: targets for crop improvement. Journal of Experimental Botany 65, 6155–6166. [DOI] [PubMed] [Google Scholar]
- Materechera S, Alston A, Kirby J, Dexter A. 1992. Influence of root diameter on the penetration of seminal roots into a compacted subsoil. Plant and Soil 144, 297–303. [Google Scholar]
- May OL, Kasperbauer MJ. 1999. Genotypic variation for root penetration of a soil pan. Journal of Sustainable Agriculture 13, 87–94. [Google Scholar]
- Oladokun MAO, Ennos AR. 2006. Structural development and stability of rice Oryza sativa L. var. nerica 1. Journal of Experimental Botany 57, 3123–3130. [DOI] [PubMed] [Google Scholar]
- Pietola L, Smucker AJM. 1998. Fibrous carrot root responses to irrigation and compaction of sandy and organic soils. Plant and Soil 200, 95–105. [Google Scholar]
- Pollen N, Simon A. 2005. Estimating the mechanical effects of riparian vegetation on stream bank stability using a fiber bundle model. Water Resources Research 41, W07025. doi: 10.1029/2004WR003801. [Google Scholar]
- Price AH, Steele KA, Moore BJ, Barraclough PP, Clark LJ. 2000. A combined RFLP and AFLP linkage map of upland rice (Oryza sativa L.) used to identify QTLs for root-penetration ability. Theoretical and Applied Genetics 100, 49–56. [Google Scholar]
- R Development Core Team. 2014. R: A language and environment for statistical computing R Foundation for Statistical Computing [WWW Document]. R Found. Stat. Comput., R Foundation for Statistical Computing. URL http://www.r-project.org
- Samson BK, Hasan M, Wade LJ. 2002. Penetration of hardpans by rice lines in the rainfed lowlands. Field Crops Research 76, 175–188. [Google Scholar]
- Scholefied D, Hall D. 1985. Constricted growth of grass roots through rigid pores. Plant and Soil 85, 153–162. [Google Scholar]
- Striker GG, Insausti P, Grimoldi AA, Leon RJC. 2006. Root strength and trampling tolerance in the grass Paspalum dilatatum and the dicot Lotus glaber in flooded soil. Functional Ecology 20, 4–10. [Google Scholar]
- Striker GG, Insausti P, Grimoldi AA, Vega AS. 2007. Trade-off between root porosity and mechanical strength in species with different types of aerenchyma. Plant, Cell and Environment 30, 580–589. [DOI] [PubMed] [Google Scholar]
- Taylor HM, Gardner HR. 1960. Use of wax substrates in root penetration studies. Soil Science Society of America Proceedings 24, 79–81. [Google Scholar]
- Whalley WR, Dodd IC, Watts CW, Webster CP, Phillips AL, Andralojc J, White RP, Davies WJ, Parry MAJ. 2012. Genotypic variation in the ability of wheat roots to penetrate wax layers. Plant and Soil 364, 171–179. [Google Scholar]
- Whiteley G, Hewitt J, Dexter A. 1982. The buckling of plant roots. Physiologia Plantarum 54, 333–342. [Google Scholar]
- Whitmore AP, Whalley WR. 2009. Physical effects of soil drying on roots and crop growth. Journal of Experimental Botany 60, 2845–2857. [DOI] [PubMed] [Google Scholar]
- York LM, Nord EA, Lynch JP. 2013. Integration of root phenes for soil resource acquisition. Frontiers in Plant Science 4, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu LX, Ray JD, O’Toole JC, Nguyen HT. 1995. Use of wax-petrolatum layers for screening rice root penetration. Crop Science 35, 684–687. [Google Scholar]
- Zheng HG, Babu RC, Pathan MS, Ali L, Huang N, Courtois B, Nguyen T. 2000. Quantitative trait loci for root-penetration ability and root thickness in rice: comparison of genetic backgrounds. Genome 43, 53–61. [PubMed] [Google Scholar]
- Zhu J, Brown KM, Lynch JP. 2010. Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant, Cell and Environment 33, 740–749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.