Abstract
Significance: Diabetes is an important risk factor for the development of heart failure (HF). Given the increasing prevalence of diabetes in the population, strategies are needed to reduce the burden of HF in these patients. Recent Advances: Diabetes is associated with several pathologic findings in the heart including dysregulated metabolism, lipid accumulation, oxidative stress, and inflammation. Emerging evidence suggests that mitochondrial dysfunction may be a central mediator of these pathologic responses. The development of therapeutic approaches targeting mitochondrial biology holds promise for the management of HF in diabetic patients. Critical Issues: Despite significant data implicating mitochondrial pathology in diabetic cardiomyopathy, the optimal pharmacologic approach to improve mitochondrial function remains undefined. Future Directions: Detailed mechanistic studies coupled with more robust clinical phenotyping will be necessary to develop novel approaches to improve cardiac function in diabetes. Moreover, understanding the interplay between diabetes and other cardiac stressors (hypertension, ischemia, and valvular disease) will be of the utmost importance for clinical translation of scientific discoveries made in this field. Antioxid. Redox Signal. 22, 1515–1526.
Introduction
The prevalence of diabetes continues to increase in the Western world. Cardiovascular disease remains the leading cause of morbidity and mortality in patients with this metabolic condition. In addition to its effects in promoting atherosclerosis, there is evidence that diabetes can directly affect the myocardium, a condition frequently referred to as “diabetic cardiomyopathy” (diabetic CM) (86). Despite the clear association between heart failure (HF) and diabetes, specific diagnostic criteria for diabetic CM do not exist.
The most common clinical features associated with diabetic CM are left ventricular hypertrophy (LVH) and diastolic dysfunction; however, these findings are commonly seen in many forms of HF (22, 24, 25, 40, 83). Although early diastolic dysfunction is reversible with improvements in systemic metabolism, continued metabolic stress on the heart can lead to symptomatic HF, most commonly HF with preserved ejection fraction (HFpEF). This is particularly true in patients with other associated conditions such as hypertension (HTN), ischemic heart disease, or aortic stenosis where the presence of diabetes accentuates the cardiac hypertrophic response and worsens LV function (3, 10, 41, 44, 60). Diabetes is also extremely common in patients who have HF with reduced EF (HFrEF), with a prevalence approaching 40% in many HF registries and clinical trials. It is unclear whether the development of systolic dysfunction can occur solely as a consequence of diabetes or whether additional cardiac insults are necessary (Fig. 1). Irrespective of this, the presence of diabetes portends a worse prognosis in those with HFrEF (37). Currently, there are limited data regarding the optimal treatment strategy to prevent diabetic cardiac disease or to manage diabetes in patients with established systolic or diastolic cardiac dysfunction. Of interest, intensive blood glucose control does not reduce the incidence of HF in diabetic patients (28, 77).
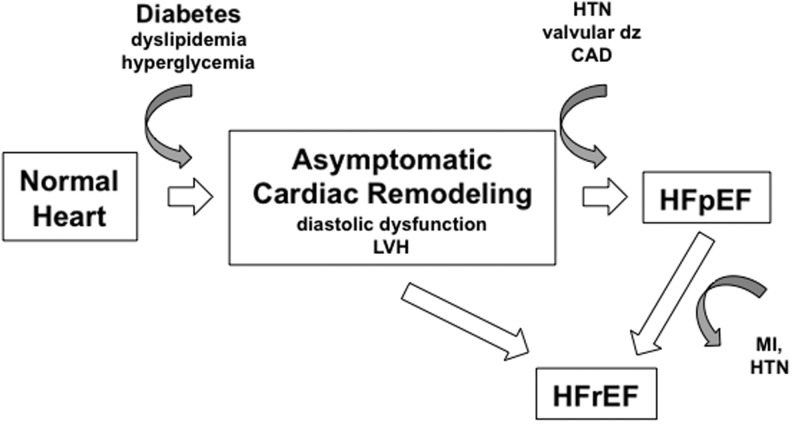
FIG. 1.
Model of diabetic heart failure (HF) progression. The diabetic metabolic environment promotes adverse cardiac remodeling, including the development of left ventricular hypertrophy (LVH) and diastolic dysfunction. This stage is often asymptomatic. Over time and often in the face of other co-morbidities, heart failure with either preserved ejection fraction (HFpEF) or reduced ejection fraction (HFrEF) can occur.
It remains controversial whether diabetes is sufficient to produce HF or rather acts to sensitize the myocardium to other insults (i.e., HTN, ischemia, valve disease). This issue notwithstanding, there is clear evidence that diabetes impacts cardiac metabolism and mitochondrial function. Moreover, many of the pathologic hallmarks of diabetic CM, including lipid accumulation, oxidative stress, inflammation, cell death, and impaired energetics, are associated with abnormal mitochondria. In this article, the current evidence implicating mitochondrial dysfunction in diabetic CM will be reviewed with an emphasis on potential approaches to modulate mitochondrial biology for therapeutic benefit.
Cardiac Mitochondria in Diabetes
When considering the impact of diabetes on cardiac mitochondrial function, it is useful to distinguish early adaptive alternations from the maladaptive responses that define the later stages of the disease. Under normal circumstances, the heart is a metabolic omnivore and is capable of using diverse substrates to supply its energetic needs, including glucose, fatty acids, ketone bodies, and lactate. In nondiabetic conditions, the heart generates ∼70% of its ATP from the oxidation of fatty acids (FA) with most of the remainder coming from glucose metabolism. In contrast, diabetes is a state of nutrient excess in which the levels of circulating glucose and FAs are increased (19, 53). A consequence of excessive FA delivery and the resultant insulin resistance is a shift in cardiac metabolism further toward the use of FAs. In part, this metabolic shift is orchestrated by increased expression of the nuclear receptor transcription factor PPARα and its coactivators PGC-1α/β (30, 36). PPARα-regulated genes include those involved in FA uptake (CD36, FATP1), β-oxidation (MCAD, LCAD, VLCAD), and triglyceride (TAG) synthesis (DGAT) and, therefore, this metabolic program initially helps buffer the excess lipids. In addition, animal models have shown that in the diabetic heart, PGC-1α and β activate a mitochondrial biogenic program that expands the cardiac mitochondrial pool (30, 69). Similar data exist in human subjects where it has been shown using PET metabolic imaging that diabetics have increased rates of fatty acid oxidation (FAO) and myocardial oxygen consumption compared with nondiabetic controls (45, 78).
Although the upregulation of lipid metabolic pathways in the setting of nutrient excess may initially be adaptive, sustained activation of this metabolic program can be detrimental. Consistent with this notion, mice engineered to overexpress PPARα in cardiac myocytes develop contractile dysfunction over time that is exacerbated by a high-fat diet (36). There are several potential explanations as to why sustained activation of lipid metabolic pathways in cardiac myocytes can lead to a decline in cardiac function, and these will be discussed in the ensuing sections.
To understand the mechanisms by which increased lipid flux can negatively impact cardiomyocyte function, it is first necessary to review the basics of FA metabolism. On entry into a cardiomyocyte, FAs are esterified by acetyl-CoA synthetase enzymes to generate FA-CoA molecules. The FA-CoAs can be converted to TAG for storage in neutral lipid droplets (LDs), used for de novo phospholipid or sphingolipid biosynthesis, or transported to the mitochondria for oxidation. The FA-CoAs that are delivered to the mitochondria are converted to acylcarnitines by carnitine palmitolyl transferase 1 (CPT1), a process that facilitates entry of the FAs into the mitochondrial matrix. The FA-carnitines are subsequently converted back to FA-CoAs by CPT2 present on the inner mitochondrial membrane. These intramitochondrial FA-CoAs undergo β-oxidation, generating acetyl-CoA for entry into the tricarboxcylic acid (TCA) cycle.
Along with chronic increases in the delivery of FA substrates to the mitochondria for β-oxidation, several pathologic events can occur. For one, excess flux through the electron transport chain (ETC) can increase mitochondrial membrane potential (MMP), especially when β-oxidation outpaces the energetic needs of the cell and ADP levels are reduced. Increased MMP along with accumulation of NADH and TCA intermediates can have a negative impact on TCA cycle flux (55). There is also evidence that over time TCA cycles intermediates can also be depleted in diabetes (51). In either situation, the process of β-oxidation can exceed the capacity of the downstream oxidative pathways, thereby uncoupling FAO from mitochondrial oxidative phosphorylation (OX-PHOS). A consequence of this imbalance is the accumulation of FAO intermediates, including FA-CoAs and FA-carnitines (51, 72). Acetyl-CoA levels also increase, which inhibits the pyruvate dehydrogenase complex and limits oxidative glucose metabolism (8). In addition, redox metabolites such as NADH can accumulate, leading to reductive stress (47). The backlog of these metabolites can be toxic to the cell and may contribute to cardiomyocyte death and dysfunction. Consistent with the concept of imbalance between β-oxidation and mitochondrial respiration, FAO intermediates, such as acylcarnitines, are elevated in animal models and humans with diabetes (11, 73, 91).
As the duration of diabetes increases, mitochondrial oxidative capacity begins to decline (15). The resultant imbalance between lipid uptake and oxidation further worsens the accumulation of FA-CoAs in the cell. The backlogged FA-CoA molecules are diverted toward nonoxidative fates in the cell such as diacylglycerol (DAG) and TAG synthesis and the production of sphingolipids such as ceramides. In line with this pathology, intracellular accumulation of LDs and ceramides are hallmarks of the diabetic heart (76, 96). Current evidence indicates that the storage of FAs in the form of TAG is cardioprotective in the setting of lipid overload (62–64). This concept is also supported by studies of perilipin 5, a LD protein that regulates the breakdown of TAGs into FFAs. Loss of perilipin 5 in cardiomyocytes leads to uncontrolled lipolysis, mitochondrial FA overload, and cardiomyocyte dysfunction; whereas overexpression prevents LD lipolysis, increases intracellular TAG, and protects myocytes from contractile dysfunction (52, 98). In concert, these results argue that LDs may be beneficial by reducing the generation of toxic lipid species and preventing excessive mitochondrial FA flux (71, 76). Another potential benefit of LD storage is that regulated, low-level lipolysis could provide a source of FFA for occasions when other energetic substrates are limiting.
A sustained increase in mitochondrial FA flux also produces pathology through the generation of excessive reactive oxygen species (ROS), although the mechanisms of this response remain controversial. When FAs are present in excess of energetic demand (i.e., a low ADP environment), the reducing equivalents generated by lipid oxidation are more likely to produce free radical oxygen due to reduced forward flux of electrons through the ETC. In addition, the first step of β-oxidation involves the transfer of electrons directly to the ETC flavoprotein via acyl-CoA dehydrogenases, which can also exacerbate downstream superoxide production (79). Conversely, FAO can be protective against oxidant stress because the oxidation of lipids, in comparison to carbohydrates, generates more reducing equivalents. These reducing equivalents act to replenish mitochondrial ROS-scavenging molecules, including reduced glutathione and mitochondrial thioredoxin (Trx2) (95). How does this balance become disrupted to produce a net increase in oxidative stress during diabetes? One possible mechanism is related to the ability of LCFA and their partially oxidized derivatives to directly impair the function of the ETC (1). As such, the reducing equivalents generated by the oxidation of glucose and FA are even more likely to generate superoxide, which could overwhelm the antioxidant defenses. Moreover, diabetes impairs the function of superoxide dismutase enzymes along with glutathione and Trx2, further amplifying the ROS burden (93, 107).
Irrespective of the mechanism, there is strong evidence demonstrating that increased oxidative stress is a common feature observed in cardiac tissue from humans or animals with diabetic CM (38, 104). Recent data obtained with mitochondria isolated from atrial cardiomyocytes of diabetic patients also demonstrated respiratory defects and amplified ROS generation during OX-PHOS compared with nondiabetic control mitochondria (5, 70). Interestingly, while mitochondria isolated from obese patients also have defects in respiration, only diabetic mitochondria produce higher levels of ROS (70). These findings suggest that as metabolic disease progresses, mitochondrial-derived oxidative stress increases (4, 95, 99). Adding insult to injury, the ability of cardiomyocytes to clear dysfunctional, ROS-producing mitochondria through mitophagy may also be impaired (49). Mitophagy is a specialized form of autophagy that acts to remove damaged mitochondria that are particularly prone to ROS generation. There is conflicting evidence about the impact of metabolic stress on cardiomyocyte mitophagy, but it is enticing to speculate that dysregulation of this clearance mechanism may further exacerbate oxidative stress. This topic is discussed in greater detail in the article by Kubli and Gustafsson in this Forum. Regardless of the source, excess ROS can damage proteins, nucleic acids, and lipids, leading to cardiomyocyte dysfunction and death.
In response to oxidative stress or PPARα activation, mitochondria upregulate protein expression of uncoupling proteins (UCPs), in particular UCP2 and UCP3 (74). UCPs are also induced in the diabetic heart, presumably as an adaptive mechanism to reduce mitochondrial ROS production, although this is controversial (14). Mechanistically, UCPs uncouple proton leak across the mitochondrial membrane from ATP synthesis, thereby dissipating the MMP. In this way, the generation of mitochondrial superoxide through complex I of the ETC is decreased. However, there is an energetic cost to mitochondrial uncoupling. Dissipation of the proton motive force across the mitochondrial membrane leads to diminished capacity for ATP production, which reduces mitochondrial efficiency. Therefore, sustained activation of mitochondrial uncoupling may adversely affect cardiac energetics and myocyte contractile function. It also remains possible that increasing uncoupled respiration could enhance ROS generation from complex III of the ETC, which would be maladaptive. Definitive experiments to tease out the role of UCPs in diabetic HF remain to be performed. In support of UCPs contributing to the metabolic derangements in diabetes, mitochondria isolated from diabetic mouse hearts have elevated rates of oxygen consumption, increased uncoupled respiration, and reduced efficiency (13). Similar phenotypes have been observed in the hearts of diabetic patients through the use of positron emission tomography (PET) metabolic imaging (59).
Another important aspect of the metabolic remodeling that occurs in the diabetic heart is the loss of substrate flexibility. It is well known that FAO yields the most ATP per mole of substrate utilized; however, this occurs at an increased oxygen cost compared with glucose oxidation. The metabolic milieu present in diabetes not only increases FAO but also impairs the ability of cardiac myocytes to utilize other energetic substrates (glucose, lactate, and ketone bodies). This metabolic inflexibility can be problematic in situations where oxygen is limiting, such as ischemia, where glucose is a preferred energetic substrate. This may be part of the reason that diabetics are more prone to HF after myocardial infarction compared with their nondiabetic counterparts (32, 50, 92).
Mitochondrial Dysfunction as a Unifying Theme of Diabetic CM
As discussed in the previous section, multiple pathologic mechanisms have been proposed to explain cardiac dysfunction in patients with diabetes. This complexity has made it challenging to identify the optimal approach to prevent or delay the progression of HF in patients with diabetes. One of the challenges that must be overcome to translate scientific discovery into therapeutics will be to identify key nodal points that underlie the pathology of diabetic myocardial disease. An attractive hypothesis is that diabetes-induced mitochondrial dysfunction is a central event in the pathobiology of diabetic HF. This hypothesis will be explored in the next section.
Several reproducible pathologic features have been observed in the hearts of diabetic patients and in animal models of diabetic CM. These include increased rates of FAO, myocardial lipid accumulation, myocyte cell death, oxidative stress, inflammation, and fibrosis (17, 86). Mitochondria are positioned to be an important regulator of all of these responses. This concept will be reviewed in the next section by subdividing the pathogenesis of diabetic CM into three stages: compensated, transitional, and decompensated (Figs. 2–4).
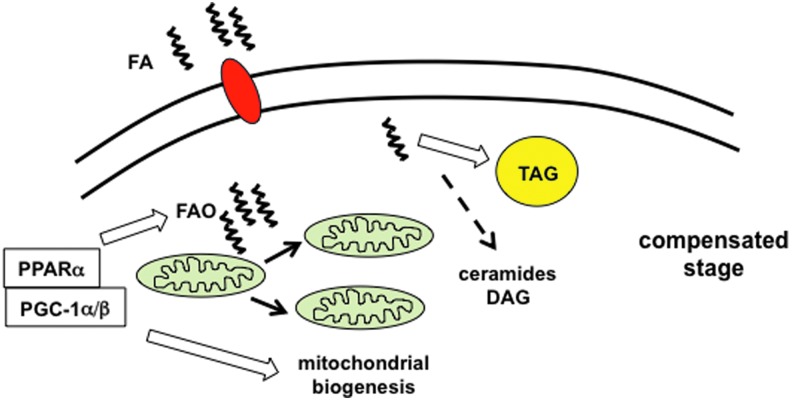
FIG. 2.
The compensated phase of diabetic cardiomyopathy. During the compensated stage of diabetic CM, excess fatty acids (FA) trigger activation of PPARα and PGC-1α/β, which serve to increase fatty acid oxidation (FAO), triglyceride (TAG) synthesis, and mitochondrial biogenesis. The shuttling of excess FA into TAG prevents increased generation of toxic lipid species such as diacylglyerol (DAG) and ceramides, minimizing lipotoxicity. During this phase, cardiac function remains normal; however, cardiomyocyte reliance on FA oxidation for energy generation is increased. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
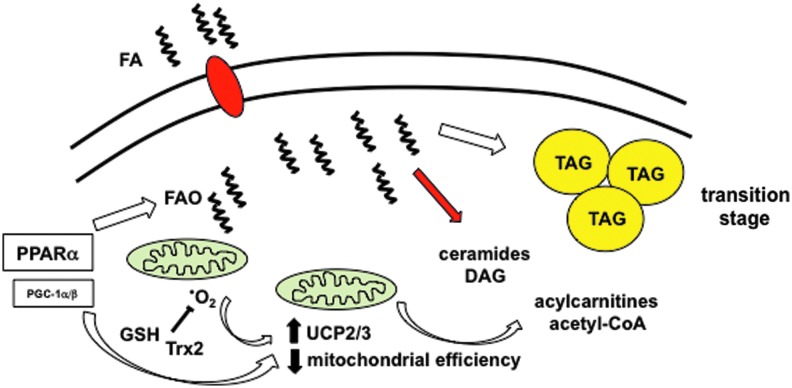
FIG. 3.
The transition phase of diabetic cardiomyopathy. The transition stage of diabetic CM results from sustained increases in lipid uptake and FAO. Elevated mitochondrial FA flux leads to reactive oxygen species (ROS) generation (•O2) and upregulation of uncoupling proteins (UCPs), both of which decrease mitochondrial efficiency and the accumulation of FAO metabolites such as acylcarnitines and acetyl-CoA. Mitochondrial ROS scavengers such as glutathione (GSH) and thioredoxin2 (Trx2) minimize oxidative stress. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
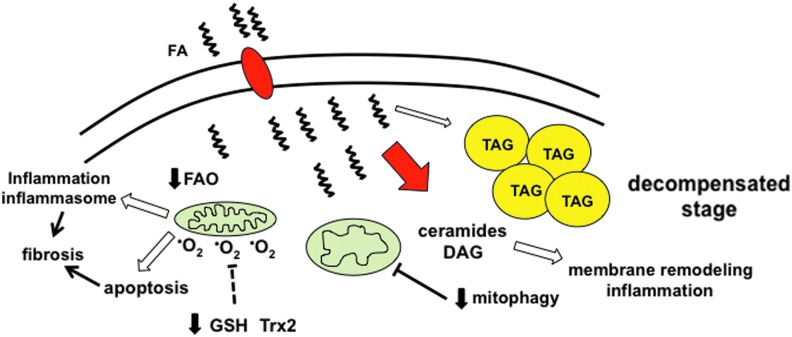
FIG. 4.
The decompensated phase of diabetic cardiomyopathy. With continued metabolic stress, cardiac cells enter a decompensated stage during which further mitochondrial dysfunction and ROS generation worsen the imbalance between lipid uptake and oxidation, which overwhelms TAG synthesis pathways and promotes the generation of toxic lipid species such as ceramides and DAG. Excessive ROS production overwhelms the scavenger machinery, leading to oxidative stress. Together, ROS and lipid accumulation trigger inflammation/inflammasome activation and cell death, both of which can contribute to contractile dysfunction and cardiac fibrosis. In addition, mitophagy is impaired, which can lead to further accumulation of ROS-generating mitochondria. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The compensated stage of diabetic CM can be viewed as a phase of metabolic remodeling shaped by changes in energetic substrate delivery and utilization (Fig. 2). Diabetic patients have increased levels of circulating FAs and lipoproteins. In response to this lipid load, cardiomyocytes adapt by upregulating the expression of genes involved in FA utilization, while simultaneously reducing the expression of glucose metabolic genes. In large part, this transcriptional response is orchestrated by the transcription factor PPARα (34). The expression of the metabolic transcriptional co-activators PGC-1α and β is also upregulated in the insulin-resistant heart, triggering mitochondrial biogenesis and further enhancing mitochondrial oxidative capacity (69). In concert, this gene expression program enhances the hearts' ability to oxidize and store FAs. However, an important consequence of this compensatory response is that it reduces metabolic flexibility, which can be detrimental in the face of ischemia or other changes in substrate availability. Moreover, it is important to consider that this lipid-metabolic program is designed to turn on and off rapidly in response to environmental cues such as fasting. Therefore, when high rates of mitochondrial FA delivery and oxidation are sustained, these organelles become overwhelmed and additional pathology develops.
The transition phase of diabetic CM is driven primarily by sustained increases in FA flux through cardiac mitochondria (Fig. 3). A high rate of mitochondrial respiration in excess of energetic demand leads to an increase in MMP, a reduction in TCA flux, and promotes ROS generation. Moreover, reduced TCA flux in the setting of continued β-oxidation of FAs results in the accumulation of incomplete FAO metabolites such as acylcarnitines and acetyl-CoA. These molecules modulate cell signaling events and directly interfere with mitochondrial respiratory activity (1, 81). In addition, decreased flux of acetyl-CoA molecules through the TCA cycle can deplete the free CoA pool, impairing several other CoA-dependent biochemical reactions in the cell (23). Myocardial FA-CoAs will also accumulate as a consequence of the imbalance between lipid uptake and oxidation. To reduce lipotoxicity, cardiomyocytes divert many of the excess FAs into TAG synthesis pathways, where they can be stored in neutral LDs. Simultaneously, increased rates of mitochondrial FA flux promote ROS generation, particularly when energetic ADP stores are low (i.e., in the energy replete state). In the short term, this is counterbalanced by the induction of UCPs and ROS scavengers, which mitigate oxidant stress but reduce cardiac efficiency.
The decompensated phase of diabetic CM occurs when the adaptive measures described earlier are overwhelmed (Fig. 4). In essence, it is the metabolic equivalent of a “perfect storm.” Mitochondria are at the center of this transition. The combination of UCP upregulation, acylcarnitine accumulation, and oxidative stress leads to a progressive decrease in mitochondrial respiratory capacity. Declining FAO capacity further exacerbates the imbalance between lipid uptake and β-oxidation, which overwhelms the buffering capacity of TAG synthesis pathways. As a consequence, esterified FAs are channeled to alternate fates in the cell, including the formation of ceramides, phospholipids, and DAG. Ceramides and phospholipids that are rich in saturated FAs remodel ER and mitochondrial membranes, resulting in dysregulated calcium handling, worsened mitochondrial respiration, and further ROS generation.
To add insult to injury, the ability of cardiomyocytes to remove damaged, ROS-producing mitochondria through mitophagy also appears to be impaired in the diabetic heart. The net effect of this mitochondrial stress is the release of inflammatory cytokines/chemokines and/or the induction of myocyte cell death. In particular, mitochondrial damage and oxidative stress activates the NLRP3 inflammasome, increasing IL-1β release (90, 109). This inflammatory complex can also be activated in recruited macrophages through an FFA-dependent mechanism (100, 101). Once initiated, this inflammatory response can further propagate cardiac injury. Inflammation also suppresses the expression of PGC-1α and β in cardiac myocytes, resulting in a feed-forward downward spiral of mitochondrial dysfunction, increased ROS generation, and cell death (84). Ultimately, these events create a pro-fibrotic environment, a pathologic hallmark of more advanced diabetic CM.
Although it useful to sub-divide diabetic CM into discrete pathologic stages, it is important to acknowledge that the natural history of diabetic HF in humans has been difficult to delineate. It is likely that the compensated stage of diabetic myocardial disease is likely to have a long latency period during which there is no clinical evidence of cardiac dysfunction. The cardiac pathology that characterizes this stage appears to be reversible with correction of the systemic metabolic abnormities (59). However, if the metabolic stress is sustained and/or additional cardiac damage occurs from ischemia, HTN, or valve disease, overt cardiomyopathy ensues.
Mitochondria as a Therapeutic Target in Diabetic CM
Research over the past 40 years has shown us that the pathophysiologic mechanisms linking diabetes and HF are complex. This fact combined with the nonspecific clinical definition of diabetic CM has hindered the development of novel therapies for this condition. However, the central role of mitochondria in the pathogenesis of diabetic heart disease suggests that therapies aimed at modulating mitochondrial stress may have potential for treating this condition. Although there is only limited clinical evidence investigating such approaches, this section will explore the possibilities of targeting mitochondria in diabetic heart disease.
Metabolic modulation
As discussed earlier, substrate metabolism in the diabetic heart is shifted toward the uptake and utilization of FAs. Over time, excess mitochondrial FAO can have adverse consequences on cardiomyocyte function. Therefore, interfering with this process has the potential for therapeutic benefit. Myocardial FAO is upregulated in response to excess lipid delivery; therefore, one approach that is used to interrupt this cycle would be by reducing lipid delivery to and/or uptake by cardiomyocytes. This can be accomplished via lifestyle changes involving dietary modifications, exercise, and weight loss (Fig. 5). Alternatively, in morbidly obese patients, bariatric surgery is also an effective means of reducing adiposity and circulating lipoprotein levels. Weight loss through either of these means has been shown to reverse the cardiometabolic alternations and diastolic dysfunction associated with metabolic disease (59). It is also worth noting that weight loss and exercise are associated with improved insulin sensitivity, which may also improve mitochondrial function directly (9, 46).
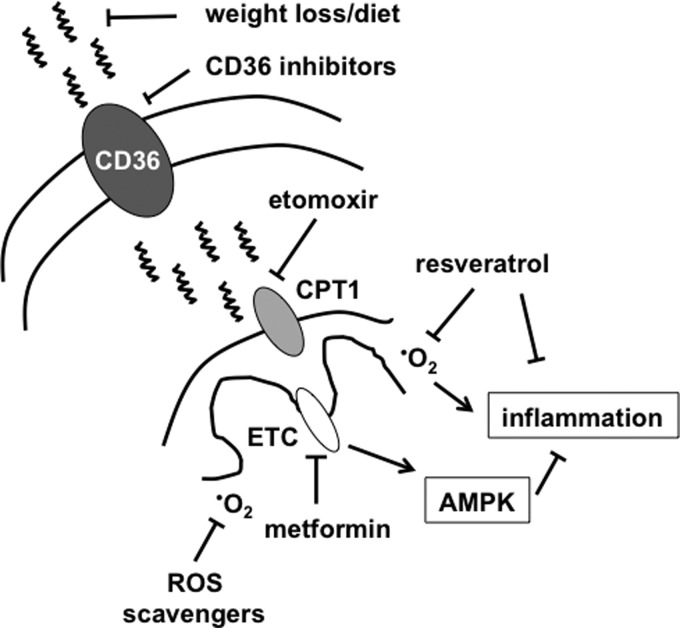
FIG. 5.
Targeting mitochondrial FA flux in diabetic heart failure. FA and lipoprotein uptake is facilitated by FA transporters such as CD36. Reducing the level of FAs in circulation with diet and exercise or inhibiting FA uptake into cardiomyocytes can reduce FA stress on the mitochondria. Other potential strategies to reduce mitochondrial oxidative overload include CPT1 inhibitors (etomoxir), electron transport chain (ETC) inhibitors/AMPK activators (metformin), or antioxidants (scavengers, resveratrol).
In addition to decreasing circulating lipids, there are pharmacologic approaches to inhibit the uptake of FAs that may hold promise. CD36 is a plasma membrane protein that plays an important role in cardiomyocte FA and lipoprotein uptake (Fig. 5). The relevance of this molecule to cardiac lipid overload has been shown using a transgenic model of diabetic CM in which cardiomyocytes are engineered to overexpress PPARα (MHC-PPARα) (36). In this system, the deletion of CD36 mice prevented the development of cardiac steatosis and cardiomyopathy (106). The same model of HF could also be rescued by lipoprotein lipase deficiency, further substantiating the importance of excess lipid delivery to this cardiomyopathy phenotype (29). Similar results have also been obtained in other models of diabetic CM using pharmacologic approaches to inhibit CD36 (6, 12). Thus, preventing excess lipid delivery as a means to reduce mitochondrial stress may be a viable approach to reverse or prevent early stages of diabetic CM. However, the ubiquitous expression and pleotropic functions of CD36 mandate extensive testing to ensure safety of CD36 inhibitors in humans, especially for long-term treatment.
Another strategy to reduce the damaging effects of FA flux would be to prevent the import of esterified FAs into the mitochondria. Inhibition of the mitochondrial outer membrane transporter CPT1 is one way to accomplish this objective. In support of this approach, short-term CPT1 inhibition with etomoxir prevents the development of contractile dysfunction in a rat model of diabetic CM (87). However, a potential downside of inhibiting mitochondrial FAO in the setting of excess lipid uptake would be to exacerbate cardiomyocyte lipid accumulation (26, 27). Indeed, even short-term etomoxir treatment dramatically increases cardiac lipid content in diabetic animals (87). An alternate approach to reduce mitochondrial flux is through the use of the anti-diabetic drug metformin. Metformin inhibits complex 1 of the ETC, thereby reducing oxidative flux through the mitochondria. As a result, ATP production decreases, leading to the activation of AMPK, a kinase with antioxidant and anti-inflammatory properties (105). Metformin has been shown to have cardioprotective affects in animal models of ischemia, and AMPK is believed to be an important mediator of this effect (31). In contrast to etomoxir, metformin does not lead to excess cardiac lipid accumulation despite reducing mitochondrial oxidative flux. One potential explanation may be related to the ability of metformin to inhibit de novo lipogenesis in the liver, thereby reducing FA delivery to the heart (39). There is a growing body of evidence that metformin may be beneficial for the treatment of HF in the setting of diabetes (33, 43, 75, 80, 82); however, the majority of human study data comes from nonrandomized retrospective analyses. Future investigation will be necessary to determine whether reducing mitochondrial FA flux, and in particular metformin, improves cardiac function and/or outcomes in diabetics. The MET-DIME trial is an ongoing, randomized clinical study designed to address this question (54). Together, these approaches could help slow the evolution from the compensated to transitional phases of diabetic CM.
Antioxidant therapies
Oxidative stress has been implicated in the pathogenesis of many diabetic complications. In the diabetic heart, both increased ROS production and downregulation of antioxidant defenses appear to be responsible for the oxidative stress burden. Based on this evidence, oxidative stress pathways have become an attractive target for diabetes complications. However, several challenges have been encountered with this approach. For one, ROS are not always pathologic and also participate in physiologic cell signaling. Therefore, antioxidants have the potential to impact normal cell function and produce unwanted side effects. In addition, there are numerous compounds with anti-oxidant properties; however, it remains unclear which agents are the most effective at alleviating oxidative stress in diabetics.
Most human and animal model data come from studies using ROS scavenger-based therapies, including superoxide dismutase mimetics, to reduce oxidative damage. Although there is some evidence of efficacy, most of this data was obtained from streptozotocin (STZ)-induced models of diabetes where nonmitochondrial ROS may be more prominent. Moreover, the issue of whether to use general or mitochondrial-targeted antioxidants is still an open question. In addition to ROS-scavenging approaches, targeting events upstream of ROS production or augmenting endogenous antioxidant responses may also have promise and warrant further investigation.
Current data regarding the benefit of antioxidant therapy in diabetic cardiovascular disease are mixed. Studies with natural antioxidants such as vitamin E, vitamin C, and α-lipoic acid have yielded promising results in animal models of diabetes, particularly when STZ is used to induce hyperglycemia (7, 57). However, when investigated in humans, these antioxidants either alone or in combination have not shown consistent beneficial effects on serum metabolic parameters or the incidence of cardiovascular disease (65, 108). The discrepancies between mouse and man may reflect the fact that STZ-induced diabetes is a poor model of the human disease and/or that more potent or selective antioxidant approaches will be necessary to achieve clinical benefits in patients.
In addition to general antioxidants, there are several mitochondrial-targeted ROS scavengers that have potential for use in the treatment of diabetic CM. Initial enthusiasm for this approach came from data demonstrating that transgenic overexpression of mitochondrial superoxide dismutase could prevent diabetes-induced contractile dysfunction in isolated cardiomyocytes (89). Although the investigators used a genetic model of type 1 diabetes in lieu of STZ, these animals still had profound hyperglycemia. More recently, pharmacologic approaches designed to reduce mitochondrial ROS have been employed, including the compounds mitotempol and MitoQ. Mitotempol is a superoxide dismutase mimetic that has potent antioxidant properties in cell culture (58). Surprisingly, there have been relatively little data about the effectiveness of this compound in in vivo models of diabetes. MitoQ is a version of the antioxidant coenzymeQ that is targeted to mitochondria. As with mitotempol, MitoQ can reduce mitochondrial oxidative stress in cell culture. In addition, in a mouse model of type 1 diabetes, MitoQ was shown to attenuate renal tubular injury, which is another important complication of diabetes (20). The only cardiac data with this agent come from a rat model of ischemia-reperfusion injury where MitoQ was shown to reduce myocyte injury and improve contractile function (2). Interestingly, in this study, the use of an untargeted antioxidant did not protect the heart from ischemic damage. There is little published evidence about the use of MitoQ preclinical models of diabetic HF. The paucity of recent publications on the use of mitochondrial antioxidants in diabetic cardiac disease and the lack of efficacy data in type 2 diabetic models suggest that strategies targeting events upstream or downstream mitochondrial ROS may hold more promise to improve cardiac function in diabetes.
The flavonoids are naturally occurring compounds that act upstream of mitochondria and have shown promise in models of diabetes, potentially as a consequence of their antioxidant properties. The poster child of this molecular class is resveratrol, a flavonoid with pleotropic affects on mitochondria, metabolism, and ROS scavenging. Mechanistically, resveratrol activates the deacetylase situin 1 (SIRT1), which has many targets, including the metabolic coactivator protein PGC-1α (18). Deacetylation increases PGC-1α's transcriptional activity, upregulating genes involved in FA metabolism and ROS scavenging (35). In animal models of diabetes complications, resveratrol has been shown to protect against pathology, including cardiomyopathy (94). However, it should be noted that most of this data also comes from STZ-induced diabetes models. Therefore, the translatability of these findings to diabetic humans remains to be determined. This issue notwithstanding, the multifaceted effects of resveratrol place it among the more promising of the antioxidant-based therapies for the prevention and/or treatment of diabetic myocardial disease.
As mentioned earlier, the study of antioxidants for the treatment of diabetes complications in vivo has predominantly occurred in mouse models of type 1 diabetes. Of these, STZ is the most frequently used diabetic model, largely due to its ease of inducing hyperglycemia and lack of need for breeding. However, STZ treatment, without insulin supplementation, produces profound hyperglycemia and weight loss due to a near complete loss of insulin. Thus, the STZ phenotype more closely resembles the very ill type 1 diabetic who is not receiving treatment with insulin, rather than the overweight type 2 diabetic patient on oral medications. This is relevant to the study of antioxidants, because very high glucose levels could significantly alter the molecular origin and severity of oxidative stress. For example, it would be expected that STZ-treated, hyperglycemic mice would have increased total oxidative stress with a greater contribution from nonmitochondrial ROS pathways (i.e., RAGE/NADPH oxidase) (21). Thus, the data obtained with antioxidant therapies using type 1 diabetic models may not be generalizable to pathology of type 2 diabetes in animals or humans. Future studies of antioxidant compounds will need to incorporate more models of type 2 diabetes, with an emphasis on diet-induced obesity. If successful, antioxidant approaches could be useful during both the transitional and decompensated phases of diabetic CM.
Inflammation
In the decompensated phase of diabetic CM, mitochondrial dysfunction and oxidative stress lead to the damage of proteins, lipids, and nucleic acids, resulting in myocyte death and/or the elaboration of inflammatory cytokines. Cardiac inflammation and leukocyte recruitment is increased in both animal models and humans with diabetes and HF (48, 85, 88, 102, 103). Of particular relevance to mitochondrial-derived inflammation is an inflammatory complex known as the inflammasome (Fig. 6). It is now established that mitochondrial damage, particularly in the context of toll-like receptor activation with release of mitochondrial DNA and/or ROS, can activate the NLRP3 inflammasome, leading to the cleavage and release of IL-1β and IL-18 through a caspase 1-dependent mechanism (42, 90, 109). Emerging evidence suggests that the NLRP3 inflammasome is hyperactivated in diabetes and contributes to inflammatory damage in the heart and other tissues (56, 66, 68, 100, 101). Interestingly, excess FFA, hyperglycemia, and ischemic stress have been shown to promote inflammasome assembly by producing mitochondrial and/or lysosome damage (97, 100, 101).
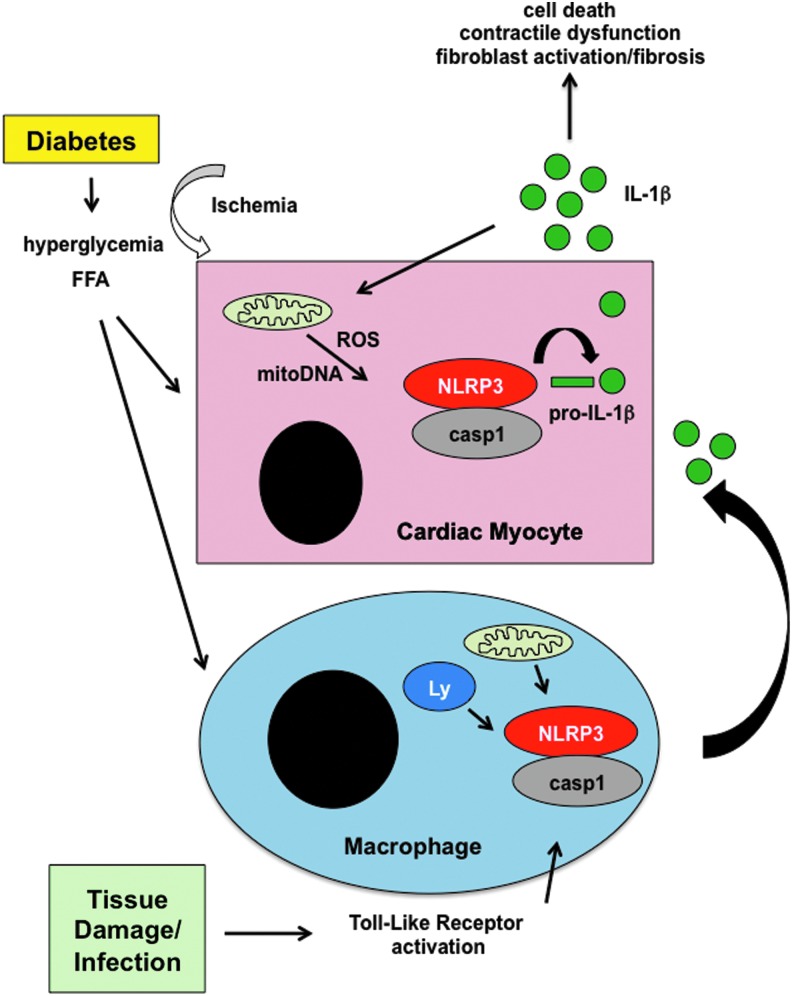
FIG. 6.
Activation of the inflammasome in the heart in response to mitochondrial stress. In response to the metabolic stress of diabetes, including excess FFA and hyperglycemia, damaged mitochondria release excess ROS and oxidized DNA. This response can be exacerbated by ischemia. These danger signals can promote the assembly of the NLRP3 inflammasome, including caspase 1 (casp1), which cleaves pro-IL-1β, to release the mature cytokine. On release, IL-1β can promote additional inflammation, cardiac injury, and fibrosis. Moreover, in the diabetic environment, activation of toll-like receptors by tissue damage or infection can trigger mitochondrial and lysosomal (Ly) stress, leading to inflammasome activation in recruited macrophages and further amplifying the IL-1 response. The excessive release of cytokines exacerbates mitochondrial dysfunction and ROS generation, leading to cardiomyocyte damage and contractile dysfunction.
In the context of myocardial injury, the inflammasome can be activated in both myocytes and infiltrating monocytes/macrophages (67). The elaboration of IL-1 can further increase ROS generation and mitochondrial dysfunction, accelerating the cardiomyopathic process. For these reasons, the NLRP3 inflammasome is an attractive target to mitigate the inflammatory response associated with mitochondrial damage and metabolic stress that occurs in diabetes. In support of this concept, there are preclinical data demonstrating a protective effect of inflammasome inhibition in several models of cardiac injury (16, 66, 67, 103). Although these data are provocative, further translational investigation is needed. Additional research focusing on the role of diabetes in altering interplay between cardiac macrophages and myocytes should be explored.
The future of therapeutics for diabetic CM
The lack of diagnostic criteria for diabetic CM has made it challenging to investigate the human disease and to translate basic science into practice. Whether such criteria can be developed remains to be determined. However, without clear inclusion criteria for the study of diabetic CM, it will be impossible to enroll appropriate patient populations for therapeutic trails. As such, the intersection between diabetes and other cardiac stressors (ischemic, HTN, and renal disease) is perhaps a more tenable and relevant area for investigation. Along these lines, the influence of diabetes on HFpEF was recently reported in a subset of patients from the RELAX trial (61). In this analysis, diabetic patients had increased LV mass, more co-morbidities, higher rates of HF hospitalizations, and worse exercise capacity compared with nondiabetics with HFpEF. Serum markers of inflammation, fibrosis, and oxidative stress were also elevated in the diabetic cohort. Studies such as this argue that understanding how metabolic stress alters HF progression irrespective of the etiology will be relevant to patients with diabetes. To achieve this goal, improved animals models that incorporate common co-morbidities and more rigorous clinical investigation coupled with tissues analyses will be necessary.
Conclusion
The number of patients with diabetes and cardiomyopathy will continue to increase for the foreseeable future. Therefore, novel strategies to prevent diabetes-induced cardiac damage are needed. Unfortunately, the lack of clear diagnostic criteria for diabetic CM in combination with sub-optimal diabetic animal models has limited progress in this area. That being said, mitochondrial dysfunction is a common theme present in virtually all human and animal studies of diabetes complications. Moreover, mitochondrial pathology can explain many of the findings associated with diabetic heart disease, including altered cardiac metabolism, lipid accumulation, oxidative stress, and inflammation. For these reasons, targeting mitochondrial dysfunction and/or its sequela are attractive therapeutic targets for improving cardiac function in patients with HF and diabetes. However, additional studies from human tissue and relevant animals models will be required to develop appropriate strategies for intervention. In particular, investigating diabetes in combination with other cardiac co-morbidities will be important to move the field forward in a clinically relevant manner. The importance of preventative medicine should also be emphasized, especially since diet and exercise can reverse early stages of disease. Together, such approaches have the potential to substantially reduce the morbidity and mortality associated with diabetes in patients with HF.
Abbreviations Used
- CPT1
carnitine palmitoyl transferase 1
- DAG
diacylglycerol
- diabetic CM
diabetic cardiomyopathy
- FA
fatty acid
- FAO
fatty acid oxidation
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HTN
hypertension
- LVH
left ventricular hypertrophy
- MMP
mitochondrial membrane potential
- OX-PHOS
oxidative phosphorylation
- PET
positron emission tomography
- ROS
reactive oxygen species
- TAG
triacylglercerol
- TCA
tricarboxylic acid
- Trx2
thioredoxin 2
- UCP
uncoupling protein
Acknowledgments
This work was funded by NIH KO8 HL09837302 and P30 DK020579.
References
- 1.Abdul-Ghani MA, Muller FL, Liu Y, Chavez AO, Balas B, Zuo P, Chang Z, Tripathy D, Jani R, Molina-Carrion M, Monroy A, Folli F, Van Remmen H, and DeFronzo RA. Deleterious action of FA metabolites on ATP synthesis: possible link between lipotoxicity, mitochondrial dysfunction, and insulin resistance. Am J Physiol Endocrinol Metab 295: E678–E685, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, and Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J 19: 1088–1095, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Aguilar D, Deswal A, Ramasubbu K, Mann DL, and Bozkurt B. Comparison of patients with heart failure and preserved left ventricular ejection fraction among those with versus without diabetes mellitus. Am J Cardiol 105: 373–377, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aliciguzel Y, Ozen I, Aslan M, and Karayalcin U. Activities of xanthine oxidoreductase and antioxidant enzymes in different tissues of diabetic rats. J Lab Clin Med 142: 172–177, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, and Neufer PD. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J Am Coll Cardiol 54: 1891–1898, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angin Y, Steinbusch LK, Simons PJ, Greulich S, Hoebers NT, Douma K, van Zandvoort MA, Coumans WA, Wijnen W, Diamant M, Ouwens DM, Glatz JF, and Luiken JJ. CD36 inhibition prevents lipid accumulation and contractile dysfunction in rat cardiomyocytes. Biochem J 448: 43–53, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Aydemir-Koksoy A, Bilginoglu A, Sariahmetoglu M, Schulz R, and Turan B. Antioxidant treatment protects diabetic rats from cardiac dysfunction by preserving contractile protein targets of oxidative stress. J Nutr Biochem 21: 827–833, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Batenburg JJ. and Olson MS. Regulation of pyruvate dehydrogenase by fatty acid in isolated rat liver mitochondria. J Biol Chem 251: 1364–1370, 1976 [PubMed] [Google Scholar]
- 9.Belke DD, Betuing S, Tuttle MJ, Graveleau C, Young ME, Pham M, Zhang D, Cooksey RC, McClain DA, Litwin SE, Taegtmeyer H, Severson D, Kahn CR, and Abel ED. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest 109: 629–639, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bella JN, Devereux RB, Roman MJ, Palmieri V, Liu JE, Paranicas M, Welty TK, Lee ET, Fabsitz RR, and Howard BV. Separate and joint effects of systemic hypertension and diabetes mellitus on left ventricular structure and function in American Indians (the Strong Heart Study). Am J Cardiol 87: 1260–1265, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Bene J, Marton M, Mohas M, Bagosi Z, Bujtor Z, Oroszlan T, Gasztonyi B, Wittmann I, and Melegh B. Similarities in serum acylcarnitine patterns in type 1 and type 2 diabetes mellitus and in metabolic syndrome. Ann Nutr Metab 62: 80–85, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Bessi VL, Labbe SM, Huynh DN, Menard L, Jossart C, Febbraio M, Guerin B, Bentourkia M, Lecomte R, Carpentier AC, Ong H, and Marleau S. EP 80317, a selective CD36 ligand, shows cardioprotective effects against post-ischaemic myocardial damage in mice. Cardiovasc Res 96: 99–108, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Boudina S. and Abel ED. Mitochondrial uncoupling: a key contributor to reduced cardiac efficiency in diabetes. Physiology (Bethesda) 21: 250–258, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Boudina S, Han YH, Pei S, Tidwell TJ, Henrie B, Tuinei J, Olsen C, Sena S, and Abel ED. UCP3 regulates cardiac efficiency and mitochondrial coupling in high fat-fed mice but not in leptin-deficient mice. Diabetes 61: 3260–3269, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, and Abel ED. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes 56: 2457–2466, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Bracey NA, Beck PL, Muruve DA, Hirota SA, Guo J, Jabagi H, Wright JR, Jr., Macdonald JA, Lees-Miller JP, Roach D, Semeniuk LM, and Duff HJ. The Nlrp3 inflammasome promotes myocardial dysfunction in structural cardiomyopathy through interleukin-1beta. Exp Physiol 98: 462–472, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Bugger H. and Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 57: 660–671, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canto C. and Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol 20: 98–105, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cascio G, Schiera G, and Di Liegro I. Dietary fatty acids in metabolic syndrome, diabetes and cardiovascular diseases. Curr Diabetes Rev 8: 2–17, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Chacko BK, Reily C, Srivastava A, Johnson MS, Ye Y, Ulasova E, Agarwal A, Zinn KR, Murphy MP, Kalyanaraman B, and Darley-Usmar V. Prevention of diabetic nephropathy in Ins2(+/)(-)(AkitaJ) mice by the mitochondria-targeted therapy MitoQ. Biochem J 432: 9–19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daffu G, del Pozo CH, O'Shea KM, Ananthakrishnan R, Ramasamy R, and Schmidt AM. Radical roles for RAGE in the pathogenesis of oxidative stress in cardiovascular diseases and beyond. Int J Mol Sci 14: 19891–19910, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dandamudi S, Slusser J, Mahoney DW, Redfield MM, Rodeheffer RJ, and Chen HH. The prevalence of diabetic cardiomyopathy: a population-based study in Olmsted County, Minnesota. J Card Fail 20: 304–309, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davaapil H, Tsuchiya Y, and Gout I. Signalling functions of coenzyme A and its derivatives in mammalian cells. Biochem Soc Trans 42: 1056–1062, 2014 [DOI] [PubMed] [Google Scholar]
- 24.de Simone G, Palmieri V, Bella JN, Celentano A, Hong Y, Oberman A, Kitzman DW, Hopkins PN, Arnett DK, and Devereux RB. Association of left ventricular hypertrophy with metabolic risk factors: the HyperGEN study. J Hypertens 20: 323–331, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Devereux RB, Roman MJ, Paranicas M, O'Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, and Howard BV. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation 101: 2271–2276, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Dhalla NS, Elimban V, and Rupp H. Paradoxical role of lipid metabolism in heart function and dysfunction. Mol Cell Biochem 116: 3–9, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Djouadi F, Brandt JM, Weinheimer CJ, Leone TC, Gonzalez FJ, and Kelly DP. The role of the peroxisome proliferator-activated receptor alpha (PPAR alpha) in the control of cardiac lipid metabolism. Prostaglandins Leukot Essent Fatty Acids 60: 339–343, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, and Huang GD. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 360: 129–139, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Duncan JG, Bharadwaj KG, Fong JL, Mitra R, Sambandam N, Courtois MR, Lavine KJ, Goldberg IJ, and Kelly DP. Rescue of cardiomyopathy in peroxisome proliferator-activated receptor-alpha transgenic mice by deletion of lipoprotein lipase identifies sources of cardiac lipids and peroxisome proliferator-activated receptor-alpha activators. Circulation 121: 426–435, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duncan JG, Fong JL, Medeiros DM, Finck BN, and Kelly DP. Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-alpha/PGC-1alpha gene regulatory pathway. Circulation 115: 909–917, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Messaoudi S, Rongen GA, and Riksen NP. Metformin therapy in diabetes: the role of cardioprotection. Curr Atheroscler Rep 15: 314, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Estep JD. and Aguilar D. Diabetes and heart failure in the post-myocardial infarction patient. Curr Heart Fail Rep 3: 164–169, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Eurich DT, McAlister FA, Blackburn DF, Majumdar SR, Tsuyuki RT, Varney J, and Johnson JA. Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: systematic review. BMJ 335: 497, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finck BN, Han X, Courtois M, Aimond F, Nerbonne JM, Kovacs A, Gross RW, and Kelly DP. A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci U S A 100: 1226–1231, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finck BN. and Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest 116: 615–622, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, and Kelly DP. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest 109: 121–130, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.From AM, Leibson CL, Bursi F, Redfield MM, Weston SA, Jacobsen SJ, Rodeheffer RJ, and Roger VL. Diabetes in heart failure: prevalence and impact on outcome in the population. Am J Med 119: 591–599, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, and Anversa P. Myocardial cell death in human diabetes. Circ Res 87: 1123–1132, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen ZP, O'Neill HM, Ford RJ, Palanivel R, O'Brien M, Hardie DG, Macaulay SL, Schertzer JD, Dyck JR, van Denderen BJ, Kemp BE, and Steinberg GR. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med 19: 1649–1654, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galderisi M, Anderson KM, Wilson PW, and Levy D. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study). Am J Cardiol 68: 85–89, 1991 [DOI] [PubMed] [Google Scholar]
- 41.Gerdts E, Okin PM, Omvik P, Wachtell K, Dahlof B, Hildebrandt P, Nieminen MS, and Devereux RB. Impact of diabetes on treatment-induced changes in left ventricular structure and function in hypertensive patients with left ventricular hypertrophy. The LIFE study. Nutr Metab Cardiovasc Dis 19: 306–312, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Gross O, Thomas CJ, Guarda G, and Tschopp J. The inflammasome: an integrated view. Immunol Rev 243: 136–151, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, Ji SY, Nunez D, Ramachandran A, Anaya-Cisneros M, Tian R, and Lefer DJ. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res 104: 403–411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herlitz J, Malmberg K, Karlson BW, Ryden L, and Hjalmarson A. Mortality and morbidity during a five-year follow-up of diabetics with myocardial infarction. Acta Med Scand 224: 31–38, 1988 [DOI] [PubMed] [Google Scholar]
- 45.Herrero P, Peterson LR, McGill JB, Matthew S, Lesniak D, Dence C, and Gropler RJ. Increased myocardial fatty acid metabolism in patients with type 1 diabetes mellitus. J Am Coll Cardiol 47: 598–604, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Huang X, Vaag A, Hansson M, and Groop L. Down-regulation of insulin receptor substrates (IRS)-1 and IRS-2 and Src homologous and collagen-like protein Shc gene expression by insulin in skeletal muscle is not associated with insulin resistance or type 2 diabetes. J Clin Endocrinol Metab 87: 255–259, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Ido Y. Pyridine nucleotide redox abnormalities in diabetes. Antioxid Redox Signal 9: 931–942, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Ko HJ, Zhang Z, Jung DY, Jun JY, Ma Z, Jones KE, Chan SY, and Kim JK. Nutrient stress activates inflammation and reduces glucose metabolism by suppressing AMP-activated protein kinase in the heart. Diabetes 58: 2536–2546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobayashi S. and Liang Q. Autophagy and mitophagy in diabetic cardiomyopathy. Biochim Biophys Acta 1852: 252–261, 2015 [DOI] [PubMed] [Google Scholar]
- 50.Kouvaras G, Cokkinos D, and Spyropoulou M. Increased mortality of diabetics after acute myocardial infarction attributed to diffusely impaired left ventricular performance as assessed by echocardiography. Jpn Heart J 29: 1–9, 1988 [DOI] [PubMed] [Google Scholar]
- 51.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, and Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7: 45–56, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Kuramoto K, Okamura T, Yamaguchi T, Nakamura TY, Wakabayashi S, Morinaga H, Nomura M, Yanase T, Otsu K, Usuda N, Matsumura S, Inoue K, Fushiki T, Kojima Y, Hashimoto T, Sakai F, Hirose F, and Osumi T. Perilipin 5, a lipid droplet-binding protein, protects heart from oxidative burden by sequestering fatty acid from excessive oxidation. J Biol Chem 287: 23852–23863, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laakso M, Voutilainen E, Sarlund H, Aro A, Pyorala K, and Penttila I. Serum lipids and lipoproteins in middle-aged non-insulin-dependent diabetics. Atherosclerosis 56: 271–281, 1985 [DOI] [PubMed] [Google Scholar]
- 54.Ladeiras-Lopes R, Fontes-Carvalho R, Bettencourt N, Sampaio F, Gama V, and Leite-Moreira AF. METformin in DIastolic Dysfunction of MEtabolic syndrome (MET-DIME) trial: rationale and study design: MET-DIME trial. Cardiovasc Drugs Ther 28: 191–196, 2014 [DOI] [PubMed] [Google Scholar]
- 55.LaNoue KF, Bryla J, and Williamson JR. Feedback interactions in the control of citric acid cycle activity in rat heart mitochondria. J Biol Chem 247: 667–679, 1972 [PubMed] [Google Scholar]
- 56.Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ, and Jo EK. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes 62: 194–204, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li CJ, Lv L, Li H, and Yu DM. Cardiac fibrosis and dysfunction in experimental diabetic cardiomyopathy are ameliorated by alpha-lipoic acid. Cardiovasc Diabetol 11: 73, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lim S, Rashid MA, Jang M, Kim Y, Won H, Lee J, Woo JT, Kim YS, Murphy MP, Ali L, Ha J, and Kim SS. Mitochondria-targeted antioxidants protect pancreatic beta-cells against oxidative stress and improve insulin secretion in glucotoxicity and glucolipotoxicity. Cell Physiol Biochem 28: 873–886, 2011 [DOI] [PubMed] [Google Scholar]
- 59.Lin CH, Kurup S, Herrero P, Schechtman KB, Eagon JC, Klein S, Davila-Roman VG, Stein RI, Dorn Ii GW, Gropler RJ, Waggoner AD, and Peterson LR. Myocardial oxygen consumption change predicts left ventricular relaxation improvement in obese humans after weight loss. Obesity (Silver Spring) 19: 1804–1812, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lindman BR, Arnold SV, Madrazo JA, Zajarias A, Johnson SN, Perez JE, and Mann DL. The adverse impact of diabetes mellitus on left ventricular remodeling and function in patients with severe aortic stenosis. Circ Heart Fail 4: 286–292, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lindman BR, Davila-Roman VG, Mann DL, McNulty S, Semigran MJ, Lewis GD, de Las Fuentes L, Joseph SM, Vader J, Hernandez AF, and Redfield MM. Cardiovascular Phenotype in HFpEF Patients With or Without Diabetes: A RELAX Trial Ancillary Study. J Am Coll Cardiol 64: 541–549, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr., Ory DS, and Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A 100: 3077–3082, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu L, Shi X, Bharadwaj KG, Ikeda S, Yamashita H, Yagyu H, Schaffer JE, Yu YH, and Goldberg IJ. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J Biol Chem 284: 36312–36323, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu L, Yu S, Khan RS, Homma S, Schulze PC, Blaner WS, Yin Y, and Goldberg IJ. Diacylglycerol acyl transferase 1 overexpression detoxifies cardiac lipids in PPARgamma transgenic mice. J Lipid Res 53: 1482–1492, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, and Dagenais GR. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA 293: 1338–1347, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Luo B, Li B, Wang W, Liu X, Xia Y, Zhang C, Zhang M, Zhang Y, and An F. NLRP3 Gene Silencing Ameliorates Diabetic Cardiomyopathy in a Type 2 Diabetes Rat Model. PLoS One 9: e104771, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mezzaroma E, Toldo S, Farkas D, Seropian IM, Van Tassell BW, Salloum FN, Kannan HR, Menna AC, Voelkel NF, and Abbate A. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci U S A 108: 19725–19730, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mirza RE, Fang MM, Ennis WJ, and Koh TJ. Blocking IL-1beta induces a healing-associated wound macrophage phenotype and improves healing in type-2 diabetes. Diabetes 62: 2579–2587, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mitra R, Nogee DP, Zechner JF, Yea K, Gierasch CM, Kovacs A, Medeiros DM, Kelly DP, and Duncan JG. The transcriptional coactivators, PGC-1alpha and beta, cooperate to maintain cardiac mitochondrial function during the early stages of insulin resistance. J Mol Cell Cardiol 52: 701–710, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Montaigne D, Marechal X, Coisne A, Debry N, Modine T, Fayad G, Potelle C, El Arid JM, Mouton S, Sebti Y, Duez H, Preau S, Remy-Jouet I, Zerimech F, Koussa M, Richard V, Neviere R, Edme JL, Lefebvre P, and Staels B. Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation 130: 554–564, 2014 [DOI] [PubMed] [Google Scholar]
- 71.Mullen TD. and Obeid LM. Ceramide and apoptosis: exploring the enigmatic connections between sphingolipid metabolism and programmed cell death. Anticancer Agents Med Chem 12: 340–363, 2012 [DOI] [PubMed] [Google Scholar]
- 72.Muoio DM. and Neufer PD. Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab 15: 595–605, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muoio DM, Noland RC, Kovalik JP, Seiler SE, Davies MN, DeBalsi KL, Ilkayeva OR, Stevens RD, Kheterpal I, Zhang J, Covington JD, Bajpeyi S, Ravussin E, Kraus W, Koves TR, and Mynatt RL. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab 15: 764–777, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murray AJ, Anderson RE, Watson GC, Radda GK, and Clarke K. Uncoupling proteins in human heart. Lancet 364: 1786–1788, 2004 [DOI] [PubMed] [Google Scholar]
- 75.Nichols GA, Koro CE, Gullion CM, Ephross SA, and Brown JB. The incidence of congestive heart failure associated with antidiabetic therapies. Diabetes Metab Res Rev 21: 51–57, 2005 [DOI] [PubMed] [Google Scholar]
- 76.Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, Tuinei J, Homma S, Jiang XC, Abel ED, and Goldberg IJ. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res 49: 2101–2112, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, and Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358: 2560–2572, 2008 [DOI] [PubMed] [Google Scholar]
- 78.Peterson LR, Saeed IM, McGill JB, Herrero P, Schechtman KB, Gunawardena R, Recklein CL, Coggan AR, DeMoss AJ, Dence CS, and Gropler RJ. Sex and type 2 diabetes: obesity-independent effects on left ventricular substrate metabolism and relaxation in humans. Obesity (Silver Spring) 20: 802–810, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rindler PM, Crewe CL, Fernandes J, Kinter M, and Szweda LI. Redox regulation of insulin sensitivity due to enhanced fatty acid utilization in the mitochondria. Am J Physiol Heart Circ Physiol 305: H634–E643, 2013 [DOI] [PubMed] [Google Scholar]
- 80.Romero SP, Andrey JL, Garcia-Egido A, Escobar MA, Perez V, Corzo R, Garcia-Domiguez GJ, and Gomez F. Metformin therapy and prognosis of patients with heart failure and new-onset diabetes mellitus. A propensity-matched study in the community. Int J Cardiol 166: 404–412, 2013 [DOI] [PubMed] [Google Scholar]
- 81.Rutkowsky JM, Knotts TA, Ono-Moore KD, McCoin CS, Huang S, Schneider D, Singh S, Adams SH, and Hwang DH. Acylcarnitines activate pro-inflammatory signaling pathways. Am J Physiol Endocrinol Metab 306: E1378–E1387, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sasaki H, Asanuma H, Fujita M, Takahama H, Wakeno M, Ito S, Ogai A, Asakura M, Kim J, Minamino T, Takashima S, Sanada S, Sugimachi M, Komamura K, Mochizuki N, and Kitakaze M. Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase. Circulation 119: 2568–2577, 2009 [DOI] [PubMed] [Google Scholar]
- 83.Schannwell CM, Schneppenheim M, Perings S, Plehn G, and Strauer BE. Left ventricular diastolic dysfunction as an early manifestation of diabetic cardiomyopathy. Cardiology 98: 33–39, 2002 [DOI] [PubMed] [Google Scholar]
- 84.Schilling J, Lai L, Sambandam N, Dey CE, Leone TC, and Kelly DP. Toll-like receptor-mediated inflammatory signaling reprograms cardiac energy metabolism by repressing peroxisome proliferator-activated receptor {gamma} coactivator-1 (PGC-1) signaling. Circ Heart Fail 4: 474–482, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schilling JD, Machkovech HM, Kim AH, Schwendener R, and Schaffer JE. Macrophages modulate cardiac function in lipotoxic cardiomyopathy. Am J Physiol Heart Circ Physiol 303: H1366–H1373, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schilling JD. and Mann DL. Diabetic cardiomyopathy: bench to bedside. Heart Fail Clin 8: 619–631, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schmitz FJ, Rosen P, and Reinauer H. Improvement of myocardial function and metabolism in diabetic rats by the carnitine palmitoyl transferase inhibitor Etomoxir. Horm Metab Res 27: 515–522, 1995 [DOI] [PubMed] [Google Scholar]
- 88.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, and Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. Faseb J 18: 1692–1700, 2004 [DOI] [PubMed] [Google Scholar]
- 89.Shen X, Zheng S, Metreveli NS, and Epstein PN. Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes 55: 798–805, 2006 [DOI] [PubMed] [Google Scholar]
- 90.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, and Arditi M. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36: 401–414, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Son NH, Yu S, Tuinei J, Arai K, Hamai H, Homma S, Shulman GI, Abel ED, and Goldberg IJ. PPARgamma-induced cardiolipotoxicity in mice is ameliorated by PPARalpha deficiency despite increases in fatty acid oxidation. J Clin Invest 120: 3443–3454, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stone PH, Muller JE, Hartwell T, York BJ, Rutherford JD, Parker CB, Turi ZG, Strauss HW, Willerson JT, Robertson T, et al. . The effect of diabetes mellitus on prognosis and serial left ventricular function after acute myocardial infarction: contribution of both coronary disease and diastolic left ventricular dysfunction to the adverse prognosis. The MILIS Study Group. J Am Coll Cardiol 14: 49–57, 1989 [DOI] [PubMed] [Google Scholar]
- 93.Styskal J, Van Remmen H, Richardson A, and Salmon AB. Oxidative stress and diabetes: what can we learn about insulin resistance from antioxidant mutant mouse models? Free Radic Biol Med 52: 46–58, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thirunavukkarasu M, Penumathsa SV, Koneru S, Juhasz B, Zhan L, Otani H, Bagchi D, Das DK, and Maulik N. Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: Role of nitric oxide, thioredoxin, and heme oxygenase. Free Radic Biol Med 43: 720–729, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tocchetti CG, Caceres V, Stanley BA, Xie C, Shi S, Watson WH, O'Rourke B, Spadari-Bratfisch RC, Cortassa S, Akar FG, Paolocci N, and Aon MA. GSH or palmitate preserves mitochondrial energetic/redox balance, preventing mechanical dysfunction in metabolically challenged myocytes/hearts from type 2 diabetic mice. Diabetes 61: 3094–3105, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van de Weijer T, Schrauwen-Hinderling VB, and Schrauwen P. Lipotoxicity in type 2 diabetic cardiomyopathy. Cardiovasc Res 92: 10–18, 2011 [DOI] [PubMed] [Google Scholar]
- 97.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, and Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 17: 179–188, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang H, Sreenivasan U, Gong DW, O'Connell KA, Dabkowski ER, Hecker PA, Ionica N, Konig M, Mahurkar A, Sun Y, Stanley WC, and Sztalryd C. Cardiomyocyte-specific perilipin 5 overexpression leads to myocardial steatosis and modest cardiac dysfunction. J Lipid Res 54: 953–965, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wassmann S, Wassmann K, and Nickenig G. Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension 44: 381–386, 2004 [DOI] [PubMed] [Google Scholar]
- 100.Weber KJ. and Schilling JD. Lysosomes integrate metabolic-inflammatory crosstalk in primary macrophage inflammasome activation. J Biol Chem 289: 9158–9171, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, and Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 12: 408–415, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Westermann D, Rutschow S, Van Linthout S, Linderer A, Bucker-Gartner C, Sobirey M, Riad A, Pauschinger M, Schultheiss HP, and Tschope C. Inhibition of p38 mitogen-activated protein kinase attenuates left ventricular dysfunction by mediating pro-inflammatory cardiac cytokine levels in a mouse model of diabetes mellitus. Diabetologia 49: 2507–2513, 2006 [DOI] [PubMed] [Google Scholar]
- 103.Westermann D, Van Linthout S, Dhayat S, Dhayat N, Escher F, Bucker-Gartner C, Spillmann F, Noutsias M, Riad A, Schultheiss HP, and Tschope C. Cardioprotective and anti-inflammatory effects of interleukin converting enzyme inhibition in experimental diabetic cardiomyopathy. Diabetes 56: 1834–1841, 2007 [DOI] [PubMed] [Google Scholar]
- 104.Wold LE, Ceylan-Isik AF, and Ren J. Oxidative stress and stress signaling: menace of diabetic cardiomyopathy. Acta Pharmacol Sin 26: 908–917, 2005 [DOI] [PubMed] [Google Scholar]
- 105.Wu SB, Wu YT, Wu TP, and Wei YH. Role of AMPK-mediated adaptive responses in human cells with mitochondrial dysfunction to oxidative stress. Biochim Biophys Acta 1840: 1331–1344, 2014 [DOI] [PubMed] [Google Scholar]
- 106.Yang J, Sambandam N, Han X, Gross RW, Courtois M, Kovacs A, Febbraio M, Finck BN, and Kelly DP. CD36 deficiency rescues lipotoxic cardiomyopathy. Circ Res 100: 1208–1217, 2007 [DOI] [PubMed] [Google Scholar]
- 107.Yuan Y, Jiao X, Lau WB, Wang Y, Christopher TA, Lopez BL, Ramachandrarao SP, Tao L, and Ma XL. Thioredoxin glycation: a novel posttranslational modification that inhibits its antioxidant and organ protective actions. Free Radic Biol Med 49: 332–338, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yusuf S, Dagenais G, Pogue J, Bosch J, and Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 342: 154–160, 2000 [DOI] [PubMed] [Google Scholar]
- 109.Zhou R, Yazdi AS, Menu P, and Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469: 221–225, 2011 [DOI] [PubMed] [Google Scholar]