Abstract
Retinal disease is the major cause of irreversible blindness in developed countries. Transplantation of photoreceptor precursor cells (PPCs) derived from human embryonic stem cells (hESCs) is a promising and widely applicable approach for the treatment of these blinding conditions. Previously, it has been shown that after transplantation into the degenerating retina, the percentage of PPCs that undergo functional integration is low. The factors that inhibit PPC engraftment remain largely unknown, in part, because so many adverse factors could be at play during in vivo experiments. To advance our knowledge in overcoming potential adverse effects and optimize PPC transplantation, we have developed a novel ex vivo system. Harvested neural retina was placed directly on top of cultured retinal pigment epithelial (RPE) cells from a number of different sources. To mimic PPC transplantation into the subretinal space, hESC-derived PPCs were inserted between the retinal explant and underlying RPE. Explants cocultured with hESC-derived RPE maintained normal gross morphology and viability for up to 2 weeks, whereas the explants cultured on ARPE19 and RPE-J failed by 7 days. Furthermore, the proportion of PPCs expressing ribbon synapse-specific proteins BASSOON and RIBEYE was significantly higher when cocultured with hESC-derived RPE (20% and 10%, respectively), than when cocultured with ARPE19 (only 6% and 2%, respectively). In the presence of the synaptogenic factor thrombospondin-1 (TSP-1), the proportion of BASSOON-positive and RIBEYE-positive PPCs cocultured with hESC-derived RPE increased to ∼30% and 15%, respectively. These data demonstrate the utility of an ex vivo model system to define factors, such as TSP-1, which could influence integration efficiency in future in vivo experiments in models of retinal degeneration.
Introduction
Retinal diseases such as age-related macular degeneration (AMD), diabetic retinopathy, and (to a lesser extent) retinitis pigmentosa as a group are major causes of blindness in the developed world, with over 14 million people blind or severely visually impaired from AMD alone.1 Since photoreceptors and retinal pigment epithelium (RPE) have no regenerative capacity, the tissue damage in these retinal diseases is irreversible. Although gene therapy approaches have been initiated for a few specific retinal diseases,2 for the vast majority this approach would not be relevant. Cell transplantation to replace lost tissue, however, offers the most promising approach in reversing blindness attributable to these conditions.
Significant advances in cell transplantation have been reported both in vitro and in vivo. Seminal work exposing human embryonic stem cells (hESCs) to growth factors and modulators of signaling pathways that mimic normal retinal development has been extremely successful in directing hESCs either toward RPE3,4 or neural retinal cell fate.5,6 Using these techniques, it has been shown that most types of cells found in the neural retina can be generated, including ganglion cells, amacrine cells, horizontal cells, bipolar cells, and photoreceptor cells.4–6 Transplanting hESC-derived cells7 or cells obtained from fetal retinal tissue,8 umbilical cord tissue,9 human adult stem cells,10,11 or reprogrammed-induced pluripotent stem cells12 into retinal disease models has shown significant promise. Results, however, from some studies suggest that although transplantation promotes photoreceptor cell survival and migration, the transplanted cells fail to express retinal-specific markers.13,14 One explanation might be that specific developmental cues are missing from the host transplant environment; alternatively, perhaps, only certain types of photoreceptor precursor cells (PPCs) can fully differentiate in vivo.
Numerous challenges still remain before these early preclinical and clinical proof-of-concept studies can translate into larger clinical trials focused on quantifying functional benefits. One particular aspect that has been recently highlighted is the low percentage of PPCs that survive and functionally integrate after injection into the retina, with an attrition rate reported to be as high as 22–40%.15–19 This may be due to problems of immune rejection or that we are not using the optimal type of PPC in transplantation work.16,17 Equally, anatomical factors and the complications and difficulties of retinal surgery could also be contributing to this high transplantation attrition rate. With so many potential contributors identifying those at work and their relative importance will be challenging in any in vivo model systems. On the basis of the above evidence, we have therefore elected to develop a novel ex vivo model system using the rodent and human retina to study PPC integration with less confounding variables at play.
Materials and Methods
RPE cell lines
All tissue culture reagents were from Life Technologies, unless noted otherwise. The rat RPE cell line RPE-J was a generous gift from Nabi et al.20 Briefly, cells were grown at a temperature of 33°C in a medium containing high-glucose Dulbecco's modified Eagle's medium (DMEM) supplemented with 4% fetal bovine serum (FBS), 1% l-glutamine, 1% penicillin/streptomycin solution, and 1% nonessential amino acids (NEAA). To obtain a differentiated RPE phenotype, 3–3.5×105 cells/cm2 were plated on a growth factor-reduced Matrigel™ (BD Biosciences)-coated 24 mm Transwell polyester filter (Corning) and grown for 6–7 days at 33°C followed by a 48-h growth at 40°C in growth media supplemented with 10 nM all-trans-retinoic acid (Sigma). The human RPE cell line ARPE-1921 was maintained in high-glucose DMEM supplemented with 10% FBS, 1% l-glutamine, and 1% penicillin/streptomycin at 37°C. To obtain a differentiated RPE phenotype,22 1–1.5×105 cell/cm2 were plated on Matrigel-coated Transwell as above, except that FBS was reduced to 1%. Cells were allowed to differentiate for 8–10 weeks.
Human RPE derived from hESCs (hESC-RPE) was generated using published protocols23,24 Briefly, the hESC line WA09 (WiCell Research Institute)25 was allowed to spontaneously mature in standard hESC media (7–10 days). The medium was then changed to differentiation media, which consisted of knockout DMEM (koDMEM), 15% knockout serum replacer (KOSR), 1% l-glutamine, 1% penicillin/streptomycin solution, 1% NEAA, and 0.1 mM β-mercaptoethanol (β-ME; Sigma). The medium was changed every 1–2 days and cells were not passaged further. After 6–8 weeks, clusters of polygonal-shaped cells with brown pigment were clearly visible. The pigmented colonies were manually scraped and transferred to the Transwell filter coated with 0.1% gelatin (Stem Cell Technologies) and expanded in the RPE medium (koDMEM, 7% KOSR, 5% FBS, 1% l-glutamine, 1% penicillin/streptomycin solution, 1% NEAA, and 0.1 mM β-ME).
Human and animal tissue
Human tissue was obtained with written informed consent and complied with the Declaration of Helsinki. Human donor eyes had no known ocular pathology and were used under the guidelines and regulation of the Research Ethics Board at the University of British Columbia. Donors were 54, 59, and 72 years of age (n=3). The average time from death to enucleation was 4, 2.5, and 4 h, respectively. The time from death to retinal harvest was ∼11 h in each case. Rodent retinal tissue from the S334ter model of retinal degeneration26 was carried out with the approval of the Animal Care Committee at the University of British Columbia and in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Retinal explants harvesting and coculture with RPE
Human and rodent ocular tissue was processed in ice-cold phosphate-buffered saline (PBS) supplemented with 1% antibiotic–antimycotic solution (Life Technologies). The cornea, lens, and vitreous were excised and the neurosensory retina (minus the RPE) was teased off the globe after it was detached from the optic nerve. Each retina was cut into four equal parts and was transferred to the back of a wet 12-mm polytetrafluoroethylene (PTFE) Millicell tissue culture insert (Millipore) with the photoreceptor side up using a pipette tip. The dissection medium was aspirated forcing the explants to flatten and limit their movement. RPE cells growing in a Transwell tissue culture insert were washed twice with the Neurobasal-A medium and transferred to a new six-well plate (Nunc) containing 1.35 mL/well of explant medium (Neurobasal-A medium, 2% B-27 supplement, 1% N-2 supplement, 0.4% l-glutamine, and 1% penicillin–streptomycin).27 All Neurobasal-A media were aspirated from the Transwell before the Millicell insert was carefully turned upside down and positioned on top of the RPE cells so that the photoreceptor side of the retina was touching the RPE. Dropwise addition of 150 μL explant medium was distributed on exposed cells. Half of the explant medium was changed the next day and every other day afterward for the duration of the experiment (up to 2 weeks).
Coculture of PPCs with retinal explant and RPE
PPCs were labeled with 20 μM CellTrace™ Far Red DDAO-SE (Life Technologies) according to the manufacturer's protocol. PPCs were then harvested with Accutase (Stem Cell Technologies), incubated with trypan blue (Stem Cell Technologies) to count live cells, and then suspended to 2.5–3×105 live cells in 10 μL explant medium per explant. In experiments that included thrombospondin-1 (TSP-1; Sigma), cells were prepared in the explant medium that contained 5 μg/mL TSP-1.28 For each explant, labeled PPCs were placed on top of the RPE cells before the Millicell insert was positioned on top.
Preparation of cocultures for cryosectioning
Tissues were washed twice with ice-cold PBS and then fixed with cold 4% paraformaldehyde for 4 h at 4°C. This was removed by two, 5-min PBS washes before infiltration with 30% sucrose for 18–24 h at 4°C. Sucrose was replaced with cryoprotectant (Polyfreeze; PolySciences, Inc.) and incubated at −80°C for 18–24 h. On a layer of dry ice, tissue was excised directly into a 15×15-mm cryo mold (Tissue-Tek) containing liquid Polyfreeze using a disposable scalpel. The mold was immediately refrozen at −80°C for at least several hours. Cryostat sections of 14–16 μm thickness were transferred to Superfrost Plus microscope slides (Fisher Scientific). The slides were processed immediately for immunohistochemistry or stored at −80°C for future use.
Immunohistochemistry and detection of apoptosis
Slides were washed in PBS thrice to remove Polyfreeze. The following antibodies were used: the rabbit monoclonal anti-BASSOON antibody (1:200; Cell Signaling Technology); mouse monoclonal anti-RIBEYE antibody (1:250; BD Transduction Laboratories); mouse monoclonal anti-rhodopsin antibody (1:500; Abcam); and the goat anti-rabbit Alexa 488 and goat anti-mouse Alexa 488 secondary antibodies (1:500; Life Technologies). Briefly, slides were incubated for 1 h in the blocking solution (BASSOON: 5% normal goat serum, 0.3% Triton-X-100 in PBS; RIBEYE: 2% normal goat serum, 1% bovine serum albumin (BSA), 0.1% Triton-X-100 in PBS; rhodopsin, 2% normal goat serum, 0.2% Triton-X-100 in PBS) followed by overnight incubation at 4°C with a primary antibody in an antibody dilution buffer (1% BSA, 0.3% Triton X-100 in PBS for BASSOON, and blocking solution for RIBEYE and rhodopsin). After rinsing 3×5 min with PBS, sections were incubated with the appropriate fluorescently conjugated secondary antibody in an antibody dilution buffer for 2 h at room temperature, rinsed, and counterstained for 5 min with 10 μg/mL Hoechst 33342 (Sigma). All slides were rinsed twice with PBS and mounted in Fluoromount-G (SouthernBiotech). Confocal images were acquired using a Zeiss LSM 510 META confocal laser scanning system. To detect levels of apoptotic cell death, the ApopTag® Peroxidase In Situ Apoptosis Detection Kit (Millipore) was used according to the manufacturer's instructions.
Fluorescence-activated cell sorting
To separate the coculture components, the exposed surface between the inserts was washed once with PBS, which was then replaced with 0.5 mL of 0.05% trypsin-EDTA (Life Technologies). The coculture was incubated at 37°C for 7 min. To wash residual cells from the Millicell filter and to assist with tissue dissociation, the insert was removed, the trypsin fluid passed two to three times over the bottom of the filter, and then pipetted up and down. This was followed by inactivation of the trypsin by the addition of 25 μL of FBS. The cell suspension was centrifuged at 300×g for 7 min and the pellet resuspended in 0.5 mL FACSmax cell dissociation solution (AMS Biotechnology). The centrifugation and resuspension were repeated once more and the suspension was filtered through a 40-μm cell strainer (Fisher Scientific). Fluorescently labeled PPCs were captured with a flow cytometer (BD Biosciences Influx Sorter; BD Biosciences) into a cold RLT disruption buffer (Qiagen) for RNA extraction.
Reverse transcription quantitative polymerase chain reaction
Total RNA from fluorescence-activated cell sorting (FACS)-isolated cells was extracted using the RNeasy Micro Kit (Qiagen) according to the manufacturer's instructions. Starting with 1 μg of total RNA, reverse transcription was achieved with the iScript™ cDNA Synthesis Kit (Bio-Rad). RT-qPCR used TaqMan® primer/probe sets for BASSOON (target gene) and GAPDH (reference gene) with the TaqMan Fast Advanced Master Mix (Applied Biosystems). To determine the fold change in BASSOON expression, compared with PPCs before coculture, we used the TaqMan primer/probe system and the ViiA™ 7 Real Time PCR system (Applied Biosystems) as described.25 Data were analyzed by the comparative CT method.29
Statistical analysis
Cells expressing BASSOON or RIBEYE were counted from three independent experiments and the percentages of positive cells were compared between groups using a paired Student's t-test.
Results
Development of the neural retina-RPE ex vivo coculture system
The ex vivo explant system was based initially using previously established media conditions.27 Early experiments, however, established that culture at 37°C was optimal to culture at 34°C as previously reported.27 In addition, in our hands, previous culture protocols proved inadequate because of neurosensory retinal explant movement relative to the culture plate, especially at times of media replenishment. This would prove inadequate when studying PPC integration into the neurosensory retina since it would damage functional connections that form between PPCs and neurosensory retina. We therefore adapted the technique using an alternative tissue culture apparatus,30 where we found that polyester and PTFE membranes were preferable to mixed cellulose ester membranes in the culture apparatus (Fig. 1). We found that when the cellulose ester membranes were wet, they were too opaque to clearly visualize the RPE cells when viewing through an inverted microscope. Therefore, we used polyester membranes in the Transwell insert supporting the RPE monolayer. Furthermore, the neural retina adhered to the PFTE membranes more securely than with mixed cellulose ester membranes, especially when the Millicell insert was inverted. With this combination of membranes and inserts, reproducible cultures were obtained.
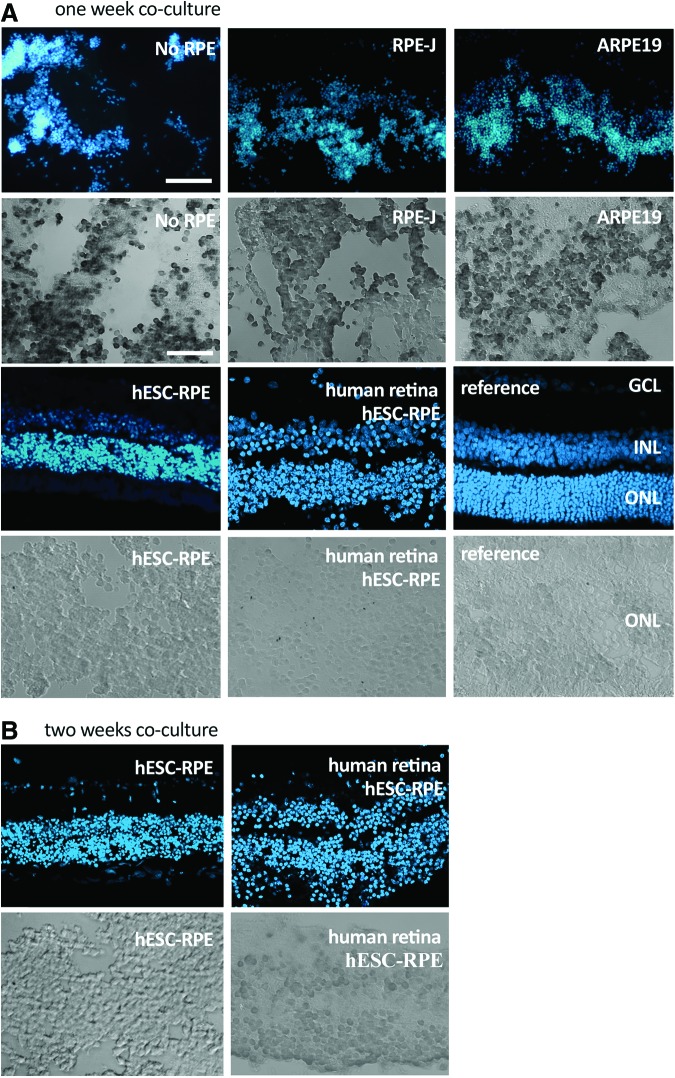
FIG. 1.
Representation of the ex vivo coculture system (not to scale). Top: retinal pigment epithelium (RPE) is grown on the Transwell insert (polyester membrane) and above it is a Millicell insert (PFTE membrane) that has neural retina attached to it, with the photoreceptor surface facing the apical surface of the RPE. For testing integration of photoreceptor precursor cells (PPCs), they were pipetted onto the RPE surface before the retinal explant was added into the culture. Bottom: representative low-magnification image of PPCs (red) between a retinal explant and hESC-RPE. Color images available online at www.liebertpub.com/tea
Whole-tissue survival of explanted, S334ter rat or normal human neurosensory retina in the ex vivo explant system was assessed by comparing the gross architecture of explanted tissue with fresh tissue. Significant improvement in tissue survival was obtained if the mutant rat neurosensory retina was cocultured with a monolayer of cells acting as a substitute RPE (Fig. 2). Without this monolayer, the neurosensory retina deteriorated within just 3–4 days with significant disorganization of retinal architecture by 7 days (Fig. 2A). Although some preservation of the retinal structure was seen with RPE substitutes RPE-J and ARPE19 (Fig. 2A), it was only with pigment epithelial cells derived from hESCs that preservation of tissue was seen at 7 days (Fig. 2A). Interestingly, the best preservation of retinal architecture (with clear preservation of all three nuclear layers) appeared to be when human rather than rodent retinal explants were cocultured with the hESC-RPE. To determine the relative extent of cell death in the explants, we used TUNEL staining on histological sections through the retina. In explants with no RPE, or coculturing with either RPE-J or ARPE19 cells, almost all imaged cell nuclei were TUNEL positive. However, in comparison, we saw very few retinal TUNEL-positive cells when the hESC-RPE was cocultured with neural retina from either rat or human (Fig. 2A), mirroring the better preservation of retinal lamination previously observed. Since the best retinal explant survival at 7 days was correlated with the use of hESC-RPE, we further extended the coculture period to 2 weeks using this type of RPE support (Fig. 2B). Again, we found that all three layers of the retinal architecture were still preserved in both human and rat explants and that there were very few TUNEL-positive photoreceptor nuclei present. These results suggest that human and rodent neurosensory retinal survival ex vivo is enhanced through coculture with hESC-RPE.
FIG. 2.
Survival of explanted human and rat neural retina. Sections through neural retina cocultured with different sources of RPE (no RPE, RPE-J, ARPE19, or human ESC-RPE) at 1 week (A; n=2) and 2 weeks (B; n=2). Blue fluorescent signal highlights the different nuclear layers of the retina in comparison to a fresh tissue section (reference); GCL, ganglion cell layer; INL, inner nuclear layer; ONL, photoreceptor outer nuclear layer. Scale bar=40 μm. The grayscale sections below each immunofluorescent image are high-resolution TUNEL images of the ONL of the coculture above it. Scale bar=50 μm. All cocultures are with rat neural tissue except for the panels labeled as human sections. Color images available online at www.liebertpub.com/tea
Coculturing the ex vivo system with PPCs
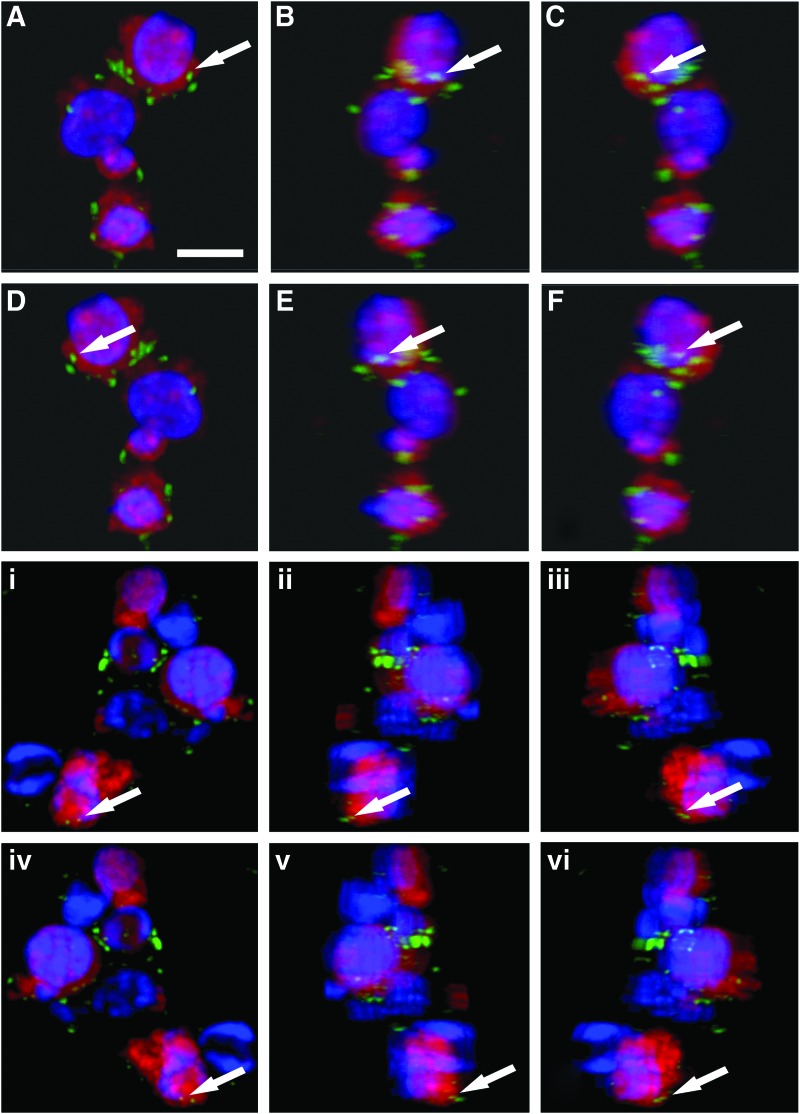
We previously developed a protocol for rapidly differentiating PPCs from hESCs.25 To investigate the maturation of these PPCs, they were prelabeled with a fluorescent tag and then placed between the hESC-RPE and neural retina of the coculture system, mimicking subretinal injection of PPCs in the in vivo environment. At the end of the culture period, we sectioned the cocultures and subjected sections to immunohistochemical analysis or collected and assessed the PPCs using FACS analysis. As seen in previous studies, the PPCs did not show structural signs of maturation (e.g., the formation of outer segments or a cilium). Immunocytochemistry, however, showed that the rhodopsin photopigment was detectable within some PPCs by 1 week (Fig. 3), whereas there was negligible rhodopsin expression in PPCs cultured in standard differentiation media.25 This suggested that there was an accelerated maturation of PPCs within the coculture system.
FIG. 3.
Confocal images of PPCs showing rhodopsin expression (green fluorescence). PPCs cocultured with rodent neurosensory retina (A–F) or human retina (i–vi) for 1 week and identified by red CellTrace dye (nuclei detected with Hoechst 33342). To confirm that rhodopsin expression was within and not on-top of or behind PPCs, confocal images were processed so as to show individual groups of PPCs rotated through 360° (images A–F and i–vi). The arrow points to a specific rhodopsin signal. Scale bar=7 μm. Color images available online at www.liebertpub.com/tea
Testing PPC integration into the neural retina using the ex vivo model system
It has been suggested that functional integration of stem cell-derived photoreceptor precursors is mostly achieved within 7 days of transplantation.31 We therefore used an endpoint of 7 days to study functional integration of PPCs in our coculture system (neurosensory retina on top of hESC-derived pigment epithelial cells). We chose as our functional endpoint and sign of PPC-retinal integration the expression of proteins important in the development of ribbon synapses. This is known to be an early event in the formation of functional synapses between developing photoreceptors and second-order neurons in the neurosensory retina.32 The two key proteins in photoreceptor ribbon synapse formation are BASSOON and RIBEYE.33 Immunohistochemical localization of BASSOON and RIBEYE proteins demonstrated noteworthy expression in transplanted PPCs after just 4 days (Fig. 4). This expression was quantified at day 7 when either the rat or human neurosensory retina was used in the coculture system (Table 1). Only 2% of PPCs were found to be BASSOON positive in PPCs kept in standard differentiation media.
FIG. 4.
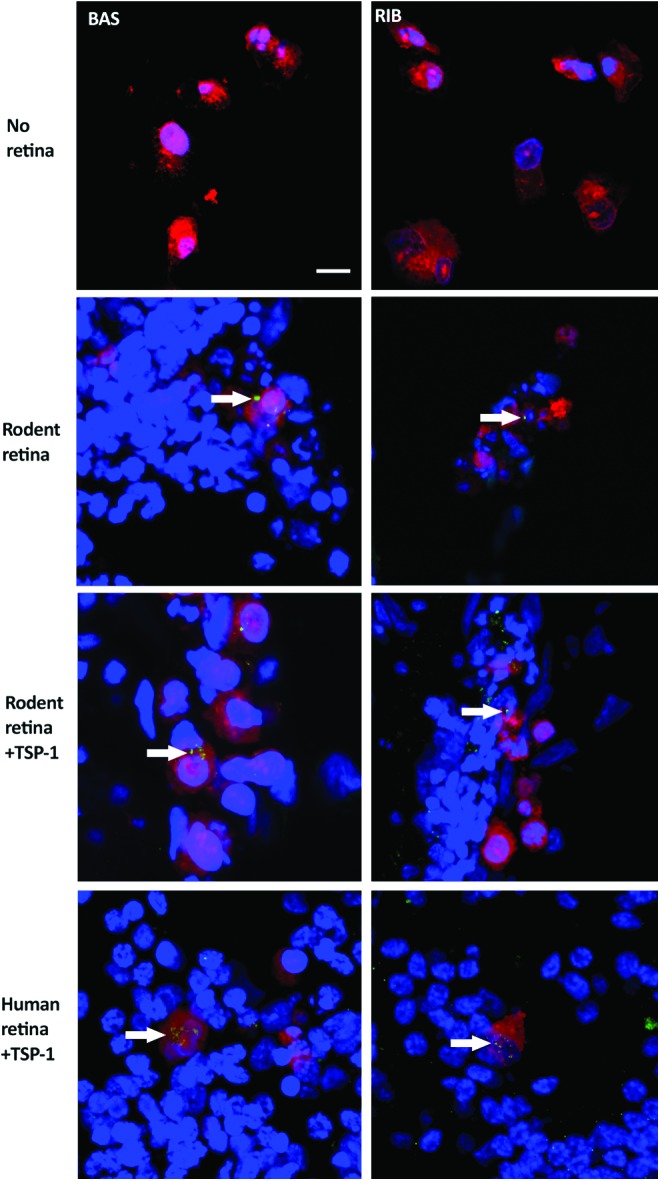
PPCs showing BASSOON and RIBEYE expression after 4 days of coculture. In rodent and human retinal explant coculture, confocal images of PPCs labeled with CellTrace™ Far Red, and BASSOON (BAS) and RIBEYE (RIB) localization are shown in green (arrows). When PPCs are incubated in standard tissue culture conditions, they do not express either BASSOON or RIBEYE. To stimulate synapse formation, cocultures were treated with thrombospondin-1 (TSP-1). Scale bar=10 μm. Color images available online at www.liebertpub.com/tea
Table 1.
Quantitative Immunohistochemical Expression of Ribbon Synapse Proteins BASSOON and RIBEYE in Human Photoreceptor Precursor Cells (n=3)
| BASSOON | RIBEYE | |||
|---|---|---|---|---|
| Conditions | TSP-1–ve (%) | TSP-1+ve (%) | TSP-1–ve (%) | TSP-1+ve (%) |
| A—Media only | 2 | — | 0 | — |
| B—Rodent retina ARPE19 | 6 | — | 2 | — |
| C—Rodent retina, Hrpe | 20 | 28 | 10 | 15 |
| D—Human retina, hRPE | 21 | 36 | 13 | 18 |
Percentage of cells expressing protein after 1-week exposure to A—culture media only; B—rodent retina and ARPE19 cells; C—rodent retina and human-RPE; D—human retina and human-RPE. Results were compared with and without exposure to thrombospondin-1. Results suggest that most BASSOON/RIBEYE was expressed after PPC exposure to human retinal explant, human RPE, and TSP-1.
PPC, photoreceptor precursor cells; RPE, retinal pigment epithelial; TSP-1, thrombospondin-1.
When PPCs were cocultured with neurosensory retina and ARPE19 cells, there was a negligible increase (to 6% of PPCs). A much more noticeable 10-fold increase in BASSOON-positive PPCs (∼20% of PPCs) was, however, seen in cocultures of neurosensory retina and hESC-RPE. This enhanced BASSOON expression was confirmed with qRT-PCR where there was only a 2.4±0.16-fold increase in BASSOON expression when PPCs were exposed to an extended (7 day) period in differentiation media, but a 4.9±0.39-fold increase was seen when PPCs were cocultured with rodent neurosensory retina and hESC-RPE. Similar results were obtained when assessing RIBEYE expression. We found no evidence of RIBEYE expression in PPCs exposed to an extended period in differentiation media. In coculture with neurosensory retina and hESC-RPE, however, ∼10–13% of PPCs were RIBEYE positive. Interestingly, when PPC coculture with neurosensory retina and hESC-RPE was extended from 4 to 7 days, the distribution of RIBEYE remained unchanged (random spots within cells). The expression of BASSOON, however, seemed to change from a random distribution to more focal ring-like formations (Fig. 5).
FIG. 5.
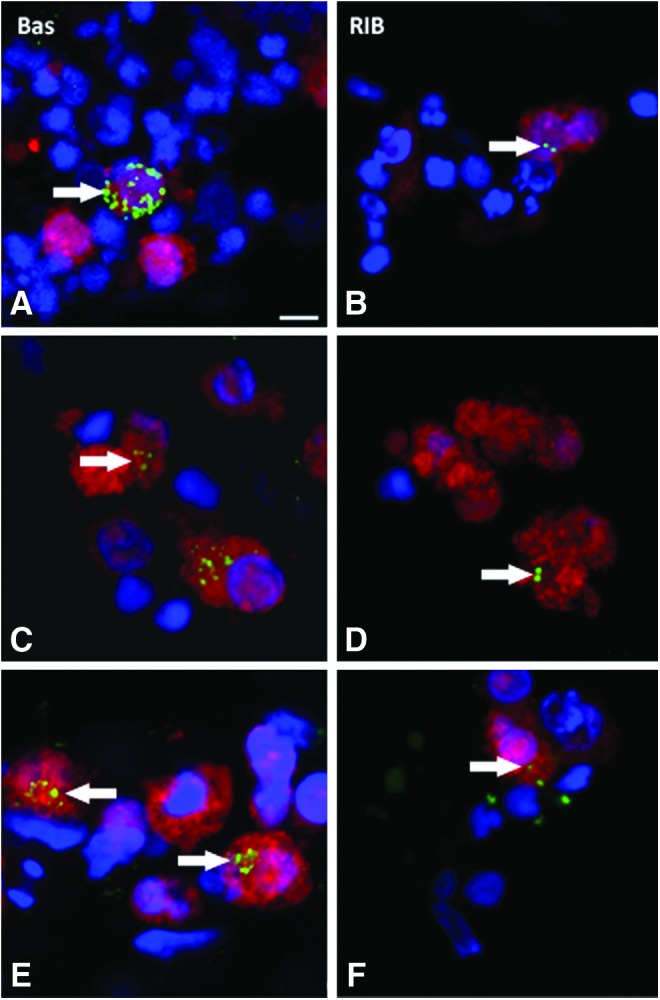
PPCs cocultured with human retina for 1 week, confocal images comparing BASSOON (BAS) and RIBEYE (RIB) at 4 days (A, B) with cells after coculture for 1 week (C, D) and also after 1 week with added thrombospondin (E, F). Changes in the expression pattern of BASSOON at 4 days (random) and 1 week (sphere-like) suggest intracellular trafficking of ribbon synapse proteins. Arrows indicate the localization of BASSOON and RIBEYE. Scale bar=5 μm. Color images available online at www.liebertpub.com/tea
To determine whether trophic factors could augment the expression of ribbon synapse proteins, TSP-1 was added to culture media. This is a secreted glycoprotein in the extracellular matrix that mediates synapse formation in the developing central nervous system.28 In the presence of TSP-1, the number of PPCs expressing BASSOON and RIBEYE proteins was increased (Table 1 and Fig. 5); however, this effect was most significant for BASSOON-positive cells (30–36% positive cells; p<0.05).
Discussion
Successful photoreceptor cell replacement therapy is dependent on numerous factors, including limiting damage caused by the transplantation surgery, effective migration, and integration of the donor cells into the host retinal tissue, differentiation and maturation of the transplanted cells into the appropriate cell type, synapse connection with second-order neurons, restoration of visual function, and long-term survival of the replacement cells. Although the common denominator in different retinal degenerations is photoreceptor cell death, other changes in the degenerative retinal environment will additionally contribute to the success or failure of the cell replacement therapy.34 Isolating each of these problems for further study is difficult in the context of an in vivo model.
A significant challenge that still remains is the need to increase the number of PPCs that undergo functional integration into the degenerate retina. Therefore, we set out to develop an ex vivo culture system that could be used to test a variety of factors that might improve the functional integration of transplanted PPCs, by reducing the number of external confounding factors (e.g., immune rejection, surgical damage to the host environment, and damage to cells through injection process). We have designed a robust system that gave reproducible cultures. Our results suggest that using this novel coculture system, mutant rodent and particularly human neurosensory retina can maintain its gross laminar architecture and show minimal signs of cell death for at least 14 days.
This appeared to be comparable with previously reported studies,27 but with the added advantage that fixed immobile explants could be maintained to better study functional integration.
The key elements found that led to success in neurosensory explant survival included culture conditions at 37°C (rather than 34°C, Johnson et al.27), using different tissue culture insert membranes and most importantly, coculture with the pigmented epithelium. The best results were obtained with hESC-RPE. That these cells might be the most competent functionally is not entirely unexpected since others have already commented on the limitations of RPE-J and ARPE19 cell lines as true surrogates for RPE. For example, the RPE-J line does not express CRALBP suggesting there would be defective visual cycle processing between the photoreceptors and RPE.35 The ARPE-19 line has compromised barrier and polarization defects21 and does not express bestrophin, Rpe65, and transducin, all key RPE-specific functional proteins.23 Our results suggest that RPE-J and ARPE-19 cell lines likely fail to secrete the necessary cues or provide the necessary architectural support required for photoreceptor survival and in that sense could therefore be considered as compromised pigment epithelium similar to the pigment epithelium deficits seen as either primary or secondary phenomena in many retinal dystrophies.36,37
This finding that viable pigment epithelium enhances effective integration of PPCs into neurosensory retina could have implications for future in vivo studies. Our results suggest that the compromised RPE in retinal dystrophy patients may, in part, explain the limited PPC integration seen in some in vivo studies.15 It could therefore be concluded that in future studies, pigment epithelial integrity should also be addressed concurrently during photoreceptor cell replacement.
The development of ribbon synapse is the first event in the formation of synaptic connections in the retina.32 In the rodent, this is initiated at about P6 and is completed when the eyes open at P14.38 The assembly of photoreceptor ribbon synapses involves the formation and trafficking of sphere-like protein aggregates of the ribbon matrix proteins BASSOON, RIBEYE, Piccolo, and RIM1.38 In animal models lacking RIBEYE, the ribbon synapse fails to develop properly and the optokinetic response driven from photoreceptor connectivity to second-order neurons is lost,39 highlighting the critical role of ribbon synapse formation in visual function. We used BASSOON and RIBEYE as markers in further assessing functional integration of PPCs into neurosensory retina. The spherical collections of BASSOON within maturing PPCs seen in our study may reflect the intracellular trafficking of ribbon synapse proteins, which further suggests that PPCs are attempting to form functional ribbon synapses. We also further saw that this could be enhanced by thrombospondin suggesting that this extracellular signaling molecule may have a role in future in vivo studies.
Previous studies have suggested that lengthy incubation in culture media is required before signs of significant PPC maturation, such as rhodopsin photopigment expression, are seen.40 It was therefore interesting to see in our system that significant rhodopsin expression could be detected in PPCs with exposure to ex vivo neurosensory retina and a supporting pigment epithelium layer. This suggests that supplementary cues for PPC maturation are being provided either by the explanted neurosensory retina and/or the hESC-derived pigment epithelium. It is currently not known which stage of PPC development is the best to use in transplantation work. This accelerated maturation phenomena might therefore be taken advantage of in future work to manufacture PPCs for transplantation at a more advanced stage of development.
In conclusion, our novel ex vivo system cannot be used to assess the roles of all confounding variables in PPC transplantation. For example, it cannot fully assess the role of immune rejection. It does, however, give us an opportunity to isolate other adverse conditions (such as the influence of host tissue on PPC maturation) giving results that can provide a more rational approach to innovation in future in vivo work.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research Team Grant (No. 222728) and the Sharon Stewart Trust Team Grant.
Disclosure Statement
No competing financial interests exist.
References
- 1.Gehrs K.M., Anderson D.H., Johnson L.V., and Hageman G.S. Age-related macular degeneration emerging pathogenetic and therapeutic concepts. Ann Med 38, 450, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boye S.E., Boye S.L., Lewin A.S., and Hauswirth W.W. A comprehensive review of retinal gene therapy. Mol Ther 21, 509, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Idelson M., Alper R., Obolensky A., Ben-Shushan E., Hemo I., Yachimovich-Cohen N., Khaner H., Smith Y., Wiser O., Gropp M., Cohen M.A., Even-Ram S., Berman-Zaken Y., Matzrafi L., Rechavi G., Banin E., and Reubinoff B. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell 5, 396, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Lu B., Malcuit C., Wang S., Girman S., Francis P., Lemieux L., Lanza R., and Lund R. Long-term safety and function of RPE from human embryonic stem cells in preclinical models of macular degeneration. Stem Cells 27, 2126, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Lamba D.A., Karl M.O., Ware C.B., and Reh T.A. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci U S A 103, 12769, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osakada F., Ikeda H., Mandai M., Wataya T., Watanabe K., Yoshimura N., Akaike A., Sasai Y., and Takahashi M. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol 26, 215, 2008 [DOI] [PubMed] [Google Scholar]
- 7.La Torre A., Lamba D.A., Jayabalu A., and Reh T.A. Production and transplantation of retinal cells from human and mouse embryonic stem cells. Methods Mol Biol 884, 229, 2012 [DOI] [PubMed] [Google Scholar]
- 8.MacLaren R.E., Pearson R.A., MacNeil A., Douglas R.H., Salt T.E., Akimoto M., Swaroop A., Sowden J.C., and Ali RR. Retinal repair by transplantation of photoreceptor precursors. Nature 444, 203, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Lund R.D., Wang S., Lu B., Girman S., Holmes T., Sauvé Y., Messina D.J., Harris I.R., Kihm A.J., Harmon A.M., Chin F.Y., Gosiewska A., and Mistry S.K. Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells 25, 602, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Wohl S.G., Schmeer C.W., and Isenmann S. Neurogenic potential of stem/progenitor-like cells in the adult mammalian eye. Prog Retin Eye Res 31, 213, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Inoue T., Coles B.L., Dorval K., Bremner R., Bessho Y., Kageyama R., Hino S., Matsuoka M., Craft C.M., McInnes R.R., Tremblay F., Prusky G.T., and van der Kooy D. Maximizing functional photoreceptor differentiation from adult human retinal stem cells. Stem Cells 28, 489, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips M.J., Wallace K.A., Dickerson S.J., Miller M.J., Verhoeven A.D., Martin J.M., Wright L.S., Shen W., Capowski E.E., Percin E.F., Perez E.T., Zhong X., Canto-Soler M.V., and Gamm D.M. Blood-derived human iPS cells generate optic vesicle-like structures with the capacity to form retinal laminae and develop synapses. Invest Ophthalmol Vis Sci 53, 2007, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizumoto H., Mizumoto K., Whiteley S.J., Shatos M., Klassen H., and Young M.J. Transplantation of human neural progenitor cells to the vitreous cavity of the Royal College of Surgeons rat. Cell Transplant 10, 223, 2001 [PubMed] [Google Scholar]
- 14.Chacko D.M., Das A.V., Zhao X., James J., Bhattacharya S., and Ahmad I. Transplantation of ocular stem cells: the role of injury in incorporation and differentiation of grafted cells in the retina. Vision Res 43, 937, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Singh M.S., Charbel Issa P., Butler R., Martin C., Lipinski D.M., Sekaran S., Barnard A.R., and MacLaren R.E. Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proc Natl Acad Sci U S A 110, 1101, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pop-Fanea L., Vallespin S.N., Hutchison J.M., Forrester J.V., Seton H.C., Foster M.A., and Liversidge J. Evaluation of MRI for in vivo monitoring of retinal damage and detachment in experimental ocular inflammation. Magn Reson Med 53, 61, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Cheng H., Nair G., Walker T.A., Kim M.K., Pardue M.T., Thulé P.M., Olson D.E., and Duong T.Q. Structural and functional MRI reveals multiple retinal layers. Proc Natl Acad Sci U S A 103, 17525, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu G., Seiler M.J., Mui C., Arai S., Aramant R.B., de Juan E., Jr., and Sadda S. Photoreceptor differentiation and integration of retinal progenitor cells transplanted into transgenic rats. Exp Eye Res 80, 515, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Thomas B.B., Arai S., Ikai Y., Qiu G., Chen Z., Aramant R.B., Sadda S.R., and Seiler M.J. Retinal transplants evaluated by optical coherence tomography in photoreceptor degenerate rats. J Neurosci Methods 151, 186, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Nabi I.R., Mathews A.P., Cohen-Gould L., Gundersen D., and Rodriguez-Boulan E. Immortalization of polarized rat retinal pigment epithelium. J Cell Sci 104, 37, 1993 [DOI] [PubMed] [Google Scholar]
- 21.Dunn K.C., Aotaki-Keen A.E., Putkey F.R., and Hjelmeland L.M. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res 62, 155, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Ahmado A., Carr A.J., Vugler A.A., Semo M., Gias C., Lawrence J.M., Chen L.L., Chen F.K., Turowski P., da Cruz L., and Coffey P.J. Induction of differentiation by pyruvate and DMEM in the human retinal pigment epithelium cell line ARPE-19. Invest Ophthalmol Vis Sci 52, 7148, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Klimanskaya I., Hipp J., Rezai K.A., West M., Atala A., and Lanza R. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells 6, 2172, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Lund R.D., Wang S., Klimanskaya I., Holmes T., Ramos-Kelsey R., Lu B., Girman S., Bischoff N., Sauvé Y., and Lanza R. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells 8, 189, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Yanai A., Laver C.R., Joe A.W., Viringipurampeer I.A., Wang X., Gregory-Evans C.Y., and Gregory-Evans K. Differentiation of human embryonic stem cells using size-controlled embryoid bodies and negative cell selection in the production of photoreceptor precursor cells. Tissue Eng Part C Methods 19, 755, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J., Makino C.L., Peachey N.S., Baylor D.A., and Simon M.I. Mechanisms of rhodopsin inactivation in vivo as revealed by a COOH-terminal truncation mutant. Science 267, 374, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Johnson T.V., and Martin K.R. Development and characterization of an adult retinal explant organotypic tissue culture system as an in vitro intraocular stem cell transplantation model. Invest Ophthalmol Vis Sci 49, 3503, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Christopherson K.S., Ullian E.M., Stokes C.C., Mullowney C.E., Hell J.W., Agah A., Lawler J., Mosher D.F., Bornstein P., and Barres B.A. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120, 421, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Livak K.J., and Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25, 402, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Carr A.J., Vugler A., Lawrence J., Chen L.L., Ahmado A., Chen F.K., Semo M., Gias C., da Cruz L., Moore H.D., Walsh J., and Coffey P.J. Molecular characterization and functional analysis of phagocytosis by human embryonic stem cell-derived RPE cells using a novel human retinal assay. Mol Vis 15, 283, 2009 [PMC free article] [PubMed] [Google Scholar]
- 31.Warre-Cornish K., Barber A.C., Sowden J.C., Ali R.R., and Pearson R.A. Migration, integration and maturation of photoreceptor precursors following transplantation in the mouse retina. Stem Cells Dev 23, 941, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nuhn J.S., and Fuerst P.G. Developmental localization of adhesion and scaffolding proteins at the cone synapse. Gene Expr Patterns 16, 36, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tom Dieck S., Altrock W.D., Kessels M.M., Qualmann B., Regus H., Brauner D., Fejtová A., Bracko O., Gundelfinger E.D., and Brandstätter J.H. Molecular dissection of the photoreceptor ribbon synapse: physical interaction of Bassoon and RIBEYE is essential for the assembly of the ribbon complex. J Cell Biol 168, 825, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.West E.L., Pearson R.A., Tschernutter M., Sowden J.C., and MacLaren R.E., and Ali R.R. Pharmacological disruption of the outer limiting membrane leads to increased retinal integration of transplanted photoreceptor precursors. Exp Eye Res 86, 601, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.West K.A., Yan L., Miyagi M., Crabb J.S., Marmorstein A.D., Marmorstein L., and Crabb JW. Proteome survey of proliferating and differentiating rat RPE-J cells. Exp Eye Res 73, 479, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Pfeffer B.A., and Philp N.J. Cell culture of retinal pigment epithelium: special issue. Exp Eye Res 126, 1, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Bonilha V.L. Retinal pigment epithelium (RPE) cytoskeleton in vivo and in vitro. Exp Eye Res 126, 38, 2014 [DOI] [PubMed] [Google Scholar]
- 38.Regus-Leidig H., Tom Dieck S., Specht D., Meyer L., and Brandstätter J.H. Early steps in the assembly of photoreceptor ribbon synapses in the mouse retina: the involvement of precursor spheres. J Comp Neurol 512, 814, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Wan L., Almers W., and Chen W. Two ribeye genes in teleosts: the role of Ribeye in ribbon formation and bipolar cell development. J Neurosci 25, 941, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakano T., Ando S., Takata N., Kawada M., Muguruma K., Sekiguchi K., Saito K., Yonemura S., Eiraku M., and Sasai Y. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771, 2012 [DOI] [PubMed] [Google Scholar]