Abstract
Appropriate selection of scaffold architecture is a key challenge in cartilage tissue engineering. Gap junction-mediated intercellular contacts play important roles in precartilage condensation of mesenchymal cells. However, scaffold architecture could potentially restrict cell–cell communication and differentiation. This is particularly important when choosing the appropriate culture platform as well as scaffold-based strategy for clinical translation, that is, hydrogel or microtissues, for investigating differentiation of chondroprogenitor cells in cartilage tissue engineering. We, therefore, studied the influence of gap junction-mediated cell–cell communication on chondrogenesis of bone marrow-derived mesenchymal stromal cells (BM-MSCs) and articular chondrocytes. Expanded human chondrocytes and BM-MSCs were either (re-) differentiated in micromass cell pellets or encapsulated as isolated cells in alginate hydrogels. Samples were treated with and without the gap junction inhibitor 18-α glycyrrhetinic acid (18αGCA). DNA and glycosaminoglycan (GAG) content and gene expression levels (collagen I/II/X, aggrecan, and connexin 43) were quantified at various time points. Protein localization was determined using immunofluorescence, and adenosine-5′-triphosphate (ATP) was measured in conditioned media. While GAG/DNA was higher in alginate compared with pellets for chondrocytes, there were no differences in chondrogenic gene expression between culture models. Gap junction blocking reduced collagen II and extracellular ATP in all chondrocyte cultures and in BM-MSC hydrogels. However, differentiation capacity was not abolished completely by 18αGCA. Connexin 43 levels were high throughout chondrocyte cultures and peaked only later during BM-MSC differentiation, consistent with the delayed response of BM-MSCs to 18αGCA. Alginate hydrogels and microtissues are equally suited culture platforms for the chondrogenic (re-)differentiation of expanded human articular chondrocytes and BM-MSCs. Therefore, reducing direct cell–cell contacts does not affect in vitro chondrogenesis. However, blocking gap junctions compromises cell differentiation, pointing to a prominent role for hemichannel function in this process. Therefore, scaffold design strategies that promote an increasing distance between single chondroprogenitor cells do not restrict their differentiation potential in tissue-engineered constructs.
Introduction
Damage to articular cartilage due to injuries or diseases frequently leads to a loss of the tissue's structure and function. This causes pain and impaired mobility for affected people and places a significant financial burden on healthcare systems and economies worldwide. Despite major advances in cartilage tissue engineering aimed at growing the patient's own cells on scaffolding materials for subsequent re-transplantation, the reliable, functional repair or regeneration of articular cartilage remains a challenge.1 Key challenges in cartilage tissue engineering include the appropriate selection of cell source and scaffold architecture.
Multipotent mesenchymal stromal cells from human bone marrow (BM-MSC) and chondrocytes isolated from articular cartilage biopsies are currently the main sources for chondroprogenitor cells. However, we are still lacking appropriate culture protocols to initiate stable chondrogenic phenotypes of these cell types in vitro. For example, articular chondrocytes, in particular, when harvested from clinically relevant elderly patients, rapidly dedifferentiate during ex vivo propagation and lose their chondrogenic phenotype.2 Unfortunately, this process seems only partially reversible under in vitro conditions as seen by sustained high levels of collagen type I, a marker of inferior fibrocartilage formation.2,3 BM-MSCs, on the other hand, tend to differentiate in a process more reminiscent of endochondral ossification, including the onset of tissue hypertrophy and calcification.4 To overcome some of these issues and to support the ex vivo differentiation of chondroprogenitor cells and the formation of functional cartilage tissue, a wide range of approaches for cell culture have been developed that could broadly be classified into scaffold-/matrix-based and biomaterial-free techniques.5,6 Among the former, hydrogels are very popular matrices for cartilage tissue engineering, as they provide a three-dimensional (3D) culture environment favorable for chondrogenic differentiation.7–9 The simplest and most widely used scaffold-free method is micromass pellet or microtissue culture, in which cell pellets are formed by centrifugation, allowing extensive cell–cell communication at the start of the culture.10,11 This may be important, in particular, in the early stages of cell differentiation, considering the importance of cell–cell contacts to initiate chondrogenesis during precartilage condensation of mesenchymal cells in skeletogenesis.12 However, the architecture of hydrogels, where the majority of cells are spatially separated by the hydrogel matrix, seems to inherently restrict direct cell–cell contacts and could potentially affect chondrogenic differentiation.
Intercellular communication during early chondrogenesis occurs via cell adhesion molecules, such as N-cadherin and neural cell adhesion molecule, or gap junctions.13,14 While the first two molecules disappear in the course of chondrogenesis,13 gap junctions can still be found in mature cartilage.15 Gap junctions are intercellular channels formed by connexins that allow for rapid exchange of ions, metabolites, and other small-molecule messengers.16 They are expressed in virtually all tissues and are involved in various cellular functions, including cell growth, differentiation, and death. One half of a gap junction channel that is not connected to an adjacent cell can still be functional and is termed a hemichannel. The major member of the connexin protein family in human cartilage is connexin 43 (Cx43, expressed by the GJA1 gene).17 Apart from the role of gap junctions in mesenchymal cell differentiation, connexin hemichannels also seem to be involved in chondrocyte mechanotransduction.18 However, the role of gap junctional communication during the different stages of chondroprogenitor differentiation and its overall contribution to cartilage tissue formation is still less well defined. This is important in considering the appropriate culture platform, that is, hydrogel based or microtissues, for the different cell types.
This study was, therefore, aimed at comparing the differentiation potential of human articular chondrocytes and BM-MSCs in hydrogel and micromass culture systems with a particular focus on the influence of gap junction-mediated cell–cell interactions on in vitro chondrogenesis. We employed sodium alginate hydrogels and micromass pellets as 3D differentiation models that either restricted or supported direct intercellular contacts. We also used a pharmaceutical inhibitor of gap junction activity to test the importance of this form of cell–cell communication during various stages of chondroprogenitor differentiation and the formation of cartilaginous tissue in vitro.
Materials and Methods
Cell isolation and expansion
Articular cartilage was collected with institutional ethics approval from consenting patients (four male donors, age 26–50 years) undergoing either limb amputations or reconstructions of the anterior cruciate ligament. Cartilage specimens were frozen in Optimal Cutting Temperature compound (OCT; Sakura, Finetek, Tokyo, Japan) for histological analysis. Chondrocytes were isolated from full-thickness cartilage of femoral condyles or grooves as previously described.19 Chondrocytes were propagated on tissue culture plastic (3000 cells/cm2) in chondrocyte basal medium (low-d-glucose Dulbecco's Modified Eagle Medium with 4 mM L-alanyl-L-glutamine, 1 mM sodium pyruvate, 10 mMN-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) (HEPES), 0.1 mM nonessential amino acids, 50 U/mL penicillin/50 μg/mL streptomycin (Pen/Strep; all from Life Technologies, Carlsbad, CA), 0.1 mM L-ascorbic acid 2-phosphate, and 0.4 mM L-proline (both Sigma Aldrich, St. Louis, MO)) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT). Cells were used in subsequent experiments after passage 2 (approximately five to six population doublings).
Mesenchymal stromal cells were isolated from bone marrow aspirates obtained under appropriate ethical approval from the iliac crest of consenting patients (one female+two male donors, age 22–30 years) undergoing spinal fusion surgery. Total bone marrow cells were plated in α-MEM supplemented with 10% FBS, Pen/Strep, and 1 ng/mL fibroblast growth factor 2 (FGF-2; Millipore, Billerica, MA). BM-MSCs were initially seeded at 100 cells/cm2, subsequently plated at 1000 cells/cm2, and used between passages 3 and 4.
All cells were maintained at 37°C in a humidified 5% CO2/95% air CO2 incubator with medium refreshed twice per week. Cells were passaged when subconfluent with 0.25% trypsin with 1 mM ethylenediamine tetraacetic acid (trypsin/EDTA; Life Technologies) at 37°C for 5 min and counted by trypan blue (Life Technologies) exclusion in a hemocytometer.
Chondrogenic differentiation
Chondrogenic potential was evaluated using either micromass pellet or alginate hydrogel cultures. Pellets of both chondrocytes and BM-MSCs were formed by centrifugation.19 Briefly, cells resuspended in a chondrogenic media were transferred into V-bottom-shaped, 96-well microplates (AXYGEN Scientific, Union City, CA) at 2×105 cells in 0.2 mL media per well and centrifuged at 600 g for 5 min. After 3 days, cells had condensed into micropellets that could be lifted from the bottom of the wells and transferred into 48-well plates containing 0.5 mL chondrogenic media. Cells were also encapsulated in 2% w/v sodium alginate (Pronova UP LVG, Novamatrix/FMC BioPolymers, Sandvika, Norway) at 107 cells/mL.20 Briefly, the cell alginate suspension was transferred into wells (height: 1.5 mm, diameter: 4 mm, volume: ∼20 μL) of a stainless steel custom-made mold, placed between sterile filter papers, and soaked in 102 mM CaCl2 for 10 min. Hydrogel constructs were then washed with serum-free basal medium twice for 5 min and transferred into 48-well plates containing 0.5 mL chondrogenic media. Cells were cultivated in serum-free high-glucose chondrocyte basal medium supplemented with 1.25 mg/mL bovine serum albumin, 10−7 M dexamethasone, (all from Sigma), 1% v/v ITS, and 10 ng/mL transforming growth factor type beta 1 (TGF-β1; both Life Technologies) and kept under reduced (5%) oxygen tension in a ProOx C-Chamber (Biospherix, Redfield, NY) inside a cell culture incubator. To reversibly block gap junction activity, 80 μM of 18-α glycyrrhetinic acid (18αGCA; Sigma) was added to the chondrogenic media of selected constructs at various time intervals. This concentration for 18αGCA is well less than what has been reported to be toxic to human fibroblasts in long-term experiments but potent enough to reduce intercellular junctional communication in albumin-containing cultures.21 Cultures were maintained till 9 days with media refreshed every 3 days and conditioned media was stored at −80°C for further analysis. Photographs of pellets were taken with a phase-contrast microscope (NIKON, Tokyo, Japan) at day 4 and 9 of culture, and pellet sizes were measured using ImageJ (National Institutes of Health, Bethesda, MD).
Quantification of glycosaminoglycans and DNA
Cells in constructs were harvested for glycosaminoglycan (GAG) and DNA quantification by incubation with 0.5 mg/mL proteinase K (Life Technologies) in a phosphate buffer (20 mM Na2HPO4, 30 mM NaH2PO4.H2O, and 5 mM EDTA, pH 7.1) at 60°C overnight.
The GAG content of conditioned media as well as digested alginate samples and cell pellets was quantified using the 1,9-dimethylmethylene blue dye (DMMB) assay (pH 1.5).22 Absorbances at 525 and 595 nm were measured in a Benchmark Plus microplate spectrophotometer (Bio-Rad Laboratories, Hercules, CA), and the concentrations were calculated using the ratio of absorbances, compared with a quadratic standard curve prepared from chondroitin sulfate C (Sigma). Data from the first media change were excluded to allow for diffusion of unbound alginate from gels groups.
The Quant-iT™ PicoGreen® dsDNA assay (Life Technologies) was used to quantify DNA content in digested samples, with fluorescence (485 nm excitation, 520 nm emission) measured using a POLARstar OPTIMA fluorescence micro-plate reader (BMG Labtech, Offenburg, Germany).
Ribonucleic acid extraction and real-time reverse transcriptase-polymerase chain reaction
To measure absolute expression levels of selected genes of interest, real-time reverse transcriptase-polymerase chain reaction (qRT-PCR) was used. Ribonucleic acid (RNA) was isolated using the PureLink™ RNA Micro Kit (Life Technologies) according to the manufacturer's instructions, which included mechanic disruption of cell pellets with pestles and dissolution of alginate hydrogels in 10 mM HEPES, 50 mM sodium EDTA, and 150 mM NaCl for 10 min, followed by sample homogenization with a 20-gauge needle and the removal of genomic DNA by column treatment with DNase I (Life Technologies).
First-strand complementary DNA (cDNA) synthesis was performed using the SuperScript™ III first-strand synthesis supermix for qRT-PCR (Life Technologies) with no more than 300 ng of total RNA in 20 μL reactions following the manufacturer's protocol.
Primers were either used as previously published for COL1A1, COL2A1, COL10A1, ACAN, RPL13A, and B2M23 or designed using PrimerBank24 and Primer BLAST (NCBI, Bethesda, MD) for GJA1 (5′→3′ F: GACCGGATAGTCAAGTTCGTAGC, R: GCAGGAGCTGTCCACGTAG), and TBP (5′→3′ F: GAGCCAAGAGTGAAGAACAGTC, R: CATCACAGCTCCCCACCATATT). All PCR reactions were done in duplicate in 10 μL volumes in a 7500 PCR system (Applied Biosystems, Foster City, CA). Reactions contained 1× SYBR® Green PCR Master Mix (Applied Biosystems), 200 nM of each forward and reverse primers, and 0.3 μL of undiluted cDNA and started with 10 min denaturation at 95°C followed by 40 cycles of 95°C for 15 s and 60°C for 30 s. The cycle threshold (Ct) value of each gene was normalized to the geometric mean of the housekeeping genes B2M, RPL13A, and TBP using the comparative Ct method (2−ΔCt).
Immunofluorescence staining analysis
To determine the expression of proteins of interest, samples from alginate constructs, pellets, and native cartilage were frozen in OCT (Sakura) and sectioned. Fresh-frozen sections were fixed in 100% cold acetone for 15 min and then hydrated in 50 mM BaCl2/100 mM Tris HCl (pH 7.3) buffer for 1 h. Antigen retrieval was only included in the collagen I and II staining procedures by applying 0.1% w/v hyaluronidase (Sigma) in phosphate-buffered saline (PBS) for 20 min at 37°C. Sections were then probed overnight at 4°C in PBS with 2% donkey or goat serum (2% serum/PBS; Jackson ImmunoResearch, West Grove, PA) and one of the following antibodies: anti-collagen I (mouse IgG2a, 1:300, I-8H5; MP Biomedicals, Solon, OH), anti-collagen II (mouse IgG1, 1:200, II-II6B3; Developmental Studies Hybridoma Bank [DSHB]), anti-aggrecan (mouse IgG1, 1:300, 969D4D11; Life Technologies), anti-connexin 43 (rabbit IgG, 1:200, C6219; Sigma), or isotype controls (rabbit IgG, ABCAM/Sapphire, Redfern, NSW; mouse IgG, Jackson; both 1:1000).
Subsequently, samples were incubated in 2% serum/PBS with a mix of either AlexaFluor®488-labeled goat anti-mouse (IgG1-specific) and AlexaFluor®594-labeled goat anti-mouse (IgG2a-specific) or AlexaFluor488-labeled donkey anti-mouse IgG and AlexaFluor594-labeled donkey anti-rabbit IgG (all minimal x-reaction with other species, 1:150, Jackson) and 5 μg/mL 4′,6-diamidino-2-phenylindole (DAPI; Sigma) for 1 h at room temperature. Images were captured with fixed exposure times on an Axio Imager.A1 microscope with epi-fluorescence attachment (Carl Zeiss, Jena, Germany).
Adenosine-5′-triphosphate assay
To quantify adenosine-5′-triphosphate (ATP) released into media, samples were taken at day 9 of chondrogenic culture, heated for 5 min at 95°C to inactivate ATPases, and stored at −80°C. The ATP Determination kit (Life Technologies) was used according to the manufacturer's instructions, and luminescence was measured in 10 cycles of 2 s detections using the POLARstar micro-plate reader, immediately after addition of the luciferase reaction mix to the media samples. ATP concentrations were calculated from averaged luminescence measurements compared with a quadratic standard curve of ATP provided with the assay kit and further normalized to the average DNA content of samples from each treatment group and donor at day 9 of culture.
Statistical analysis
Statistical analyses were performed using SPSS Statistics v18 (IBM, Somers, NY). To test for differences between the cell culture types as well as control and 18αGCA-treated samples in alginate or pellet models, data from gene expression, biochemical, and ATP assays were transformed using the binary logarithm to improve normality distribution of the data sets and tested by analysis of variance (ANOVA) using a general linear model including Dunnett's T3 post hoc test for multiple comparisons between groups. To test the effect of intermittent blocking of gap junctional communication on chondrogenic differentiation, gene expression data from day 3, 6, and 9 of pellet cultures were subjected to ANOVA using a general linear model, including Tukey's HSD post hoc test. Statistically significant differences were considered to be present at p<0.05.
Results
Effect of cell–cell interactions and gap junction blocking on gene expression during chondrogenic differentiation
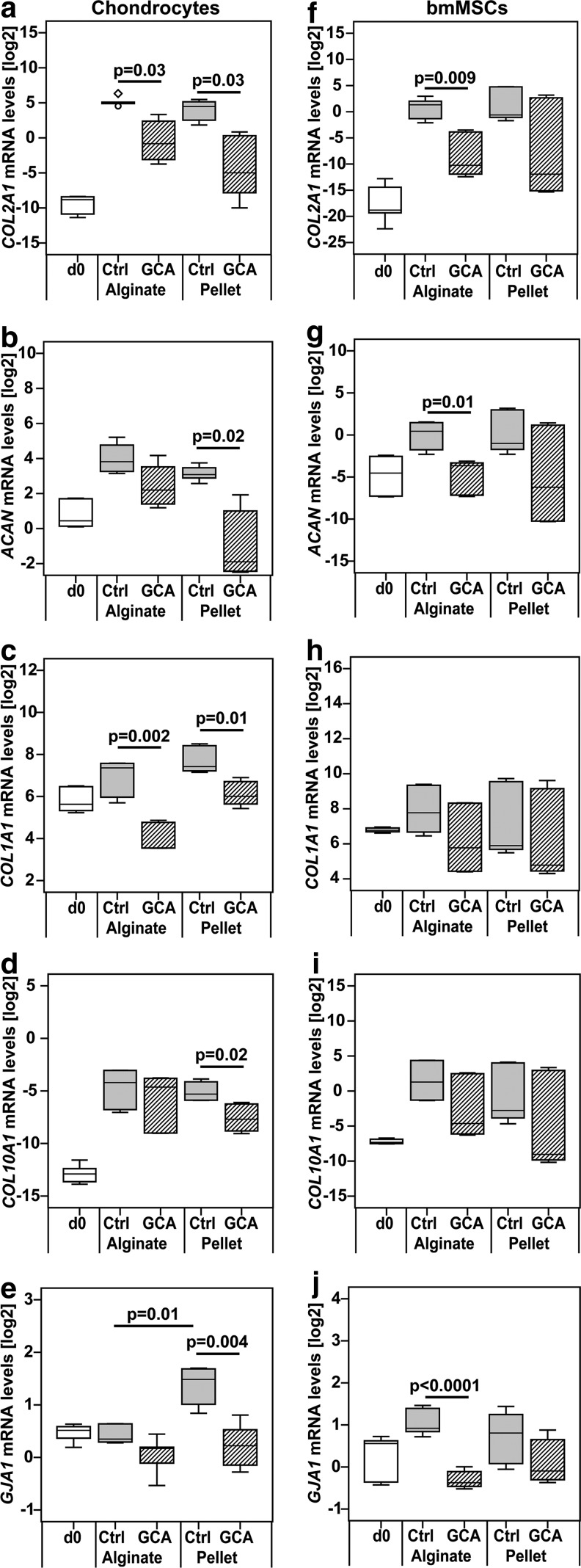
To study the importance of cell–cell interactions during differentiation of chondroprogenitor cells in vitro, expanded chondrocytes and BM-MSCs were cultured in hydrogel or micromass pellets, which either restricted or allowed extensive direct cell–cell interactions, respectively. In addition, gap junctional communication was blocked using the pharmaceutical compound 18αGCA. After 9 days of differentiation culture, the expression of the chondrogenic marker collagen type II (COL2A1) was significantly lower in 18αGCA-treated chondrocytes in both culture types compared with untreated controls (p=0.03; Fig. 1a). However, blocking gap junctions reduced the mRNA levels of aggrecan (ACAN), another chondrogenic marker, in chondrocyte pellets (p=0.02) but not in alginate cultures (p=0.19; Fig. 1b). Similar to COL2A1, transcription of the dedifferentiation marker collagen type I, COL1A1, was hampered by 18αGCA in chondrocyte alginate (p=0.002) and pellet cultures (p=0.01) in comparison to controls (Fig. 1c). Chondrocytes exhibited decreased transcription of collagen type X (COL10A1), a marker for tissue hypertrophy and calcification, only when cultured in pellets in the presence of a gap junction blocker (p=0.02; Fig. 1d). The gene expression of the major gap junction protein (GJA1) connexin 43 was elevated in untreated chondrocyte pellets compared with hydrogel cultures (p=0.01), but decreased only in pellets on 18αGCA treatment (p=0.004; Fig. 1e). In MSCs, gap junction blocking reduced mRNA levels of COL2A1 (p=0.009) and ACAN (p=0.01) in alginate cultures compared with their respective controls, and although similar trends were observed in pellets, they failed to reach significance (COL2A1: p=0.3; ACAN: p=0.39; Fig. 1f, g). Transcription levels of both COL1A1 and COL10A1 were not significantly different between 18αGCA-treated MSC alginate and pellet samples and control groups (Fig. 1h, i). In line with the chondrogenic markers, GJA1 expression was only lower in treated MSC alginate cultures (p<0.0001) versus controls but unchanged in pellets in response to gap junction blocking (p=0.52; Fig. 1j). The mRNA levels of the matrix molecules (COL2A1, ACAN, COL1A1, and COL10A1) were not significantly different between untreated alginate and pellet cultures in either chondrocytes or MSCs (Fig. 1).
FIG. 1.
Gene expression levels of (a, f) collagen II (COL2A1), (b, g) aggrecan (ACAN), (c, h) collagen I (COL1A1), (d, i) collagen X (COL10A1), and (e, j) connexin 43 (GJA1) in (a–e) human articular chondrocytes or (f–j) BM-MSCs after treatment with the gap junction blocker 18αGCA. Cells were differentiated in alginate hydrogels or pellet cultures in the absence (Ctrl) or presence of 18αGCA (GCA). The gene expression levels were measured at day 0 (d0) and day 9 of culture with real-time reverse transcriptase-polymerase chain reaction, then normalized to housekeeping genes using the comparative deltaCt method, and transformed into the binary logarithm (log2). For clarity, statistical significance was only shown for comparisons between 18αGCA-treated groups and controls as well as alginate Ctrl versus pellet Ctrl (n=6 from three donors of each cell type). 18αGCA, 18-α glycyrrhetinic acid; BM-MSC, bone marrow-derived mesenchymal stromal cell.
Effect of cell–cell interactions and gap junction blocking on DNA and matrix production during chondrogenic differentiation
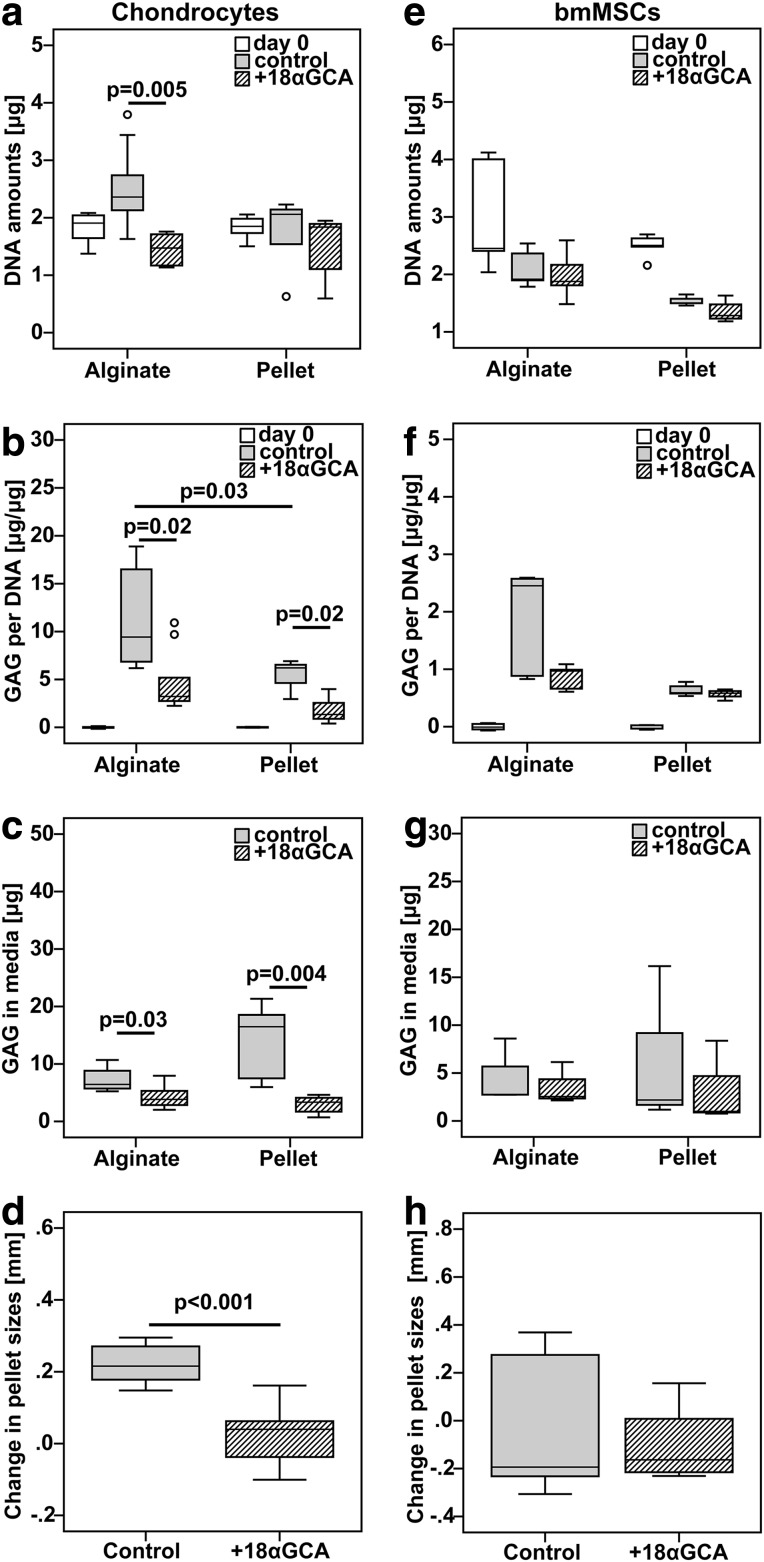
To study the importance of cell–cell interactions and gap junctional communication on matrix production of chondroprogenitor cells in vitro, we compared the DNA and GAG production of chondrocytes and BM-MSCs cultured for 9 days in hydrogel or micromass pellets in the presence or absence of 18αGCA. Blocking of gap junctions decreased DNA content (p=0.005), GAG per DNA (p=0.02), and the GAG in media (p=0.03) as well as the size (p<0.001) of chondrocyte pellets compared with untreated groups (Fig. 2a–d). Similarly, GAG per DNA in constructs as well as the GAG secreted into the media was also lower in chondrocyte alginate cultures treated with 18αGCA versus controls (Fig. 2b, c). However, DNA content was not different between these two groups. The GAG per DNA accumulated by chondrocytes over 9 days was higher in untreated alginate cultures in comparison to pellets (p=0.03; Fig. 2b). In contrast to chondrocytes, DNA amounts, GAG per DNA, GAG in media, and pellet sizes were not different between BM-MSCs cultured in the presence and absence of the gap junction blocker in either alginate or pellet cultures (Fig. 2e–h). DNA and GAG synthesis of BM-MSCs was also comparable between alginate and pellet control cultures (Fig. 2e–g).
FIG. 2.
Effect of gap junction blocking on DNA and GAG synthesis of (a–d) human articular chondrocytes and (e–h) BM-MSCs in alginate hydrogel and micromass pellet cultures. Cells of both types were cultured under identical chondrogenic conditions in the absence (control) or the presence of the gap junction blocker 18-α glycyrrhetinic acid (+18αGCA). The amounts of (a, e) DNA and (b, f) GAG per DNA were measured in constructs collected before (day 0) and at the end of the culture period. The cumulative amount of (c, g) secreted GAG was measured in conditioned media from day 6 and 9 of culture. The (d, h) change in pellet sizes was measured based on photographs taken with a phase-contrast microscope at day 4 and 9 of culture. For clarity, statistical significance was only shown for comparisons between 18αGCA-treated groups and controls or alginate Ctrl versus pellet Ctrl (chondrocytes from three donors: n=7 pellets, n=9 alginate constructs, n=15 pellets for size measurements; MSC from two donors: n=5 pellets or alginate constructs, n=9 pellets from three donors for size measurements). GAG, glycosaminoglycan.
Effect of cell–cell interactions and gap junction blocking on protein expression
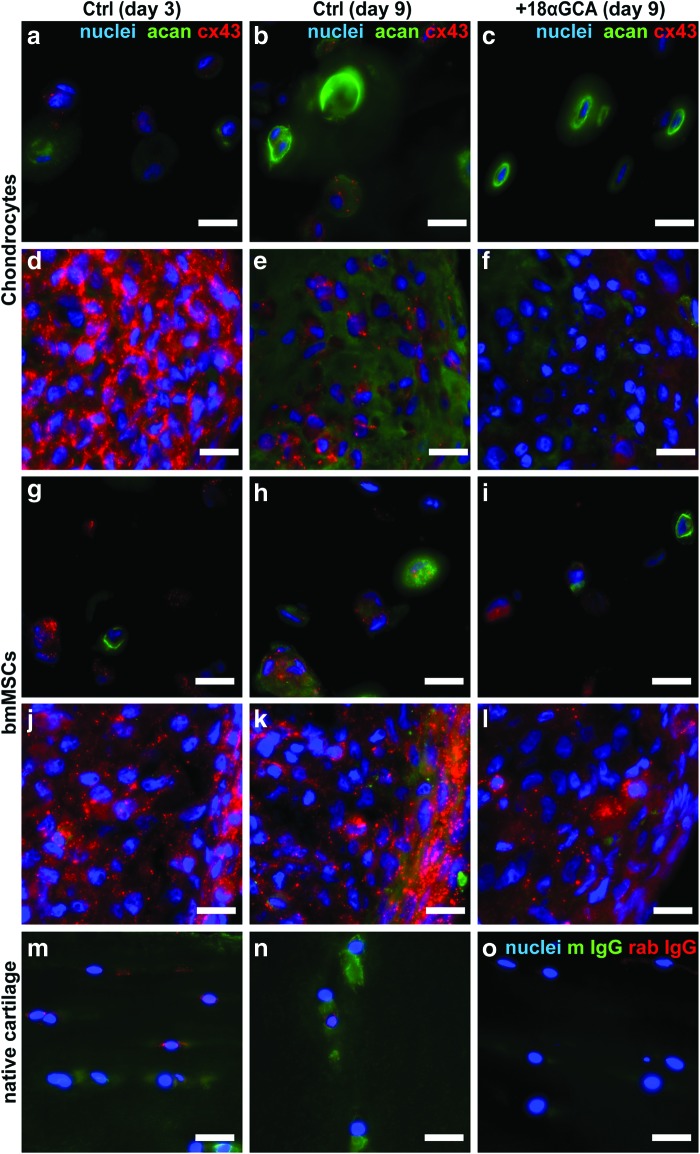
The deposition of extracellular matrix after 9 days in differentiation culture under varying levels of gap junctional communication was assessed by probing with antibodies specific for collagen type I and II. Exposure to the gap junction blocker reduced the staining intensity for both the chondrogenic collagen type II and the dedifferentiation marker collagen type I in all culture models with chondrocytes (Fig. 3a–d). Compared with control pellets, sections from 18αGCA-treated chondrocyte pellets revealed overall higher cell density and less matrix at day 9 of culture and unusual islands of intense collagen I staining (Fig. 3c, d). Similar to chondrocytes, 18αGCA-treated MSCs showed less immunoreactivity against collagen I compared with untreated controls (Fig. 3e–h). Collagen type II staining was weak in both culture types with BM-MSCs and only limited to single cells in the untreated controls (Fig. 3e–h). Inherent differences in cell seeding densities between alginate and pellets cultures preclude a direct comparison of their matrix qualities based on immunofluorescence staining.
FIG. 3.
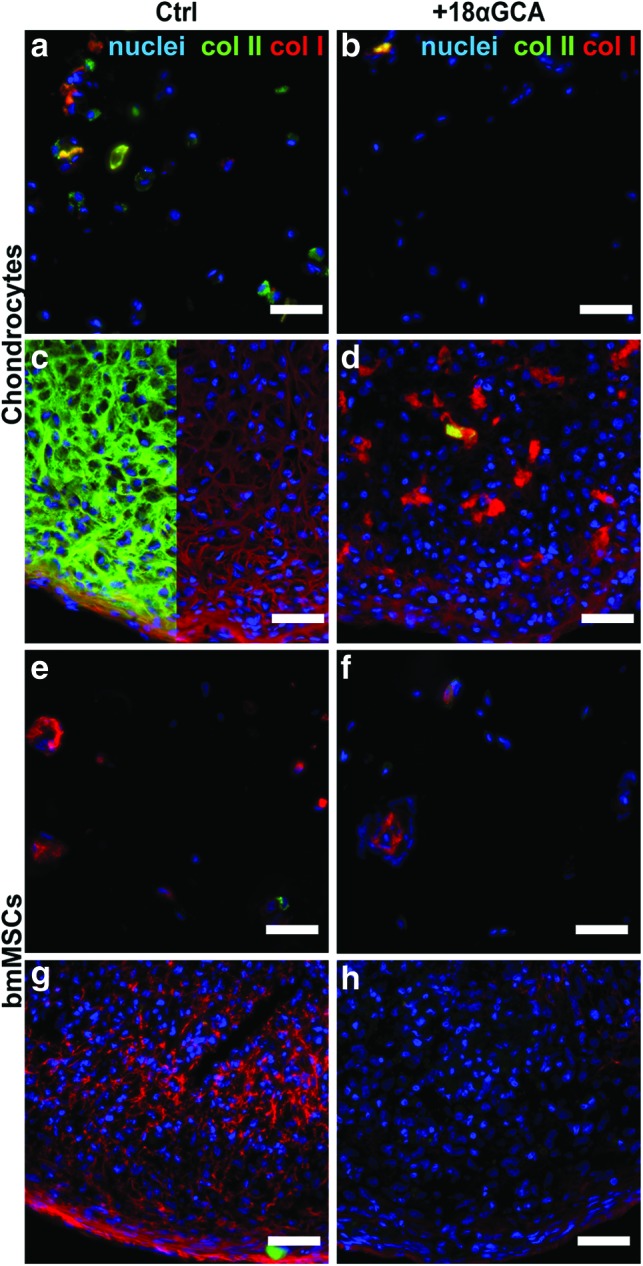
Expression of collagen type II and type I from (a–d) human articular chondrocytes and (e–h) BM-MSCs in (a, b, e, f) alginate hydrogel and (c, d, g, h) micromass pellet cultures. Cells of both types were cultured under identical chondrogenic conditions in the (a, c, e, g) absence (Ctrl) or the (b, d, f, h) presence of the gap junction blocker 18-α glycyrrhetinic acid (+18αGCA). Representative samples were processed for fresh-frozen tissue immunohistology at day 9, simultaneously probed with antibodies against collagen type II (col II, green) or collagen type I (col I, red), and counterstained with DAPI (blue). In panel c, col II signal was omitted in the right half of the image for better visibility of col I staining. Scale bars=50 μm. DAPI, 4′,6-diamidino-2-phenylindole. Color images available online at www.liebertpub.com/tea
Sections from the various groups at day 3 and 9 of culture were also probed with antibodies for the cartilaginous matrix protein, aggrecan, and the gap junction protein connexin 43 (Fig. 4). All groups revealed immunoreactive regions for aggrecan that were confined to the extracellular matrix. At day 9, untreated alginate and pellet controls with chondrocytes and BM-MSCs showed slightly more intense immunoreactivity for aggrecan and connexin 43 compared with 18αGCA-treated groups (Fig. 4b, c, e, f, h, i, k, l). There were no clear differences in connexin 43 staining between day 3 and 9 of chondrocytes or BM-MSCs in alginate cultures (Fig. 4a, b, g, h). However, chondrocyte pellets showed more intense, punctate staining throughout all regions at the earlier compared with the later time point, which coincided with higher cell density and lower immunoreactivity for aggrecan at day 3 compared to day 9 (Fig. 4d, e). In contrast, with BM-MSCs, areas of more intense, clustered staining for connexin 43 appeared to be localized to the outer rim of pellet sections at both time points (Fig. 4j, k). Control sections from the superficial and middle/deep zone of human native cartilage revealed extracellular matrix positive for aggrecan as well as localized cellular staining for connexin 43, which was seemingly less intense compared with pellet cultures (Fig. 4m, n). Stainings with IgG isotype control antibodies from mouse and rabbit proved negligible unspecific background reactivity (Fig. 4o).
FIG. 4.
Expression of aggrecan and connexin 43 from (a–f) human articular chondrocytes and (g–l) BM-MSCs in (a, b, c, g, h, i) alginate hydrogel and (d, e, f, j, k, l) micromass pellet cultures. Cells of both types were cultured for (a, d, g, j) 3 days or (b, c, e, f, h, i, k, l) 9 days under identical chondrogenic conditions in the (a, b, d, e, g, h, j, k) absence (Ctrl) or the (c, f, i, l) presence of the gap junction blocker 18-α glycyrrhetinic acid (+18αGCA). Representative samples were processed for fresh-frozen tissue immunohistology, simultaneously probed with antibodies against aggrecan (acan, green) or connexin 43 (cx43, red), and counterstained with DAPI (blue). Sections from the (m, o) superficial or (n) the middle zone of native human articular cartilage either (m, n) stained for aggrecan/connexin 43 or (o) probed with unspecific antibodies against mouse IgG (m IgG) and rabbit IgG (rab IgG) are shown for comparison. Scale bars=20 μm. Color images available online at www.liebertpub.com/tea
Effect of cell culture models and gap junction blocking on extracellular ATP levels
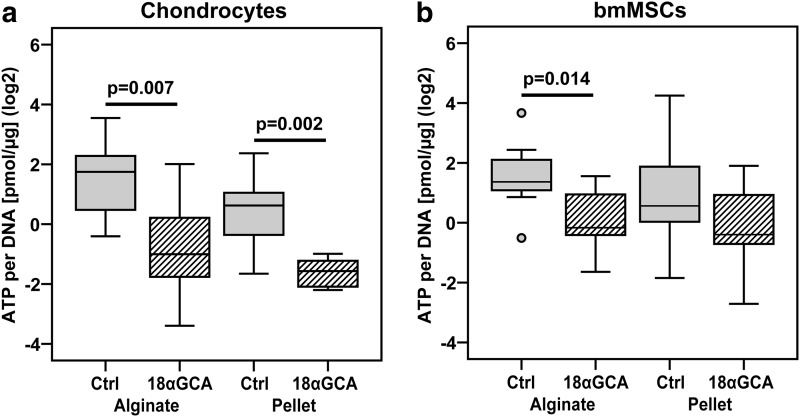
Connexin hemichannels have been implicated in the release of ATP into the extracellular environment.18 Therefore, we measured ATP levels in the conditioned media from chondrocytes and BM-MSCs cultured in alginate or pellets in the presence or absence of 18αGCA between days 6 and 9 and normalized these values to the average DNA content of samples from each treatment group and donor at day 9 of culture. Gap junction blocking decreased the amount of ATP in media normalized to DNA of chondrocyte alginate (p=0.007) and pellet cultures (p=0.002; Fig. 5a). The same effect was observed for BM-MSCs in alginate cultures (p=0.014) but not in pellets (p=0.89; Fig. 5b). ATP amounts in media per DNA were not different between alginate and pellet control groups of chondrocytes (p=0.18) nor BM-MSCs (p=0.7; Fig. 5a, b).
FIG. 5.
Effect of gap junction blocking on ATP secretion of (a) human articular chondrocytes and (b) BM-MSCs in alginate hydrogel and micromass pellet cultures. Cells of both types were cultured under identical chondrogenic conditions in the absence (Ctrl) or the presence of the gap junction blocker 18-α glycyrrhetinic acid (+18αGCA). The amount of secreted ATP was measured in conditioned media from day 9 of culture, normalized to the average DNA content of samples from each treatment group and donor at day 9 of culture, and transformed into the binary logarithm (log2) (n=8–12 from two donors of each cell type). ATP, adenosine-5′-triphosphate.
Discussion
Intercellular communication via gap junctions is an important driver of mesenchymal condensation in early chondrogenic differentiation.12 However, it is far from clear whether direct cell-to-cell contacts and the exchange of messenger molecules through gap junctions also have an essential role in the differentiation of chondroprogenitor cells in ex vivo engineered cartilage constructs. Furthermore, scaffold architecture and composition play a critical role not only in modulating chondrogenic differentiation and diffusion of nutrients,25,26 but also in controlling cell distribution in 3D.27 However, the influence of 3D cell distribution and concomitant cell–cell communication on the differentiation capacity of chondroprogenitor cells offered by either high-density microtissue versus more discrete hydrogel culture platforms for cartilage tissue engineering has also not been well established.
Our data show that differentiating chondrocytes or BM-MSCs in micromass cell pellets brought no advantage compared with alginate hydrogel cultures in terms of the cell's chondrogenic marker expression and GAG synthesis. Our findings are in agreement with other studies, in which neither human chondrocytes28 nor human BM-MSCs29 showed clear differences in chondrogenic marker expression when differentiated in either pellet or alginate cultures. This suggests that promoting direct intercellular contacts may not be critical for the differentiation of chondroprogenitor cells in vitro.
Despite this, we still found that blocking gap junctional intercellular communication using the pharmaceutical inhibitor 18αGCA negatively affected chondrogenic differentiation, as evident in a reduction in GAG synthesis and in the expression of the important chondrocyte marker, collagen type II (Figs. 1 and 2). Interestingly, 18αGCA treatment did not altogether abolish the expression of chondrogenic marker molecules and seemed to affect chondrocytes and BM-MSCs to a different extent depending on the cell culture model. Aggrecan mRNA levels of chondrocytes, for example, were only significantly reduced in pellets but not in alginate, which may be explained by the higher connexin 43 expression in the former compared with the latter (Fig. 1). In contrast, while there was a strong impact on COL2A1 and aggrecan expressions in BM-MSC alginate cultures, reduction in these markers was less severe in BM-MSC pellets. It should be noted that there were large donor-to-donor variations for BM-MSCs in terms of their chondrogenic potential, which may explain the high standard deviations in the mRNA and biochemical data sets and could be—at least in part—blamed for the difficulties in detecting significant effects for this cell type.
Another interesting difference between the two cell types was that 18αGCA treatment had the most profound effect in the first few days of chondrocyte differentiation in pellets; however, chondrogenesis of BM-MSCs appeared to be disturbed only past day 3 (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea). This has also been observed in mesenchymal cells from chicken limb buds, which only showed a discernable response to 18αGCA treatment 48 h after initiation of chondrogenic differentiation.30 It could, therefore, be speculated that BM-MSCs, the more immature chondroprogenitor cell type as evidenced by relatively low COL2A1 and ACAN expression at the start of the differentiation, respond only to impediments of their gap junction function after the chondrogenic phenotype has been established. It is interesting to note in this regard that connexin 43 protein expression was, indeed, highest at day 3 of chondrocyte pellet culture whereas BM-MSC pellets appeared to exhibit higher connexin 43 levels at day 9 than day 3 (Fig. 4). In addition, our results and those of other groups indicate that connexin 43 mRNA and protein levels decrease in cells in response to 18αGCA at concentrations higher than 20 μM.31 This raises the question about the mechanism of action of the gap junction blocker 18αGCA, which does not seem to be fully elucidated to date. We only observed minor effects on cell numbers in treated cultures. DNA levels in 18αGCA samples were not different from controls except for chondrocyte alginate cultures, in which case DNA quantities of the 18αGCA group were still at similar levels as measured at day 0 of culture (Fig. 2). Some evidence points to the incorporation of 18αGCA into the cell membrane, where it disrupts the formation of gap junction plaques and possibly induces a conformational change of connexin closing the gap junctions.32 Furthermore, it has been proposed that glycyrrhetinic acid interferes with the interaction between connexin 43 and phosphatases or kinases, altering connexin phosphorylation and leading to reversible closure of gap junction and reduced gap junction intercellular communication.31,33 Therefore, the main mode of actions of 18αGCA is most likely a combination of decrease in connexin expressions and interference with opening of gap junctions.
A role for gap junctional intercellular communication in cell differentiation has been established for a number of cell types, including epithelial and mesenchymal cells.30,34 Our study shows for the first time that chondrogenesis is even hampered by 18αGCA in alginate cultures with already limited cell–cell contacts. This suggests that the effect of gap junction-mediated crosstalk on chondrocyte differentiation is mainly exerted through the formation and activity of hemichannels. Hemichannels are aqueous membrane pores that allow for the controlled release of small messenger molecules (e.g., ATP, prostaglandin E2, ions) for autocrine/paracrine cellular communication in response to a range of stimuli.35 In chondrocytes, connexin hemichannels seem to be involved in the release of intracellular ATP into the extracellular space after cyclic loading, thereby modulating purinergic and calcium signaling pathways.18,36 In our study, we found, indeed, reduced ATP levels in the media of almost all groups treated with a gap junction blocker compared with their controls (Fig. 5). Only media samples from BM-MSC pellets showed no differences for this nucleotide, which may be explained by ATP release from dying or dead cells that were observed at relatively high levels in these cultures (Fig. 2e). In summary, our data clearly point to a broader, important role for hemichannel activity and ATP release in chondrogenic differentiation even in the absence of mechanical stimuli. Indeed, a study on the mouse chondrogenic cell line ATDC5 revealed synchronized ATP oscillations during early differentiation that appeared gap junction dependent and positively affected prechondrogenic condensation.37 However, the signals driving gap junctional ATP release in the absence of cyclic mechanical cues still remain elusive.
In this regard, it is tempting to speculate about the potential use of small-molecule messengers, such as ATP, as relatively inexpensive media supplements to improve quality of engineered cartilage constructs. However, it may not be as simple as continuously adding ATP to culture media. When human BM-MSCs and bovine chondrocytes were differentiated in agarose hydrogels, the presence of ATP did not enhance matrix contents and brought only minor improvements to mechanical properties of the constructs.38 Therefore, the role for extracellular ATP in chondrogenic differentiation is likely to be complex and requires further investigation.
Conclusions
Alginate hydrogel and microtissue culture systems are equally suited to study the in vitro differentiation of articular chondrocytes and bMSCs. Immediate cell-to-cell interactions offered by micromass culture do not play a significant role in the in vitro chondrogenesis of expanded human chondrocytes and human BM-MSCs compared with hydrogel culture. Therefore, restricting direct intercellular communication through scaffold architecture should not affect the chondrogenic potential of isolated cells in scaffold–based cartilage repair strategies. However, for the first time to our knowledge, our study demonstrates the importance of connexin hemichannels in gap junction-mediated crosstalk between chondrocytes during differentiation that goes beyond their involvement in chondrocyte mechanotransduction, and appears to control the release of small molecules, such as ATP, for autocrine and paracrine signaling. Further studies need to address the specific role for connexin hemichannels in coordinating or regulating chondrogenic differentiation and identify the specific cues that drive hemichannel activity. This will give new insights into the complex processes of chondrogenesis and potentially reveal new important factors and strategies to improve current approaches in cartilage tissue engineering.
Supplementary Material
Acknowledgments
The authors wish to acknowledge funding from the AO Foundation Research Fund Start-up Grant (S-08-81W; T.B.F.W., K.S.), Royal Society of New Zealand Rutherford Discovery Fellowship (T.B.F.W.), the European Union Seventh Framework Program (skelGEN [FP7/2007–2013] [FP7/2007–2011]) under grant agreement n° [318553], New Zealand Lotteries Health Research Grant scheme (T.B.F.W., K.S.), and the Australian Research Council (K.S., T.J.K.). They also thank Prof. Ross Crawford as well as Dr. Andrew Vincent and Dr. Graham Ingles for supplying tissue samples from surgeries performed at the Prince Charles Hospital, Brisbane and Christchurch Hospital, respectively. They wish to acknowledge Dr. Simon Ströbel for his assistance with gap junction blocking experiments. The antibody against collagen type II (II-II6B3), developed by T.F Linsenmayer, was obtained from the DSHB developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA.
Disclosure Statement
No competing financial interests exist.
References
- 1.Demoor M., Ollitrault D., Gomez-Leduc T., Bouyoucef M., Hervieu M., Fabre H., Lafont J., Denoix J.M., Audigie F., Mallein-Gerin F., Legendre F., and Galera P. Cartilage tissue engineering: molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochim Biophys Acta 1840, 2414, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Schrobback K., Klein T.J., Crawford R., Upton Z., Malda J., and Leavesley D.I. Effects of oxygen and culture system on in vitro propagation and redifferentiation of osteoarthritic human articular chondrocytes. Cell Tissue Res 347, 649, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Levett P.A., Melchels F.P., Schrobback K., Hutmacher D.W., Malda J., and Klein T.J. A biomimetic extracellular matrix for cartilage tissue engineering centered on photocurable gelatin, hyaluronic acid and chondroitin sulfate. Acta Biomater 10, 214, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Pelttari K., Winter A., Steck E., Goetzke K., Hennig T., Ochs B.G., Aigner T., and Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum 54, 3254, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Klein T.J., Malda J., Sah R.L., and Hutmacher D.W. Tissue engineering of articular cartilage with biomimetic zones. Tissue Eng Part B Rev 15, 143, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodfield T.B., Bezemer J.M., Pieper J.S., van Blitterswijk C.A., and Riesle J. Scaffolds for tissue engineering of cartilage. Crit Rev Eukaryot Gene Expr 12, 209, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Benya P.D., and Shaffer J.D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 30, 215, 1982 [DOI] [PubMed] [Google Scholar]
- 8.Klein T.J., Rizzi S.C., Reichert J.C., Georgi N., Malda J., Schuurman W., Crawford R.W., and Hutmacher D.W. Strategies for zonal cartilage repair using hydrogels. Macromol Biosci 9, 1049, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Mauck R.L., Yuan X., and Tuan R.S. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage 14, 179, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Schon B.S., Schrobback K., van der Ven, M., Stroebel S., Hooper G.J., and Woodfield T.B. Validation of a high-throughput microtissue fabrication process for 3D assembly of tissue engineered cartilage constructs. Cell Tissue Res 347, 629, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Bhumiratana S., Eton R.E., Oungoulian S.R., Wan L.Q., Ateshian G.A., and Vunjak-Novakovic G. Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation. Proc Natl Acad Sci U S A 111, 6940, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLise A.M., Fischer L., and Tuan R.S. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage 8, 309, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Coelho C.N., and Kosher R.A. Gap junctional communication during limb cartilage differentiation. Dev Biol 144, 47, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Tavella S., Raffo P., Tacchetti C., Cancedda R., and Castagnola P. N-CAM and N-cadherin expression during in vitro chondrogenesis. Exp Cell Res 215, 354, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Mayan M.D., Gago-Fuentes R., Carpintero-Fernandez P., Fernandez-Puente P., Filgueira-Fernandez P., Goyanes N., Valiunas V., Brink P.R., Goldberg G.S., and Blanco F.J. Articular chondrocyte network mediated by gap junctions: role in metabolic cartilage homeostasis. Ann Rheum Dis 74, 275, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herve J.C., and Derangeon M. Gap-junction-mediated cell-to-cell communication. Cell Tissue Res 352, 21, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Mayan M.D., Carpintero-Fernandez P., Gago-Fuentes R., Martinez-de-Ilarduya O., Wang H.Z., Valiunas V., Brink P., and Blanco F.J. Human articular chondrocytes express multiple gap junction proteins: differential expression of connexins in normal and osteoarthritic cartilage. Am J Pathol 182, 1337, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia M., and Knight M.M. Cyclic loading opens hemichannels to release ATP as part of a chondrocyte mechanotransduction pathway. J Orthop Res 28, 510, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Schrobback K., Malda J., Crawford R.W., Upton Z., Leavesley D.I., and Klein T.J. Effects of oxygen on zonal marker expression in human articular chondrocytes. Tissue Eng Part A 18, 920, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Jeon J.E., Schrobback K., Hutmacher D.W., and Klein T.J. Dynamic compression improves biosynthesis of human zonal chondrocytes from osteoarthritis patients. Osteoarthritis Cartilage 20, 906, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Davidson J.S., Baumgarten I.M., and Harley E.H. Reversible inhibition of intercellular junctional communication by glycyrrhetinic acid. Biochem Biophys Res Commun 134, 29, 1986 [DOI] [PubMed] [Google Scholar]
- 22.Farndale R.W., Buttle D.J., and Barrett A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 883, 173, 1986 [DOI] [PubMed] [Google Scholar]
- 23.Schrobback K., Wrobel J., Hutmacher D.W., Woodfield T.B., and Klein T.J. Stage-specific embryonic antigen-4 is not a marker for chondrogenic and osteogenic potential in cultured chondrocytes and mesenchymal progenitor cells. Tissue Eng Part A 19, 1316, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spandidos A., Wang X., Wang H., and Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res 38, D792, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malda J., Woodfield T.B., van der Vloodt F., Kooy F.K., Martens D.E., Tramper J., van Blitterswijk C.A., and Riesle J. The effect of PEGT/PBT scaffold architecture on oxygen gradients in tissue engineered cartilaginous constructs. Biomaterials 25, 5773, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Miot S., Woodfield T., Daniels A.U., Suetterlin R., Peterschmitt I., Heberer M., van Blitterswijk C.A., Riesle J., and Martin I. Effects of scaffold composition and architecture on human nasal chondrocyte redifferentiation and cartilaginous matrix deposition. Biomaterials 26, 2479, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Woodfield T.B., Van Blitterswijk C.A., De Wijn J., Sims T.J., Hollander A.P., and Riesle J. Polymer scaffolds fabricated with pore-size gradients as a model for studying the zonal organization within tissue-engineered cartilage constructs. Tissue Eng 11, 1297, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Caron M.M., Emans P.J., Coolsen M.M., Voss L., Surtel D.A., Cremers A., van Rhijn L.W., and Welting T.J. Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthritis Cartilage 20, 1170, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Yang I.H., Kim S.H., Kim Y.H., Sun H.J., Kim S.J., and Lee J.W. Comparison of phenotypic characterization between “alginate bead” and “pellet” culture systems as chondrogenic differentiation models for human mesenchymal stem cells. Yonsei Med J 45, 891, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Zhang W., Green C., and Stott N.S. Bone morphogenetic protein-2 modulation of chondrogenic differentiation in vitro involves gap junction-mediated intercellular communication. J Cell Physiol 193, 233, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Guo Y., Martinez-Williams C., Gilbert K.A., and Rannels D.E. Inhibition of gap junction communication in alveolar epithelial cells by 18alpha-glycyrrhetinic acid. Am J Physiol 276, L1018, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Goldberg G.S., Moreno A.P., Bechberger J.F., Hearn S.S., Shivers R.R., MacPhee D.J., Zhang Y.C., and Naus C.C. Evidence that disruption of connexon particle arrangements in gap junction plaques is associated with inhibition of gap junctional communication by a glycyrrhetinic acid derivative. Exp Cell Res 222, 48, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Chung T.H., Wang S.M., Chang Y.C., Chen Y.L., and Wu J.C. 18beta-glycyrrhetinic acid promotes src interaction with connexin43 in rat cardiomyocytes. J Cell Biochem 100, 653, 2007 [DOI] [PubMed] [Google Scholar]
- 34.El-Sabban M.E., Sfeir A.J., Daher M.H., Kalaany N.Y., Bassam R.A., and Talhouk R.S. ECM-induced gap junctional communication enhances mammary epithelial cell differentiation. J Cell Sci 116, 3531, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Saez J.C., Schalper K.A., Retamal M.A., Orellana J.A., Shoji K.F., and Bennett M.V. Cell membrane permeabilization via connexin hemichannels in living and dying cells. Exp Cell Res 316, 2377, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Pingguan-Murphy B., El-Azzeh M., Bader D.L., and Knight M.M. Cyclic compression of chondrocytes modulates a purinergic calcium signalling pathway in a strain rate- and frequency-dependent manner. J Cell Physiol 209, 389, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Kwon H.J., Ohmiya Y., Honma K.I., Honma S., Nagai T., Saito K., and Yasuda K. Synchronized ATP oscillations have a critical role in prechondrogenic condensation during chondrogenesis. Cell Death Dis 3, e278, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gadjanski I., Yodmuang S., Spiller K., Bhumiratana S., and Vunjak-Novakovic G. Supplementation of exogenous adenosine 5′-triphosphate enhances mechanical properties of 3D cell-agarose constructs for cartilage tissue engineering. Tissue Eng Part A 19, 2188, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.