Abstract
Background: In the last 50 years, the use of medical implants has increased dramatically. Failure of implanted devices and biomaterials is a significant source of morbidity and increasing healthcare expenditures. An important cause of implant failure is the host inflammatory response. Recent evidence implicates extracellular ATP as an important inflammatory signaling molecule. A major pathway for release of cytoplasmic ATP into the extracellular space is through connexin hemichannels, which are the unpaired constituents of gap junction intercellular channels. Blockade of hemichannels of the connexin 43 (Cx43) isoform has been shown to reduce inflammation and improve healing. We have developed a Cx43 mimetic peptide (JM2) that targets the microtubule-binding domain of Cx43. The following report investigates the role of the Cx43 microtubule-binding domain in extracellular ATP release by Cx43 hemichannels and how this impacts early inflammatory events of the foreign body reaction.
Methods: In vitro Cx43 hemichannel-mediated ATP release by cultured human microvascular endothelial cells subjected to hypocalcemic and normocalcemic conditions was measured after application of JM2 and the known hemichannel blocker, flufenamic acid. A submuscular silicone implant model was used to investigate in vivo ATP signaling during the early foreign body response. Implants were coated with control pluronic vehicle or pluronic carrying JM2, ATP, JM2+ATP, or known hemichannel blockers and harvested at 24 h for analysis.
Results: JM2 significantly inhibited connexin hemichannel-mediated ATP release from cultured endothelial cells. Importantly, the early inflammatory response to submuscular silicone implants was inhibited by JM2. The reduction in inflammation by JM2 was reversed by the addition of exogenous ATP to the pluronic vehicle.
Conclusions: These data indicate that ATP released through Cx43 hemichannels into the vasculature is an important signal driving the early inflammatory response to implanted devices. A vital aspect of this work is that it demonstrates that targeted molecular therapeutics, such as JM2, provide the capacity to regulate inflammation in a clinically relevant system.
Introduction
The last 50 years have seen a steady increase in the number and types of implantable medical devices and biomaterials placed in the human body. These devices include hernia mesh, pacemakers, and silicone implants, with the technology collectively representing an $85 billion industry.1–3 Overall, the use of implants for clinical applications has substantially increased patient survival and quality of life.4–6 However, implant failure and associated complications are significant sources of patient morbidity and increased healthcare costs.7–9
The immune response secondary to tissue damage, hemostasis, and introduction of foreign material is an important contributing factor in device-related complications.10,11 The acute phase of this response is characterized by neutrophil (polymorphonuclear leukocyte [PMN]) infiltration, activation, and release of hydrolytic enzymes and reactive oxygen species. These processes play an essential function in immune defense against infection as well as in providing an initiating step in subsequent tissue regeneration. However, these same processes damage surrounding tissues, corrode implants, and can destroy implanted cells.9–13 The effect of infiltrating PMNs is not limited to the acute phase of the foreign body response. PMNs at the implant site generate signals that regulate the behavior and phenotype of subsequently arriving macrophages.14–16 Macrophages are responsible for regulating chronic responses to foreign bodies, such as implant encapsulation, which can lead to device failure.3,17,18 Therefore, the fate of implanted medical devices may be determined during the first hours and days after surgical placement.
The inflammatory response encompasses a series of signaling pathways that induce and regulate infection prevention, the clearing of debris, and elimination of foreign material.19 Cytokines released by immune cells are widely accepted as key signals in inflammatory processes.20 However, recent evidence supports a parallel pathway of purine-mediated (aka purinergic) signaling as a critical modulator of inflammation. Notably, extracellular ATP has been shown to be a mediator of multiple early inflammatory mechanisms, including neutrophil trafficking.21,22
An important mechanism of ATP release occurs through connexin hemichannels expressed at the cell surface by many cell types, including endothelial cells, fibroblasts, and leukocytes. Connexin hemichannels are the undocked constituents of gap junction (GJ) intercellular channels that reside in an adjacent membrane domain called the perinexus.23,24 GJ intercellular channels are capable of transferring ions, messengers, and other molecules, 1000 Da or less, between the cytoplasm of adjacent cells.25 Conversely, hemichannels provide a tightly regulated conduit for passage of small molecules and ions, including ATP, into the extracellular space.26–28 It is well established that GJs, and their connexin subunits, function in various aspects of the tissue injury response and wound healing processes.29–32 Reports for such functions include roles for intercellular spread of injury signals (bystander effect), inflammation and chronic wounds, cardiac preconditioning, and formation of granulation tissue.31,33–35
A variety of signals and environmental conditions, including low extracellular calcium and shear stresses, induce hemichannel opening and ATP release.36–38 The connexin 43 (Cx43) isoform is the most ubiquitously expressed connexin and is present in cells and tissues significant to the foreign body response, including vascular endothelial cells, fibroblasts, and smooth muscle.39 Interestingly, Cx43 expression increases in blood vessels in response to tissue damage.40 Several studies specifically implicate Cx43 hemichannels in a variety of injuries and inflammatory pathways by demonstrating that modulation of Cx43 hemichannels correlates with reduced inflammatory infiltrate and improves healing.30–32,34 However, a direct link between Cx43 hemichannel function, purinergic signaling, and early inflammation during the foreign body response has yet to be established. This study provides the first evidence that these events are intimately related.
The novel peptide, JM2, was developed as a cell-permeable tool to investigate Cx43 function and is based on the juxtamembrane region of Cx43—a sequence that incorporates the microtubule-binding domain (Fig. 1).41 Microtubules have been implicated in trafficking of connexins to the cell surface,42–44 and the work by Lo and colleagues demonstrates that deletion of the microtubule-binding domain inhibits Cx43 channel functions.45 Therefore, we hypothesized that targeting the juxtamembrane domain would reduce Cx43 hemichannel-mediated ATP release and consequently inhibit the inflammatory response. To test this hypothesis, we used an in vitro model of connexin hemichannel-mediated ATP release in cultured human microvascular endothelial cells (HMVECs) and an in vivo model of early inflammation during the foreign body response. The results support a model in which inflammatory cells are targeted to implanted materials by ATP released from microvascular endothelial Cx43 hemichannels. These observations have significant implications for efforts to improve clinical outcomes associated with implanted medical devices, biomaterials, and tissue-engineered constructs.
FIG. 1.
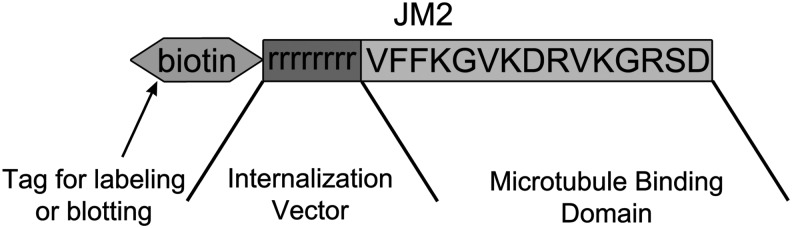
Structure of the JM2 peptide. The peptide comprises the connexin 43 (Cx43) C-terminal amino acids 231–245 (VFFKGVKDRVKGRSD), which include the microtubule-binding sequence, attached to a poly-d-arginine internalization vector. A biotin tag is attached to the N-terminus for identification and labeling.
Materials and Methods
Peptides and cell culture
Peptides were produced at American Peptide Company (Sunnyvale, CA) or Petron, Inc., (Daejeon, Korea) and comprised a biotin tag, a poly-d-arginine internalization vector,46 and the Cx43 C-terminal amino acids 231–245 (VFFKGVKDRVKGRSD), which include the microtubule-binding sequence (amino acids 234–24341), biotin-rrrrrrrr-VFFKGVKDRVKGRSD, for targeting. HMVECs (Lonza, Allendale, NJ) were cultured in EGM™-2 MV (Lonza) according to the manufacturer's instructions and used for experiments at passage 5 or below.
ATP release assay
HMVECs were cultured to confluence on 96-well plates and treated for 2 h with either vehicle (H2O), 50 μM JM2, or 50 μM flufenamic acid (FFA; Abcam, Cambridge, MA). Following the 2 h incubation, the media were replaced with either Hank's balanced salt solution buffered with 25 mM HEPES (HBSS-HEPES) plus Ca2+ and Mg2+ or HBSS-HEPES containing 4 mM EGTA and without Ca2+ and Mg2+ for 5 min. One hundred micromolars of ARL 67156 (Sigma-Aldrich Corp., St. Louis, MO) was also added to the buffers to prevent ATP hydrolysis. After the 5-min incubation, the buffer was collected and analyzed for ATP levels using an ATP bioluminescent assay kit (Sigma-Aldrich Corp.) on a BioTek Synergy HT microplate reader (BioTek Instruments, Inc., Winooski, VT). Four separate experiments were performed with three replicates for each treatment.
Implant preparation
Silicone implants (nonreinforced, vulcanized, 40 durometer, matte-finished clinical-grade silicone sheeting; Specialty Manufacturing, Saginaw, MI) were cut to a uniform 0.5×0.5×5 mm size. Implants were coated with 25% pluronic F127 solution (Sigma-Aldrich Corp.), which is a thermoreversible gel liquid at 4°C and solid above 30°C,47 and served as a vehicle to deliver treatments. Control and treatment solutions were prepared the day of the surgery and filtered through an enclosed 0.2-μm system (Millipore Corp., Darmstadt, Germany). Immediately before implantation, silicone rods were coated with control pluronic or pluronic-carrying treatments. Experimental groups included the following added to the pluronic vehicle: 180 μM JM2 peptide alone; 1 mM ATP alone (Sigma-Aldrich Corp.); 180 μM JM2+1 mM ATP; 1 mM FFA; and 200 μM mefloquine (MFQ; Sigma-Aldrich Corp).
Surgery
Animals were housed in accordance with IUCAC guidelines. Two hundred fifty to 350 g male Sprague-Dawley rats were anesthetized with 3.5% isoflurane, maintained with 2.5%, and provided 0.03 mg/kg buprenorphine for analgesia. The left hind leg was clipped, prepped, and draped in a sterile manner using povidone-iodine surgical scrub solution. A 1.5-cm longitudinal incision was made over the region of the biceps femoris muscle, the midpoint of which was 32–34 mm from the ankle joint. Skin flaps were raised using blunt dissection to expose a small portion of the underlying muscle. A longitudinal 4-mm incision, 33 mm from the ankle joint, was then made in the biceps femoris using microscissors. Dissection was then carefully continued through the biceps femoris to the underlying vastus muscle group to create a pocket for the coated implant in the fascial plane between muscles. The implant (nonreinforced, vulcanized, 40 durometer, matte-finished clinical-grade silicone sheeting cut to a uniform 0.5×0.5×5 mm size; Specialty Manufacturing) was positioned with the long axis perpendicular to the fibers of the biceps femoris and parallel to those of the vastus muscle group. Once the implant was properly seated, the muscle was closed with two simple interrupted sutures of 4-0 prolene (Ethicon, Inc., Somerville, NJ). The wound was irrigated with warmed normal saline and the skin was closed with GLUture® tissue glue (Abbott Laboratories, Abbot Park, IL). At 24 h, the animals were anesthetized as described and the implants with surrounding muscle were removed, washed of blood and debris with cold sterile normal saline solution, placed in 4% paraformaldehyde (Sigma-Aldrich Corp.), and cooled on ice for fixation. To reduce bias introduced by personnel responsible for tissue sectioning and cell counting, animals were identified only by a unique number.
Histology
Tissue was fixed overnight with 4% paraformaldehyde at 4°C, sectioned in 6-μm-thick slices with a microtome ensuring full sections of the implant pocket were obtained, and then placed on slides for staining with hematoxylin and eosin (H&E) following a standard protocol. Morphologic analysis of the implant interface, implant pocket, inflammatory infiltrate, and adjacent muscle was performed using an Olympus BX63 microscope (Olympus America, Inc., Center Valley, PA) with 10×and 40×objectives. To reduce confounding by the dorsally located sutures, the ventral portion of the implant pocket adjacent to the underlying vastus muscle group was analyzed for all samples and only sections with an intact implant–muscle interface of at least 500 μm were included in the analysis.
Cellular infiltrate quantitative analysis
Inflammation was measured as a function of the leukocyte number with cell counting performed on the H&E-stained tissue sections. The implant interface was divided into 100 μm lengths to define regions for cell counting, three of which were selected at random. To account for tissue distortion caused by sectioning, boundaries of the regions for counting included the 100 μm length of the implant interface, a corresponding 100 μm length of vastus muscle not involved with inflammation, and perpendicular lines of varying length intersecting the ends of the two 100 μm lengths. Cells in the defined regions were manually counted using cellSens software (Olympus Corp.). All cells thought to be neutrophils, monocytes, macrophages, or other granulocytes were included, while spindle-shaped cells, thought likely to be fibroblasts, were excluded. Section averages were calculated for each animal and then grouped by treatment arm for statistical analysis.
Infiltrate cellular composition analysis
To calculate the proportion of the inflammatory infiltrate comprising PMNs, counts of total mature neutrophils and total infiltrating cell numbers (which included monocytes, macrophages, and other granulocytes) were measured in 3 high-powered fields per 500 μm length of implant interface. PMNs were identified by their characteristic morphology and differential staining in H&E using cellSens software (Olympus Corp.). The fraction of PMNs for each tissue section was calculated by dividing total PMN numbers by total infiltrating cell numbers. This fraction was used to estimate the number of PMNs per 100 μm length of implant–muscle interface for each animal.
Statistical analysis
For each in vivo experimental arm, animal data were pooled and average cell numbers and standard deviations were calculated. Using IBM SPSS Statistics for Windows (IBM Corp., Armonk, NY), a one-way ANOVA test was performed for the results of the ATP release assay and animal implant model. A p<0.05 was considered significant. Post hoc Bonferroni tests were used to detect significance between groups.
Results
Targeting the Cx43 microtubule-binding domain significantly reduces ATP release in a hemichannel-dependent manner
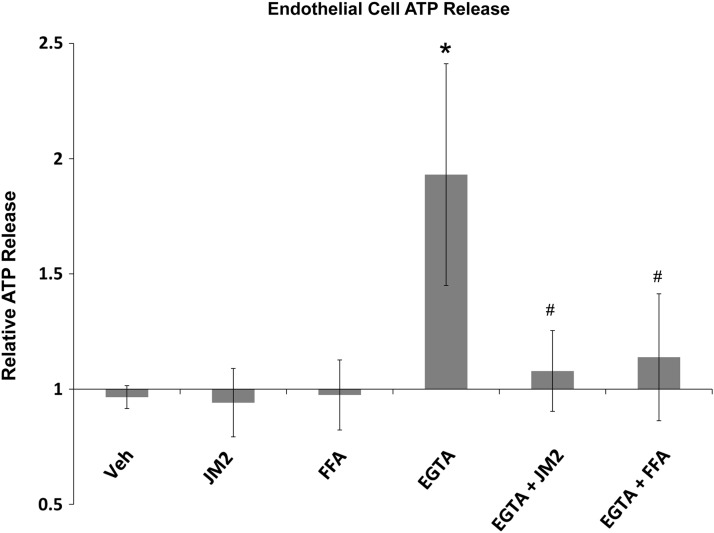
In this study, we endeavored to examine the link between extracellular ATP and inflammation in the foreign body response. Connexin hemichannels, and in particular Cx43, are major contributors to the release of purinergic signals that attract inflammatory cells in other injury types.48,49 Chemical inhibitors of connexins (such as FFA and MFQ, respectively) affect other channel types, including pannexin channels and ion channels.50–53 Nonspecific inhibition by these inhibitors may have pleiotropic effects. In addition, FFA has a direct effect on inflammation by inhibiting prostaglandin production.54 Therefore, we developed a peptide mimetic of the juxtamembrane region of the Cx43 carboxyl-terminus, JM2, to specifically target Cx43-β-tubulin interaction, which has been shown to be necessary for Cx43 channel function (Fig. 1).45 To test whether the peptide affected connexin-mediated ATP release, HMVECs were subjected to low-Ca2+ conditions. Chelation of calcium with EGTA subjects the endothelial cells to hypocalcemic conditions, which is known to cause connexin hemichannel-mediated release of ATP.55 Consistent with this, our results show a statistically significant increase (p<0.01) in ATP detected in the media of EGTA-treated HMVECs (Fig. 2). The amount of ATP detected in the normocalcemic media was not affected by treatment with FFA or JM2. However, in low-calcium conditions, the addition of FFA or JM2 to the media significantly attenuated the increased ATP release (p<0.05) observed with EGTA treatment alone.
FIG. 2.
Targeting Cx43/microtubule interaction inhibits ATP release by microvascular endothelial cells. Human microvascular endothelial cells exposed to low calcium induced by EGTA chelation display significantly enhanced ATP release over baseline (vehicle control). JM2 and flufenamic acid (FFA) alone had no effect on ATP release; however, when applied in hypocalcemic conditions, JM2 and FFA prevent ATP increases induced by low calcium. *p<0.01 versus all normocalcemic treatments; #p<0.05 EGTA versus JM2+EGTA and FFA+EGTA. Comparisons between all other groups were not statistically significant. Error bars represent standard deviation.
Known blockers of connexin hemichannels reduce early inflammation surrounding silicone implants
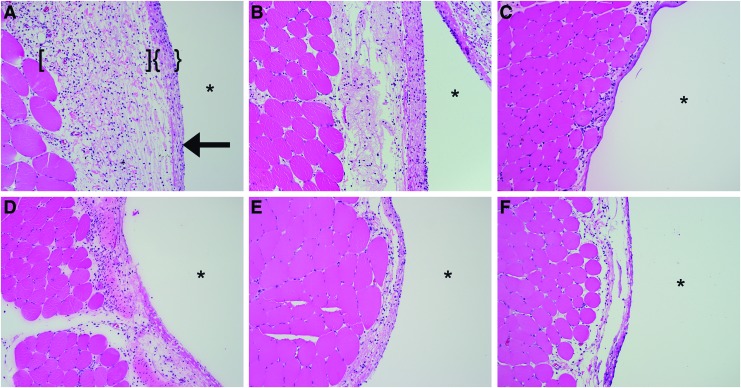
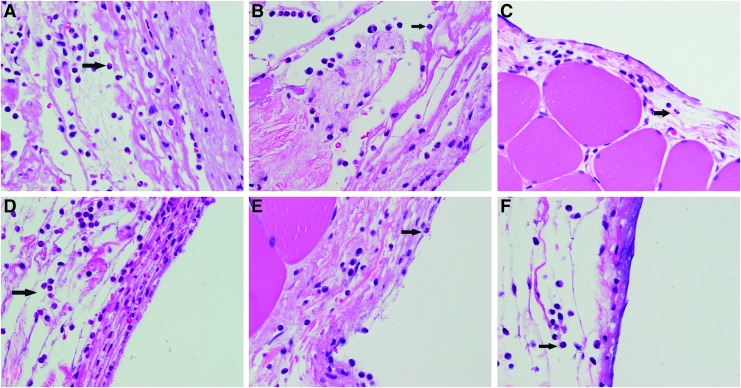
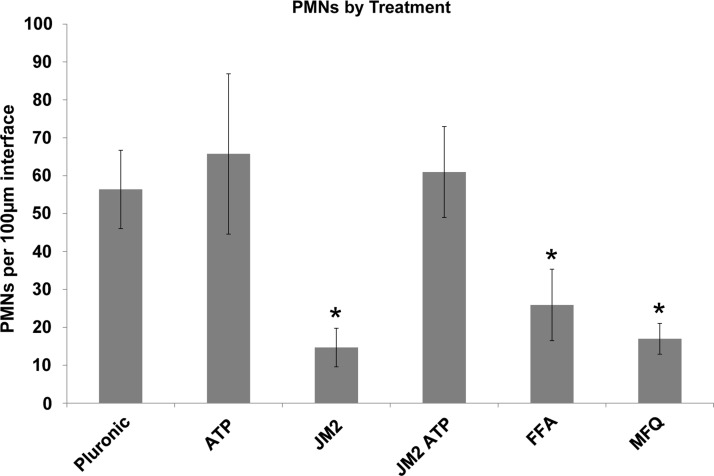
H&E bright-field imaging of the muscle tissue–silicone implant interface at 24 h revealed a mixed cellular infiltrate of neutrophils, monocytes, macrophages, other granulocytes, and fibroblasts in a dense eosinophilic layer of extracellular matrix in control conditions (Fig. 3). Neutrophils were the most abundant inflammatory cell type present in control specimens, although notable populations of monocytes and macrophages were observed (Fig. 4). In contrast, the channel blockers, FFA and MFQ, were found to decrease the intensity of the total inflammatory infiltrate compared with control (16.3% and 35.6%, respectively). Reductions observed in total inflammatory cell numbers were significant for the MFQ group (p<0.05), but not for the FFA group (p>0.05) (Fig. 5). However, reductions in PMN populations compared with control were statistically significant (p<0.01) in both FFA- and MFQ-treated animals (Figs. 6 and 7).
FIG. 3.
JM2 improves the histologic appearance of the implant/muscle interface. Hematoxylin and eosin-stained tissue sections were imaged at 10×magnification. (A) Pluronic vehicle control, (B) 1 mM ATP, (C) 180 μM JM2, (D) 180 μM JM2+1 mM ATP, (E) 200 μM FFA, and (F) 200 μM mefloquine (MFQ). * indicates implant pocket; arrows indicate implant interface. Application of JM2, FFA, and MFQ reduced formation of matrix surrounding implants and reduced cellularity of the compact matrix (indicated by {}) and loose matrix (indicated by []) adjacent to implant pockets. Note that JM2, FFA, and MFQ groups had smaller numbers of infiltrating inflammatory cells around implants. Color images available online at www.liebertpub.com/tea
FIG. 4.
JM2 reduces the number of infiltrating polymorphonuclear leukocytes (PMNs). Hematoxylin and eosin-stained tissue sections were imaged at 40×magnification. (A) Pluronic vehicle control, (B) pluronic containing 1 mM ATP, (C) pluronic containing 180 μM JM2, (D) pluronic containing 180 μM JM2+1 mM ATP, (E) Pluronic containing 200 μM FFA, and (F) Pluronic containing 200 μM MFQ. Arrows indicate cells representative of PMNs. Inhibition of ATP signaling with JM2, FFA, or MFQ reduced populations of infiltrating PMNs as at 24 h. PMNs, polymorphonuclear leukocytes.
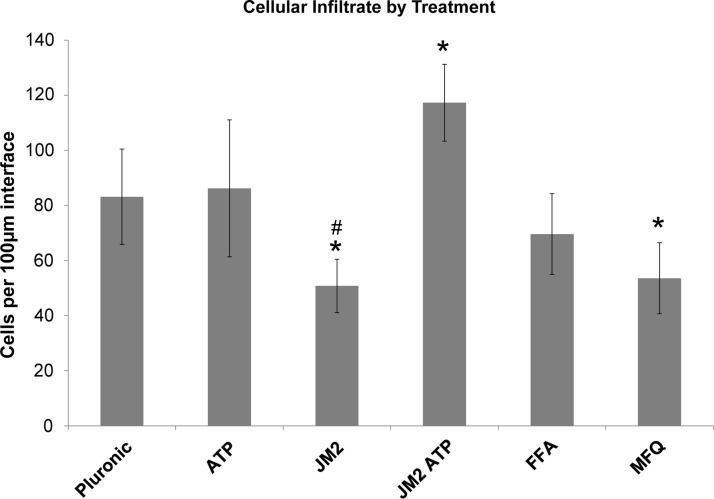
FIG. 5.
Inflammatory infiltrate in response to silicone implants at 24 h is significantly reduced by JM2. Cell numbers in interface regions defined by 100 μm lengths were counted and are presented as averages per experimental group. *p<0.05 versus control pluronic; #p<0.001 versus JM2+ATP. Error bars represent standard deviation.
FIG. 6.
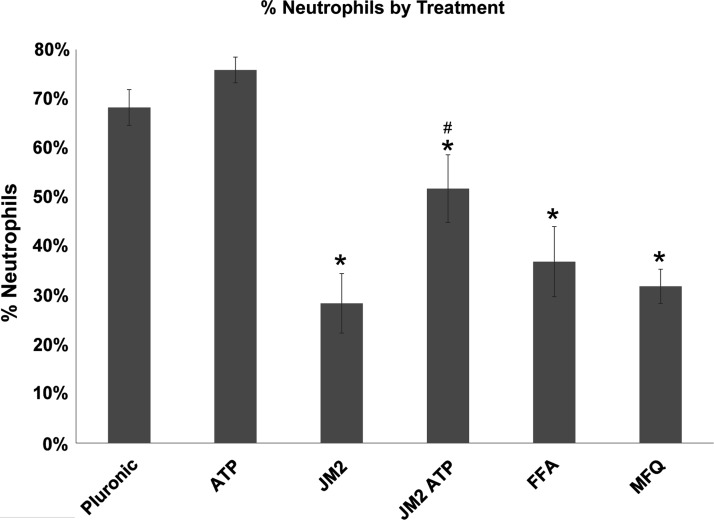
Targeting Cx43 channels reduces the proportion of inflammatory infiltrate comprising neutrophils. Average percent of infiltrates identified as PMNs for each experimental group. *p<0.001 versus control pluronic or ATP groups; #p<0.01 versus all other groups. Error bars represent standard deviation.
FIG. 7.
Connexin channel blockers significantly reduce the number of infiltrating neutrophils at the implant interface. Neutrophils were counted in high-resolution (40×) images of the implant interface and, based on the total number of infiltrating cells, counted in low-resolution images (Fig. 4); the number of neutrophils per 100 μM of implant interface was estimated for each section. Connexin channel inhibitors, JM2, FFA, and MFQ, significantly reduce the number of infiltrating neutrophils at the implant interface *p<0.01. Error bars represent standard deviation.
JM2 inhibits and reduces inflammation surrounding silicone implants in an ATP-dependent manner
MFQ and FFA are promiscuous inhibitors known to block not only connexin hemichannels but also pannexin and other ion channels.51,52,56,57 In contrast, the Cx43 microtubule-binding motif is specific to Cx43 and not conserved among other connexin isoforms, let alone other channel types.41 Therefore, we used JM2 to determine the contribution of Cx43 to early inflammation in the foreign body response. Morphological and cellular infiltrate features were comparable between control implants and those treated with pluronic gel containing 1 mM ATP (Figs. 3 and 4). By comparison, the presence of 180 μM JM2 in the pluronic coating dramatically reduced the inflammatory infiltrate compared with control and ATP groups (Figs. 3 and 4). This was confirmed by cell counts, which indicated a statistically significant (p<0.05) reduction in inflammatory cells of 38.9% and 41.1% compared with control and ATP groups, respectively (Fig. 5). The matrix interface present at the implant surface of JM2-treated animals appeared less dense than ATP or control groups. Importantly, the effects of JM2 alone were reversed by addition of 1 mM ATP with 180 μM JM2, resulting in a dramatic increase in the inflammatory response compared with JM2 (p<0.001), control (p<0.01), and ATP alone (p<0.05). Compounding this effect, quantitative analysis of infiltrate composition at 24 h confirmed that PMNs represented the largest fraction of cells in the control, ATP, and JM2+ATP groups, while in JM2-treated animals, PMNs only represented 28.4% of the total infiltrate (Figs. 6 and 7). Taking cellular composition into account, treatment with JM2, FFA, or MFQ yielded statistically significant (p<0.01) reductions in PMNs of 74%, 54%, and 69.9%, respectively. These results demonstrate that the JM2 peptide inhibits early inflammation during the foreign body response, indicating that purinergic signaling through Cx43 hemichannels is a major contributor to targeting of neutrophils to implant sites.
Discussion
This study represents the first demonstration that extracellular ATP is a crucial factor in determining the intensity of acute inflammation during the foreign body response to sterile implants. Specifically, we found that endothelial cell ATP release through Cx43 hemichannels could be attenuated by targeting the Cx43 microtubule-binding domain. Application of the Cx43 mimetic peptide used to target this domain reduced inflammation around silicone implants and this effect could be rescued by addition of exogenous ATP. Taken together, these findings suggest that ATP release from endothelial cells mediates recruitment of inflammatory cells to sites of surgical implantation.
This model is supported by evidence in the literature. Early inflammatory events following surgical placement of medical devices and biomaterials have been shown to initiate processes responsible for implant-associated complications, dysfunction, or failure.3,18,58,59 Shortly after placement, a provisional matrix forms on the implant surface comprising proteins derived from bodily fluids in the immediate vicinity.60,61 This coating serves as a recognition site for recruitment, adherence, and activation of PMNs (60, 61). These activated neutrophils release cytokines that attract additional neutrophils, monocytes, and macrophages.14,16,58–60,62 The ensuing release of reactive oxygen species and enzymes damages implanted materials and kills healthy surrounding native tissue.10 Downstream consequences of this response may contribute to the formation of a fibrotic contractile capsule.3,18,30
However, the initial signals targeting PMNs to the implant site are not fully defined.10–12 This process is regulated by a complex array of cellular signals released by multiple cell types. Recent research has demonstrated that purinergic signals participate in regulation of inflammatory processes. In vitro work in the 1990s by Bodin and Burnstock showed that under experimental conditions of shear and inflammation, endothelial cells were found to release ATP into the surrounding media.63 Chen et al. found that neutrophil chemotaxis occurred in an ATP-dependent manner.22 Additional work by this group showed that in an animal model of sepsis, PMN activation correlated with serum levels of ATP.64 Furthermore, activation of ATP receptors after intraperitoneal injection of Escherichia coli increased the neutrophil response.65 Relevant to sterile implant placement, work by Kubes and colleagues has demonstrated that extracellular ATP generated in a sterile injury plays a key role in targeting PMNs to damaged tissues.21 Knockout of CD39, which degrades ATP to AMP, was shown in mice to increase tissue damage induced by ischemia and increase the number of acutely infiltrating inflammatory cells, indicating that inhibiting enzymatic clearance of ATP produces a proinflammatory environment.66 The findings of these studies confirm that purinergic signals, in particular extracellular ATP, direct neutrophil responses to inflammatory stimuli.
Permeability of connexin hemichannels to ATP is regulated by a number of physiologic, metabolic, and mechanical conditions. Tight regulation of connexin hemichannels assures a low open probability, maintaining low membrane permeability in connexin-expressing cells.67 However, connexin hemichannels have been shown to open in response to a variety of stimuli, including mechanical stress and ischemic conditions.20 Mechanical stress to cellular components of tissues is an unavoidable consequence of injury even in the context of sterile surgical procedures. An in vitro model of mechanical stress by Gomes et al. entailed physically depressing the cellular membrane of bovine corneal endothelial cells, known to express Cx43 hemichannels, with a glass pipette, inducing ATP release through connexin hemichannels.68 Plated astrocytes subjected to mechanical stimulation induced ATP release, again, through Cx43 hemichannels.69 Ponsaerts et al. found that the Cx43 hemichannel opening was regulated by interactions with the cytoskeleton.70 Although these results were observed in in vitro models, they indicate that connexin hemichannels, in particular of the Cx43 isoform, open and release ATP under conditions that are encountered during inflammation.
We and others have demonstrated that connexins are fundamental to wound healing responses. Seminal work by Qiu et al. established this role for connexins in wound healing by showing that knockdown of Cx43 expression accelerated healing and reduced scarring.32 Subsequent work by our laboratories has solidified the importance of Cx43 in the injury response. Application of a connexin mimetic peptide significantly improved the wound healing response to dermal injuries in mice.31 More salient to the present study, we demonstrated that connexin channel function is an important mediator of the inflammatory and encapsulation response to a foreign body.30 In that study, a peptide designed to block Cx43/ZO-1 interaction was used (ACT1). Importantly, ACT1 was shown to sequester hemichannels by incorporation into GJ plaques as intercellular channels.23 This indicated that hemichannel function plays a role in the inflammatory response to an implant with downstream effects on encapsulation, but the specific link between hemichannel function and the foreign body response was not clear.
Taken together, the evidence that purinergic signals initiate early inflammation, connexin hemichannels are conduits for ATP release, and Cx43 plays a key role in wound healing and the foreign body response indicates that Cx43 hemichannels are a principle pathway for inflammation-inducing purinergic signaling following an injury. Therefore, in the present study, we sought to test this hypothesis, and our results demonstrate that inhibition of connexin-mediated ATP release significantly reduces inflammatory cell infiltrates around implants during the acute phase of the response. Importantly, we found that the most effective means of limiting inflammation was by specifically inhibiting ATP release through Cx43 hemichannels using JM2, a peptide targeting the unique Cx43 microtubule-binding motif.41 The significance of this finding is that it provides a specific target for therapeutic intervention. Others have linked connexins, purinergic signaling, and inflammation in a model of spinal cord injury49; however, this is the first demonstration in an implant model.
We note that the combinatorial treatment of JM2 and ATP did not fully rescue the reduced relative contribution of neutrophils observed in JM2-treated implants (Fig. 6). This may reflect a secondary effect of JM2 on GJ communication. Specifically, Lo and colleagues have demonstrated that targeting the Cx43 microtubule-binding domain reduces GJ communication.45 Therefore, while addition of ATP to JM2 treatments might rescue reduced ATP signaling, JM2 may also reduce cell death signals communicated directly through GJs—the bystander effect.71 The resulting decrease in cellular debris could further dampen the neutrophil response.
Another pertinent consideration for our findings is the macrophage phenotype. Macrophages enter the implant site soon after PMNs and participate in both the acute and chronic immune responses.61 During an inflammatory response, neutrophils and macrophages release signals that regulate each other's behavior.59,72,73 PMNs affect the recruitment and behavior of macrophages, including phenotypic polarization.58,59,74 Macrophage polarization is an important factor in determining whether the host's response to an implant will be damaging or regenerative, as demonstrated by recent findings.75 Badylak and colleagues have shown that macrophage phenotypes influence the immune response to an implanted material.74 Resolution of inflammation, indicated by neutrophil apoptosis and phagocytosis by macrophages, promotes an anti-inflammatory macrophage phenotype.72,76–78 On the other hand, persistent activation of neutrophils leads to continued recruitment and production of proinflammatory cytokines promoting proinflammatory macrophage phenotypes.58,74,79,80 These findings, in the context of our results, suggest that inhibition of Cx43 hemichannel ATP release may promote anti-inflammatory macrophage phenotypes after implant placement, reducing implant failure and associated complications.
A major obstacle to successful implantation of tissue-engineered constructs and cell-based therapies is the host immune response.20,81–83 Once implanted, the actions of the acute immune response jeopardize the integrity of scaffolds and the survival of implanted cells. The clinical success of these constructs depends on adequate delivery and survival of engineered tissues and cells. Technologies that act locally and promote integration and regeneration of the site rather than continued inflammation may potentially increase the success of engineered tissues. Selective local inhibition of Cx43 hemichannel-mediated ATP release may provide an option to increase cell viability by reducing the intensity of the neutrophil response and promoting anti-inflammatory signaling, thereby promoting survival of cellular constructs.
Importantly, the effects of the peptide are likely limited in duration to the time frame of the early inflammatory response. We have found that a similar Cx43-based peptide, when applied to the murine heart in a methyl cellulose patch formulation, is detectable in ventricular muscle within the first 24 h and is mostly gone by 48 h.84 We have also previously shown that neutrophil infiltration in a rat submuscular implant model is greatest at 24 h.30 Therefore, cell-penetrating Cx43-based peptides represent effective tools to augment the initial neutrophil-dominated stages of the innate inflammatory response without impacting the proliferative and remodeling phases of wound healing.
Cx43 hemichannels are increasingly recognized as components of inflammatory signaling mechanisms.85 As such, the implications of this study and others showing that endothelial cell release of ATP is an important factor in a variety of pathologic conditions extend far beyond the immune response to an implant. Further studies will likely shed light on the role played by connexin-mediated purinergic signaling in infection, sustained immunity, the endothelial dysfunction of shock, and reperfusion injury, among others.
Concluding Remarks
The use of medical implants has grown tremendously in the past 50 years and continues to do so.1,2 Implant dysfunction and failure are major sources of patient morbidity and increased healthcare costs.8 The response to an implant is a complex series of events that includes tissue damage, hemostasis, immune activation, debris clearance, abatement, and tissue regeneration versus chronic inflammation.86 Driving this sequence is a web of various autocrine and paracrine cellular signals, with recent evidence pointing to extracellular ATP as an important component in the signaling milieu.20 This study contributes to this growing body of evidence, with the novel finding that purinergic signaling is a key component of early inflammation during the foreign body response. Moreover, our results show that this response is likely mediated by Cx43 hemichannel release of ATP. Importantly, these data support previous findings that a new class of Cx43-based peptidomimetic drugs is a highly efficacious route to treating injuries, while limiting off-target side effects.30,31,34,35,84 These novel molecular therapies may be vital in improving patient tolerance of implanted devices and engineered tissues.
Acknowledgments
Funding was provided by the National institutes of Health, National Institute of Dental and Craniofacial Research NIDCR-1 R01 DE019355. M.J.Y. was the principal investigator. This study used the services of the Morphology, Imaging, and Instrumentation Core, which is supported by NIH-NIGMS P30 GM103342 to the South Carolina COBRE for Developmentally Based Cardiovascular Diseases.
Disclosure Statement
No competing financial interests exist.
References
- 1.Ratner B.D., and Bryant S.J. Biomaterials: where we have been and where we are going. Annu Rev Biomed Eng 6, 41, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Allied Market Research. www.alliedmarketresearch.com Medical Implants Market—Growth, Global Share, Industry Overview, Analysis, Trends Opportunities and Forecast 2012–2020. 2014
- 3.DiEgidio P., Friedman H.I., Gourdie R.G., Riley A.E., Yost M.J., and Goodwin R.L. Biomedical implant capsule formation: lessons learned and the road ahead. Ann Plast Surg 73, 451, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Krishnan N.M., Chatterjee A., Rosenkranz K.M., Powell S.G., Nigriny J.F., and Vidal D.C. The cost effectiveness of acellular dermal matrix in expander-implant immediate breast reconstruction. J Plast Reconstr Aesthet Surg 67, 468, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Bond M., Mealing S., Anderson R., Elston J., Weiner G., Taylor R.S., et al. The effectiveness and cost-effectiveness of cochlear implants for severe to profound deafness in children and adults: a systematic review and economic model. Health Technol Assess 13, 1, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Epstein A.E., DiMarco J.P., Ellenbogen K.A., Estes N.A., 3rd, Freedman R.A., Gettes L.S., et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol 51, e1, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Nguyen M.T., Berger R.L., Hicks S.C., Davila J.A., Li L.T., Kao L.S., et al. Comparison of outcomes of synthetic mesh vs suture repair of elective primary ventral herniorrhaphy: a systematic review and meta-analysis. JAMA Surg 149, 415, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Poulose B.K., Shelton J., Phillips S., Moore D., Nealon W., Penson D., et al. Epidemiology and cost of ventral hernia repair: making the case for hernia research. Hernia 16, 179, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Bazaka K.J., MV. Implantable devices: issues and challenges. Electronics 2, 1, 2013 [Google Scholar]
- 10.Labow R.S., Meek E., and Santerre J.P. Neutrophil-mediated biodegradation of medical implant materials. J Cell Physiol 186, 95, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Sutherland K., Mahoney J.R., 2nd, Coury A.J., and Eaton J.W. Degradation of biomaterials by phagocyte-derived oxidants. J Clin Invest 92, 2360, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye Q., Harmsen M.C., van Luyn M.J., and Bank R.A. The relationship between collagen scaffold cross-linking agents and neutrophils in the foreign body reaction. Biomaterials 31, 9192, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Bennet W., Sundberg B., Groth C.G., Brendel M.D., Brandhorst D., Brandhorst H., et al. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes 48, 1907, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Janardhan K.S., Sandhu S.K., and Singh B. Neutrophil depletion inhibits early and late monocyte/macrophage increase in lung inflammation. Front Biosci 11, 1569, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Soehnlein O., Xie X., Ulbrich H., Kenne E., Rotzius P., Flodgaard H., et al. Neutrophil-derived heparin-binding protein (HBP/CAP37) deposited on endothelium enhances monocyte arrest under flow conditions. J Immunol 174, 6399, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Soehnlein O., Zernecke A., Eriksson E.E., Rothfuchs A.G., Pham C.T., Herwald H., et al. Neutrophil secretion products pave the way for inflammatory monocytes. Blood 112, 1461, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franz S., Rammelt S., Scharnweber D., and Simon J.C. Immune responses to implants—a review of the implications for the design of immunomodulatory biomaterials. Biomaterials 32, 6692, 2011 [DOI] [PubMed] [Google Scholar]
- 18.MacLauchlan S., Skokos E.A., Meznarich N., Zhu D.H., Raoof S., Shipley J.M., et al. Macrophage fusion, giant cell formation, and the foreign body response require matrix metalloproteinase 9. J Leukoc Biol 85, 617, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singer A.J., and Clark R.A. Cutaneous wound healing. N Engl J Med 341, 738, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Rhett J.M., Fann S.A., and Yost M.J. Purinergic signaling in early inflammatory events of the foreign body response: modulating extracellular ATP as an enabling technology for engineered implants and tissues. Tissue Eng Part B Rev 20, 392, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald B., Pittman K., Menezes G.B., Hirota S.A., Slaba I., Waterhouse C.C., et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330, 362, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Chen Y., Corriden R., Inoue Y., Yip L., Hashiguchi N., Zinkernagel A., et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314, 1792, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Rhett J.M., Jourdan J., and Gourdie R.G. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol Biol Cell 22, 1516, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhett J.M., Ongstad E.L., Jourdan J., and Gourdie R.G. Cx43 associates with Na(v)1.5 in the cardiomyocyte perinexus. J Membr Biol 245, 411, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans W.H., and Martin P.E. Gap junctions: structure and function (Review). Mol Membr Biol 19, 121, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Orellana J.A., Froger N., Ezan P., Jiang J.X., Bennett M.V., Naus C.C., et al. ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J Neurochem 118, 826, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Froger N., Orellana J.A., Calvo C.F., Amigou E., Kozoriz M.G., Naus C.C., et al. Inhibition of cytokine-induced connexin43 hemichannel activity in astrocytes is neuroprotective. Mol Cell Neurosci 45, 37, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Garre J.M., Retamal M.A., Cassina P., Barbeito L., Bukauskas F.F., Saez J.C., et al. FGF-1 induces ATP release from spinal astrocytes in culture and opens pannexin and connexin hemichannels. Proc Natl Acad Sci U S A 107, 22659, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhett J.M., Ghatnekar G.S., Palatinus J.A., O'Quinn M., Yost M.J., and Gourdie R.G. Novel therapies for scar reduction and regenerative healing of skin wounds. Trends Biotechnol 26, 173, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Soder B.L., Propst J.T., Brooks T.M., Goodwin R.L., Friedman H.I., Yost M.J., et al. The connexin43 carboxyl-terminal peptide ACT1 modulates the biological response to silicone implants. Plast Reconstr Surg 123, 1440, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Ghatnekar G.S., O'Quinn M.P., Jourdan L.J., Gurjarpadhye A.A., Draughn R.L., and Gourdie R.G. Connexin43 carboxyl-terminal peptides reduce scar progenitor and promote regenerative healing following skin wounding. Regen Med 4, 205, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu C., Coutinho P., Frank S., Franke S., Law L.Y., Martin P., et al. Targeting connexin43 expression accelerates the rate of wound repair. Curr Biol 13, 1697, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Solan J.L., and Lampe P.D. Connexin43 phosphorylation: structural changes and biological effects. Biochem J 419, 261, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grek C.L., Rhett J.M., and Ghatnekar G.S. Cardiac to cancer: connecting connexins to clinical opportunity. FEBS Lett 588, 1349, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghatnekar G.S., Grek C.L., Armstrong D.G., Desai S.C., and Gourdie R.G. The effect of a connexin43-based peptide on the healing of chronic venous leg ulcers: a multicenter, randomized trial. J Invest Dermatol 135, 289, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faigle M., Seessle J., Zug S., El Kasmi K.C., and Eltzschig H.K. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS One 3, e2801, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guyot A., and Hanrahan J.W. ATP release from human airway epithelial cells studied using a capillary cell culture system. J Physiol 545, 199, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lohman A.W., and Isakson B.E. Differentiating connexin hemichannels and pannexin channels in cellular ATP release. FEBS Lett 588, 1379, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laird D.W. Life cycle of connexins in health and disease. Biochem J 394, 527, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coutinho P., Qiu C., Frank S., Tamber K., and Becker D. Dynamic changes in connexin expression correlate with key events in the wound healing process. Cell Biol Int 27, 525, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Giepmans B.N., Verlaan I., Hengeveld T., Janssen H., Calafat J., Falk M.M., et al. Gap junction protein connexin-43 interacts directly with microtubules. Curr Biol 11, 1364, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Martin P.E., Blundell G., Ahmad S., Errington R.J., and Evans W.H. Multiple pathways in the trafficking and assembly of connexin 26, 32 and 43 into gap junction intercellular communication channels. J Cell Sci 114, 3845, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Lauf U., Giepmans B.N., Lopez P., Braconnot S., Chen S.C., and Falk M.M. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci U S A 99, 10446, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas T., Jordan K., Simek J., Shao Q., Jedeszko C., Walton P., et al. Mechanisms of Cx43 and Cx26 transport to the plasma membrane and gap junction regeneration. J Cell Sci 118, 4451, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Francis R., Xu X., Park H., Wei C.J., Chang S., Chatterjee B., et al. Connexin43 modulates cell polarity and directional cell migration by regulating microtubule dynamics. PLoS One 6, e26379, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wender P.A., Mitchell D.J., Pattabiraman K., Pelkey E.T., Steinman L., and Rothbard J.B. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc Natl Acad Sci U S A 97, 13003, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basak R., and Bandyopadhyay R. Encapsulation of hydrophobic drugs in Pluronic F127 micelles: effects of drug hydrophobicity, solution temperature, and pH. Langmuir 29, 4350, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Takahashi T., Kimura Y., Niwa K., Ohmiya Y., Fujimura T., Yamasaki K., et al. In vivo imaging demonstrates ATP release from murine keratinocytes and its involvement in cutaneous inflammation after tape stripping. J Invest Dermatol 133, 2407, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Huang C., Han X., Li X., Lam E., Peng W., Lou N., et al. Critical role of connexin 43 in secondary expansion of traumatic spinal cord injury. J Neurosci 32, 3333, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guinamard R., Simard C., and Del Negro C. Flufenamic acid as an ion channel modulator. Pharmacol Ther 138, 272, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bruzzone R., Barbe M.T., Jakob N.J., and Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem 92, 1033, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Iglesias R., Spray D.C., and Scemes E. Mefloquine blockade of Pannexin1 currents: resolution of a conflict. Cell Commun Adhes 16, 131, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lopez-Izquierdo A., Ponce-Balbuena D., Moreno-Galindo E.G., Arechiga-Figueroa I.A., Rodriguez-Martinez M., Ferrer T., et al. The antimalarial drug mefloquine inhibits cardiac inward rectifier K+ channels: evidence for interference in PIP2-channel interaction. J Cardiovasc Pharmacol 57, 407, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Flower R., Gryglewski R., Herbaczynska-Cedro K., and Vane J.R. Effects of anti-inflammatory drugs on prostaglandin biosynthesis. Nature New Biol 238, 104, 1972 [DOI] [PubMed] [Google Scholar]
- 55.Quist A.P., Rhee S.K., Lin H., and Lal R. Physiological role of gap-junctional hemichannels. Extracellular calcium-dependent isosmotic volume regulation. J Cell Biol 148, 1063, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eskandari S., Zampighi G.A., Leung D.W., Wright E.M., and Loo D.D. Inhibition of gap junction hemichannels by chloride channel blockers. J Membr Biol 185, 93, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Cruikshank S.J., Hopperstad M., Younger M., Connors B.W., Spray D.C., and Srinivas M. Potent block of Cx36 and Cx50 gap junction channels by mefloquine. Proc Natl Acad Sci U S A 101, 12364, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Erwig L.P., and Henson P.M. Immunological consequences of apoptotic cell phagocytosis. Am J Pathol 171, 2, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fadok V.A., McDonald P.P., Bratton D.L., and Henson P.M. Regulation of macrophage cytokine production by phagocytosis of apoptotic and post-apoptotic cells. Biochem Soc Trans 26, 653, 1998 [DOI] [PubMed] [Google Scholar]
- 60.Wilson C.J., Clegg R.E., Leavesley D.I., and Pearcy M.J. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng 11, 1, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Anderson J.M., Rodriguez A., and Chang D.T. Foreign body reaction to biomaterials. Semin Immunol 20, 86, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jaillon S., Galdiero M.R., Del Prete D., Cassatella M.A., Garlanda C., and Mantovani A. Neutrophils in innate and adaptive immunity. Semin Immunopathol 35, 377, 2013 [DOI] [PubMed] [Google Scholar]
- 63.Bodin P., and Burnstock G. Increased release of ATP from endothelial cells during acute inflammation. Inflamm Res 47, 351, 1998 [DOI] [PubMed] [Google Scholar]
- 64.Sumi Y., Woehrle T., Chen Y., Bao Y., Li X., Yao Y., et al. Plasma ATP is required for neutrophil activation in a mouse sepsis model. Shock 42, 142, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lecut C., Frederix K., Johnson D.M., Deroanne C., Thiry M., Faccinetto C., et al. P2X1 ion channels promote neutrophil chemotaxis through Rho kinase activation. J Immunol 183, 2801, 2009 [DOI] [PubMed] [Google Scholar]
- 66.Hyman M.C., Petrovic-Djergovic D., Visovatti S.H., Liao H., Yanamadala S., Bouis D., et al. Self-regulation of inflammatory cell trafficking in mice by the leukocyte surface apyrase CD39. J Clin Invest 119, 1136, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bao X., Altenberg G.A., and Reuss L. Mechanism of regulation of the gap junction protein connexin 43 by protein kinase C-mediated phosphorylation. Am J Physiol Cell Physiol 286, C647, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Gomes P., Srinivas S.P., Van Driessche W., Vereecke J., and Himpens B. ATP release through connexin hemichannels in corneal endothelial cells. Invest Ophthalmol Vis Sci 46, 1208, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Stout C.E., Costantin J.L., Naus C.C., and Charles A.C. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem 277, 10482, 2002 [DOI] [PubMed] [Google Scholar]
- 70.Ponsaerts R., De Vuyst E., Retamal M., D'Hondt C., Vermeire D., Wang N., et al. Intramolecular loop/tail interactions are essential for connexin 43-hemichannel activity. FASEB J 24, 4378, 2010 [DOI] [PubMed] [Google Scholar]
- 71.Grek C.L., Matthew Rhett J., and Ghatnekar G.S. Cardiac to cancer: connecting connexins to clinical opportunity. FEBS Lett 588, 1349, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bratton D.L., and Henson P.M. Neutrophil clearance: when the party is over, clean-up begins. Trends Immunol 32, 350, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barrientos S., Stojadinovic O., Golinko M.S., Brem H., and Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen 16, 585, 2008 [DOI] [PubMed] [Google Scholar]
- 74.Brown B.N., Londono R., Tottey S., Zhang L., Kukla K.A., Wolf M.T., et al. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater 8, 978, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wolf M.T., Dearth C.L., Ranallo C.A., LoPresti S.T., Carey L.E., Daly K.A., et al. Macrophage polarization in response to ECM coated polypropylene mesh. Biomaterials 35, 6838, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eken C., Martin P.J., Sadallah S., Treves S., Schaller M., and Schifferli J.A. Ectosomes released by polymorphonuclear neutrophils induce a MerTK-dependent anti-inflammatory pathway in macrophages. J Biol chem 285, 39914, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Savill J.S., Wyllie A.H., Henson J.E., Walport M.J., Henson P.M., and Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest 83, 865, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Serhan C.N., and Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol 6, 1191, 2005 [DOI] [PubMed] [Google Scholar]
- 79.Simon H.U. Neutrophil apoptosis pathways and their modifications in inflammation. Immunol Rev 193, 101, 2003 [DOI] [PubMed] [Google Scholar]
- 80.Kumar V., and Sharma A. Neutrophils: Cinderella of innate immune system. Int Immunopharmacol 10, 1325, 2010 [DOI] [PubMed] [Google Scholar]
- 81.Naziruddin B., Iwahashi S., Kanak M.A., Takita M., Itoh T., and Levy M.F. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. Am J Transplant 14, 428, 2014 [DOI] [PubMed] [Google Scholar]
- 82.Don C.W., and Murry C.E. Improving survival and efficacy of pluripotent stem cell-derived cardiac grafts. J Cell Mol Med 17, 1355, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Terrovitis J., Stuber M., Youssef A., Preece S., Leppo M., Kizana E., et al. Magnetic resonance imaging overestimates ferumoxide-labeled stem cell survival after transplantation in the heart. Circulation 117, 1555, 2008 [DOI] [PubMed] [Google Scholar]
- 84.O'Quinn M.P., Palatinus J.A., Harris B.S., Hewett K.W., and Gourdie R.G. A peptide mimetic of the connexin43 carboxyl terminus reduces gap junction remodeling and induced arrhythmia following ventricular injury. Circ Res 108, 704, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eltzschig H.K., Sitkovsky M.V., and Robson S.C. Purinergic signaling during inflammation. N Engl J Med 367, 2322, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bryers J.D., Giachelli C.M., and Ratner B.D. Engineering biomaterials to integrate and heal: the biocompatibility paradigm shifts. Biotechnol Bioeng 109, 1898, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]