Abstract
Cartilage damage and/or aging effects can cause constant pain, which limits the patient's quality of life. Although different strategies have been proposed to enhance the limited regenerative capacity of cartilage tissue, the full production of native and functional cartilaginous extracellular matrix (ECM) has not yet been achieved. Poly(γ-glutamic acid) (γ-PGA), a naturally occurring polyamino acid, biodegradable into glutamate residues, has been explored for tissue regeneration. In this work, γ-PGA's ability to support the production of cartilaginous ECM by human bone marrow mesenchymal stem/stromal cells (MSCs) and nasal chondrocytes (NCs) was investigated. MSC and NC pellets were cultured in basal medium (BM), chondrogenic medium (CM), and CM-γ-PGA-supplemented medium (CM+γ-PGA) over a period of 21 days. Pellet size/shape was monitored with time. At 14 and 21 days of culture, the presence of sulfated glycosaminoglycans (sGAGs), type II collagen (Col II), Sox-9, aggrecan, type XI collagen (Col XI), type X collagen (Col X), calcium deposits, and type I collagen (Col I) was analyzed. After excluding γ-PGA's cytotoxicity, earlier cell condensation, higher sGAG content, Col II, Sox-9 (day 14), aggrecan, and Col X (day 14) production was observed in γ-PGA-supplemented MSC cultures, with no signs of mineralization or Col I. These effects were not evident with NCs. However, Sox-9 (at day 14) and Col X (at days 14 and 21) were increased, decreased, or absent, respectively. Overall, γ-PGA improved chondrogenic differentiation of MSCs, increasing ECM production earlier in culture. It is proposed that γ-PGA incorporation in novel biomaterials has a beneficial impact on future approaches for cartilage regeneration.
Introduction
Hyaline cartilage is the most abundant cartilaginous tissue.1 It is formed by a process known as chondrogenesis, which starts with the migration of mesenchymal progenitor cells and subsequent condensation and differentiation into resting chondrocytes.1–3 These chondrocytes synthesize two kinds of cartilage: permanent and temporary.1,3,4 Permanent or persistent hyaline cartilage serves as a principal form of skeletal tissue,1 while temporary or transient hyaline cartilage acts as templates for bone growth during endochondral ossification, a process that occurs during skeletal development.1–3 The principal functions of cartilage include shock absorption, lubrication, and articulation in movable joints.1,5,6
Cartilage damage is commonly found in clinical orthopedics.7 Potential causes and mechanisms for cartilage lesions include aging effects, genetic disorders, trauma, or tumor ablations, and commonly lead to joint swelling, cartilage erosion, bone stripping, constant pain, and severe motion restrictions, with a serious impact on the quality of life.7–10 However, due to cartilage avascularity, low cellularity, and low cell turnover, this tissue has a very limited intrinsic regenerative capacity upon injury.11
Currently, cartilage regeneration strategies rely on the use of exogenous stimuli that act as chondrogenic inducers12,13 and in different combinations of cell types and biomaterials that attempt to stimulate the regenerative capacity of cartilage.12,14,15 The use of biomaterials aims at providing microenvironmental cues that mimic the native cartilaginous tissue, either inherent to the biomaterial itself or conferred by functionalization routes accessed by the biomaterial or by its degradation products. Thus, tissue formation and regeneration should be enhanced.14,16
Natural biopolymers based on cartilage extracellular matrix (ECM), such as collagen, hyaluronic acid, and chondroitin sulfate have been proposed for cartilage regeneration, particularly as tissue-engineered (TE) scaffolds.7,17 Although these biomaterials hold potential for the latter purpose, challenges such as creating biomechanically suitable tissues that promote cell adhesion and native ECM production, and moreover, integrate well with native tissue, remain unsolved.12 Hence, the continuous discovery of promising biomaterials is always encouraged. In this regard, this study aims to start exploring the potential of poly(γ-glutamic acid) (γ-PGA) in chondrogenesis by analyzing its biological effects in the cells while in its soluble form.
PGA is a natural biopolymer with an increasing potential for different tissue regenerative approaches. Its application in the biomedical field has been growing, as observed by its use in biological adhesives, chelating agents for imaging techniques, vaccines, drug/gene delivery systems, and TE-scaffolds, particularly for bone and the nervous system.18–21
In the case of bone, γ-PGA has been combined with collagen (Col) in the form of γ-PGA/Col sponges. Thus, γ-PGA modification was shown to increase calcium deposits and apatite formation.20 In addition, both L- and D-glutamate are essential for bone mineralization, which justifies the interest in this polymer for bone regeneration.22–25 For nerve tissue regeneration, γ-PGA scaffolds were shown to favor the differentiation of induced pluripotent stem cells into neuron-lineage cells.26 Moreover, the fact that L-glutamate is a major excitatory neurotransmitter in the central and peripheral nervous system underlines the interest in γ-PGA use in this field.27–29
In cartilage, glutamate signaling was shown to tune rat and human chondrocyte behavior and enhance matrix production.30–32 γ-PGA-blended scaffolds were reported to support rat chondrocyte cultures.33 A patent describing the injection of γ-PGA for the treatment of degenerative joint diseases has also been filed.34 Nevertheless, how γ-PGA influences cell differentiation toward the chondrogenic lineage and cartilaginous ECM production has never been explored.
PGA can be chemically synthesized (α-PGA) or microbially produced (γ-PGA).18 In particular, γ-PGA is obtained from fermentation of several Bacillus strains,18,35,36 and is constituted by D- and L-glutamic acid residues.21,36,37
γ-PGA biodegradability and nonimmunogenicity make this polymer attractive for tissue regeneration.18,21 Besides chemical degradation induced by prolonged exposure to extreme pH and high temperatures,18 only γ-PGA, but not α-PGA, can be degraded by enzymatic/biochemical pathways, giving rise to glutamic acid residues.18,38,39 Lee et al. demonstrated that γ-PGA supplementation in rat diet significantly increased glutamate concentration in serum and in the brain, suggesting γ-PGA biodegradation.40 On the other hand, γ-PGA nonimmunogenicity has been proposed on the basis of the absence of an immune response in the rat following repeated injections of γ-PGA.39,41
Our team has previously described γ-PGA production by Bacillus subtilis natto and subsequent purification to yield a polymer with low molecular weight (Mw) (10–50 kDa), high purity grade (∼99.5%), and a D-/L-glutamate ratio of 50–60/50–40%.36,42 This high purity level is very attractive since commercially available γ-PGA usually has low (c.a. 70%) or unknown purity levels,33,43–45 which may hinder its application in the biomedical field, and especially when it comes to clinical translation.
In this study, the effect of γ-PGA on chondrogenic differentiation of human mesenchymal stem/stromal cells (MSCs) and redifferentiation of nasal chondrocytes (NCs) was addressed. γ-PGA was added to pellet cultures over a 21-day period. This culture system is the most common in vitro model of chondrogenesis, since it simulates early condensation of mesenchymal cells during skeletogenesis.1 It is considered a straightforward method to induce chondrogenic differentiation/redifferentiation and cartilaginous matrix production of expanded chondroprogenitor/chondrocytic cells for cartilage regeneration.6 The use of MSCs and NCs relies on their ability to produce type II collagen (Col II), sulfated glycosaminoglycans (sGAGs), and other ECM components present in functional hyaline cartilage.9,15
Materials and Methods
γ-PGA production and characterization
γ-PGA was microbially produced and purified as described in Pereira et al..36
Briefly, γ-PGA was produced by B. subtilis in E medium containing 3% L-glutamic acid and 2% citric acid46,47 at 37°C and 120 rpm for a period of 72 h. The polymer was isolated from the bacterial culture broth following several steps to eliminate the medium components and other bacterial metabolites. Polysaccharides were removed by acidic hydrolysis using 6 M H2SO4 at pH 3.0, proteins were eliminated by incubation with proteinase K, and the resultant solution followed a dialysis step to remove salts and other small molecules.
MW, purity, and stereochemical composition were evaluated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, Fourier transform infrared spectroscopy, and circular dichroism, respectively. The resulting polymer had a molecular weight ranging from 10 to 50 kDa, high purity grade (99.5%), and a D-/L-glutamate ratio of 50–60/50–40%.42 The endotoxin level of γ-PGA, which was below the limit 0.5 EU/mL,48 was controlled by the Limulus Amebocyte Lysate QCL-1000 kit (Cambrex/Lonza).
MSC isolation from bone marrow
Bone marrow aspirates from newborn vertebrae were collected at the Institute of Pathology of the University Medical Center Mainz, according to the ethical guidelines of the University Medical Center Mainz and ethical committee of the German state of Rhineland-Palatinate.
The bone marrow aspirates were mixed with red blood cell lysis buffer, containing 0.15 M ammonium chloride (Merck), 10 mM potassium bicarbonate (Merck), 0.1 mM disodium ethylenediamine tetraacetate (Sigma), pH 7.2–7.4, in a 1:9 ratio and incubated at room temperature for 3 min. After centrifugation (3 min at 390 g) and subsequent washing in phosphate-buffered saline (PBS) solution, cell pellets were resuspended in Dulbecco's modified Eagle's medium/Nutrient Mixture F-12 (DMEM/F-12; GlutaMAX™, Gibco®) cell culture medium supplemented with 10% fetal bovine serum (FBS; Sigma), 1% penicillin/streptomycin (P/S; Gibco), and 5 ng/mL basic fibroblast growth factor (bFGF; Sigma-Aldrich), and seeded on cell culture flasks in a humidified atmosphere with 5% CO2 at 37°C.
At a subconfluent to confluent stage, cells were detached with 0.05% trypsin/EDTA (Gibco) and reseeded until reaching the necessary amount for the subsequent experiments. The phenotypic characterization and multilineage differentiation potential of MSCs were characterized as described in the Supplementary data (Supplementary Data are available online at www.liebertpub.com/tea).
NC isolation from nasal cartilage
NCs were isolated from adult nasal cartilage, which was collected at the Department of Oral, Cranio-Maxillofacial, and Facial Plastic Surgery of the Medical Center of the Goethe University Frankfurt, after approval by the Ethical Committee of the Medical Center of the Goethe University Frankfurt.
Slices of cartilage were chopped into small pieces with a scalpel and digested overnight with 0.15% collagenase B (Roche) in DMEM (Sigma) with 5% FBS at 37°C. Afterward, digested tissue was filtered through a 100 μm pore sized cell strainer. Chondrocytes were then pelleted down by centrifugation and resuspended in high-glucose DMEM (4.5% glucose; Gibco) supplemented with 10% FBS, 1% N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid (HEPES buffer, 1 M; Gibco), 1% sodium pyruvate (Gibco), and 1% P/S. Additionally, 1 ng/mL transforming growth factor beta 1 (TGF-β1; R&D) and 5 ng/mL bFGF were added to the medium. NCs were expanded in monolayer culture in the culture medium mentioned above in a humidified atmosphere with 5% CO2 at 37°C.
At a subconfluent to confluent stage, cells were detached with 0.05% trypsin/EDTA (Gibco) and reseeded until reaching the necessary cell quantity for the subsequent experiments.
γ-PGA cytotoxicity assay
γ-PGA cytotoxicity for MSCs and NCs was assessed by the MTS assay (MTS, Promega), which measures the conversion of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium to formazan that can be photometrically detected (492 nm).
MSCs and NCs were seeded at 30,000 cells/cm2 in flat-bottomed 96-well plates, in 100 μL of DMEM/F-12, GlutaMAX™+10% FBS+1% P/S+5 ng/mL bFGF culture medium. After 48 h, cells were cultured in two conditions: (1) medium supplemented with γ-PGA (0.2 mg/mL) and (2) medium without γ-PGA. Metabolic activity was then assessed by MTS conversion every 24 h for a total of 72 h. A blank control comprising only medium was also included. For each cell type, two to three independent donors were selected. For each measurement, three replicates/condition were included.
Three dimensional pellet cultures of MSCs and NCs
MSCs (passage 6–7) and NCs (passage 2–4) routinely expanded were seeded in 15 mL polypropylene tubes (1×106 cells/tube/mL).
Cell pellets were formed by centrifugation (5 min at 390 g) and cultured in basal medium (BM) (DMEM-high glucose (Sigma)+10% FBS+1% P/S) in a CO2 incubator at 37°C. Tube lids were loosely maintained on top of each tube to allow gas exchange. After 24 h, the pellets were cultured in three different conditions: (1) BM; (2) chondrogenic medium (CM) (DMEM-high glucose+5 μg/mL insulin (Sigma)+5 μg/mL Transferrin (Sigma)+5 ng/mL selenous acid (Sigma)+0.1 μM dexamethasone (Sigma)+0.17 mM ascorbic 2-phosphate acid (Sigma)+1 mM sodium pyruvate (Gibco)+0.35 mM proline (Sigma)+1.25 mg/mL bovine serum albumin (BSA) (Sigma)+10 ng/mL TGF-β3 (R&D) freshly added); and (3) CM supplemented with γ-PGA (0.2 mg/mL, also freshly added) (CM+γ-PGA). The media were changed twice per week during 21 days of culture.
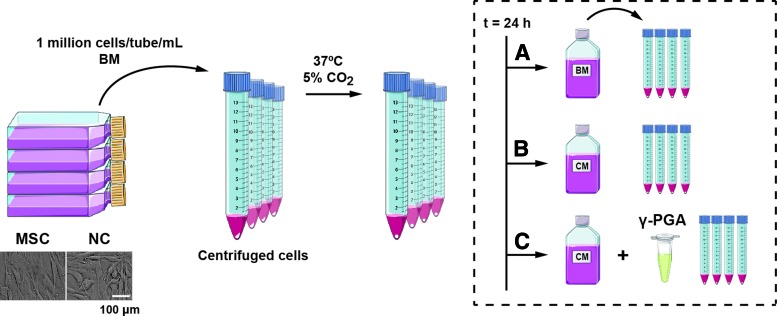
The experimental design is depicted in Figure 1.
FIG. 1.
Pellet culture experimental design. Mesenchymal stem/stromal cells (MSCs) and nasal chondrocytes (NCs) were first expanded in monolayer (bar represents 100 μm), after which they were seeded in 15 mL tubes (1 million cells/tube/mL) in basal medium (BM). Cells were centrifuged and the pellets obtained cultured at 37°C, 5% CO2. After 24 h, the pellets were cultured in three distinct conditions: (A) BM; (B) chondrogenic medium (CM); (C) CM supplemented with γ-PGA (0.2 mg/mL) during a period of 14 or 21 days. Figure uses images from Servier Medical Art. Color images available online at www.liebertpub.com/tea
Analysis of pellet size/shape distribution
Cell pellets were imaged periodically during in vitro culture using a Canon EOS 1000D Rebel XS with 18–55 mm lens (Canon Inc.) and analyzed using ImageJ software (version: 1.46r). The sizes of the pellets were depicted as the area enclosed by an ellipse. The horizontal and vertical lengths of cell pellets normalized by the length of tube base in each image were treated as the major and minor axes of each ellipse. The aspect ratios (AR) of the pellets were calculated by the quotient between horizontal and vertical lengths of cell pellets normalized by the length of tube base in each image. Image analysis was performed in ten to twelve pellets/condition (MSCs) and in six to eight pellets/condition (NCs), for three to four independent donors.
Analysis of sGAGs by histochemistry
At days 14 and 21, pellets were fixed in paraformaldehyde (PFA, 3.7%) for 15 min, washed in PBS and stored at 4°C. Subsequently, they were placed in histology cassettes, embedded in paraffin wax, cut into 2–4 μm sections, and mounted on glass slides (Super Frost color; Menzel-Gläser) or coated microscope slides (FLEX IHC Microscope Slides; Dako) compatible with a Dako Stainer plus equipment (Dako).
The slides were then immersed three times in xylene to remove paraffin and rehydrated in an alcohol series and distilled water. Paraffin-embedded pellet sections were incubated in 3% acetic acid (VWR) for 3 min and in 1% alcian blue (Chroma) solution (in 3% acetic acid, pH 2.5) for 30 min, to detect sGAGs deposition. Cell nuclei were counterstained by immersion in 0.1% nuclear fast red (VWR), 5% aluminum sulfate (VWR) solution for 10 min. After a final rinse in distilled water, dehydration in absolute ethanol, and clearing in xylene, the slides were mounted with resinous mounting medium (Entellan®; VWR).
Detection of Col II, Sox-9, Aggrecan, Col X, and Col I, by immunohistochemistry
Paraffin-embedded sections were processed for immunohistochemistry (IHC) using different antibodies: a monoclonal antibody (II-II6B3) against type II collagen (Col II, developed by Dr. Thomas F. Linsenmayer, was obtained from the Developmental Studies Hybridoma Bank under the auspices of the NICHD and maintained at the University of Iowa, Department of Biology, Iowa City, IA 52242); a polyclonal antibody (IMG-6489A) against Sox-9 (Imgenex); a monoclonal antibody (AHP0022) against aggrecan (Invitrogen); a monoclonal antibody (2031501005) against type X collagen (Col X; Quartett); and a polyclonal antibody (SAB4500362) against type I collagen (Col I; Sigma).
Sections for Sox-9, aggrecan, Col X, and Col I were stained in a standardized fully automated manner by use of a Dako Stainer plus (Dako). Endogenous peroxidase was blocked with 3% hydrogen peroxide (VWR) solution for Col II, or EnVision™ FLEX Peroxidase-Blocking Reagent for Col I, for 10 min. Antigen retrieval for Col II was performed through incubation with proteinase K (Dako) for 5 min. For Sox-9, aggrecan, Col X, and Col I, heated citrate buffer (pH 6.0; Dako) was used during 35 min. The sections were then incubated with the primary antibody against Col II (1:50, 30 min), Sox-9 (1:400, 60 min), aggrecan (1:1000, 30 min), Col X (1:50, 60 min), and Col I (1:50, 60 min) in the dark. For Col II, an extra blocking step was introduced to reduce nonspecific background staining with 6 min incubation in antibody diluent with background reducing components (Dako); while for Col I a 15 min incubation in a protein block (Dako) was included.
For Col II, visualization was performed using the EnVision Detection Systems Peroxidase/DAB Rabbit/Mouse staining kit instructions from the supplier (Dako). Bound antibodies were revealed after a 30 min incubation of HRP-Polymer (Dako) in the dark and 1 min incubation with peroxidase-substrate solution (DAB+Chromogen and Substrate Buffer; Dako). For Sox-9, aggrecan, Col X, and Col I, visualization was performed using the EnVision FLEX detection system intended for use in IHC together with Dako Autostainer Instruments following the kit instructions from the supplier (Dako). Bound antibodies were revealed after a 20 min incubation of EnVision FLEX/HRP detection reagent (Dako) in the dark and two times 5 min incubation with peroxidase-substrate solution (EnVision FLEX/DAB+Chromogen and EnVision FLEX/Substrate Buffer; Dako).
As a positive control, human nasal cartilage sections or growth plate cartilage were used, while for the negative control the same pellets without primary antibody were used.
Detection of type XI collagen by immunofluorescence
Type XI collagen (Col XI) deposition on the paraffin-embedded pellet sections was analyzed by immunofluorescent (IF) staining.
Antigen retrieval for Col XI was performed through incubation of the slices with a 20 μg/mL proteinase K (Sigma-Aldrich) solution for 5 min. A blocking step was introduced to reduce nonspecific background staining with 1 h incubation in PBS containing 5% donkey serum (DS; Santa Cruz Biotechnology, Inc.) and 0.1% Triton X-100 (Sigma). The sections were then incubated (1:50) overnight at 4°C with the primary antibody against Col XI (polyclonal antibody COL11A2 (K-16); Santa Cruz Biotechnology, Inc.) in the dark. A fluorescent secondary antibody (Invitrogen) was diluted 1:200 and incubated for 1 h, also in the dark. After washing in PBS, the samples were mounted with Fluoroshield mounting medium with DAPI (Sigma).
As positive controls, human nasal cartilage sections were used, while for negative control the same pellets without primary antibody were used.
Analysis of calcium (Ca2+) deposits by histochemistry
To evaluate possible signs of mineralization, the von Kossa stain was performed. Paraffin-embedded slides obtained as described above were immersed in 5% silver nitrate solution (VWR) for 1 h under strong light and in 0.2% gold chloride (VWR) solution for 5 min, to intensify the staining. Excess silver nitrate was removed with a 5% sodium thiosulfate (VWR) solution for 5 min. Cell nuclei were counterstained with 0.1% nuclear fast red (VWR) solution for 10 min. Ascending grades of alcohol solutions were used to dehydrate the pellet sections. Finally, samples were cleared in xylene and mounted in resin.
Quantification of sGAGs
Pellets were digested overnight at 60°C in 300 μL of papain digestion buffer containing 100 mM disodium hydrogen phosphate (Roth), 5 mM EDTA (Sigma), 5 mM L-cysteine (Aldrich), and 125 μg/mL papain from papaya latex (Sigma) at pH 7.5. After vortexing and centrifugation, sGAG content was measured after reaction with 1,9-dimethyl-methylene blue zinc chloride double salt (DMMB; Sigma-Aldrich) dye reagent solution, containing 40 mM sodium chloride (NaCl; Roth), 40 mM glycine (Roth), and 46 μM DMMB, previously adjusted to pH 3.0.
Chondroitin sulfate A sodium salt from bovine trachea (Sigma) was used as standard. To normalize the results, cellularity was measured based on the DNA content using a CyQuant® kit (Invitrogen) with lambda DNA as a standard. For MSCs, 8–10 pellets/condition were digested from three independent donors. For NCs, five to six pellets/condition were digested from three independent donors.
Free active TGF-β1 quantification
The concentration of TGF-β1 in the pellet supernatants was determined after 14 and 21 days of culture. An ELISA kit (LEGEND MAX™ Free Active TGF-β1 ELISA Kit; BioLegend®) was used to quantify TGF-β1 levels, according to the manufacturer's instructions. Results were normalized to the DNA content of each pellet. Three-pellet supernatants/condition from three independent donors were selected.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (version: 5.0a). At least three independent experiments were considered for each characterization method. The parametric distribution of the data was first evaluated by the D'Agostino & Pearson omnibus normality test. Since collected data followed a nonparametric distribution, statistical analysis was performed using Wilcoxon test to compare two groups of samples or Friedman test followed by the Dunn's multiple comparison post-test to compare more than two groups of samples. A confidence interval of at least 95% was taken to define statistical significance (*p<0.05, **p<0.025, ***p<0.001).
Results
γ-PGA cytotoxicity in MSC and NC 2D cultures
γ-PGA is a polyamino acid that was microbially produced in our lab36 and has been used by us on the basis of its ability to form polyelectrolyte complexes with cationic polymers, such as chitosan (Ch), by electrostatic interaction.42,49 γ-PGA/Ch multilayer films (prepared with [γ-PGA]=0.2 mg/mL) were not cytotoxic for human fibroblasts in culture.42
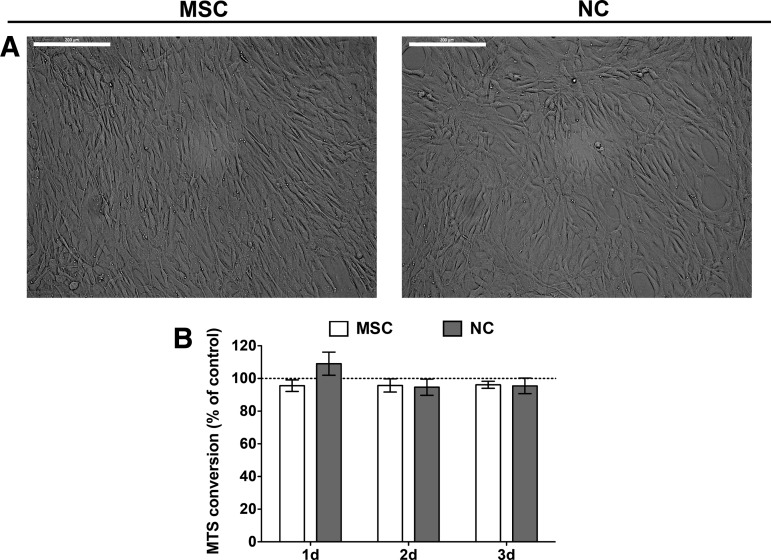
In this study, the cytotoxicity of γ-PGA in both MSC and NC cultures was addressed. First, images of MSC and NC cultures showed that both cell types presented a characteristic spindle-shaped morphology, which is their normal aspect in 2D conditions (Fig. 2A). Moreover, cell metabolic activity was evaluated during a 72 h period after γ-PGA addition and compared with control cultures (without the polymer) (Fig. 2B). The results show that γ-PGA was not cytotoxic for either cell type at the selected concentration (0.2 mg/mL). γ-PGA concentration was maintained at 0.2 mg/mL to conduct the following studies, since it is the polyion concentration of the polyelectrolyte complexation processes optimized by our team.36,42,49
FIG. 2.
(A) MSC (left) and NC (right) morphology in monolayer culture (bar represents 300 μm). (B) γ- PGA cytotoxicity in MSC (white bars) and NC (gray bars) cultures. Metabolic activity assessed by MTS assay of γ-PGA-supplemented cultures was compared with cell cultures in basal culture conditions for each time point. Results are shown as mean±SD from two to three independent donors. No statistical differences were detected using the nonparametric Wilcoxon test.
Effect of γ-PGA on the size and shape of 3D pellet chondrogenic cultures of MSCs and NCs
To promote the production of cartilaginous ECM, MSC and NC cultures were performed in three dimensional (3D) conditions. Both cell types can form cell aggregates, known as pellets, after centrifugation. The cultures were performed in CM, which is a high-glucose medium with standard chondrogenic inducers (described in the Materials and Methods section), and in γ-PGA-supplemented CM (CM+γ-PGA) ([γ-PGA]=0.2 mg/mL) during 21 days of culture. Pellet cultures in the presence of BM, without any chondrogenic inducer, were performed as control.
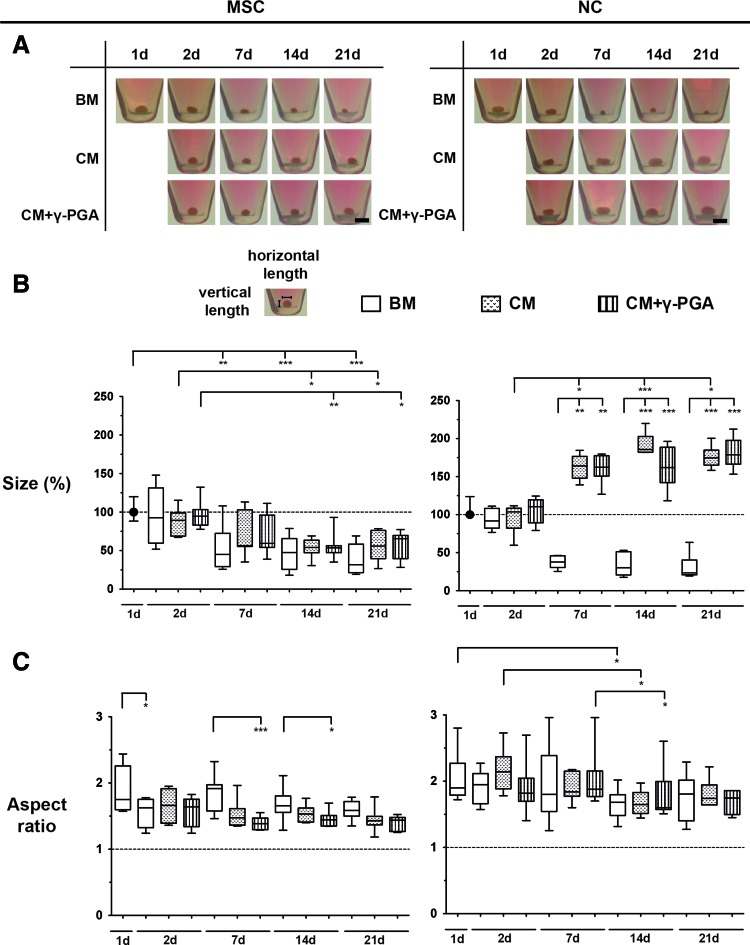
Both MSCs and NCs formed pellets within 24 h after cell seeding, and their size and shape were monitored during time in culture (Fig. 3A). The variations in size and shape of the pellets can be related with cell condensation, cell proliferation or death, and ECM production, as suggested by other authors.4
FIG. 3.
(A) Evolution of size and shape of MSC and NC pellets cultured over a 21-day period. One representative donor and pellet of each cell type is presented here. Bar represents 0.2 cm. (B) The size (ellipse area) of cell pellets was calculated from the images above over time in culture for all the conditions tested: BM (white bars), CM (bars with dots), or CM supplemented with γ-PGA (CM+γ-PGA, bars with stripes). For each experiment, areas were compared to the areas of pellets from day 1. (C) The aspect ratio (horizontal/vertical length) of the same cell pellets was also calculated. Results are represented as box plots (MSCs: seven pellets/condition, three independent donors; NCs: six pellets/condition, four independent donors). Statistical significance was set at (*p<0.05, **p<0.025, ***p<0.001), using the Friedman paired test followed by the Dunn's multiple comparison post-test. Color images available online at www.liebertpub.com/tea
To analyze the differences in pellet size in the cultures with and without γ-PGA, their surface area was quantified. Figure 3B shows size (compared to pellets at day 1, in %) median±interquartile range (IQR, difference between the 75th and 25th percentiles) of MSC and NC pellets cultured under the different conditions. In both chondrogenic conditions, MSC pellet size significantly decreased (*p<0.05) with time in culture, reaching 56.0%±36.7% and 65.5%±30.1% for CM and CM+γ-PGA, respectively, after 21 days. In BM, pellet size also showed a pronounced decrease (***p<0.001), reaching 31.7%±37.0% of their initial size.
In chondrogenic cultures of NCs, the pellet size increased, reaching 174.6%±19.8% (*p<0.05) and 178.7%±31.2% after 21 days for CM and CM+γ-PGA, respectively. In BM, NC pellet size decreased with time in culture (reaching 23.4%±19.5% after 21 days).
In addition, the pellet AR was quantified to analyze the differences in pellet shape in the cultures with and without γ-PGA.
Figure 3C shows AR median±interquartile range of MSC and NC pellets cultured under the different conditions. In the case of MSCs, from day 7 until the end of the culture, the AR of MSC pellets cultured in both chondrogenic conditions was always lower than those cultured in BM (decrease of 0.1–0.3 times). The major difference was observed after 7 days when AR of MSC pellets cultured with CM+γ-PGA was 1.4±0.2 (***p<0.001). On the other hand, the AR of NC chondrogenic pellets was higher (ranging from 1.1 to 1.4 times higher) and more variable than those of MSC pellets, although the general trend toward AR decrease with time in culture was also observed. In CM+γ-PGA conditions, the AR of NC pellets significantly decreased over time until day 14 (1.7±0.4) (*p<0.05). In addition, no significant differences were found between the different culture conditions at each time point.
Overall, morphological analysis of cell pellets suggests that cell condensation was observed in both MSC and NC pellets: MSC pellets became denser with culture time and adopted a spheroid shape (lower size and lower AR), while NC chondrogenic pellets became larger translucent discs (greater size and higher AR).
Analysis of sGAG and TGF-β1 production by MSC and NC 3D pellet cultures
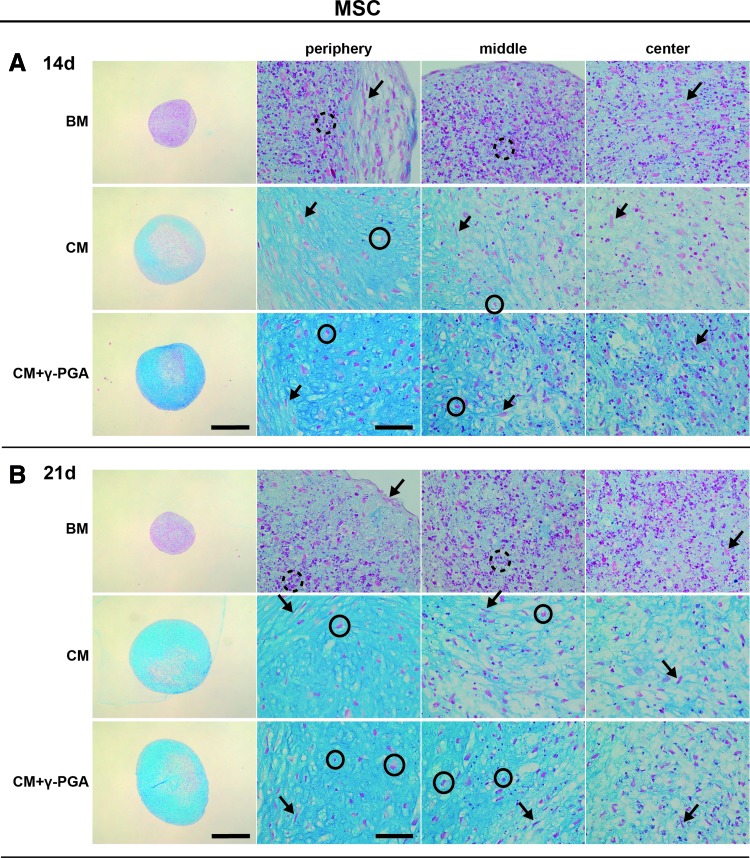
To evaluate how γ-PGA could have an impact on the production of cartilaginous matrix deposition, both MSC and NC 3D cultures were analyzed after 14 and 21 days of culture for sGAG deposition either in pellet sections or in the whole pellets after digestion. In pellet sections, alcian blue staining allowed the visualization of sGAG deposition (light blue, represented in darker shades of grey within pellets ECM) within the sections (Figs. 4 and 5 show representative images of MSC and NC pellets cultured with BM, CM and CM+γ-PGA).
FIG. 4.
Alcian blue/nuclear fast red histological staining of paraffin sections of MSC pellets cultured for (A) 14, and (B) 21 days in the three culture conditions tested: BM, CM, or CM supplemented with γ-PGA (CM+γ-PGA). Sulfated glycosaminoglycan (sGAG) deposition was observed by the presence of blue staining, here represented in darker shades of grey within pellets ECM. One representative donor of each cell type is presented here. For each time point: the first column presents whole pellet images (bars represent 500 μm). The three columns to the right are detailed images of distinct regions of the cell pellet: peripheral, middle, and center of the pellets. The bars represent 50 μm. The dashed black circles locate pyknotic/necrotic bodies, black arrows the fibrous/spindle cell shape, and the black circles highlight round-shaped morphologies. Color images available online at www.liebertpub.com/tea
FIG. 5.
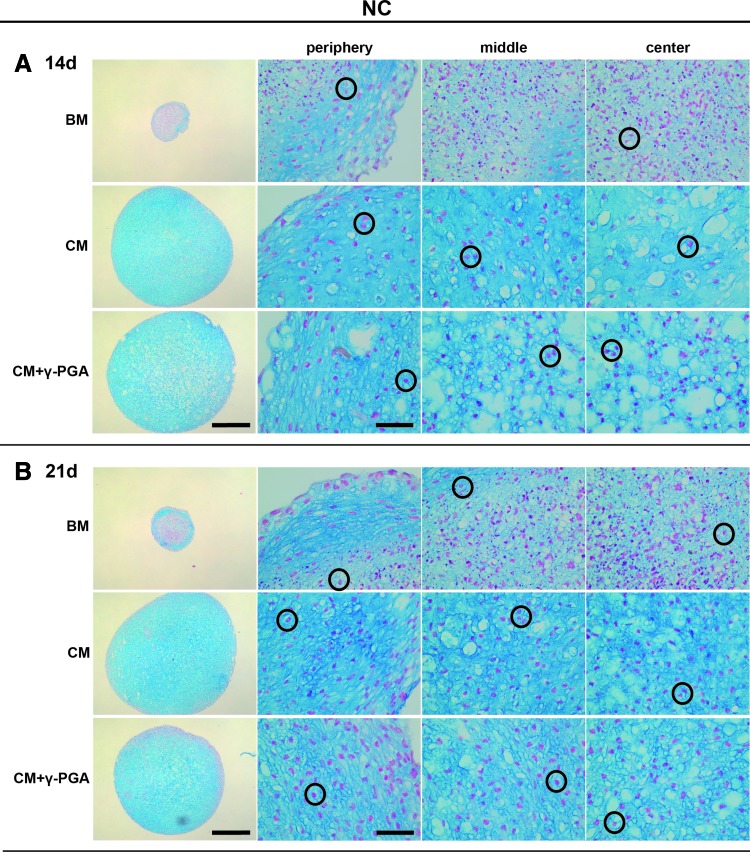
Alcian blue/nuclear fast red histological staining of paraffin sections of NC pellets cultured for (A) 14, and (B) 21 days in the three culture conditions tested: BM, CM, or CM supplemented with γ-PGA (CM+γ-PGA). sGAG deposition was observed by the presence of blue staining, here represented in darker shades of grey within pellets ECM. One representative donor of each cell type is presented here. For each time point: the first column presents whole pellet images (bars represent 500 μm). The three columns to the right are detailed images of distinct regions of the cell pellet: peripheral, middle, and center of the pellets. The bars represent 50 μm. Black circles highlight round-shaped cell morphologies. Color images available online at www.liebertpub.com/tea
The presence of pyknotic/necrotic bodies (dashed black circles), as depicted elsewhere,50,51 clearly distinguished MSC pellets in BM from chondrogenic conditions (Fig. 4). This fact suggests that cells without chondrogenic inducers present low viability and poor capacity to form matrix. On the other hand, in both chondrogenic conditions, alcian blue staining revealed sGAG deposition in MSC pellets, with the majority of cells exhibiting a fibrous/spindle shape (black arrows), while others presented round-shaped morphology (black circles). sGAG deposits appeared to be more frequently accumulated in the pellet periphery after 21 days of culture. Importantly, when γ-PGA was supplemented to the medium, a more homogenous sGAG deposition was observed in MSC pellets, in particular in the center.
In the case of NC pellets, cells exhibited typical round-shaped chondrocyte morphology with lacunae formation (Fig. 5, black circles). The sGAG deposition appeared to be higher than in MSC pellets, and distributed throughout the whole sectional areas. However, in these cultures, γ-PGA did not substantially increase sGAG deposition.
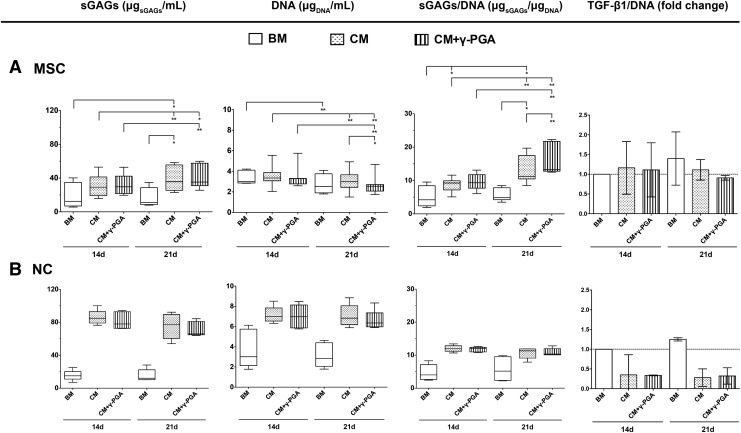
The histochemical analysis was corroborated by sGAG quantification in whole pellets (Fig. 6). In MSC cultures, sGAGs slightly increased from basal levels in both chondrogenic conditions at day 14, this being more evident after 21 days of culture, in both CM (35.7±30.5 μgsGAGs/mL) and in CM+γ-PGA (35.3±26.7 μgsGAGs/mL), without significant differences existing between them. When sGAG deposition was normalized to the DNA content of each pellet, the potential of γ-PGA to increase sGAG deposition became more evident. Thus, after 21 days of culture, sGAGs/DNA significantly increased from the basal level (4.8±3.6 μgsGAGs/μgDNA) to CM (11.3±6.4 μgsGAGs/μgDNA) (*p<0.05) and from CM to CM+γ-PGA (13.3±8.9 μgsGAGs/μgDNA) (**p<0.025), which clearly demonstrates the potential of γ-PGA to enhance ECM synthesis.
FIG. 6.
Biochemical analysis of sGAG deposition and DNA content in (A) MSC and (B) NC pellets, cultured for 14 and 21 days in the three conditions tested: BM (white bars), chondrogenic (CM, bars with dots), or CM supplemented with γ-PGA (CM+γ-PGA, bars with stripes). Results are represented as box plots (MSC: eight pellets/condition, three independent donors; NC: five pellets/condition, three independent donors). Statistical significance was set at *p<0.05, (**p<0.025), using the Friedman paired test followed by the Dunn's multiple comparison post-test.
In the case of NC pellets, the sGAG quantification was also in accordance with the histological observations. In general, sGAGs were much higher in NC than in MSC cultures. However, no significant differences were observed between the different culture media, despite the increase in sGAG production observed from basal to chondrogenic conditions (ranging from 12.1±12.0 μgsGAGs/mL to 84.9±13.8 μgsGAGs/mL and to 78.0±20.0 μgsGAGs/mL in CM and CM+γ-PGA, respectively). This trend was also observed when sGAGs were normalized to the DNA content of each pellet.
TGF-β1 has been predominantly and consistently associated with an enhancement of cell condensation in the initial stages of chondrogenesis,52–55 which are critical for the subsequent stages of chondrogenesis.56 Although no statistically significant differences could be observed between the different culture conditions, the analysis of endogenous TGF-β1 shows that pellets cultured under basal conditions tended to present higher TGF-β1 concentration values that did not decrease with culture time. This effect was more evident in NC pellets.
Analysis of Col II deposition of MSC and NC 3D pellet cultures
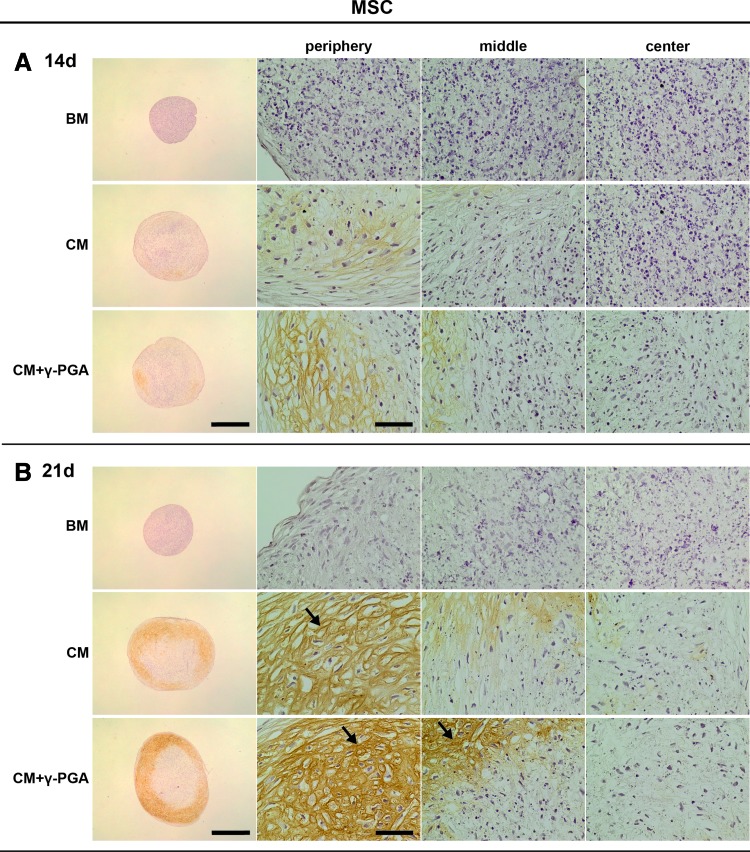
Col II is widely recognized as a definitive attribute of cartilage. Immunohistochemical analysis for Col II allows the assessment of its deposition, an outcome achieved by cells that undergo full hyaline chondrogenic differentiation.50 Figures 7 and 8 show representative images of Col II immunostaining in MSC and NC pellets, respectively. In MSC pellets cultured in CM, Col II staining was observed in some areas of the pellets (brown, represented in darker shades of grey with pellets ECM), equivalent to those in which more sGAGs were deposited. Of interest was the presence of higher fibrillar labeling (black arrows) in both chondrogenic conditions but more pronounced in the presence of γ-PGA at day 21. No Col II was detected in BM, as expected.
FIG. 7.
Type II collagen (Col II) deposition (brown, here represented in darker shades of grey within pellets ECM) assessed by immunohistochemistry (IHC) of paraffin sections of MSC pellets cultured for (A) 14, and (B) 21 days in the three culture conditions tested: BM, CM, or CM supplemented with γ-PGA (CM+γ-PGA). One representative donor of each cell type is presented here. For each time point: the first column presents whole pellet images (bars represent 500 μm). The three columns to the right are detailed images of distinct regions of the cell pellet: peripheral, middle, and center of the pellets. The bars represent 50 μm. Black arrows point to fibrillar Col II. Color images available online at www.liebertpub.com/tea
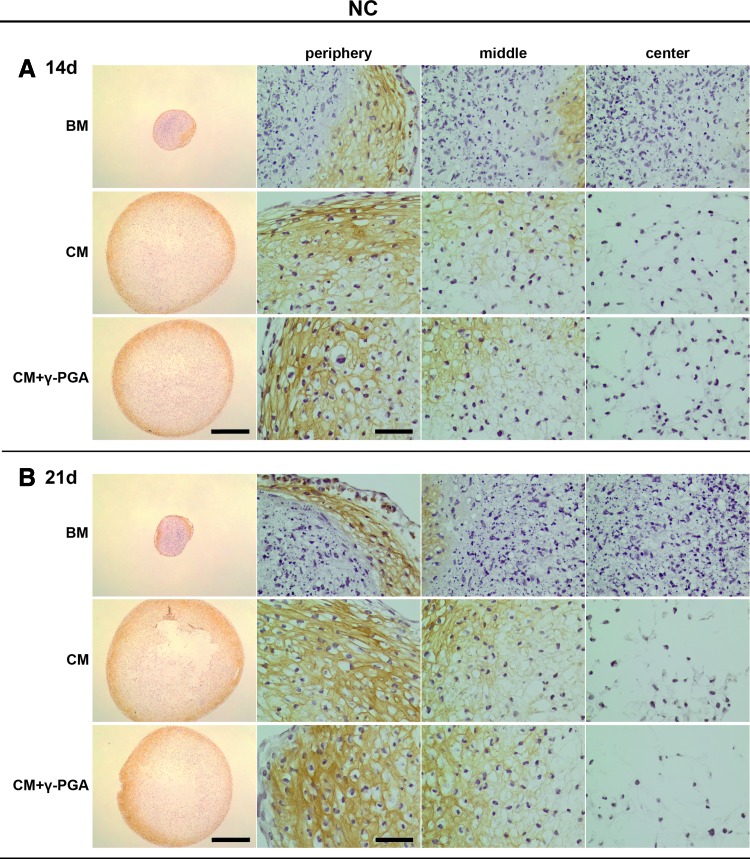
FIG. 8.
Col II deposition (brown, here represented in darker shades of grey within pellets ECM) assessed by IHC of paraffin-sections of NC pellets cultured for (A) 14, and (B) 21 days in the three culture conditions tested: BM, CM or CM supplemented with γ-PGA (CM+γ-PGA). One representative donor of each cell type is presented here. For each time point: the first column presents whole pellet images (bars represent 500 μm). The three columns to the right are detailed images of distinct regions of the cell pellet: peripheral, middle, and center of the pellets. The bars represent 50 μm. Color images available online at www.liebertpub.com/tea
In the case of NC pellets, a more intense Col II staining was observed in chondrogenic conditions. Nevertheless, no noticeable differences could be seen in intensity or distribution of Col II between CM and CM-γ-PGA conditions, in line with the sGAG results. Regarding the culture in BM, Col II accumulation could be observed in the pellet outer regions, suggesting some level of neochondrogenesis in the case of fully differentiated chondrocyte cultures.
Analysis of Sox-9, aggrecan, Col XI, Col X, Col I, and calcium deposition of MSC and NC 3D pellet cultures
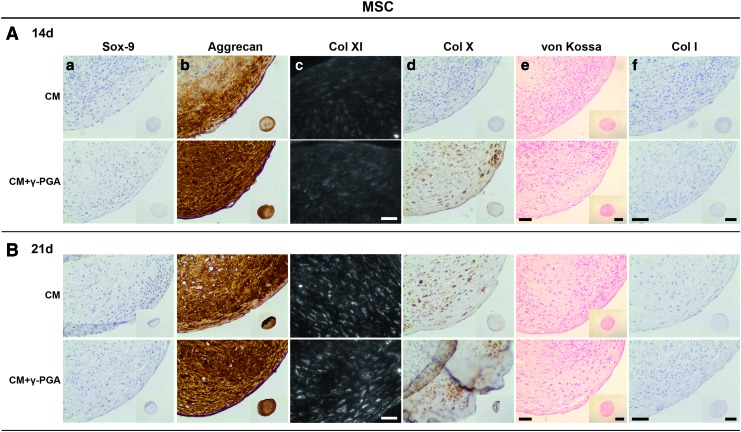
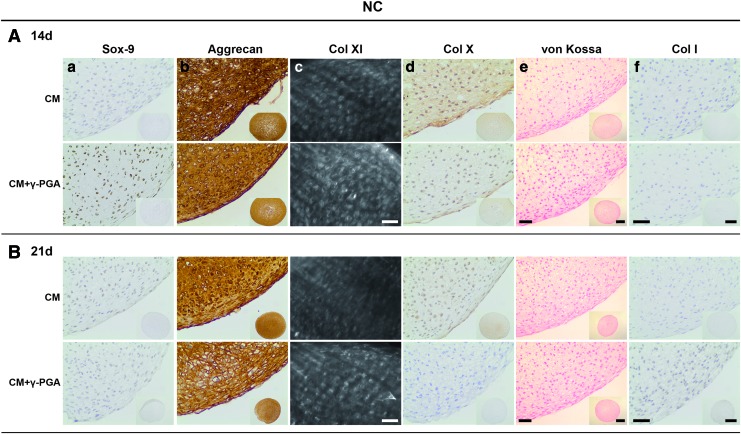
After establishing that the cultured chondrogenic 3D pellets from both cell types expressed the main attributes of an in vitro engineered cartilaginous tissue, additional elements, from different stages of the chondrogenic differentiation pathway, were procured. The aim was to further validate the chondrogenic differentiation of MSCs/NCs observed and reinforce the bridge between the NC pellets and the differentiated MSCs. Accordingly, Sox-9, aggrecan, Col XI, Col X, and Col I were analyzed (Figs. 9 and 10), and substantiated the results obtained previously.
FIG. 9.
Paraffin sections of MSC pellets cultured for (A) 14, and (B) 21 days in CM or CM supplemented with γ-PGA (CM+γ-PGA) stained for (a) Sox-9, (b) aggrecan, (c) Col XI, (d) Col X, (e) calcium salt deposits, and (f) Col I. In all the conditions tested the detection of Col I by IHC and calcium salt deposits by von Kossa/nuclear fast red histological staining could not be detected. Representative donors are presented here. For Col XI, bars represent 50 μm. For Sox-9, aggrecan, Col X and Col I, and for the calcium salt deposits, bars of the detailed images of peripheral regions of the pellets represent 50 μm, while bars of the inserted whole pellet pictures represent 500 μm. Color images available online at www.liebertpub.com/tea
FIG. 10.
Paraffin sections of NC pellets cultured for (A) 14, and (B) 21 days in CM or CM supplemented with γ-PGA (CM+γ-PGA) stained for (a) Sox-9, (b) aggrecan, (c) Col XI, (d) Col X, (e) calcium salt deposits, and (f) Col I. In all the conditions tested the detection of Col I by IHC and calcium salt deposits by von Kossa/nuclear fast red histological staining could not be detected. One representative donor is presented here. For Col XI, bars represent 50 um. For Sox-9, aggrecan, Col X, and Col I, and for the calcium salt deposits, bars of the detailed images of peripheral regions of the pellets represent 50 μm, while bars of the inserted whole pellet pictures represent 500 μm. Color images available online at www.liebertpub.com/tea
Sox-9 is a nuclear transcription factor, an early chondrogenic marker, vital for the cell condensation step.57 Sox-9 labeling (brown, represented in darker shades of grey within cells nuclei) was mainly nuclear and predominantly expressed in MSC and NC pellets at earlier time points (day 14) when γ-PGA was present (Fig. 9a). Aggrecan is the most abundant proteoglycan in hyaline cartilage, comprising about 90% of the total proteoglycan content,2,7 and it is indicative of differentiated chondrocytes.58–61 In the case of MSCs, aggrecan labeling (brown, represented in darker shades of grey with pellets ECM) was more intense in the CM+γ-PGA culture conditions at day 21, and within the pellet regions with highest sGAG and Col II deposition (Fig. 9b). The staining was visible in the pericellular space and extracellular space. With NC pellets (Fig. 10b), the labeling in the extracellular space was more evident for all culture conditions.
Col XI is a cartilaginous collagenous component (present in smaller amounts than Col II) that assists in the stabilization of the Col network by forming fibrils with Col II and interacting with other components of the ECM,7,62 particularly at an early stage of fibril formation.63,64 The labeling (white, Fig. 9c) was mainly retained at the surface of chondrocytes that were allowed to reconstitute their pericellular matrix in culture. The MSC pellets showed a trend in Col XI deposition, which was more evident with γ-PGA and after 21 days of culture.
Col X is a nonfibrillar and network-forming Col predominantly found in the calcified zone of cartilage growth plate.7,65,66 After cell differentiation/redifferentiation into resting chondrocytes, if chondrogenesis proceeds with maturation toward terminal differentiation (through the process of endochondral ossification), the chondrocytes become hypertrophic.61,67–70 Col X has been linked to hypertrophic chondrocytes.1,7,71–73 The MSC pellets presented the same trend as Col XI, aggrecan, Col II, and sGAG deposition, with a higher labeling with γ-PGA and after 21 days of culture (Fig. 9d). The NCs also showed Col X accumulation, although it decreased at day 14 for the CM+γ-PGA condition and was absent at day 21 (Fig. 10d).
Col I was used to evaluate chondrocyte dedifferentiation at the beginning of chondrogenesis,51,69,74 and/or if the cells followed endochondral ossification until expression of osteoblastic traits.75,76 Both chondroprogenitor cells and osteoblasts may produce Col I.74 However, no Col I labeling (brown, represented by darker shades of grey within pellets ECM) was seen in any of the culture conditions/time points/cell types (Figs. 9f and 10f).
Finally, to evaluate the stability of the chondrogenic phenotype achieved in the MSCs and NC pellets cultures, von Kossa staining was performed to determine the presence of a mineralized matrix (Figs. 9e and 10e). Usually, with this stain it is possible to visualize calcium deposits as black crystals. No mineralization could be seen in any of the pellets from either cell type, which indicates that γ-PGA did not induce ECM mineralization under the culture conditions tested.
Discussion
In this study we have addressed the potential of γ-PGA to promote chondrogenic differentiation of MSCs, redifferentiation of NCs, and its effect on the deposition of cartilaginous matrix in both MSC and NC cultures. γ-PGA is a biocompatible and biodegradable polymer, with attractive features for tissue regeneration, such as hydrophilicity and low-immunogenicity.18,21
The use of γ-PGA for bone and nervous system regeneration has already been reported.20,26 However, γ-PGA potential for cartilage regeneration is far from being explored. Chang et al. developed γ-PGA-graft-chondroitin sulfate-blend-poly(ɛ-caprolactone) scaffolds that were shown to support rat articular chondrocyte culture for 4 weeks.33 Prescott patented γ-PGA injection for the treatment of degenerative joint diseases.34 Nevertheless, none of these studies has clarified γ-PGA potential for the process of chondrogenesis.
In this study, γ-PGA cytotoxicity was first excluded in both MSCs and NCs; two cell populations of interest for cartilage regeneration. To achieve this, we used γ-PGA produced and characterized in-house by our group, as previously described.36
Human neonatal bone marrow-derived MSCs were used due to their superior proliferation rate and differentiation capabilities resembling human growth plate cartilage, when compared to aged adult bone marrow-derived MSC.77,78 As a source of differentiated cartilage cells, NCs were selected on account of their high degree of proliferation and stability of hyaline-cartilage phenotype, with increasing capacity for ECM production.79 Although articular chondrocytes are the most studied chondrocyte source, NCs have the advantage of being able to be isolated under minimally invasive conditions with reduced site morbidity,6,73,79 to be highly proliferative cells, with elevated and more reproducible chondrogenic capacity (both in vitro and ectopically in vivo), with increased capacity for ECM production (apparently donor age-independent79 and not affected by a joint pathological condition).80,81
The absence of toxic effects in the presence of γ-PGA was in accordance with the previous results observed in fibroblast cultures carried out in the presence of γ-PGA/Ch multilayered films.42 In the literature, the absence of γ-PGA toxic effects (obtained from another strain, Bacillus licheniformis) after repeated injections of this polymer into rodents was also reported.39,41
In 3D pellet cultures, MSCs cultured in the presence of γ-PGA displayed early packing, suggesting earlier cell condensation, an essential natural trigger of the chondrogenic differentiation cascade of events.56 Decreased active TGF-β1 values were measured in the supernatants of chondrogenic MSCs (day 21) and NCs (days 14 and 21), thus substantiating that the absence of CM delays the initiation of cell condensation.52–55
MSC chondrogenic differentiation was also analyzed by the deposition of proteoglycan-rich ECM and Col II, as typically described.3,50 The standard chondrogenic recipes include the use of TGF-β,3,4,9,60 also present in the CM cocktail of this study. Nevertheless, under these conditions the distribution of sGAGs and Col II was shown to be heterogeneous within the pellet, perhaps on account of differences in oxygen tension and nutrient diffusion throughout the spheroids.50,51,81 On the other hand, when γ-PGA was supplemented in the culture medium, sGAG and Col II deposition increased significantly, and both matrix components were more evenly distributed within the 3D structures.
Aggrecan and Col XI, other elements of bona fide cartilage ECM2,7,62 shared the same trend, possibly accompanying Col II synthesis and deposition. Aggrecan staining was visible in the pericellular space, as expected for cultured pellets,82 although the extracellular space was also stained (a feature so far only described for cultured cartilage explants82); the pericellular labeling of Col XI was also an expected finding.62,69,83,84
The early chondrogenic marker, Sox-9, showed a nuclear staining pattern, as typically observed in normal cartilage.85,86 The labeling was more evident at day 14, which can also be explained by the importance of Sox-9 for the activation of genes that encode several cartilaginous ECM proteins (such as COL2A1,87–89 COL11A1,90 and aggrecan91), before cartilaginous ECM deposition,56 in addition to its key role in cell condensation.57 This way, the occurrence of more intense chondrogenic differentiation features in the presence of γ-PGA and at different stages of the process can be reinforced, since this labeling (inherently associated with an early stage of chondrogenesis–cell condensation stage) was also accompanied by more intense staining of alcian blue, Col II, aggrecan, and Col XI.
In the case of NCs, the γ-PGA effect was not so evident. NC pellets were able to synthesize cartilage-committed matrix even under basal conditions, without chondrogenic inducers. Although the ECM production increased under chondrogenic stimulation, no differences were detected after γ-PGA addition. sGAG distribution was homogeneous from the periphery of the pellets to their cores. The same was observed in aggrecan and Col XI deposition. Nevertheless, Sox-9 labeling was also increased at day 14 in the presence of γ-PGA.
While entering the step of cell maturation toward terminal differentiation,61,67–70 Col X increase in MSCs presents evidence of cell hypertrophy (Col X is able to differentiate chondroprogenitor cells from terminally differentiated hypertrophic chondrocytes,65,92 as described in the literature11,71,73,79,93,94), although with no mineralization after 21 days of culture.3,72 Moreover, as expected, NC pellets presented mild Col X accumulation in some pellet regions, but also did not mineralize.79 The absence of Col X labeling at day 21 in pellets cultured with γ-PGA-supplemented medium suggests that γ-PGA may prevent cells hypertrophy at a later stage.
Wang et al.95 observed an inhibition of chondral mineralization in organotypic cultured mouse metatarsals in the presence of exogenous glutamate, while the contrary was observed for those cultured in its absence. This was achieved by a higher incidence of apoptotic cells. In parallel, it is possible that the hypertrophic cells observed at day 14 have been directed to apoptosis and further hypertrophy was inhibited, hence the lack of Col X labeling at day 21 in the presence of γ-PGA. Col I labeling, which has typically been described as being intercellularly located in the peripheral region of pellets, and accompanied by a cell fibroblastic-like morphology,6,51 was absent from all pellet sections, although the periphery of MSC pellets contained cells with fibroblast-like appearance. This suggests that cell differentiation/redifferentiation was a success and/or that cells did not differentiate into osteoblasts.
Overall, the results presented here clearly demonstrate that γ-PGA, as an exogenous agent to the cocktail of chondrogenic factors, is able to support chondrogenesis at different stages of the process, whereas such an effect could not be observed with redifferentiated chondrocytes.
The mechanism of action of γ-PGA was not the focus of attention in this study and remains to be investigated. Nevertheless, some hypotheses could be formulated, based on the literature. It is expected that γ-PGA might be consumed/degraded, thus generating a glutamate pool able to activate the glutamate signaling pathway.96 In fact, glutamate signaling has been described in cartilage, namely in human articular chondrocytes,28 rat costal and articular chondrocytes,30–32 and also in mouse and human MSCs.97–99 L-glutamate, but not D-glutamate, suppressed mouse MSC proliferation and differentiation in 2D without inducing cell death.98 This differentiation inhibition was observed at the gene expression level in osteogenesis, but not in adipogenesis.99 No data have been published concerning chondrogenesis.
The results obtained by us at the protein level for sGAGs, aggrecan, Col XI, Col II, Col X, and Col I strongly suggest that γ-PGA promotes human MSC chondrogenic differentiation. In mature rat costal chondrocytes, glutamate uptake was demonstrated to occur via the glutamate aspartate transporter and glutamate transporter-1.30 Moreover, endogenous glutamate was also shown to have an antiproliferative effect.31 Nevertheless, the influence of glutamate on the production of cartilaginous ECM by mature chondrocytes was not addressed. The results obtained in this study also did not provide a clear indication for a positive role of γ-PGA in NC cultures.
In summary, the potential of γ-PGA to support human MSC chondrogenesis underlies the advantage of applying this polymer to cartilage regeneration in the future.
Conclusions
γ-PGA is a biocompatible and biodegradable polyamino acid that clearly improved MSC differentiation into the chondrogenic lineage when used as a supplement in a culture medium enriched in chondrogenic factors. This effect was observed by (1) earlier and more marked condensation of 3D pellet cultures, along with higher expression of Sox-9; (2) increased sGAG deposition; and (3) augmented production of chondrogenic ECM proteins, such as aggrecan, Col XI, Col II, and Col X. However, a γ-PGA effect with respect to supporting mature cartilage cell (NC) culture was not evident, since no differences were observed in chondrogenic markers when compared with standard CM. As exceptions, Sox-9 labeling was increased at 2 weeks of culture, whereas Col X was decreased after 2 weeks and absent after three. No mineralization was observed in MSC and NC cultures.
To our knowledge this is the first study demonstrating that γ-PGA promotes MSC chondrogenesis, which can be relevant to exploit the use of γ-PGA in cartilage regenerative strategies in the future.
Supplementary Material
Acknowledgments
This work was financed by FEDER funds through the Programa Operacional Factores de Competitividade − COMPETE, by Portuguese funds through FCT − Fundação para a Ciência e a Tecnologia in the framework of the project PEst-C/SAU/LA0002/2013 and by DISC Regeneration project (grant agreement no. NMP3-LA-2008-213904 [FP7]). Joana C. Antunes and Catarina Leite Pereira are grateful to FCT for their Ph.D. grants: SFRH/BD/48554/2008 and SFRH/BD/85779/2012. Raquel Gonçalves acknowledges FCT for her Pos-Doc grant (SFRH/BPD/85651/2012) and project NORTE-07-0124-FEDER-000005- project on “Biomedical Engineering for Regenerative Therapies and Cancer,” financed by ON.2–O Novo Norte, QREN.
The authors gratefully acknowledge Dr. Ronald E. Unger, Susanne Barth, Ulrike Hilbig, Anne Sartoris, Susana Carrilho, António Ribeiro, Daniel Vasconcelos, Ana Silva, Joana Caldeira, and Juliana Alves for technical assistance/guidance during the experiments. The authors would also like to thank Dr. Larissa Seidmann for collecting the bone marrow and Dr. Andreas Mamilos for the excellent photographic assistance.
Disclosure Statement
No competing financial interests exist.
References
- 1.Bobick B.E., Chen F.H., Le A.M., and Tuan R.S. Regulation of the chondrogenic phenotype in culture. Birth Defects Res Part C 87, 351, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Goldring M.B. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther Adv Musculoskelet Dis 4, 269, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pelttari K., Steck E., and Richter W. The use of mesenchymal stem cells for chondrogenesis. Injury 39, S58, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Dexheimer V., Frank S., and Richter W. Proliferation as a requirement for in vitro chondrogenesis of human mesenchymal stem cells. Stem Cells Dev 21, 2160, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oldershaw R.A. Cell sources for the regeneration of articular cartilage: the past, the horizon and the future. Int J Exp Pathol 93, 389, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schon B.S., Schrobback K., van der Ven M., Stroebel S., Hooper G.J., and Woodfield T.B.F. Validation of a high-throughput microtissue fabrication process for 3D assembly of tissue engineered cartilage constructs. Cell Tissue Res 347, 629, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Kuo C., Li W.-J., and Tuan R. Cartilage and ligament tissue engineering: biomaterials, cellular interactions, and regenerative strategies. In: Ratner B.D., Hoffman A., Schoen F., and Lemons J., eds. Biomaterials Science: An Introduction to Materials in Medicine. Oxford: Academic Press, 2012, pp. 1214–1236 [Google Scholar]
- 8.Benders K.E.M., van Weeren P.R., Badylak S.F., Saris D.B.F., Dhert W.J.A., and Malda J. Extracellular matrix scaffolds for cartilage and bone regeneration. Trends Biotechnol 31, 169, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Freyria A.-M., and Mallein-Gerin F. Chondrocytes or adult stem cells for cartilage repair: the indisputable role of growth factors. Injury 43, 259, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Wu L., Cai X., Zhang S., Karperien M., and Lin Y. Regeneration of articular cartilage by adipose tissue derived mesenchymal stem cells: perspectives from stem cell biology and molecular medicine. J Cell Physio 228, 938, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Giovannini S., Diaz-Romero J., Aigner T., Heini P., Mainil-Varlet P., and Nesic D. Micromass co-culture of human articular chondrocytes and human bone marrow mesenchymal stem cells to investigate stable neocartilage tissue formation in vitro. Eur Cell Mater 20, 245, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Huey D.J., Hu J.C., and Athanasiou K.A. Unlike bone, cartilage regeneration remains elusive. Science 338, 917, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinert A.F., Ghivizzani S.C., Rethwilm A., Tuan R.S., Evans C.H., and Noeth U. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res Ther 9, 213, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toh W.S., Spector M., Lee E.H., and Cao T. Biomaterial-mediated delivery of microenvironmental cues for repair and regeneration of articular cartilage. Mol Pharm 8, 994, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Vinatier C., Mrugala D., Jorgensen C., Guicheux J., and Noel D. Cartilage engineering: a crucial combination of cells, biomaterials and biofactors. Trends Biotechnol 27, 307, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Lutolf M.P., and Hubbell J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol 23, 47, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Vinatier C., Bouffi C., Merceron C., Gordeladze J., Brondello J.-M., Jorgensen C., Weiss P., Guicheux J., and Noël D. Cartilage tissue engineering: towards a biomaterial-assisted mesenchymal stem cell therapy. Curr Stem Cell Res Ther 4, 318, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buescher J.M., and Margaritis A. Microbial biosynthesis of polyglutamic acid biopolymer and applications in the biopharmaceutical, biomedical and food industries. Crit Rev Biotechnol 27, 1, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Kuo Y.-C., and Chang Y.-H. Differentiation of induced pluripotent stem cells toward neurons in hydrogel biomaterials. Colloids Surf B Biointerfaces 102, 405, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki T., Kuramoto A., Hirakawa A., Shirosaki Y., and Ohtsuki C. Biomineralization on chemically synthesized collagen containing immobilized poly-gamma-glutamic acid. Dent Mater J 32, 544, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Shih I.L., Van Y.T., and Shen M.H. Biomedical applications of chemically and microbiologically synthesized poly(glutamic acid) and poly(lysine). Mini Rev Med Chem 4, 179, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Brown J.L., Kumbar S.G., and Laurencin C.T. Bone tissue engineering. In: Ratner B.D., Hoffman A., Schoen F., and Lemons J., eds. Biomaterials Science: An Introduction to Materials in Medicine. Oxford: Academic Press, 2012, pp. 1194–1214 [Google Scholar]
- 23.Fujisawa R., and Tamura M. Acidic bone matrix proteins and their roles in calcification. Front Biosci (Landmark Ed) 17, 1891, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Hunter G.K., and Goldberg H.A. Modulation of crystal-formation by bone phosphoproteins - role of glutamic acid-rich sequences in the nucleation of hydroxiapatite by bone sialoprotein. Biochem J 302, 175, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugino A., Miyazaki T., and Ohtsuki C. Apatite-forming ability of polyglutamic acid hydrogels in a body-simulating environment. J Mater Sci Mater Med 19, 2269, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Kuo Y.-C., and Chung C.-Y. TATVHL peptide-grafted alginate/poly(gamma-glutamic acid) scaffolds with inverted colloidal crystal topology for neuronal differentiation of iPS cells. Biomaterials 33, 8955, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Hinoi E., Takarada T., Ueshima T., Tsuchihashi Y., and Yoneda Y. Glutamate signaling in peripheral tissues. Eur J Biochem 271, 1, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Salter D.M., Wright M.O., and Millward-Sadler S.J. NMDA receptor expression and roles in human articular chondrocyte mechanotransduction. Biorheology 41, 273, 2004 [PubMed] [Google Scholar]
- 29.Skerry T.M., and Genever P.G. Glutamate signalling in non-neuronal tissues. Trends Pharmacol Sci 22, 174, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Hinoi E., Wang L.Y., Takemori A., and Yoneda Y. Functional expression of particular isoforms of excitatory amino acid transporters by rodent cartilage. Biochem Pharmacol 70, 70, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Piepoli T., Mennuni L., Zerbi S., Lanza M., Rovati L.C., and Caselli G. Glutamate signaling in chondrocytes and the potential involvement of NMDA receptors in cell proliferation and inflammatory gene expression. Osteoarthritis Cartilage 17, 1076, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Wang L.Y., Hinoi E., Takemori A., and Yoneda Y. Release of endogenous glutamate by AMPA receptors expressed in cultured rat costal chondrocytes. Biol Pharm Bull 28, 990, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Chang K.-Y., Cheng L.-W., Ho G.-H., Huang Y.-P., and Lee Y.-D. Fabrication and characterization of poly(gamma-glutamic acid)-graft-chondroitin sulfate/polycaprolactone porous scaffolds for cartilage tissue engineering. Acta Biomater 5, 1937, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Prescott A.G. Methods for treating joint pain using poly-gamma-glutamic acid. U.S. Patent No. US 2006/0234192 A1, 2006
- 35.Bovarnick M. The formation of extracellular d(-)-glutamic acid polypeptide by Bacillus subtilis. J Biol Chem 145, 415, 1942 [Google Scholar]
- 36.Pereira C.L., Antunes J.C., Goncalves R.M., Ferreira-da-Silva F., and Barbosa M.A. Biosynthesis of highly pure poly-gamma-glutamic acid for biomedical applications. J Mater Sci Mater Med 23, 1583, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Ashiuchi M., Shimanouchi K., Nakamura H., Kamei T., Soda K., Park C., Sung M.H., and Misono H. Enzymatic synthesis of high-molecular-mass poly-gamma-glutamate and regulation of its stereochemistry. Appl Environ Microbiol 70, 4249, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edde B., Rossier J., Lecaer J.P., Desbruyeres E., Gros F., and Denoulet P. Posttranslational glutamylation of alpha-tubulin. Science 247, 83, 1990 [DOI] [PubMed] [Google Scholar]
- 39.Prescott A.G., Stock L.R. Method for producing medical and commercial grade poly-gamma-glutamic acid of high molecular weight. Crescent Innovations Inc; 2005 [Google Scholar]
- 40.Lee H., Chang M.-J., and Kim S.-H. Effects of poly-gamma-glutamic acid on serum and brain concentrations of glutamate and GABA in diet-induced obese rats. Nutr Res Pract 4, 23, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prodhomme E.J.F., Tutt A.L., Glennie M.J., and Bugg T.D.H. Multivalent conjugates of poly-gamma-D-glutamic acid from Bacillus licheniformis with antibody F(ab') and glycopeptide Ligands. Bioconjugate Chem 14, 1148, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Antunes J.C., Pereira C.L., Molinos M., Ferreira-da-Silva F., Dessi M., Gloria A., Ambrosio L., Gonçalves R.M., and Barbosa M.A. Layer-by-layer self-assembly of chitosan and poly(gamma-glutamic acid) into polyelectrolyte complexes. Biomacromolecules 12, 4183, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Park B.G., Kang H.-S., Lee W., Kim J.S., and Son T.-I. Reinforcement of pH-responsive gamma-poly(glutamic acid)/chitosan hydrogel for orally administrable colon-targeted drug delivery. J Appl Polym Sci 127, 832, 2013 [Google Scholar]
- 44.Serizawa T., Goto H., Kishida A., Endo T., and Akashi M. Improved alternate deposition of biodegradable naturally occurring polymers onto a quartz crystal microbalance. J Polym Sci Part A Polym Chem 37, 801, 1999 [Google Scholar]
- 45.Tsao C.T., Chang C.H., Lin Y.Y., Wu M.F., Wang J.-L., Han J.L., and Hsieh K.H. Antibacterial activity and biocompatibility of a chitosan-gamma-poly(glutamic acid) polyelectrolyte complex hydrogel. Carbohydr Res 345, 1774, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Goto A., and Kunioka M. Biosynthesis and hydrolysis of poly(gamma-glutamic acid) from bacillus-subtilis IFO3335. Biosci Biotechnol Biochem 56, 1031, 1992 [DOI] [PubMed] [Google Scholar]
- 47.Kubota H., Matsunobu T., Uotani K., Takebe H., Satoh A., Tanaka T., and Taniguchi M. Production of poly(gamma-glutamic acid) by bacillus-subtilis F-2-01. Biosci Biotechnol Biochem 57, 1212, 1993 [DOI] [PubMed] [Google Scholar]
- 48.U.S. Department of Health and Human Services/Food and Drug Administration/Center for Drug Evaluation and Research/Center for Biologics Evaluation and Research/Center for Veterinary Medicine/Center for Devices and Radiological Health/Office of Regulatory Affairs. Guidance for industry: Pyrogen and endotoxins testing: questions and answers, 2012, pp. 1–10
- 49.Goncalves R.M., Antunes J.C., and Barbosa M.A. Mesenhymal stem cell recruitment by stromal derived factor-1 delivery systems based on chitosan/poly(gamma-glutamic acid) polyelectrolyte complexes. Eur Cell Mater 23, 249, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Grogan S.P., Barbero A., Winkelmann V., Rieser F., Fitzsimmons J.S., O'Driscoll S., Martin I., and Mainil-Varlet P. Visual histological grading system for the evaluation of in vitro-generated neocartilage. Tissue Eng 12, 2141, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Ichinose S., Tagami M., Muneta T., and Sekiya I. Morphological examination during in vitro cartilage formation by human mesenchymal stem cells. Cell Tissue Res 322, 217, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Chimal-Monroy J., and de Leon L.D. Expression of N-cadherin, N-CAM, fibronectin and tenascin is stimulated by TGF-beta 1, beta 2, beta 3 and beta 5 during the formation of precartilage condensations. Int J Dev Biol 43, 59, 1999 [PubMed] [Google Scholar]
- 53.Denker A.E., Nicoll S.B., and Tuan R.S. Formation of cartilage-like spheroids by micromass cultures of murine C3H10T1/2 cells upon treatment with transforming growth-factor-beta-1. Differentiation 59, 25, 1995 [DOI] [PubMed] [Google Scholar]
- 54.Leonard C.M., Fuld H.M., Frenz D.A., Downie S.A., Massage J., and Newman S.A. Role of transforming growth-factor-beta in chondrogenic pattern-formation in the embryonic limb–stimulation of mesenchymal condensation and fibronectin gene-expression by exogenous TGF-beta and evidence for endogenous TGF-beta-like activity. Dev Biol 145, 99, 1991 [DOI] [PubMed] [Google Scholar]
- 55.Tuli R., Tuli S., Nandi S., Huang X.X., Manner P.A., Hozack W.J., Keith G., Danielson K.G., Hall D.J., and Tuan R.S. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogenactivated protein kinase and Wnt signaling cross-talk. J Biol Chem 278, 41227, 2003 [DOI] [PubMed] [Google Scholar]
- 56.DeLise A.M., Fischer L., and Tuan R.S. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage 8, 309, 2000 [DOI] [PubMed] [Google Scholar]
- 57.Bi W.M., Deng J.M., Zhang Z.P., Behringer R.R., and de Crombrugghe B. Sox9 is required for cartilage formation. Nat Genet 22, 85, 1999 [DOI] [PubMed] [Google Scholar]
- 58.Diaz-Romero J., Nesic D., Grogan S.P., Hein P., and Mainil-Varlet P. Immunophenotypic changes of human articular chondrocytes during monolayer culture reflect bona fide dedifferentiation rather than amplification of progenitor cells. J Cell Physiol 214, 75, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Pelttari K., Winter A., Steck E., Goetzke K., Hennig T., Ochs B.G., Aigner T., and Richter W. Quantitative analysis of gene expression in human articular cartilage from normal and osteoarthritic joints. Osteoarthritis Cartilage 9, 112, 2001 [DOI] [PubMed] [Google Scholar]
- 60.Spencer G.J., McGrath C.J., and Genever P.G. Chondrogenic potential of bone marrow- and adipose tissue-derived adult human mesenchymal stem cells. Biomed Mater Eng 20, 145, 2010 [DOI] [PubMed] [Google Scholar]
- 61.Zuscik M.J., Hilton M.J., Zhang X., Chen D., and O'Keefe R.J. Regulation of chondrogenesis and chondrocyte differentiation by stress. J Clin Invest 118, 429, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruggiero F., Petit B., Ronziere M.C., Farjanel J., Hartmann D.J., and Herbage D. Composition and organization of the collagen network produced by fetal bovine chondrocytes cultured at high-density. J Histochem Cytochem 41, 867, 1993 [DOI] [PubMed] [Google Scholar]
- 63.Oxford J.T., DeScala J., Morris N., Gregory K., Medeck R., Irwin K., Oxford R., Brown R., Mercer L., and Cusack S. Interaction between amino propeptides of type XI procollagen alpha 1 chains. J Biol Chem 279, 10939, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yingst S., Bloxham K., Warner L.R., Brown R.J., Cole J., Kenoyer L., Knowlton W.B., and Oxford J.T. Characterization of collagenous matrix assembly in a chondrocyte model system. J Biol Chem 90A, 247, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aigner T., Reichenberger E., Bertling W., Kirsch T., Stoss H., and Vondermark K. Type-X collagen expression in osteoarthritic and rheumatoid articular-cartilage. Virchows Arch B Cell Pathol Incl Mol Pathol 63, 205, 1993 [DOI] [PubMed] [Google Scholar]
- 66.Girkontaite I., Frischholz S., Lammi P., Wagner K., Swoboda B., Aigner T., and Von der Mark K. Immunolocalization of type X collagen in normal fetal and adult osteoarthritic cartilage with monoclonal antibodies. Matrix Biol 15, 231, 1996 [DOI] [PubMed] [Google Scholar]
- 67.Blitterswijk C., Thomsen P., Lindahl A., Hubbell J., Williams D.F., Cancedda R., de Bruijn J.D., and Sohier J. Tissue Engineering. London: Academic Press, 2008 [Google Scholar]
- 68.Caplan A. Why are MSCs therapeutic? New data: new insight. J Pathol 217, 318, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krug D., Klinger M., Haller R., Hargus G., Buening J., Rohwedel J., and Kramer J. Minor cartilage collagens type IX and XI are expressed during embryonic stem cell-derived in vitro chondrogenesis. Ann Anat 195, 88, 2013 [DOI] [PubMed] [Google Scholar]
- 70.Uccelli A., Moretta L., and Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 8, 726, 2008 [DOI] [PubMed] [Google Scholar]
- 71.Johnstone B., Hering T.M., Caplan A.I., Goldberg V.M., and Yoo J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 238, 265, 1998 [DOI] [PubMed] [Google Scholar]
- 72.Pelttari K., Winter A., Steck E., Goetzke K., Hennig T., Ochs B.G., Aigner T., and Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum 54, 3254, 2006 [DOI] [PubMed] [Google Scholar]
- 73.Scotti C., Tonnarelli B., Papadimitropoulos A., Scherberich A., Schaeren S., Schauerte A., Lopez-Rios J., Zeller R., Barbero A., and Martin I. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci U S A 107, 7251, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Binette F., McQuaid D.P., Haudenschild D.R., Yaeger P.C., McPherson J.M., and Tubo R. Expression of a stable articular cartilage phenotype without evidence of hypertrophy by adult human articular chondrocytes in vitro. J Orthop Res 16, 207, 1998 [DOI] [PubMed] [Google Scholar]
- 75.Jaehn K., Richards R.G., Archer C.W., and Stoddart M.J. Pellet culture model for human primary osteoblasts. Eur Cell Mater 20, 149, 2010 [DOI] [PubMed] [Google Scholar]
- 76.Ko J.-Y., Kim K.-I., Park S., and Im G.-I. In vitro chondrogenesis and in vivo repair of osteochondral defect with human induced pluripotent stem cells. Biomaterials 35, 3571, 2014 [DOI] [PubMed] [Google Scholar]
- 77.Moroni L., and Fornasari P.M. Human mesenchymal stem cells: A bank perspective on the isolation, characterization and potential of alternative sources for the regeneration of musculoskeletal tissues. J Cell Physiol 228, 680, 2013 [DOI] [PubMed] [Google Scholar]
- 78.van Gool S.A., Emons J.A.M., Leijten J.C.H., Decker E., Sticht C., van Houwelingen J.C., Goeman J.J., Kleijburg C., Scherjon S.A., Gretz N., Wit J.M., Rappold G., Post J.N., and Karperien M. Fetal mesenchymal stromal cells differentiating towards chondrocytes acquire a gene expression profile resembling human growth plate cartilage. Plos One 7, e44561, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hellingman C.A., Verwiel E.T.P., Slagt I., Koevoet W., Poublon R.M.L., Nolst-Trenite G.J., Baatenburg de Jong R.J., Jahr H., and van Osch G.J. Differences in cartilage-forming capacity of expanded human chondrocytes from ear and nose and their gene expression profiles. Cell Transplant 20, 925, 2011 [DOI] [PubMed] [Google Scholar]
- 80.Asawa Y., Ogasawara T., Takahashi T., Yamaoka H., Nishizawa S., Matsudaira K., Mori Y., Takato T., and Hoshi K. Aptitude of auricular and nasoseptal chondrocytes cultured under a monolayer or three-dimensional condition for cartilage tissue engineering. Tissue Eng Part A 15, 1109, 2009 [DOI] [PubMed] [Google Scholar]
- 81.Scotti C., Osmokrovic A., Wolf F., Miot S., Peretti G.M., Barbero A., and Martin I. Response of human engineered cartilage based on articular or nasal chondrocytes to interleukin-1 beta and low oxygen. Tissue Eng Part A 18, 362, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Z.J., McCaffery J.M., Spencer R.G.S., and Francomano C.A. Hyaline cartilage engineered by chondrocytes in pellet culture: histological, immunohistochemical and ultrastructural analysis in comparison with cartilage explants. J Anat 205, 229, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Keene D.R., Oxford J.T., and Morris N.P. Ultrastructural-localization of collagen type-II, type-IX, and type-XI in the growth-plate of human rib and fetal bovine epiphyseal cartilage–type-XI collagen is restricted to thin fibrils. J Histochem Cytochem 43, 967, 1995 [DOI] [PubMed] [Google Scholar]
- 84.Swoboda B., Holmdahl R., Stoss H., and Vondermark K. Cellular heterogeneity in cultured human chondrocytes identified by antibodies specific for alpha-2(XI) collagen chains. J Cell Biol 109, 1363, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grogan S.P., Miyaki S., Asahara H., D'Lima D.D., and Lotz M.K. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther 11, R85, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shen B., Wei A., Whittaker S., Williams L.A., Tao H., Ma D.D.F., and Diwan A.D. The role of BMP-7 in chondrogenic and osteogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in vitro. J Cell Biochem 109, 406, 2010 [DOI] [PubMed] [Google Scholar]
- 87.Bell D.M., Leung K.K.H., Wheatley S.C., Ng L.J., Zhou S., Ling K.W., Sham M.H., Koopman P., Tam P.P., and Cheah K.S. SOX9 directly regulates the type-II collagen gene. Nat Genet 16, 174, 1997 [DOI] [PubMed] [Google Scholar]
- 88.Lefebvre V., Huang W.D., Harley V.R., Goodfellow P.N., and de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha 1(II) collagen gene. Mol Cell Biol 17, 2336, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ng L.J., Wheatley S., Muscat G.E.O., Conway-Campbell J., Bowles J., Wright E., Bell D.M., Tam P.P., Cheah K.S., and Koopman P. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev Biol 183, 108, 1997 [DOI] [PubMed] [Google Scholar]
- 90.Bridgewater L.C., Walker M.D., Miller G.C., Ellison T.A., Holsinger L.D., Potter J.L., Jackson T.L., Chen R.K., Winkel V.L., Zhang Z., McKinney S., and de Crombrugghe B. Adjacent DNA sequences modulate Sox9 transcriptional activation at paired Sox sites in three chondrocyte-specific enhancer elements. Nucleic Acids Res 31, 1541, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sekiya I., Tsuji K., Koopman P., Watanabe H., Yamada Y., Shinomiya K., Nifuji A., and Noda M. SOX9 enhances aggrecan gene promoter/enhancer activity and is up-regulated by retinoic acid in a cartilage-derived cell line, TC6. J Biol Chem 275, 10738, 2000 [DOI] [PubMed] [Google Scholar]
- 92.Aigner T., Loos S., Muller S., Sandell L.J., Unni K.K., and Kirchner T. Cell differentiation and matrix gene expression in mesenchymal chondrosarcomas. Am J Pathol 156, 1327, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dickhut A., Pelttari K., Janicki P., Wagner W., Eckstein V., Egermann M., and Richter W. Calcification or dedifferentiation: requirement to lock mesenchymal stem cells in a desired differentiation stage. J Cell Physiol 219, 219, 2009 [DOI] [PubMed] [Google Scholar]
- 94.Ichinose S., Yamagata K., Sekiya I., Muneta T., and Tagami M. Detailed examination of cartilage formation and endochondral ossification using human mesenchymal stem cells. Clin Exp Pharmacol Physiol 32, 561, 2005 [DOI] [PubMed] [Google Scholar]
- 95.Wang L., Hinoi E., Takemori A., Nakamichi N., and Yoneda Y. Glutamate inhibits chondral mineralization through apoptotic cell death mediated by retrograde operation of the cystine/glutamate antiporter. J Biol Chem 281, 24553, 2006 [DOI] [PubMed] [Google Scholar]
- 96.Spencer G.J., McGrath C.J., and Genever P.G. Current perspectives on NMDA-type glutamate signalling in bone. Int J Biochem Cell Biol 39, 1089, 2007 [DOI] [PubMed] [Google Scholar]
- 97.Danielyan L., Schaefer R., Schulz A., Ladewig T., Lourhmati A., Buadze M., Schmitt A.L., Verleysdonk S., Kabisch D., Koeppen K., Siegel G., Proksch B., Kluba T., Eckert A., Köhle C., Schöneberg T., Northoff H., Schwab M., and Gleiter C.H. Survival, neuron-like differentiation and functionality of mesenchymal stem cells in neurotoxic environment: the critical role of erythropoietin. Cell Death Differ 16, 1599, 2009 [DOI] [PubMed] [Google Scholar]
- 98.Iemata M., Takarada T., Hinoi E., Taniura H., and Yoneda Y. Suppression by glutamate of proliferative activity through glutathione depletion mediated by the Cystine/Glutamate antiporter in mesenchymal C3H10T1/2 stem cells. J Cell Physiol 213, 721, 2007 [DOI] [PubMed] [Google Scholar]
- 99.Takarada-Iemata M., Takarada T., Nakamura Y., Nakatani E., Hori O., and Yoneda Y. Glutamate preferentially suppresses osteoblastogenesis than adipogenesis through the cystine/glutamate antiporter in mesenchymal stem cells. J Cell Physiol 226, 652, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.