Abstract
Objective
To report the rare but distinct clinical and neuropathological phenotype of non-familial, rapidly progressive parkinsonism and dementia associated with frontotemporal lobar degeneration with motor neuron disease (FTLD-MND).
Methods
Subjects included two 70-year-old women presenting with rapidly progressive severe postural instability, axial-predominant parkinsonism, oculomotor dysfunction and frontal-predominant dementia with language impairment and pseudobulbar palsy. One had diffuse weakness without signs of lower motor neuron disease. Post-mortem evaluations included immunohistochemistry with antiphospho-TAR DNA-binding protein 43 (TDP-43) and genetic analysis of the TARDBP and PGRN genes.
Results
Subjects died within 14 months from symptom onset. TDP-43-positive neuronal intracytoplasmic inclusions were prominent in the primary motor cortex, granule cell layer of the hippocampus, and several cranial and spinal cord nuclei. TDP-43 globular glial inclusions (GGI) were identified in one case. There were no mutations in PGRN or TARDBP genes.
Conclusions
FTLD-MND due to TDP-43-proteinopathy should be considered in patients with rapidly progressive parkinsonism and dementia phenotype, especially when aphasia and/or weakness are also present.
Rapidly progressive dementia with parkinsonism has been associated with several pathological substrates of neurodegeneration including frontotemporal lobar degeneration (FTLD) with or without motor neuron disease (MND), Alzheimer’s disease, diffuse Lewy body disease, corticobasal degeneration (CBD), progressive supranuclear palsy (PSP) and Creutzfeldt–Jakob disease.1 Traditionally, Parkinsonian phenotypes with frontal-predominant dementia have been considered highly predictive of τ-positive neuropathology.2,3
FTLD is the most common anatomic correlate of progressive frontotemporal dementia and parkinsonism. Tauopathies (FTLD-τ) and TAR DNA-binding protein 43 (TDP-43)-proteinopathies (FTLD-TDP), associated with the accumulation of either hyperphosphorylated τ or TDP-43 protein, respectively, account for 50% of all cases of FTLD (4). Neuropathological subtypes have been identified within the FTLD-τ subgroup (Pick’s disease, progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), argyrophilic grain disease (AGD), multiple system tauopathy with presenile dementia (MSTD), white-matter tauopathy with globular glial inclusions (WMT-GGI)), and the FTLD-TDP subgroup.4,5
We report two cases of sporadic parkinsonism and frontotemporal dementia with unusually rapidly progressive disease course (within 14 months) associated with weakness and progressive non-fluent aphasia due to FTLD-TDP with MND. One case had a peculiar oligodendroglial pathological phenotype with globular glial inclusions resembling those of WMT-GGI5 and multiple system atrophy (MSA).6
REPORT OF CASES
Patient 1
A 70-year-old woman suffered backward falls and shortly thereafter slowed and hypophonic voice, word-finding difficulty, difficulty reading, dysphagia and diffuse weakness. On examination, 9 months after symptom onset, she had dementia (Mini-Mental State Examination=15/30; Frontal Assessment Battery=8/18; abnormal clock drawing test), pseudobulbar affect, non-fluent aphasia with agrammatism and impaired repetition. Supranuclear vertical gaze paresis, apraxia of eyelid closing, frontal release signs, diffuse hyper-reflexia and limb weakness, marked postural and gait impairment were observed (see online video, segment 1). There was no tongue or muscle atrophy or fasciculations. An MRI of the brain revealed mild diffuse cortical atrophy, predominantly affecting frontal and temporal lobes (figure 1A–C). EMG of the lower extremities revealed widespread fibrillations. She died of bronchopneumonia 14 months after symptom onset.
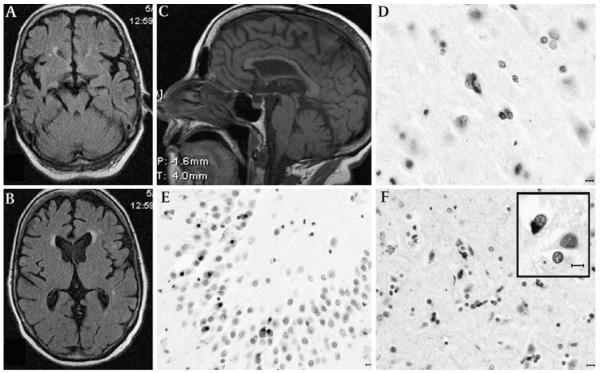
Figure 1.
Magnetic MRI and post-mortem findings of patient 1. Axial sequences at the midbrain (A) and thalamic (B) levels showing mild temporal- and frontal-predominant cortical atrophy; mid-sagittal T1 weighted MRI (C) demonstrates mild thinning of the body and genu of the corpus callosum with relative preservation of the splenium, suggesting a reduction of interhemispheric frontotemporal fibres; midbrain appears of normal size. TAR DNA-binding protein 43 (TDP-43) immunohistochemistry shows granular cytoplasmic deposits in the primary motor cortex (D). Neuronal cytoplasmic inclusions are abundant in the granule cell layer of the hippocampus (E). The subcortical white matter showed abundant pTDP-43 oligodendroglial inclusions (F). Some oligodendroglial inclusions had typical appearance of globular inclusions (GGI, inset). Horizontal bar=10 μm.
The weight of the fixed brain was 1250 g. Mild diffuse cortical atrophy and mildly depigmented substantia nigra were noted. Microscopically, moderate to severe neuronal loss, gliosis and superficial microvacuolar changes were observed in the prefrontal, primary motor and temporal cortices and, to a lesser extent, in the parietal cortex and cingulate gyrus. There was severe loss of Betz cells. Mild neuronal loss and gliosis were observed in the hippocampus. In the brainstem, mild to moderate neuronal loss, gliosis and neuromelaninladen macrophages were seen in the substantia nigra and locus coeruleus, and moderate loss of neurons in the interstitial nucleus of the medial longitudinal fasciculus (iMLF), motor dorsal nucleus of the vagus nerve and hypoglossal nucleus, where Bunina bodies were present.
Phosphorylated TDP-43 immunohistochemistry revealed neuronal cytoplasmic inclusions (NCI) in several regions, including the iMLF, most abundant in the granule cell layer of the hippocampus (figure 1D, E). Abundant pTDP-43 pathology in the form of oligodendroglial cytoplasmic inclusions was seen in the lower cortical layers and, more extensively, in the subcortical white matter (figure 1F). Oligodendroglial inclusions had a perinuclear, crescent, triangular or oval appearance, resembling that of Papp–Lantos inclusions of MSA or oligodendroglial inclusions of WMT-GGI.5,6 They measured about the size of the nucleus in longest dimension and they were ubiquitin-positive but not visualised with H&E or silver-staining, τ- or α-synuclein immunohistochemistry. τ-Pathology was limited to a few granular deposits in neurons of the subiculum. Immunohistochemistry for α-synuclein and β-amyloid protein was negative.
Patient 2
A 70-year-old, right-handed woman developed apathy, bradykinesia, falls, non-fluent aphasia, severe hypophonia and dysphagia. She had a history of dementia in two maternal uncles. On first examination, 9 months after symptom onset, she had dysexecutive cognitive impairment (Mini-Mental Status Examination=26/30; Frontal Assessment Battery=8/18), speech in staccato, slow and moderate dysprosody, glabellar reflex and saccadic pursuit, left hand ideomotor apraxia to intransitive gestures, severe axial (more than limb) rigidity, bradykinesia, generalised hyper-reflexia with clonus, bilateral Babinski and Hoffman signs, and slow, short-stepped gait associated with marked postural instability (see online video, segment 2). No atrophy or fasciculations were noted. Neuropsychological evaluation showed a Dementia Rating Scale-2 test score below the fifth percentile. Brain MRI revealed mild insular atrophy (figure 2A–C), and EEG displayed left temporal intermittent rhythmic delta activity. EMG/NCV showed no evidence of motor neuron disease. CSF examination was normal as 14-3-3, and cultures, including fungal and AFB, were negative. Her symptoms rapidly progressed. Four months later, she was mute, combative and unable to stand during hospitalisation. She died less than 14 months after symptom onset.
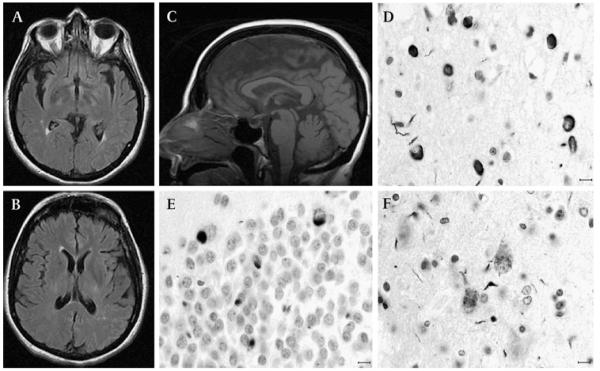
Figure 2.
MRI and post-mortem findings of patient 2. Axial fluid-attenuated inversion recovery sequences at the lower (A) and higher thalamic (B) levels show mild insular enlargement with no overt frontal atrophy; mid-sagittal T1 weighted MRI (C) demonstrates mild thinning of the body of the corpus callosum with normal-appearing brainstem and cerebellar volume. TAR DNA-binding protein 43 immunohistochemistry shows neuronal cytoplasmic inclusions, some with ring-like perinuclear appearance, in the frontal cortical layer II (D) and dentate gyrus of the hippocampus (E). Granular and occasional skein-like inclusions are seen in a few residual upper motor neurons (F). Horizontal bar=10 μm.
The weight of the fresh brain was 1199 g. There was mild to moderate atrophy of the frontal lobes and mild atrophy of the parietal and occipital lobes and parahippocampal gyrus. Microscopically, severe neuronal loss and mild gliosis were observed in the superior frontal, middle frontal and cingulate gyri and, to a lesser extent, in the superior and middle temporal gyri, superior parietal lobe and occipital cortex. Microvacuolar changes were observed in the upper frontal and temporal cortical layers and less extensively in the superior parietal lobule. Moderate neuronal loss was observed in the CA2 sector of the hippocampus and subiculum, and, more widespread, in the entorhinal and transentorhinal cortices. In the subcortical nuclei, moderate to severe neuronal loss and gliosis were noted in the caudate nucleus, putamen, globus pallidus and amygdala, substantia nigra, locus coeruleus, reticular formation, motor dorsal nucleus of the vagus nerve, hypoglossal nucleus, and inferior olivary nucleus, as well as to a lesser extent in the thalamus and subthalamic nucleus. The cerebellum displayed mild loss of Purkinje cells and dentate nucleus neurons. Mild to moderate loss of motor neurons was seen in the spinal cord. There was extensive gliosis of the subcortical white matter and internal capsule.
TDP-43 immunohistochemistry revealed abundant NCI in the dentate gyrus of the hippocampus with ring-like perinuclear appearance in the frontal and temporal cortical layer II, and, to a much lesser extent, in the parietal cortex (figure 2D, E), with granular and occasional skein-like inclusions in a few residual upper motor neurons (figure 2F). A few dystrophic neurites were recognised in the caudate nucleus, and less extensively in the putamen and amygdala. NCIs were identified in a few remaining lower motor neurons in the spinal cord. τ-Immunoreactive neurofibrillar tangles were seen in the CA1 sector of the hippocampus, entorhinal and transentorhinal cortices, and inferior temporal cortex. No α-synuclein or β-amyloid protein deposits were observed.
DNA analysis did not show mutations in PGRN or TARDBP genes in either case. Western blot analysis did not reveal the presence of abnormal, protease-resistant prion protein (PrP27-30).
DISCUSSION
We report two cases of an atypical PSP-like syndrome associated with FTLD-MND and TDP-43-proteinopathy. There is striking similarity in the clinical phenotype which is characterised by atypical and rapidly progressive parkinsonism and frontal dementia. Both subjects presented with clinical features of PSP and MND, had a rapidly progressive clinical course and died within 14 months from clinical onset. Although our patients had an older age at onset (70 years) than the median of the five cases with FTLD-MND reported within the rapidly progressive dementia series from the Mayo Clinic (50 years), it is important to note that four of these five FTLD-MND cases presented with personality and behavioural abnormalities in their mid-40s.1 Our patients’ presentations were clinically closer to that of the 67-year-old FTLD-MND woman with gait disturbances.1 Collectively, these seven cases would suggest that a non-behavioural, axial-predominant phenotype of FTLD-MND may be the more common in older subjects. Finally, although the tremorless, hypokinetic-rigid syndrome with early falls and oculomotor impairment described here suggested PSP, weakness and early pyramidal signs, severe language deficits (eg, progressive non-fluent aphasia (PNFA)) and rapid progression excluded the clinical diagnosis of possible or probable PSP, as per current criteria.7 Recent work, however, suggests that PNFA can rarely be part of the clinical spectrum of PSP (PSP-PNFA).8
The association of clinical PSP and MND has been described occurring either in the setting of an underlining tauopathy, as in atypical PSP with corticospinal degeneration (aPSP-CSD),9 or TDP-43 proteinopathy.10,11 Despite clinical similarities and common TDP-43 proteinopathy, the two cases present different neuropathological features. Noteworthy is the observation of abundant TDP-43 oligodendroglial pathology in the lowest cortical layers and subcortical white matter of subject 1, with some of the inclusions being reminiscent of τ-ir GGI of WMT-GGI and aPSP-CSD. This may represent a newly identified TDP-43 variant of the spectrum of clinicopathological syndromes associated with GGI white matter pathology, which includes the tauopathies WMT-GGI, aPSP-CSD and sporadic MSTD, as well as the α-synucleinopathy MSA.5,6,9 Patient 2’s clinicopathological presentation resembles that of a 66-year-old man with rapidly progressive MND and PSP-like features, recently described by McCluskey et al.10 Beyond the more overt bulbar and limb weakness of that case, which fulfilled the diagnosis of ALS, the development of hypokinesia, rigidity and early postural instability were highly suggestive of PSP. In summary, our observations further support considering FTLD-MND with TDP-43 pathology in patients with a rapidly progressive PSP-like disorder, especially when accompanied by aphasia, dysarthria, and early-onset MND. The neuropathological substrate of this phenotype is heterogeneous and may include a newly identified variant of oligodendrogliopathy characterised by globular TDP-43-ir cytoplasmic inclusions.
Acknowledgments
Funding This work was sponsored in part by research grants NIH PHS AG010133 (BG) and R01 PAS-03-092 NIA (IL).
Footnotes
Competing interests None.
Patient consent Obtained.
Contributors AJE and SS contributed equally and should be considered co-first authors.
Provenance and peer review Not commissioned; externally peer reviewed.
Additional videos are published online only. To view these files please visit the journal online (http://jnnp.bmj.com).
REFERENCES
- 1.Josephs KA, Ahlskog JE, Parisi JE, et al. Rapidly progressive neurodegenerative dementias. Arch Neurol. 2009;66:201–7. doi: 10.1001/archneurol.2008.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodges JR, Davies RR, Xuereb JH, et al. Clinicopathological correlates in frontotemporal dementia. Ann Neurol. 2004;56:399–406. doi: 10.1002/ana.20203. [DOI] [PubMed] [Google Scholar]
- 3.Josephs KA, Petersen RC, Knopman DS, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006;66:41–8. doi: 10.1212/01.wnl.0000191307.69661.c3. [DOI] [PubMed] [Google Scholar]
- 4.Cairns NJ, Bigio EH, Mackenzie IR, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kovacs GG, Majtenyi K, Spina S, et al. White matter tauopathy with globular glial inclusions: a distinct sporadic frontotemporal lobar degeneration. J Neuropathol Exp Neurol. 2008;67:963–75. doi: 10.1097/NEN.0b013e318187a80f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome) J Neurol Sci. 1989;94:79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- 7.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele–Richardson–Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Williams DR, Lees AJ. Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. Lancet Neurol. 2009;8:270–9. doi: 10.1016/S1474-4422(09)70042-0. [DOI] [PubMed] [Google Scholar]
- 9.Josephs KA, Katsuse O, Beccano-Kelly DA, et al. Atypical progressive supranuclear palsy with corticospinal tract degeneration. J Neuropathol Exp Neurol. 2006;65:396–405. doi: 10.1097/01.jnen.0000218446.38158.61. [DOI] [PubMed] [Google Scholar]
- 10.McCluskey LF, Elman LB, Martinez-Lage M, et al. Amyotrophic lateral sclerosis-plus syndrome with TAR DNA-binding protein-43 pathology. Arch Neurol. 2009;66:121–4. doi: 10.1001/archneur.66.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paviour DC, Lees AJ, Josephs KA, et al. Frontotemporal lobar degeneration with ubiquitin-only-immunoreactive neuronal changes: broadening the clinical picture to include progressive supranuclear palsy. Brain. 2004;127:2441–51. doi: 10.1093/brain/awh265. [DOI] [PubMed] [Google Scholar]