Abstract
As the first line of defense, innate immunity plays an important role in protecting the host against pathogens. Innate lymphoid cells (ILCs) are emerging as important effector cells in the innate immune system and the cell type that regulates immune and tissue homeostasis. ILC2s are a subset of ILCs and are characterized by their capacity to produce large quantities of type 2 cytokines and certain tissue growth factors. In animal models, lung ILC2s are involved in allergic airway inflammation induced by exposure to allergens even in the absence of CD4+ T cells and are likely responsible for tissue repair and recovery after respiratory virus infection. ILC2s are also identified in various organs in humans, and the numbers are increased in mucosal tissues from patients with allergic disorders. Further investigations of this novel cell type will provide major conceptual advances in our understanding of the mechanisms of asthma and allergic diseases.
Keywords: Innate lymphoid cells, asthma, allergy, cytokines
INTRODUCTION
Innate immunity plays an important role in protecting the host against pathogens such as bacteria, viruses, and parasites. Innate lymphoid cells (ILCs) are emerging as important effector cells of the innate immune system that are involved in pathogen clearance, lymphoid organogenesis, and tissue remodeling. These cells are derived from a common lymphoid progenitor, exhibit lymphoid morphology, lack rearranged antigen receptors, and express no conventional lymphocyte or dendritic cell (DC) phenotypic markers.
Based on their cytokine production profiles and the transcription factors utilized for their development and functions, ILCs have been recently categorized into three groups: group 1 ILCs (ILC1s), group 2 ILCs (ILC2s), and group 3 ILCs (ILC3s) (Spits et al., 2013). ILC1s comprise IFN-γ-secreting ILCs that likely use transcription factor T-bet for lineage commitment. ILC2s comprise type 2 cytokine-producing ILCs that require transcription factor GATA3 for their development and function. ILC3s comprise IL-17- and/or IL-22-producing ILCs that are dependent on transcription factor RORγt for lineage specification. In this review, we will specifically focus on ILC2s, especially ILC2s in the lung, and discuss their functional roles in allergic airway diseases.
General features of ILC2s
ILC2s are considered to be the counterpart of Th2-type CD4+ T cells in the adaptive immune system. They characteristically produce type 2 cytokines, such as IL-5 and IL-13. ILC2s were first described in mice in the early 2000s as non-B/non-T cells that secrete IL-5 and IL-13 in response to IL-25 (Fort et al., 2001; Hurst et al., 2002). A subsequent study showed that these IL-25-responsive ILCs play important roles in Nippostrongylus brasiliensis worm expulsion (Fallon et al., 2006). In 2010, ILC2s were characterized in detail by three groups, and they were independently named as natural helper cells, nuocytes, and innate helper 2 cells (Moro et al., 2010; Neill et al., 2010; Price et al., 2010). They were later named group 2 ILCs (ILC2s) in a consensus report (Spits et al., 2013).
Generally, mouse ILC2s are negative for classical cell surface markers for T cells, B cells, natural killer (NK) cells, myeloid cells, and DCs, including CD3, CD4, CD8, CD5, CD19, B220, TCR, NK1.1, Ter119, Gr-1, Mac-1, CD11c, CD14, and CD16/32; thus, they are designated lineage-negative (Lin−). Mouse ILC2s do express ST2 (IL-33 receptor), CD127 (IL-7R α-chain), ICOS, CD117 (c-kit), Thy1, IL-17RB (IL-25 receptor), CD44 and CD25 (IL-2R α-chain). Mouse ILC2s are widely distributed in the tissues, including fat-associated lymphoid clusters (FALC), mesenteric and mediastinal lymph nodes, liver, spleen, intestine, bone marrow, visceral adipose tissue and lung. Developmentally, ILC2s arise from common lymphoid precursors in the bone morrow and require common IL-2 receptor γ-chain (cγ), transcription factor inhibitor of DNA binding 2 (Id2), nuclear orphan receptor RORα and transcription factor GATA3 for their development and differentiation (Hoyler et al., 2012; Moro et al., 2010; Wong et al., 2012; Yang et al., 2011). Mature mouse ILC2s are activated to produce type 2 cytokines, including IL-4, IL-5, IL-9 and IL-13, in response to the cytokines, such as IL-25, IL-33 and thymic stromal lymphopoietin (TSLP) (Kim et al., 2013; Mjosberg et al., 2012; Moro et al., 2010; Neill et al., 2010; Price et al., 2010), that are devived from epithelial cells and certain immune cells.
Initial studies on mouse ILC2s demonstrated their critical roles in innate immunity against a variety of organisms. For example, ILC2s play critical roles in protective immunity against helminth infection (Moro et al., 2010; Neill et al., 2010; Price et al., 2010), in influenza-induced lung inflammation and airway hyperreactivity (AHR) (Chang et al., 2011), and in respiratory epithelial repair after influenza infection (Monticelli et al., 2011). ILC2s and their cytokines also play pathological roles in allergen-induced airway inflammation (Barlow et al., 2012; Bartemes et al., 2012; Halim et al., 2012; Kim et al., 2012) and skin inflammation (Kim et al., 2013; Roediger et al., 2013). Some homeostatic and tissue remodeling roles for ILC2s have been reported, including eosinophil homeostasis (Molofsky et al., 2013; Nussbaum et al., 2013) and hepatic fibrosis (McHedlidze et al., 2013).
Multipotent progenitor type 2 (MPPtype2) cells are likely a special subset of ILC2s. These cells were originally discovered in the gut-associated lymphoid tissue of IL-25-treated mice (Saenz et al., 2010); they are also found in blood, lymph nodes, lung and the peritoneal cavity (Saenz et al., 2013). Unlike other ILC2s, MPPtype2 cells display a multipotent capacity to differentiate into monocyte/macrophage and granulocyte lineages (Saenz et al., 2010). In addition, MPPtype2 cells can present antigens to T cells and promote Th2-type differentiation. A recent study demonstrated that MPPtype2 cells are predominantly activated by IL-25, but not IL-33, and exhibit distinct transcriptional profiles and developmental requirements as compared to ILC2s (Saenz et al., 2013), suggesting that MPPtype2 cells and classical ILC2s are distinct subsets.
Lung ILC2s at resting condition
ILC2s are normally resident in the lungs of naïve animals. In the lungs of naïve mice, ILC2s are Lin− and generally express various cell surface markers, including CD117, CD122 (IL-2R β-chain), CD25, CD127, Ly5.2, Thy1, Sca-1, ST2, CD69, CD9, CD38, MHC class II, CD44 and ICOS (Bartemes et al., 2012; Halim et al., 2012; Monticelli et al., 2011; Price et al., 2010). Some heterogeneity in the expression of cell surface molecules is also observed among the studies, likely due to differences in the experimental models and housing conditions of the animals. Combinations of these cell markers are used to identify and isolate ILC2s among the Lin− populations in the lung of naïve mice (Figure 1A). Importantly, lung ILC2s are present in Rag2−/− mice and ST2−/− mice (i.e. deficient in IL-33R), suggesting that they do not require TCR recombination or IL-33 for their development. In contrast, mice that are deficient in IL-2 receptor common γ-chain (cγ), IL-7R α-chain or transcription regulator Id2 lack mature ILC2s, consistent with their dependency on IL-7 and Id2 for their development.
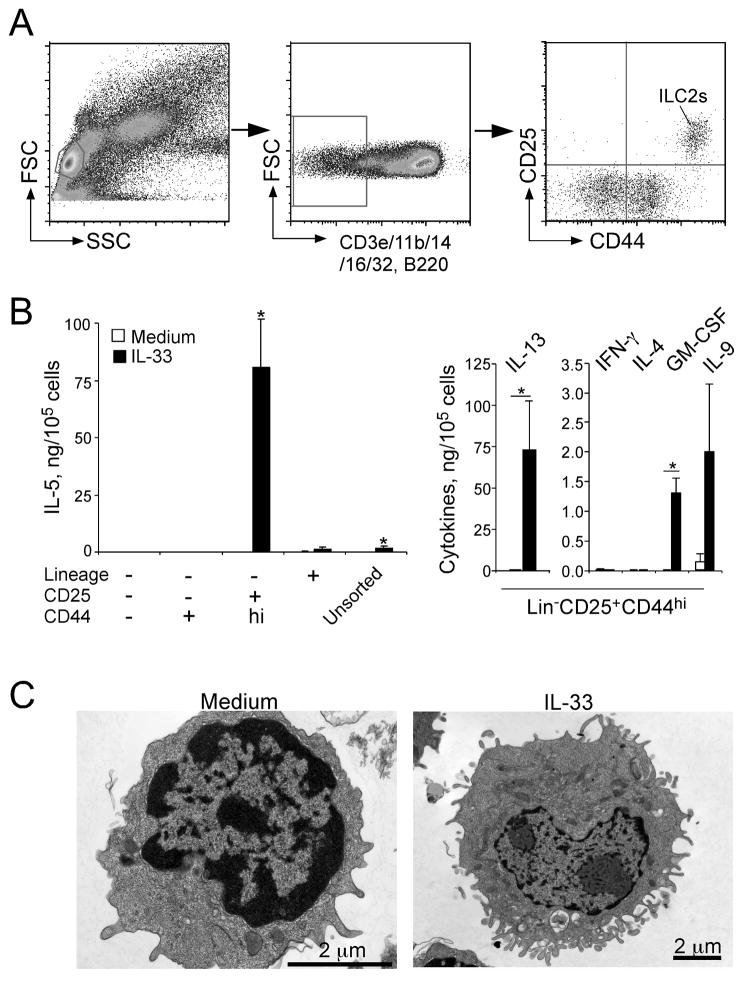
Figure 1.
Lung ILC2s respond vigorously to IL-33 and produce a large quantity of IL-5 and IL-13 in vitro. (A) Gating strategy and identification of ILC2s in lung single cell suspensions from naïve BALB/c mice. (B) Four populations of lung cells, including Lin+ cells, Lin−CD25−CD44− cells, Lin−CD25−CD44+ cells and Lin−CD25+CD44hi cells (i.e. ILC2s) were isolated from naïve BALB/c mice by FACS sorting. Sorted and unsorted lung cells were cultured with medium alone or with IL-33, and the levels of cytokines in the supernatants were measured by ELISA. (C) Morphology of lung ILC2s. Lung ILC2s were cultured with medium alone or IL-33 and examined under electron microscopy. Original magnifications; 25,000x (medium alone, left) and 12,000x (IL-33, right).
Lung ILC2s are a rare cell population. In wild-type C57BL/6 mice, lung ILC2s represent only 0.25–1% of total live cells in the lung. ILC2s are located in collagen-rich regions close to the confluence of medium-sized blood vessels and airways, but not in alveolar areas of the lung (Nussbaum et al., 2013). Resting lung ILC2s have morphology similar to that of resting lymphocytes, with no apparent intracellular granule structures (Bartemes et al., 2012). However, once they are stimulated with IL-33, lung ILC2s increase in size and display pronounced endoplasmic reticulum and Golgi apparatus (Figure 1C).
Resting lung ILC2s also display a gene expression profile distinct from those of macrophages, DCs, CD4+ T cells, NK cells, γδT cells, and regulatory T cells (Treg) in the lung. More specifically, ILC2s show high mRNA expression levels of Gata3, Rora, Cd69, Il2ra, Il2rg, Il4ra, Il7r, Il17rb, Il1rl1, Il5, and Il13 (Halim et al., 2012). IL-5 and IL-13 transcripts were also detected in resting lung ILC2s in cytokine reporter mice (Ikutani et al., 2012; Nussbaum et al., 2013; Price et al., 2010). At the protein level, ELISAs could not detect IL-5 and IL-13 in the culture supernatants of naïve and resting lung ILC2s (Bartemes et al., 2012; Halim et al., 2012). However, ELISPOT assays revealed IL-5-producing lung ILC2s when cultured in medium alone (Nussbaum et al., 2013), suggesting constitutive but minimal production of IL-5 by resting ILC2s. Interestingly, this constitutive expression of IL-5 by ILC2s may play a role in regulating eosinophil homeostasis in various organs (Molofsky et al., 2013; Nussbaum et al., 2013). Some controversies exist as to expression of IL-4. Although gene microarray analysis shows no or low expression levels of Il4 (Halim et al., 2012), IL-4 transcripts were found in ILC2s in the lungs of IL-4 reporter mice (Price et al., 2010).
Regulation and function of lung ILC2s
Exposure of lung ILC2s to cytokines and other inflammatory mediators rapidly activates their effector functions. For example, IL-33 activates lung ILC2s to produce large quantities of IL-5 and IL-13 in vitro (Bartemes et al., 2012; Halim et al., 2012) (Figure 1B). While IL-25 and TSLP do not activate lung ILC2s by themselves, they synergistically enhance cytokine production by ILC2s (Halim et al., 2012). IL-25 and IL-33 also promote expansion and/or migration of lung ILC2s, as intraperitoneal or intranasal administration of IL-25 or IL-33 increased ILC2 cell numbers in lung tissues and draining lymph nodes in vivo (Barlow et al., 2012; Price et al., 2010). IL-33 is likely more potent than IL-25 in inducing ILC2 cell expansion (Barlow et al., 2013). While stimulatory effects of TSLP on lung ILC2s have not been demonstrated, TSLP has been shown to activate skin ILC2s (Kim et al., 2013), suggesting specialization of ILC2s in different organs.
Lung ILC2 activities can also be regulated by IL-2-family cytokines. In vitro, neither IL-2 nor IL-7 alone induces significant IL-5 and IL-13 production by ILC2s. However, these two cytokines synergistically enhance IL-33- and IL-25-induced proliferation and type 2 cytokine production by lung ILC2s (Bartemes et al., 2012; Halim et al., 2012; Monticelli et al., 2011). Interestingly, IL-2 itself stimulated lung CD25+ ILCs, which have phenotypes similar to those of ILC2s, to produce type 2 cytokines and IL-9 in culture (Wilhelm et al., 2011). IL-9 produced by ILCs may have a positive feedback effect on ILCs, since lung ILCs cultured with IL-9 increased production of type 2 cytokines (Wilhelm et al., 2011). IL-9 might enhance ILC2 function by upregulating the anti-apoptotic protein BCL-3 in lung ILC2s, thereby promoting ILC2 survival (Turner et al., 2013). In addition, TL1A, a TNF superfamily member, has also been reported to induce ILC2 cell expansion (Yu et al., 2013).
Besides cytokines, lung ILC2s can be regulated by lipid mediators that are generated during allergic inflammation. In vitro, leukotriene D4 (LTD4) potently stimulates mouse lung ILC2s to produce not only IL-5 and IL-13 but also a large amount of IL-4; IL-4 is not generally produced by ILC2s when stimulated with IL-33 (Doherty et al., 2013). Intranasal administration of LTD4 led to expansion of IL-5-producing ILC2s in the lung in vivo. Furthermore, LTD4 potentiated proliferation and accumulation of ILC2s in mice exposed to the fungus Alternaria (Doherty et al., 2013). Prostaglandin D2 (PGD2) and lipoxin A4 (LXA4) have also been shown to regulate migration and functions of ILC2s that are isolated from human peripheral blood (Barnig et al., 2013; Chang et al., 2013).
Cytokines, in particular type 2 cytokines, are considered a major product of activated lung ILC2s. Lung ILC2s have been found to produce IL-4, IL-5, IL-9, IL-13 and GM-CSF protein (Bartemes et al., 2012; Halim et al., 2012; Wilhelm et al., 2011). In particular, IL-5 and IL-13 protein are produced in large quantities by ILC2s, perhaps beyond the levels that are produced by Th2-type CD4+ T cells, making these cells a unique “factory” of cytokines (Bartemes et al., 2012). IL-9 protein expression by ILC2s has been shown in culture supernatants of IL-33-stimulated lung ILC2s and in papain-treated mouse lungs (Bartemes et al., 2012; Wilhelm et al., 2011). While lung ILC2s that are stimulated with cytokines do not produce detectable amounts of IL-4 protein (Bartemes et al., 2012; Halim et al., 2012), LTD4-treated lung ILC2s produced a large amount of IL-4 (Doherty et al., 2013).
Potential crosstalk between ILC2s and other immune cells
Besides producing cytokines, ILC2s may work with other immune cells and orchestrate immune responses as a part of the immune system network. The functions of ILC2s are clearly regulated by other immune cells. For example, the major ILC2-activating cytokine IL-33 is produced not only by epithelial cells but also by several types of immune cells, including natural killer T (NKT) cells, alveolar macrophages, DCs, and mast cells (Chang et al., 2011; Gorski et al., 2013; Hsu et al., 2010; Kim et al., 2012). Theoretically, each of these IL-33-producing immune cells can regulate ILC2 functions during the immune response. Indeed, both NKT cells and alveolar macrophages have been shown to enhance IL-5 production by ILC2s in mice infected with influenza virus (Gorski et al., 2013). In addition, ILC2 cell numbers were not maintained in Rag2−/− mice infected with helminths or challenged with the protease papain (Neill et al., 2010; Wilhelm et al., 2011), suggesting that adaptive immune cells (presumably T cells) are required for ILC2 expansion, migration or survival. By multiphoton microscopy, skin ILC2s have been shown to interact physically with mast cells (Roediger et al., 2013), although the functional outcome of this interaction is unknown.
Several pieces of evidence also suggest that ILC2s affect the functions of cells in the adaptive immune system. Using a helminth infection model, Neil and colleagues showed that adoptively transferred spleen ILC2s enhance IL-13 production by mesenteric lymph node cells (presumably by T cells) from Il17br−/− mice, suggesting that ILC2s could regulate antigen-specific T cell responses (Neill et al., 2010). MPPtype2 cells have been shown to promote the proliferation and Th2 cytokine production of antigen-specific CD4+ T cells in an MHC class II- and IL-4-dependent manner (Saenz et al., 2010). Using an in vitro approach, Drake et al. recently reported that lung ILC2s enhance CD4+ T cell proliferation and Th2 cytokine production when the isolated cell populations were cultured together (Drake et al, 2013). Both OX40/OX40L interaction and ILC2-derived IL-4 appeared to play important roles in this CD4+ T cell-ILC2 interaction.
ILC2s can also interact with B cells. Moro et al. reported that FALC-derived ILC2s enhance B1, but not B2, cell proliferation in an IL-5-dependent manner (Moro et al., 2010). Production of IgA (but not IgG1, IgG2b or IgG3) antibody by splenic B cells was enhanced by ILC2s. Recently, lung ILC2s were shown to promote both B1 and B2 cell expansion and antibody production in vitro (Drake et al, 2013). In this condition, lung ILC2s enhanced production of not only IgA but also IgM, IgG1 and IgE classes of antibodies by B cells. The reasons for the differences between the observations in these studies are not totally clear but may be explained by the different tissue origins of the ILC2s (Bartemes et al., 2012; Halim et al., 2012; Moro et al., 2010). While further studies are necessary, the potential implication of these observations may be significant in furthering our understanding of the regulatory mechanisms of type 2 immunity.
Roles of ILC2s in asthma and allergic airway responses
Asthma, a major public health problem affecting millions of people worldwide, is an inflammatory disorder of the airways that is characterized by chronic inflammation, AHR and airway remodeling (Kim et al., 2010). Generally, allergic asthma is thought to be mediated by dysregulated production of Th2-type cytokines, although the involvement of other cell types, such as Th17 cells, has also been implicated (Halwani et al., 2013). The pathological roles for Th2 cytokines have been well established. IL-4 promotes IgE production by B cells. IL-5 regulates eosinophil recruitment and maturation. IL-9 and IL-13 induce mast cell differentiation and maturation. IL-13 is also important for mucus production by goblet cells, airway remodeling and development of AHR (Holgate, 2012; Kim et al., 2010).
Although CD4+ T cells are the important source of these Th2 cytokines, recent studies in mouse models demonstrate that ILC2s can also serve as a major innate source for type 2 cytokines. For example, using an influenza infection model in mice, Chang et al. showed that H3N1 influenza virus induces rapid development of neutrophilic airway inflammation and AHR independent of the adaptive immune system (Chang et al., 2011). In this model, lung ILC2s were essential for development of AHR. Indeed, depletion of ILC2s attenuated AHR, and adoptive transfer of isolated ILC2s induced AHR in Il13−/− recipients, suggesting a role for ILC2, in particular ILC2-derived IL-13. Furthermore, IL-33 derived from macrophages likely plays a key role in activation of ILC2s because ST2−/− mice failed to develop AHR or airway inflammation (Chang et al., 2011).
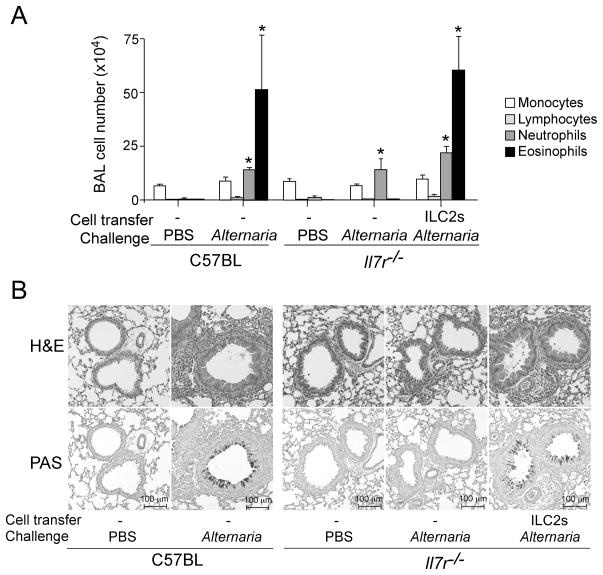
Subsequent studies established the functional importance of ILC2s in allergic airway inflammation. When mice were intranasally administered with a natural fungal allergen Alternaria alternata or a protease allergen papain, lung ILC2s produced and secreted IL-5 and IL-13, resulting in airway eosinophilia and increased mucus production (Bartemes et al., 2012; Halim et al., 2012). Furthermore, blockade of the IL-33/ST2 pathway by antibody treatment or by genetic depletion of ST2 abrogated the pathological effects of ILC2s, suggesting that IL-33 released by allergen exposure plays a key role in activating ILC2s. Because IL-7 is indispensable for development of lymphocytes and lymphoid cells, Il7r−/− mice are deficient in mature CD4+ T cells and all ILC subgroups (Walker et al., 2013). Importantly, Il7r−/− mice reconstituted with isolated lung ILC2s alone developed airway eosinophilia and pathologic changes comparable to those of wild-type mice when they were exposed to Alternaria (Figure 2), suggesting that ILC2s are sufficient to mediate allergic airway inflammation even in the absence of CD4+ T cells. Furthermore, in an OVA sensitization and challenge model, ILC2s were the major source of IL-13 together with conventional Th2-type CD4+ T cells (Barlow et al., 2012). When adoptively transferred into Il13−/− mice that are normally resistant to developing AHR, wild-type ILC2s, but not IL-13-deficient ILC2s, restored AHR and airway eosinophilia, suggesting that ILC2s are sufficient to mediate immunopathology during OVA-induced allergic airway inflammation. Similarly, IL-13-producing ILC2s were shown to be important for developing AHR in mice exposed to glycolipids (Kim et al., 2012). Together, these findings demonstrate that ILC2s can mediate allergic airway responses independent of adaptive immunity and that ILC2-derived type 2 cytokines, especially IL-5 and IL-13, play critical roles.
Figure 2.
Lung ILC2s mediate eosinophilic airway inflammation and pathology in mice exposed to fungal allergen Alternaria. (A) ILC2s were isolated from the lungs of naïve C57BL/6 mice, and they were adoptively transferred to naive Il7r−/− mice, which are deficient in mature CD4+ T cells and ILCs. Mice were then exposed intranasally three times to PBS or Alternaria extract over 6 days. Cell number and differentials in BAL fluids were determined. (B) Representative histology (upper panels: H&E staining, lower panels: PAS staining) of the mice as described in A.
IL-9 derived from ILC2s may also play roles in allergic airway inflammation. Using cytokine reporter mice, lung ILCs were shown to express IL-9 during the early phase of papain-induced airway inflammation (Wilhelm et al., 2011). Although CD4+ T cells can also produce IL-9, lung ILCs were found to be the major source of IL-9 in this model. Furthermore, addition of IL-9 to purified lung ILCs enhanced the production of IL-5, IL-6 and IL-13 by ILCs, and neutralization of IL-9 reduced the production of these cytokines in the lungs. Thus, ILC-derived IL-9 might form a positive feedback loop that amplifies production of IL-5 and IL-13 by ILCs during allergic responses.
While the studies described above clearly demonstrate the pro-inflammatory roles for lung ILC2s during allergic inflammation, potential protective roles for lung ILC2s should also be recognized. For example, in mice infected with mouse-adapted PR8 influenza virus, lung ILC2s promote airway epithelial cell repair and lung tissue homeostasis by secreting the epithelial growth factor receptor (EGFR) ligand amphiregulin, resulting in increased survival of the infected animals (Monticelli et al., 2011). Unlike the H3N1 influenza virus model (Chang et al., 2011), no role for IL-13 in immunopathology of the lung was observed. Furthermore, in mice sensitized and challeged with OVA antigen, lung ILC2s were found to be a major source of IL-22, and this IL-22 attenuated the development of allergic airway disease (Taube et al., 2011). Collectively, lung ILC2s are likely an important innate source of type 2 cytokines as well as factors that are critical for tissue repair and recovery of homeostasis. ILC2s may influence several aspects of allergic airway diseases, resulting in pathological or protective outcomes depending on the experimental conditions and disease models.
Roles of ILC2s in allergic airway diseases in humans
A major question exists as to the roles for ILC2s in allergic airway diseases in humans. The human counterpart of mouse ILC2s has been identified recently. Human ILC2s are typically Lin−, CD45+, CD127+, NKp44−, CD25+ and CD161+ (Kim et al., 2013; Mjosberg et al., 2012; Mjosberg et al., 2011; Monticelli et al., 2011). Distinct expression of two prototypic Th2-type CD4+ T cell markers, namely chemoattractant receptor-homologous molecule expressed on Th2 lymphocytes (CRTH2) and IL-33 receptor ST2, is an unique feature of human ILC2s, and these molecules are useful to differentiate human ILC2s from other human ILCs (Spits et al., 2013). Human ILC2s are reportedly found in peripheral blood, lung, bronchoalveolar lavage (BAL) fluid, nasal tissue, tonsil, gut and skin of normal healthy individuals. Similarly to mouse ILC2s, human ILC2s produce type 2 cytokines when stimulated with IL-33, IL-25, and TSLP (Kim et al., 2013; Mjosberg et al., 2012; Mjosberg et al., 2011; Monticelli et al., 2011).
The association between ILC2s and allergic airway diseases in humans was first reported by Mjosberg and colleagues who observed that the numbers of Lin−CRTH2+CD127+CD161+ ILCs were increased in nasal polyp tissues from patients with chronic rhinosinusitis (CRS) (Mjosberg et al., 2011). In a subsequent report, Shaw et al. compared ILC numbers in ethmoid sinus mucosal tissues from patients with CRS with nasal polyps (CRSwNP) and from patients with CRS without nasal polyps (CRSsNP) (Shaw et al., 2013); CRSwNP and CRSsNP are considered to be associated with polarized Th2 and Th1 cytokine production, respectively. Interestingly, patients with CRSwNP showed an increased frequency of Lin−CRTH2+CD127+CD161+ST2+ ILCs as compared to those with CRSsNP. In addition, when CD45+ lymphoid cells from sinonasal mucosa were cultured with IL-33 plus IL-2 in vitro, cells from CRSwNP patients produced more IL-13 as compared to those from CRSsNP patients, suggesting that IL-33-responsive and IL-13-producing ILC2s are increased in CRSwNP patients. In another study, Kwon and colleagues characterized the cytokines and immune cells in the pleural fluid of patients with primary spontaneous pneumothorax, a common condition causing eosinophilic pleural effusion (Kwon et al., 2013). Compared with control subjects, patients with PSP contained increased numbers of Lin−CD45+c-Kit+ CRTH2+ILC2s in their pleural effusions, and these ILC2s produced IL-5 when stimulated with IL-33 in vitro.
ILC2s can also be found in human peripheral blood. The Lin− CRTH2+CD127+CD161+ ILC2s that are comparable to airway tissue ILC2s as described above were identified in peripheral blood from normal healthy individuals, albeit at a low frequency (Mjosberg et al., 2011). Interestingly, when stimulated in vitro with IL-33 plus IL-2, peripheral blood mononuclear cells (PBMCs) from patients with allergic asthma produced significantly more IL-5 and IL-13 than those from patients with allergic rhinitis or normal individuals (Bartemes et al, 2013). Moreover, Lin−CRTH2+CD127+ ILC2 cell numbers were increased in the blood of patients with allergic asthma as compared to control groups, suggesting that ILC2s may be involved in asthma. Together, these findings suggest that ILC2s are associated with type 2 immune responses and inflammation in certain airway diseases in humans; the involvement of ILC2s in the pathology of these diseases awaits future study.
Concluding remarks
After detailed characterization of ILC2s in 2010 (Moro et al., 2010; Neill et al., 2010; Price et al., 2010), our knowledge on the biology of this novel cell type has expanded quickly. ILC2s are resident in various normal tissues. Although small in number, ILC2s likely play major roles in innate immunity and disease processes by producing large quantities of type 2 cytokines and tissue growth factors. In mice, ILC2s show both pathological and protective functions in virus- or allergen-induced allergic immune responses. In humans, a link between ILC2s and the airway diseases that are associated with type 2 immunity is being established. The identification of this unique and multifunctional cell type has provided major conceptual advances in the field.
However, the research in ILC2s is still in its infancy, and many questions remain to be addressed. For example, although the murine models suggest that ILC2s contribute to the initiation of allergic responses, little is known whether and how ILC2s are involved in the chronic phase of the immune response. At the cellular level, the interactions between ILC2s and other immune and tissue cells, such as mast cells and epithelial cells, as well the mechanisms involved in these interactions need to be studied. Our knowledge of the processes involved in migration and tissue localization of ILC2s is also limited. The roles for ILC2s in regulation and maintenance of immune homeostasis in various organs are largely unknown. Finally, the contribution of ILC2s to the severity and exacerbation of allergic airway diseases in humans provides a major question in the field. A further understanding of the biology of ILC2s and their roles in resting condition and disease status will definitely help us better understand the mechanisms of asthma and other allergic airway diseases and develop novel therapeutic options for these diseases.
References
- Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, McKenzie AN. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. Journal of Allergy and Clinical Immunology. 2012;129:191–198. e191–194. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, Bucks C, Wu X, Kane CM, Neill DR, Flynn RJ, Sayers I, Hall IP, McKenzie AN. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. The Journal of allergy and clinical immunology. 2013;132:933–941. doi: 10.1016/j.jaci.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S, Wechsler ME, Israel E, Levy BD. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Science translational medicine. 2013;5:174ra126. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. Journal of Immunology. 2012;188:1503–1513. doi: 10.4049/jimmunol.1102832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartemes KR, Fox S, Kita H. IL-33 and IL-25-responsive innate lymphoid cells are present in human peripheral blood. Journal of Immunology. 2013;190:181.1. (abstract) [Google Scholar]
- Chang JE, Doherty TA, Baum R, Broide D. Prostaglandin D2 regulates human type 2 innate lymphoid cell chemotaxis. The Journal of allergy and clinical immunology. 2013 doi: 10.1016/j.jaci.2013.09.020. Page numbers? [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, Dekruyff RH, Umetsu DT. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. The Journal of allergy and clinical immunology. 2013;132:205–213. doi: 10.1016/j.jaci.2013.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake L, Kita H. Type 2 innate lymphoid cells promote Th2 cytokine production by CD4+T cells and antibody responses by B cells. Journal of Immunology. 2013;190:183.3. (abstract) [Google Scholar]
- Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie AN. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. The Journal of experimental medicine. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- Gorski SA, Hahn YS, Braciale TJ. Group 2 innate lymphoid cell production of IL-5 is regulated by NKT cells during influenza virus infection. PLoS pathogens. 2013;9:e1003615. doi: 10.1371/journal.ppat.1003615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Halwani R, Al-Muhsen S, Hamid Q. T helper 17 cells in airway diseases: from laboratory bench to bedside. Chest. 2013;143:494–501. doi: 10.1378/chest.12-0598. [DOI] [PubMed] [Google Scholar]
- Holgate ST. Innate and adaptive immune responses in asthma. Nature medicine. 2012;18:673–683. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, Voehringer D, Busslinger M, Diefenbach A. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634–648. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CL, Neilsen CV, Bryce PJ. IL-33 is produced by mast cells and regulates IgE-dependent inflammation. PloS one. 2010;5:e11944. doi: 10.1371/journal.pone.0011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, Brieland JK, Zurawski SM, Chapman RW, Zurawski G, Coffman RL. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. Journal of immunology. 2002;169:443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- Ikutani M, Yanagibashi T, Ogasawara M, Tsuneyama K, Yamamoto S, Hattori Y, Kouro T, Itakura A, Nagai Y, Takaki S, Takatsu K. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. Journal of immunology. 2012;188:703–713. doi: 10.4049/jimmunol.1101270. [DOI] [PubMed] [Google Scholar]
- Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, Hepworth MR, Van Voorhees AS, Comeau MR, Artis D. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Trans Med. 2013;5:170ra116. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Chang YJ, Subramanian S, Lee HH, Albacker LA, Matangkasombut P, Savage PB, McKenzie AN, Smith DE, Rottman JB, DeKruyff RH, Umetsu DT. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. Journal of Allergy and Clinical Immunology. 2012;129:216–227. e211–216. doi: 10.1016/j.jaci.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, DeKruyff RH, Umetsu DT. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nature immunology. 2010;11:577–584. doi: 10.1038/ni.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon BI, Hong S, Shin K, Choi EH, Hwang JJ, Lee SH. Innate type 2 immunity is associated with eosinophilic pleural effusion in primary spontaneous pneumothorax. American journal of respiratory and critical care medicine. 2013;188:577–585. doi: 10.1164/rccm.201302-0295OC. [DOI] [PubMed] [Google Scholar]
- McHedlidze T, Waldner M, Zopf S, Walker J, Rankin AL, Schuchmann M, Voehringer D, McKenzie AN, Neurath MF, Pflanz S, Wirtz S. Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity. 2013;39:357–371. doi: 10.1016/j.immuni.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mjosberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, te Velde AA, Fokkens WJ, van Drunen CM, Spits H. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity. 2012;37:649–659. doi: 10.1016/j.immuni.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nature immunology. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, Locksley RM. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, Mitchell AJ, Tay SS, Jain R, Forbes-Blom E, Chen X, Tong PL, Bolton HA, Artis D, Paul WE, Fazekas de St Groth B, Grimbaldeston MA, Le Gros G, Weninger W. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. 2013;14:564–573. doi: 10.1038/ni.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz SA, Siracusa MC, Monticelli LA, Ziegler CG, Kim BS, Brestoff JR, Peterson LW, Wherry EJ, Goldrath AW, Bhandoola A, Artis D. IL-25 simultaneously elicits distinct populations of innate lymphoid cells and multipotent progenitor type 2 (MPPtype2) cells. J Exp Med. 2013;210:1823–1837. doi: 10.1084/jem.20122332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, Bhandoola A, Artis D. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JL, Fakhri S, Citardi MJ, Porter PC, Corry DB, Kheradmand F, Liu YJ, Luong A. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. American journal of respiratory and critical care medicine. 2013;188:432–439. doi: 10.1164/rccm.201212-2227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, Vivier E. Innate lymphoid cells--a proposal for uniform nomenclature. Nature reviews Immunology. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- Taube C, Tertilt C, Gyulveszi G, Dehzad N, Kreymborg K, Schneeweiss K, Michel E, Reuter S, Renauld JC, Arnold-Schild D, Schild H, Buhl R, Becher B. IL-22 is produced by innate lymphoid cells and limits inflammation in allergic airway disease. PloS one. 2011;6:e21799. doi: 10.1371/journal.pone.0021799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JE, Morrison PJ, Wilhelm C, Wilson M, Ahlfors H, Renauld JC, Panzer U, Helmby H, Stockinger B. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. Journal of Experimenal Mededicine 2013. 2013 Nov 18; doi: 10.1084/jem.20130071. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells - how did we miss them? Nat Rev Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, Sparwasser T, Helmby H, Stockinger B. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol. 2011;12:1071–1077. doi: 10.1038/ni.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, Barlow JL, Neill DR, Panova V, Koch U, Radtke F, Hardman CS, Hwang YY, Fallon PG, McKenzie AN. Transcription factor RORalpha is critical for nuocyte development. Nature immunology. 2012;13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Saenz SA, Zlotoff DA, Artis D, Bhandoola A. Cutting edge: Natural helper cells derive from lymphoid progenitors. Journal of immunology. 2011;187:5505–5509. doi: 10.4049/jimmunol.1102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Pappu R, Ramirez-Carrozzi V, Ota N, Caplazi P, Zhang J, Yan D, Xu M, Lee WP, Grogan JL. TNF superfamily member TL1A elicits type 2 innate lymphoid cells at mucosal barriers. Mucosal Immunol. 2013 doi: 10.1038/mi.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]