Abstract
GSK1265744 long-acting (GSK744 LA) is a strand-transfer inhibitor of HIV/SIV integrase and was shown to be an effective pre-exposure prophylaxis agent in a low-dose intrarectal SHIV rhesus macaque challenge model. Here, we examined the pharmacokinetics and efficacy of GSK744 LA as PrEP against repeat high-dose intravaginal SHIV challenge in female rhesus macaques treated with Depo-Provera which promotes viral transmission vaginally. When Depo-Provera-treated female rhesus macaques were dosed with 50 mg/kg of GSK744 LA monthly, systemic and tissue drug concentrations were lower than previously observed in male rhesus macaques. GSK744 concentrations were 5-fold lower on average in cervical tissues than rectal tissues. Eight female rhesus macaques were treated with GSK744 LA at week 0, and four female rhesus macaques served as controls. All animals received a high dose challenge of SHIV162P3 at week 1. No infection was detected in GSK744 LA-treated rhesus macaques, whereas viremia was detected 1 to 2 weeks after SHIV challenge in all control animals. The GSK744 LA-treated rhesus macaques were given a second administration of drug at week 4 and further challenged at weeks 5 and 7. GSK744 LA treatment protected 6 of 8 female rhesus macaques against three high-dose SHIV challenges, whereas all control animals became infected after the first challenge (P = 0.0003, log-rank test). These results support further clinical development of GSK744 LA for pre-exposure prophylaxis.
Introduction
The use of antiretroviral (ARV) drugs as pre-exposure prophylaxis (PrEP) has been shown to be an effective strategy for preventing HIV-1 acquisition. Daily oral emtricitabine (FTC) and tenofovir disoproxil fumarate (TDF) as PrEP prevented HIV-1 acquisition in men who have sex with men in the iPrEx trial (1). In addition, FTC/TDF and TDF alone prevented infection in heterosexual men and women in the Partners PrEP and the TDF2 trials (2, 3). And finally, TDF alone reduced HIV infection by 49% in injection drug users in the Bangkok Tenofovir Study (4). Studies performed exclusively in women have demonstrated mixed results. CAPRISA 004 utilizing 1% tenofovir gel before and after sex demonstrated protection (5), whereas no protection was observed with daily regimens in the FEM-PrEP (oral FTC/TDF) or the VOICE trials (oral FTC/TDF or TDF, or tenofovir gel) due to low adherence to study drug (6, 7). Taken together, these studies have demonstrated that efficacy of a given PrEP regimen is proportional to the degree of adherence to the intervention. We hypothesized that long-acting (LA) ARV formulations requiring less frequent dosing may improve both adherence and PrEP efficacy across groups at high-risk for HIV-1 infection.
GSK1265744 (GSK744), an analogue of dolutegravir, is a potent integrase strand transfer inhibitor (InSTI) with physiochemical properties that permit nanomilling of the crystalline free acid to a median particle size of 200 nm in the presence of surfactant, polymer, mannitol and water for injection (8). The resulting nanoparticles are essentially 100% active drug and formulated as a 200 mg/mL GSK744 LA injectable suspension. The same formulation is under evaluation in multiple clinical studies (8). In healthy volunteers, single GSK744 LA injections were well-tolerated; the most common adverse event reported was pain at the injection site (9). The apparent terminal half-life (t1/2) of GSK744 LA ranged from 21 to 50 days compared to approximately 40 hours for oral GSK744 (8, 10). The long t1/2 of GSK744 LA makes it suitable for administration every three months in the clinic.
We previously demonstrated that GSK744 LA is an effective PrEP agent against repeated low-dose intrarectal simian-human immunodeficiency virus (SHIV) challenge (11) in a model that was developed to more closely mimic human exposure and infection by HIV-1 via unprotected receptive anal intercourse (12–15). Our results showed that monthly administration of GSK744 LA beginning one week prior to intrarectal inoculation provided complete protection against eight weekly challenges (11). In a follow-up experiment, we defined the correlate of protection against intrarectal inoculation in this model as GSK744 plasma concentrations >3x protein-adjusted IC90 (PAIC90) resulting in 100% protection; concentrations ≥1x PAIC90 yielded 97% efficacy (11). These plasma concentrations are readily achievable in humans with quarterly 800 mg intramuscular (IM) injections (11). These data support the preclinical evaluation of GSK744 LA as PrEP in other transmission models. As approximately half of new HIV-1 transmissions occur in heterosexual women (16), and rectal prevention efficacy cannot predict efficacy against vaginal transmission, we evaluated the protective efficacy of GSK744 LA using an intravaginal challenge model in rhesus macaques.
Unlike the rectum, which is composed of a single layer of columnar epithelium, the vagina is a multilayered stratified squamous epithelium with a thickness that varies during the menstrual cycle (17). The multilayered epithelium provides a more substantial physical barrier to infection during repeated exposure to foreign materials as part of its normal function. Intravaginal HIV-1 transmission is typically studied in the rhesus macaque (Macaca mulatta) or pigtail macaque (Macaca nemestrina). Rhesus macaques are typically seasonal breeders, and the physical barrier of the macaque genital tract requires up to 10,000-fold more cell-free SIV to infect 100% of macaques via the vagina compared to an intravenous route (18). To increase the susceptibility of macaques to vaginal infection, pretreatment with progesterone has been used to thin the vaginal and ectocervical epithelium (19, 20). The Depo-Provera macaque model has been used to assess the efficacy of vaccines, neutralizing antibodies, ARVs, and vaginal microbicides against challenge with both SIV and SHIV (including SHIV162P3) (21–26). SHIV162P3 challenge has been most recently used in studies of topically applied ARVs, including maraviroc (27–31). Protection in these studies was time-dependent with protection waning as time increased between microbicide application and viral challenge (31). The Depo-Provera rhesus macaque model results in infection of >90% of control animals after one challenge. Conversely, pigtail macaques have a menstrual cycle similar to humans and can be infected in a repeat low-dose vaginal challenge model that has been established to resemble vaginal HIV-1 transmission without Depo-Provera pretreatment (32, 33). The variables relevant to vaginal SIV/SHIV susceptibility in the rhesus macaque and pigtail macaque models have been discussed (34). The use of pigtail macaques is limited by the low number of breeding facilities in the United States. As our primary data were generated in male rhesus macaques, we evaluated the efficacy of GSK744 LA at preventing vaginal SHIV infection in a stringent high-dose female rhesus macaque model pretreated with Depo-Provera to ensure efficient infection of control macaques. These data are complementary to the results provided by Radzio et al. demonstrating complete protection by GSK744 LA against SHIV162P3 low-dose intravaginal challenge in pigtail macaques (35), and when taken together support further testing of GSK744 LA as PrEP in high-risk women.
Results
Pharmacokinetic evaluation of GSK744 LA in Depo-Provera-treated rhesus macaques
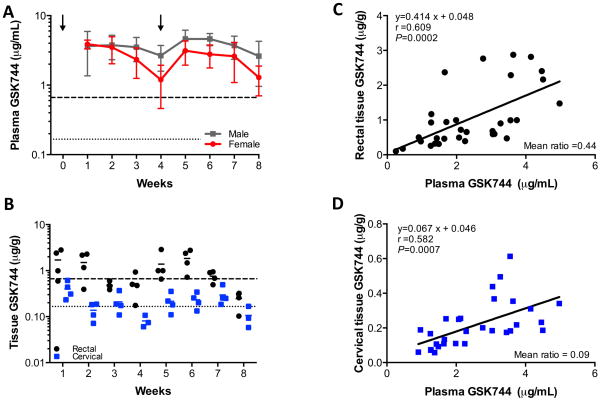
We previously evaluated the pharmacokinetic (PK) profile of GSK744 LA in male rhesus macaques and established that monthly dosing with 50 mg/kg maintained plasma concentrations similar to those achieved in healthy human volunteers dosed IM with 800 mg of GSK744 LA quarterly (11). In this study, we first explored the impact of Depo-Provera pretreatment on the GSK744 PK profile. Female rhesus macaques (n=8) were treated with Depo-Provera on weeks -3 and 2 and with 50 mg/kg of GSK744 LA on weeks 0 and 4. Mean plasma GSK744 concentrations in Depo-Provera-treated female rhesus macaques were lower than the mean plasma GSK744 concentrations observed in male rhesus macaques that protected against low-dose intrarectal challenge (Fig. 1A). When compared to male rhesus macaques, Depo-Provera-treated female rhesus macaques had comparable drug exposures following the first GSK744 LA dose (AUCDose1 = 2047 ± 772 and 1698 ± 455 μg × h/mL, respectively, P = 0.27, Mann-Whitney two-tailed t-test) (Table 1), however, the mean plasma trough concentrations (Cτ) were lower in the female macaques following the first dose (2.66 ± 1.07 and 1.20 ± 0.74 μg/mL, male and female, respectively, P = 0.007, Mann-Whitney two-tailed t-test). Following the second dose of GSK744 LA, a 38% lower AUC was observed in the female macaques compared to the male macaques (AUCDose2 = 1616 ± 591 and 2593 ± 648 μg × h/mL, respectively, P = 0.02, Mann-Whitney two-tailed t-test), whereas a lower but not statistically different Cτ was observed (1.29 ± 0.59 and 2.62 ± 1.66 μg/mL, female and male, respectively, P = 0.08, Mann-Whitney two-tailed t-test). PK studies in male rhesus macaques established that dosing with 50 mg/kg of GSK744 LA would maintain plasma concentrations ≥3x PAIC90 throughout dosing, a value that correlated with 100% protection in that model. However, in two Depo-Provera-treated female macaques, three Cτ measurements were <3x PAIC90 (Fig. S1).
Fig. 1.
GSK744 LA plasma PK profile and tissue distribution in Depo-Provera-treated female rhesus macaques. Eight female rhesus macaques were injected IM with 30 mg Depo-Provera on weeks -3 and 2, and with 50 mg/kg of GSK744 LA on weeks 0 and 4. (A) GSK744 plasma concentrations from Depo-Provera-treated female rhesus macaques (red) were compared with male rhesus macaques (black) dosed with 50 mg/kg GSK744 LA on weeks 0 and 4. Mean ± SD are shown. Dotted and dashed horizontal lines represent 1x and 4x PAIC90, respectively. (B) Rectal and cervical tissue distribution of GSK744 was assessed from pinch biopsies each week in a subset (n=4) of Depo-Provera-treated rhesus macaques. Each symbol represents tissue concentrations from an individual macaque. Solid lines represent the mean for the group. Dotted and dashed horizontal lines represent 1x and 4x PAIC90, respectively. Correlation of (C) rectal tissue GSK744 or (D) cervical tissue GSK744 and plasma GSK744 concentrations. Each symbol represents the simultaneous plasma and tissue concentrations of an individual macaque.
Table 1.
PK parameters of GSK744 in rhesus macaques dosed with 50 mg/kg GSK744 LA.
| GSK744 LA Dose | AUC (0-τ) (μg × h/mL) | Cτ (μg/mL) | Cmax (μg/mL) | Tmax (day)a | t1/2 (day)b | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 2 | |
| 50 mg/kg (male) | 2047 (772)* | 2593 (648)#,* | 2.66 (1.07)#,* | 2.62 (1.66) | 4.53 (2.12) | 5.17 (1.20)#,* | 14 (7–28) | 42 (35–56) | 8.6 (3.7) |
| 50 mg/kg (female) Depo-Provera-treated (PK study) |
1698 (455)^ | 1616 (591) | 1.20 (0.74) | 1.29 (0.59) | 4.21 (0.84)^ | 3.47 (1.45) | 10.5 (7–21) | 38.5 (35–49) | NDc |
| 50 mg/kg (female) Depo-Provera-treated (Challenge study) |
1247 (236) | 1587 (415) | 0.85 (0.51) | 1.39 (0.75) | 3.03 (0.71) | 3.45 (0.98) | 14 (7–21) | 42 (35–49) | 9.6 (7.8) |
ND: not determined
Tmax reported in median (range) and not subjected to statistical analyses
T1/2 estimated for 2nd dose only as terminal phases were long enough to estimate parameter
T1/2 not estimated for PK study as sufficient time points were not collected to estimate parameter
p<0.05 when compared with female PK study
p<0.05 when compared with female challenge study
p<0.05 when compared with female challenge study
Tissue penetration of GSK744
ARVs exhibit differential mucosal tissue penetration (36–39), a characteristic we believe to be highly relevant to the PK profile of a potential PrEP agent. We determined the GSK744 distribution in rectal and cervical tissues. In Depo-Provera-treated macaques, GSK744 penetrated rectal tissue more efficiently than cervical tissue (Fig. 1B). As observed in male macaques (11), higher plasma concentrations correlated with higher rectal tissue concentrations (Fig. 1C). In Depo-Provera-treated female macaques, the mean rectal tissue:plasma (T:P) ratio was 0.44 (range: 0.14 to 1.43). To make certain that rinsing rectal tissues in saline prior to GSK744 concentration analysis had no effect on drug concentration determinations, pinch biopsies (n=16–20) from each animal at each time point were processed in parallel with half of the biopsies subjected to a saline rinse. A linear correlation (slope = 0.88) was observed between processing methods (Fig. S2) indicating similar GSK744 values in rectal tissues irrespective of the processing method. The mean T:P ratio in unwashed rectal tissues was 0.37 (range: 0.15 to 1.13). These values were not statistically different (P = 0.6, Mann-Whitney two-tailed t-test). GSK744 concentrations were analyzed from two cervical biopsies from each individual animal at each time point (Fig. S3), and the mean concentrations were calculated (Figs. 1B and 1D). As with rectal tissue, cervical tissue GSK744 concentrations increased with plasma GSK744 concentrations (Fig. 1D). The mean cervical T:P ratio was 0.09 (range: below detection to 0.20). In some animals, GSK744 concentrations were not detectable in cervical tissues collected at weeks 4 and 8, thus not allowing for the determination of the T:P ratio.
PrEP Efficacy of GSK744 LA
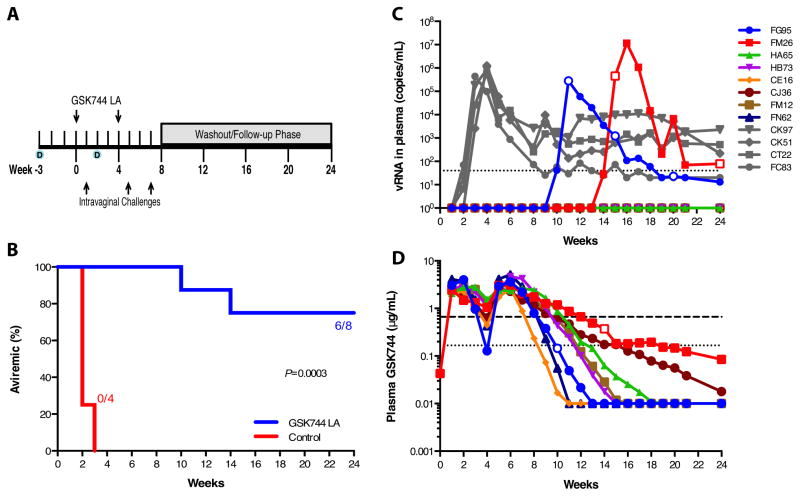
Although systemic GSK744 concentrations were lower than observed in the male rhesus macaques and GSK744 penetrated the cervical tissue with less efficiency than rectal tissue in Depo-Provera-treated female rhesus macaques, we nevertheless performed a challenge experiment to determine the efficacy of protection against SHIV infection at the observed GSK744 tissue concentrations. Twelve rhesus macaques were injected with Depo-Provera on weeks -3 and 2, and eight of the rhesus macaques were subsequently administered 50 mg/kg GSK744 LA on week 0 with the remaining 4 rhesus macaques serving as controls (Fig. 2A). Both groups of rhesus macaques were challenged by non-traumatic inoculation with high-dose 300 TCID50 SHIV162P3 on week 1. Control rhesus macaques became infected during the first challenge, with viral RNA (vRNA) detected one (n=3) to two (n=1) weeks after inoculation (Figs. 2B and 2C). GSK744 LA-treated rhesus macaques remained aviremic following the first challenge and were subsequently dosed with GSK744 LA on week 4 and similarly challenged on weeks 5 and 7 (Fig. 2A). All GSK744 LA-treated rhesus macaques remained aviremic throughout the challenge phase of the experiment. Plasma vRNA was detected in two GSK744 LA-treated macaques (FG95 and FM26), 10 and 14 weeks after the first GSK744 LA dose and 3 and 7 weeks after the last viral challenge, respectively (Fig. 2C). The GSK744 LA-treated rhesus macaques had an 8.8-fold [hazard ratio, 95% confidence interval (CI) 7.7, 1019.0] lower risk of infection compared with control rhesus macaques (P = 0.0003, log-rank test). At the time of each challenge, plasma GSK744 concentrations were 3.06, 2.93, 2.22 (13.3–18.4x PAIC90) and 2.47, 3.02, and 2.07 (12.4–18.1X PAIC90) μg/mL for FG95 and FM26, respectively (Fig. 2D). Plasma GSK744 concentrations at the time of first vRNA detection were 0.15 (<1x PAIC90) and 0.37 (≈2.2x PAIC90) μg/mL for FG95 and FM26, respectively (Fig. 2D), with cervical tissue concentrations expected to be ~10% of plasma concentrations (Fig. 1D). In the six aviremic rhesus macaques, GSK744 plasma concentrations fell below the 1x PAIC90 between weeks 9 and 16. Plasma vRNA, proviral DNA and anti-SHIV antibodies were not detected in the six aviremic rhesus macaques through week 24.
Fig. 2.
Monthly injections of GSK744 LA protect rhesus macaques against three intravaginal SHIV challenges. (A) Study design. Twelve female rhesus macaques were injected IM with 30 mg of Depo-Provera on weeks -3 and 2. Eight rhesus macaques were injected IM in the quadriceps with 50 mg/kg of GSK744 LA at two time points, weeks 0 and 4. Four Depo-treated rhesus macaques served as controls. All animals were challenged intravaginally on week 1 with 300 TCID50 of SHIV162P3. GSK744 LA-treated rhesus macaques were further challenged on weeks 5 and 7. All rhesus macaques were followed for 24 weeks. (B) Kaplan-Meier plot of GSK744 LA-treated and control rhesus macaques remaining aviremic following three intravaginal SHIV challenges. (C) Viral loads of control rhesus macaques (in grey) and GSK744 LA-treated rhesus macaques (in color). Open symbols represent samples sequenced for the integrase-coding region. Dotted line represents the LOQ, >40 SHIV RNA copies/mL plasma. (D) Plasma PK of individual GSK744 LA-treated rhesus macaques throughout the study. Open symbols represent drug concentrations at the time of first viral RNA detection. Dotted and dashed horizontal lines represent 1x and 4x PAIC90, respectively. LOQ >0.01 μg/mL
In the GSK744 LA-treated macaques that became infected, the peak viral loads (2.81 × 105 and 1.16 × 107 vRNA copies/mL plasma, FG95 and FM26, respectively) were comparable to those measured in the control rhesus macaques (mean 9.39 × 105 vRNA copies/mL plasma). Anti-SHIV antibodies were detected in the two infected GSK744 LA-treated rhesus macaques two weeks after vRNA detection compared with 2–3 weeks in the control rhesus macaques. In all infected rhesus macaques, treated and controls, proviral DNA and vRNA became detectable simultaneously (Table S1).
The t1/2 of GSK744 LA in Depo-Provera-treated female rhesus macaques in the challenge experiment was 9.6 ± 7.8 days, which was similar to 8.6 ± 3.7 days (P = 0.70, Mann-Whitney two-tailed t-test) previously observed in male rhesus macaques treated with 50 mg/kg GSK744 LA (Table 1). In the challenge experiment, a lower AUCDose1 and Cmαζ(Dose1) were observed compared to the PK experiment in female rhesus macaques pretreated with Depo-Provera (Table 1). The same rhesus macaques were used in both experiments with a 5-month washout phase between studies. Overall, these PK data demonstrate that there was some degree of inter- and intra-animal variability. With the exception of the GSK744 plasma Cτ at week 4, the PK profiles obtained in the Depo-Provera-treated rhesus macaques corresponded well with plasma concentrations observed in healthy human volunteers dosed IM with 800 mg of GSK744 LA (Fig. S4)
Integrase resistance mutants not identified in breakthrough infections
Hypothetically, when ARVs used as PrEP fail to prevent transmission, drug resistant viruses may either establish infection or emerge due to viral replication in the presence of sub-inhibitory drug concentrations. We performed consensus sequencing of the integrase-coding region one week after the first detectable viremia (weeks 11 and 15 for FG95 and FM26, respectively) and at subsequent time points as indicated by open symbols in Fig. 2C. Consensus sequencing at the time of detectable viremia revealed infection by SHIV162P3 virus lacking known resistance conferring amino acid substitutions to integrase inhibitors (Fig. S5). One mutation, E198G, was identified in FG95 at week 20. This E198G mutation did not decrease viral susceptibility to GSK744 (Table S2).
We further analyzed the integrase-coding region one week after the first detectable viremia using single-genome analysis. Approximately thirty single genomes from each infected GSK744 LA-treated rhesus macaque were analyzed providing 95% confidence that integrase variants comprising >10% of the total virus population were assessed (40). Thirty-one and 29 single genomes were obtained from FG95 (week 11) and FM26 (week 15), respectively. Two non-synonymous mutations were identified in the viral population from FG95 but none in the viral population from FM26. The two mutations identified in plasma from FG95 (P142S, I210V) were found on different genomes, and both were only identified once. These mutations did not decrease susceptibility of FG95 to GSK744 in vitro (Table S2).
Transmitted/founder (T/F) virus analyses
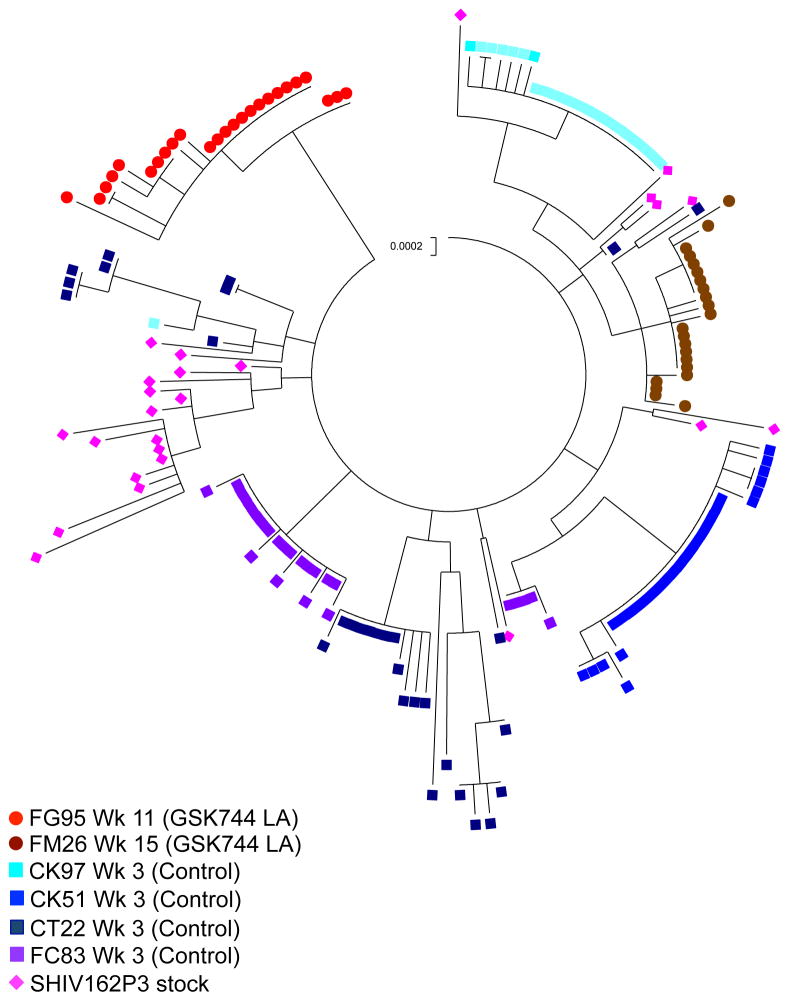
To investigate the diversity of the viral populations that infected the rhesus macaques, we compared the number of T/F viruses that established infection in the drug-treated and control rhesus macaques. The diversity of the SHIV162P3 challenge stock was characterized by generating 25 env sequences (3,102 nucleotides) using single-genome analysis as previously described (26). The SHIV162P3 challenge stock utilized in this study had a mean diversity of 0.24% (range: 0 to 0.52%), which is within the range of other challenge stocks used in such studies (24, 26, 41). From 6 infected rhesus macaques, 159 (median 26 sequences per rhesus macaque; range: 24 to 30) full-length env sequences were amplified from plasma collected within one week of first detectable viremia (Table S3). Analyzing at least 20 plasma vRNA sequences provided 95% confidence that variants representing ≥ 15% of the viral population were sampled (40). The minimum number of T/F variants was reported, but it is possible that some variants were not detected by single-genome analysis. A maximum likelihood phylogenetic tree was generated illustrating the relationships among SHIV162P3 challenge stock sequences and variants from each infected rhesus macaque (Fig. 3). The two GSK744 LA-treated rhesus macaques were each infected with a minimum of 1 T/F variant resulting in a median of 0 T/F variants in the GSK744 LA-treated group (n=8) compared with a minimum of 1 and up to 8 T/F variants in the control rhesus macaques (n=4; median T/F 2; P = 0.006, Mann-Whitney two-tailed t-test) (Fig. S6). The viral eclipse phase length may correlate with the number of T/F variants (42). The control rhesus macaque that became infected with 1 T/F variant had an eclipse phase of 2 weeks compared with an eclipse phase of 1 week in the rhesus macaques that were infected with >1 T/F variant (Table S3). The number of animals infected in this study was small; however, the number of T/F variants correlated well with what was previously observed for this model (24, 26). The transmission in GSK744 LA-treated animals limited the variants to one founder virus, suggesting a pharmacodynamic bottleneck to virus infection and subsequent transmission.
Fig. 3.
Phylogram of SHIV162P3 sequences. Maximum likelihood phylogenetic tree of SHIV162P3 sequences from viral stock and from each of the 8 infected rhesus macaques. Hypermutated sequences were excluded. Red and brown circles represent sequences from GSK744 LA-treated rhesus macaques, squares represent sequences from control rhesus macaques, and pink diamonds represent SHIV162P3 viral stock. The vertical scale bar represents 0.0002 nucleotide substitutions per site.
Discussion
In this study, we investigated the PK and protective efficacy of GSK744 LA as a PrEP agent in a high-dose vaginal challenge model. Female rhesus macaques were dosed with 50 mg/kg GSK744 LA, a dose identified in male rhesus macaques that provided plasma concentrations similar to those observed in humans (11). Overall, lower systemic exposures were observed in the Depo-Provera-treated female rhesus macaques which may be due to a drug interaction between Depo-Provera and GSK744 or perhaps a potential gender difference in the absence of Depo-Provera. PK parameters appeared to be more influenced by the second dose of Depo-Provera, including AUCDose2, CτDose1 and Cmax(Dose2) (Table 1). GSK744 is metabolized via the hepatic glucuronidation pathway, primarily by UGT1A1, with some involvement from UGT1A9 (43). Studies have shown that UGT1A1 expression is upregulated by high progesterone concentrations via a nuclear receptor, pregnane X receptor (PXR) (44). Thus, the lower systemic concentration of GSK744 may be due to increased metabolism induced by UGT1A1. That said, we do not believe that this will be a significant factor in clinical trials of GSK744 LA in women as progesterone exposures in macaque models are an order of magnitude higher than that seen in women treated with progesterone-containing birth control agents (45). However, detailed drug-drug interaction studies between GSK744 and progesterone-containing birth control agents will be a component of the drug development path.
In addition to lower systemic concentrations observed in the Depo-Provera-treated female rhesus macaques, GSK744 penetrated the cervical tissue less efficiently than it penetrated rectal tissue. However, though data are limited, tissue drug concentrations in human studies suggest that GSK744 penetrates cervical tissue slightly more efficiently than rectal tissue (46). The discrepancy in the relative GSK744 mucosal penetration may be due to species differences or possibly the influence of Depo-Provera pretreatment highlighting perhaps one limitation of this animal model. Previous studies with CCR5 inhibitors (CMPD167 and maraviroc) have shown lower drug concentrations in vaginal tissue and vaginal fluid following drug administration via vaginal rings in Depo-Provera-treated rhesus macaques compared with rhesus macaques that were not pretreated with Depo-Provera (47).
The efficacy of GSK744 LA as PrEP was demonstrated in a stringent high-dose intravaginal SHIV challenge model. Protection was observed in 6 of the 8 GSK744 LA-treated rhesus macaques against three high-dose challenges, whereas all control rhesus macaques became infected after one challenge. We did not identify known drug-resistance associated mutations in the integrase-coding region of the viruses establishing infection in the GSK744 LA-treated animals and believe that drug-sensitive virus established infection. The failure of GSK744 LA as PrEP in this model can be explained by a combination of factors including the low cervical tissue penetration of drug, approximately 10% of plasma concentration on average, the high viral inoculum used in the presence of Depo-Provera, and the mechanism of action of the integrase inhibitor. Due to the high inocula used in this model, it is likely that virus enters a number of susceptible cells locally resulting in the generation of more reverse transcribed SHIV DNA as compared to the low-dose challenge models (11, 35). Coupled with the low tissue penetration of drug, proviral DNA may have either integrated immediately or perhaps persisted pre-integration until drug decreased to concentrations permitting integration. Subsequent reductions in drug concentrations then could have allowed infection to first spread locally and then disseminate, by which time the viremia would be readily detectable. The drug effect is consistent with a genetic bottleneck in the T/F virus analysis and also accounts for the delay observed between the time of virus challenge and the subsequent appearance of viremia.
Questions remain regarding the validity of the high-dose challenge model in the rhesus macaque (33). It could be argued that the challenge doses used in this model simulating vaginal infection with HIV-1 are higher than the inoculum women are exposed to during intercourse and could therefore underestimate the potential efficacy of the candidate being tested. The result that GSK744 LA was at least 90% protective in this model may indeed underestimate its potential, at least quantitatively, and the animal models using lower inocula would suggest this is the case (35). It is worth nothing that nearly one-half of HIV-1 transmission events could be ascribed to a sex partner with acute HIV-1 infection (48), when the blood and genital-tract HIV-1 burden is the highest (49). The high-dose challenge model may also reflect HIV-1 acquisition in several high-progesterone situations including pregnancy and during injectable contraceptive use (50). This model also may mimic an important stage of the menstrual cycle when the risk of HIV/SHIV infection increases (51, 52). The relevance of this model is further supported by recent data showing that only one virus, or perhaps a few viruses, are transmitted (24), a biological feature mirroring what happens in HIV-1 infected women (40) and rhesus macaques not pretreated with Depo-Provera (53). It is notable, however, that unlike the low-dose intrarectal challenge model in which we were able to determine a systemic threshold concentration of drug at which protection is maintained, this model does not allow for such an experimental design.
In summary, our findings along with the complementary results provided by Radzio et al. demonstrating complete protection by GSK744 LA against SHIV162P3 low-dose intravaginal challenge in pigtail macaques (35), suggest that GSK744 LA is suitable for clinical testing as a next generation PrEP agent. Its activity ranged from highly to completely protective in animal models simulating HIV-1 transmission. In addition, early phase clinical trials suggest that the drug is well tolerated and its PK in man suggests that it is amenable to episodic use. Although LA formulations have improved adherence to drug regimens in a variety of settings including contraception (54), schizophrenia (55), and male hypogonadism (56), clinical trials must be performed to determine whether LA formulations will improve adherence to ARV agents used as PrEP while still demonstrating safety and efficacy. Phase 2 clinical trials to assess the safety and acceptability of GSK744 LA are underway, and should these studies prove successful, then large Phase 3 efficacy studies will soon follow.
Materials and Methods
Study design
The PK study was designed as a single-arm study in 8 female Indian rhesus macaques dosed with 50 mg/kg split into four 12.5 mg/kg injections. The primary endpoints were drug concentrations in plasma, cervical tissue and rectal tissue at time points as described below. The study was descriptive and therefore not powered. A total of 8 animals were treated. To permit tissue healing, an animal was biopsied every two weeks. Staggering the biopsies yielded tissue drug concentration results from 4 animals at each time point.
The intravaginal SHIV162P3 challenge study was a 2-arm study that included 12 female Indian rhesus macaques treated with Depo-Provera; 8 were treated with GSK744 LA as described below and 4 were controls. Animal handlers and laboratory staff performing assays were aware of each animal’s assigned study arm throughout the course of the study. Assuming that 100% of challenges would result in infection in the control animals, the study had 100% power to detect a 90% effective PrEP agent using Fisher’s exact t-test with a p-value of 0.05. The primary endpoint of the challenge study was the presence/absence of SHIV162P3 infection in all SHIV162P3-exposed animals based on the presence/absence of plasma viremia as determined by real-time RT-PCR as described below. Secondary endpoints included qualitative determinations of proviral DNA in peripheral blood mononuclear cells (PBMCs), presence of anti-SHIV antibodies in plasma, characterization of viral populations in GSK744 LA-treated animals that became infected for evidence of resistance to GSK744, and phylogenetic characterization of the SHIV162P3 stock and T/F viruses in all infected animals.
PK evaluation of GSK744 LA in rhesus macaques
Eight Indian rhesus macaques (Macaca mulatta) were treated IM with 30 mg Depo-Provera (depot medroxyprogesterone acetate) on weeks -3 and 2 and GSK744 LA on weeks 0 and 4. GSK744 LA is a 200 mg/mL nanosuspension that was administered based on body weights measured at the time of dosing (5.3 to 8.2 kg) with the 50 mg/kg dose split into four 12.5 mg/kg injections, 2 per quadriceps. Blood was collected weekly from all rhesus macaques (n=8), and rectal and cervical tissues were biopsied from a subset of rhesus macaques (n=4) each week permitting a two-week period between biopsies. Rectal biopsies (n=16–20 pieces of tissue/time point) were collected using 1.5 mm x 3 mm forceps and cervical biopsies were collected from the ectocervix (n=2 pieces of tissue/time point) using 2 mm x 4 mm forceps.
Efficacy of GSK744 LA in preventing intravaginal SHIV transmission
The efficacy of GSK744 LA against vaginal SHIV transmission was evaluated in 12 rhesus macaques treated with 30 mg Depo-Provera IM on weeks -3 and 2 and 50 mg/kg of GSK744 LA on weeks 0 and 4. Rhesus macaques were challenged by applying 300 TCID50 SHIV162P3 in 1 mL to the vaginal vault using a gastric feeding tube. Rhesus macaques remained recumbent for at least 15 minutes after inoculation. Challenges were performed on the same day with the same viral stock and inoculation method. The R5 tropic SHIV162P3 (57, 58) challenge stock was expanded and titrated in rhesus macaque PBMCs prior to this study. The SHIV162P3 challenge stock had a titer of 5180 TCID50/mL by the method of Reed and Muench (59). Systemic infection was monitored weekly for 24 weeks by detection of SHIV RNA in plasma using real-time RT-PCR assay with a sensitivity of 40 SHIV RNA copies/mL plasma as previously described (11). PBMC proviral DNA amplification was performed as previously described (11). Five replicates of each sample (0.6 to 1 μg/replicate) were analyzed. Virus-specific antibody responses were measured using a synthetic-peptide enzyme immunoassay as per the manufacturer’s instructions (Genetic Systems HIV-1/HIV-2 Plus O; Bio-Rad). The Institutional Animal Care and Use Committee (IACUC) of Tulane National Primate Research Center (TNPRC) approved all studies. TNPRC is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC # 000594). TNPRC’s OLAW animal welfare assurance number is A4499-01 and USDA registration number is 72-R-0002.
Analysis of GSK744 concentrations in plasma and tissues
Whole blood was collected using K2EDTA tubes, centrifuged to obtain plasma and stored at −80°C until analysis. Tissues were rinsed in saline (unless indicated), blotted dry, weighed, snap frozen and stored at −80°C until analysis. GSK744 concentrations were monitored by HPLC-MS/MS following protein precipitation with acetonitrile containing [13C15N2H2] GSK744 as an internal standard as previously described (11). The calibration range for GSK744 was 10 to 10,000 ng/mL for plasma or 2.5 to 1000 ng/mL for tissues. PK analyses were performed using WinNonlin Professional software (version 5.2; Pharsight Corp, Mountain View, CA) as previously described (11).
Integrase sequence analysis
Consensus integrase sequences were generated as previously described (11). Briefly, integrase-coding sequences were amplified from cell-free virus using Phusion Hot Start Flex DNA Polymerase (NEB) as per the manufacturer’s instructions with primers, macIN.F1 and macIN.R1. The product was used for nested PCR with primers, macIN.F3 and macIN.R3. Second round PCR products were sequenced by Genewiz Inc. using primers macIN.Fseq and macIN.Rseq, and sequence analysis was performed using Geneious software (version 7.1.4). The same procedure was followed for single genome analysis using cDNA diluted to provide ≤30% positive reactions representing sequences derived from a single cDNA molecule. Second round PCR products were sequenced by Genewiz Inc. using second round primers and sequencing primers.
Construction of single-cycle SIV recombinant viruses and determination of susceptibility to GSK744
To determine the susceptibility of selected viral variants to GSK744, a novel assay was developed to first, create single-cycle recombinant viruses with the SIV backbone and then, to measure drug susceptibility in vitro. Inactivation of Env expression and deletion of the integrase-coding region in full-length SIVmac239 provirus (239-FL SpX, kindly provided by Ronald Desrosiers) was achieved by ligating its two PCR products, a short fragment (1,610 bp) and a long fragment (10,196 bp). The short fragment was produced by PCR with primers F.dIN 5′-CTA GTT AGT CAA GGG CCC CAT TTT AAG GTC GGA TG-3′ (4499–4513 fused with 5466–5485 in SIVmac239) and 239.1R 5′-ACA AGA ACA TGC TAG TCT CAT TGA CCA T-3′ (7056–7034). The long fragment was produced by PCR with primers 239.2F 5′-TGA GAC TAG CAT GTT CTT GTA TAG CCC AGG-3′ (7041–7066) and R.dIN 5′-CCC TTG ACT AAC TAG GTG GTC TAT TTC TT-3′ (4513–4485), with the addition of CATG at the SpeI site within env, as underlined, introducing a stop codon resulting in a truncated env protein. The two fragments were ligated using Gibson Assembly Master Mix (NEB) and transformed into STBL2 cells (Life Technologies). A clone, Gi-3 was obtained, and used to generate single-cycle recombinant viral variants with an SIV backbone.
Pseudotyped virus with the desired integrase-coding region was obtained by first linearizing Gi-3 by digestion with PspOM I. The second round PCR product from a limiting dilution nested PCR assay generating cDNA of the integrase-coding region as detailed above was either re-amplified with the same primer pair of macIN.F3 and macIN.R3 (product size of 1,053 bp), or amplified with a primer pair of macIN.F4 5′-CTA GTT AGT CAA GGG ATT AGA CAA GTT CTC TTC-3′ (4499–4531) and macIN.R4 5′-GAC CTT AAA ATG GGG CAC ATA GCA AAC-3′(5480–5454) (982 bp), which overlap with linearized Gi-3 by 45 or 54 bp and 15 or 13 bp at the 5′ or 3′ end, respectively. Gel-purified PCR products were ligated with linear Gi-3 using Gibson Assembly Master Mix, and the resultant recombinants and pCl-VSV-G were co-transfected onto HEK239T cells using jetPRIME (Polyplus transfection, VWR). Culture supernatants were collected 48 hours after transfection, filtered, and aliquots were stored at −150°C for subsequent use. p27 concentrations of viral stocks were measured using RETROtek, SIV p27 Antigen ELISA (ZeptoMetrix)
Sensitivity to GSK744 was assessed using TZM-bl cells (1 x 104/well) plated overnight at in a 96-well plate. Cells were incubated with 20 μg of polybrene (Sigma-Aldrich) in 100 μl DMEM containing 10% FCS for 30 min at 37°C, and then incubated with either no drug or serially diluted GSK744 for 30 min at 37°C followed by the addition of pseudotyped virus (5 ng p27/per well). After 48 hours, β-galactosidase expression was measured using Galacto-Star as per the manufacturer’s instructions (Applied Biosystems). The light signal was measured using MLX microtiter Luminometer (Dynex Technologies). All assays were performed in triplicate, and the results were analyzed using GraFit 5 (Erithacus Software) with 4-parameter fitting. The GSK744 concentration inhibiting 90% luminescence (IC90) was calculated as the concentration of GSK744 that inhibited 50% luminescence (IC50) x 91/s, where ‘s’ is the slope of the fitted curve.
Single-genome analysis for T/F analysis
Viral RNA was extracted as described above. cDNA was generated as previously described using primer 11-SIVmac239-R1 (5′-CTG TAA TAA ATC CCT TCC AGT CC-3′) (26). cDNA was serially diluted and plated for nested PCR reactions amplifying the 3.1 kb env as described using primers 7-F1-SIVsm/macTatF1 (5′-CCT CCC CCT CCA GGA CTA GC-3′) and 11-SIVmac239-R1 for the first round and primers 5-R2-BKSIVsm/macEnvR261 (5′-ATG AGA CAT RTC TAT TGC CAA TTT GTA-3′) and 6-F2-envB5in (5′-TTA GGC ATC TCC TAT GGC AGG AAG AAG-3′) for the second round. cDNA dilutions were identified yielding ≤30% positive reactions (26). PCR products were sequenced by Genewiz Inc. using primers Rev14 (5′-ACC ATG TTA TTT TTC CAC ATG TTA AA-3′), For13SHIV (5′-CAG AAA GAG CAG AAG ACA GTG G-3′), Rev1200 (5′-GTG CTT GTC TTA TAT CTC CTA TTA TTT CTC-3′), For15 (5′-CAG CAC AGT ACA ATG TAC ACA TGG AA), For16SHIV (5′-TTT AAT TGT GGA GGG GAA TTT TTC TA-3′), For17 (5′-AGC AGC AGG AAG CAC TAT GGG CGC-3′), For18Bal (5′-CAT AAC AAA ATG GCT GTG GTA TAT-3′), For19Bal (5′-GGA GCC TGT TCC TCT TCA GCT ACC-3′). Single sequences from each sample were aligned using Geneious software. MEGA (version 5.0) predicted the best model of nucleotide substitution for env sequences from each animal was Hasegawa, Kishino and Yano. Phylogenetic trees were inferred by maximum likelihood analyses with 500 bootstrap replicates and rooted with SHIV162P3 env consensus sequence. Viral diversity was calculated by maximum composite likelihood methods.
Statistical Methods
PK parameters and tissue processing methods were compared using Mann-Whitney two-tailed t-test. The log-rank test was used to discern statistical differences between GSK744 LA-treated and control macaques. The hazard ratio was estimated by the log-rank model. All statistical analyses were performed using GraphPad Prism software (version 6.0).
Supplementary Material
Fig. S1. Plasma PK of individual GSK744 LA-treated rhesus macaques throughout the PK study.
Fig. S2. Correlation of rectal tissue GSK744 concentrations by processing method prior to freezing.
Fig. S3. Correlation of cervical tissue GSK744 concentrations from individual samples.
Fig. S4. GSK744 plasma concentrations from Depo-Provera-treated rhesus macaques compared with humans.
Fig. S5. Consensus sequence analysis of SHIV integrase-coding regions from plasma of infected GSK744 LA-treated macaques.
Fig. S6. Minimum number of T/F variants was estimated from plasma collected within one week of detection of viremia.
Table S1. Summary of time of detection for plasma viral RNA (vRNA), proviral DNA, and anti-SHIV antibodies (Ab).
Table S2. Susceptibility of single-cycle recombinant viruses with the integrase-coding regions of SHIV162P3 viral stock, FM26, FG95 and mutants to GSK744
Table S3. Env single-genome analysis summary.
Accessible Summary.
Taking a shot at HIV
HIV-1 transmission during vaginal intercourse remains a major public health issue. Antiretrovirals can prevent HIV-1 transmission when used as pre-exposure prophylaxis, but results are highly dependent on patient adherence to prescribed therapy. One possible solution to this limitation is the use of long-acting injectable agents. We used an animal model that simulates the heterosexual transmission of HIV-1 to women and found that GSK744 LA, a long-acting integrase inhibitor amenable to dosing every 3 months in humans, is highly protective against viral transmission. These results support further clinical trials in women at risk for HIV-1 infection.
Acknowledgments
We thank George Shaw and Ranjit Warrier for helpful discussions.
Funding: Supported by NIH grants R0-AI100724 and the Tulane National Primate Research Center grant 2P51-OD11104–52.
Footnotes
Author contributions: C.D.A., W.R.S., C.C.M., Z.H., D.D.H., and M.M. designed experiments. Y.Y. performed drug analyses. A.G., K.R.L and J.B. executed the macaque studies. C.D.A and H.M. performed viral loads, resistance sequencing, and statistical analyses. C.D.A and M.B.C. performed proviral DNA analyses. C.D.A, L.S., and K.R. performed T/F analyses. S.F. performed PK analyses. C.D.A and M.M. wrote the manuscript.
Competing interests: W.R.S., Y.Y., and S.F. are full time employees of, and hold shares in GlaxoSmithKline, Z.H. is a full time employee of, and holds shares in GlaxoSmithKline and serves on the ViiV Healthcare Board, D.D.H. is a paid consultant to GlaxoSmithKline and M.M. receives grants from GlaxoSmithKline.
Data and materials availability: The sequences can be found at GenBank KM889673-KM88910, KP127968-KP127972.
References and Notes
- 1.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapia M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernandez T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallas EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang F, McConnell JJ, Zheng JH, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, Ronald A, Tumwesigye E, Were E, Fife KH, Kiarie J, Farquhar C, John-Stewart G, Kakia A, Odoyo J, Mucunguzi A, Nakku-Joloba E, Twesigye R, Ngure K, Apaka C, Tamooh H, Gabona F, Mujugira A, Panteleeff D, Thomas KK, Kidoguchi L, Krows M, Revall J, Morrison S, Haugen H, Emmanuel-Ogier M, Ondrejcek L, Coombs RW, Frenkel L, Hendrix C, Bumpus NN, Bangsberg D, Haberer JE, Stevens WS, Lingappa JR, Celum C. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, Henderson FL, Pathak SR, Soud FA, Chillag KL, Mutanhaurwa R, Chirwa LI, Kasonde M, Abebe D, Buliva E, Gvetadze RJ, Johnson S, Sukalac T, Thomas VT, Hart C, Johnson JA, Malotte CK, Hendrix CW, Brooks JT. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 4.Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, Chiamwongpaet S, Kitisin P, Natrujirote P, Kittimunkong S, Chuachoowong R, Gvetadze RJ, McNicholl JM, Paxton LA, Curlin ME, Hendrix CW, Vanichseni S G Bangkok Tenofovir Study. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 5.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, Malahleha M, Owino F, Manongi R, Onyango J, Temu L, Monedi MC, Mak’Oketch P, Makanda M, Reblin I, Makatu SE, Saylor L, Kiernan H, Kirkendale S, Wong C, Grant R, Kashuba A, Nanda K, Mandala J, Fransen K, Deese J, Crucitti T, Mastro TD, Taylor D. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marrazzo J, Ramjee G, Nair G, Palanee T, Mkhize B, Nakabiito C, Taljaard M, Piper J, Gomez Feliciano K, Chirenje aM, Team VS. Conference on Retroviruses and Opportunistic Infections; Atlanta, GA. 2013. [Google Scholar]
- 8.Spreen WR, Margolis DA, Pottage JC., Jr Long-acting injectable antiretrovirals for HIV treatment and prevention. Current opinion in HIV and AIDS. 2013;8:565–571. doi: 10.1097/COH.0000000000000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spreen W, Williams P, Margolis D, Ford S, Crauwels H, Lou Y, Gould E, Stevens M, Piscitelli S. paper presented at the International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; Kuala Lumpur, Malaysia. 2013. [Google Scholar]
- 10.Spreen W, Min S, Ford SL, Chen S, Lou Y, Bomar M, St Clair M, Piscitelli S, Fujiwara T. Pharmacokinetics, Safety, and Monotherapy Antiviral Activity of GSK1265744, an HIV Integrase Strand Transfer Inhibitor. HIV Clin Trials. 2013;14:192–203. doi: 10.1310/hct1405-192. [DOI] [PubMed] [Google Scholar]
- 11.Andrews CD, Spreen WR, Mohri H, Moss L, Ford S, Russell-Lodrigue K, Bohm RP, Cheng-Mayer C, Hong Z, Markowitz M, Ho DD. Long-Acting Integrase Inhibitor Protects Macaques from Intrarectal Simian/Human Immunodeficiency Virus. Science. 2014 doi: 10.1126/science.1248707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subbarao S, Otten RA, Ramos A, Kim C, Jackson E, Monsour M, Adams DR, Bashirian S, Johnson J, Soriano V, Rendon A, Hudgens MG, Butera S, Janssen R, Paxton L, Greenberg AE, Folks TM. Chemoprophylaxis with tenofovir disoproxil fumarate provided partial protection against infection with simian human immunodeficiency virus in macaques given multiple virus challenges. J Infect Dis. 2006;194:904–911. doi: 10.1086/507306. [DOI] [PubMed] [Google Scholar]
- 13.Subbarao S, Ramos A, Kim C, Adams D, Monsour M, Butera S, Folks T, Otten RA. Direct stringency comparison of two macaque models (single-high vs. repeat-low) for mucosal HIV transmission using an identical anti-HIV chemoprophylaxis intervention. J Med Primatol. 2007;36:238–243. doi: 10.1111/j.1600-0684.2007.00241.x. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Lerma JG, Otten RA, Qari SH, Jackson E, Cong ME, Masciotra S, Luo W, Kim C, Adams DR, Monsour M, Lipscomb J, Johnson JA, Delinsky D, Schinazi RF, Janssen R, Folks TM, Heneine W. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS medicine. 2008;5:e28. doi: 10.1371/journal.pmed.0050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, Parren PW, Marx PA, Burton DR. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009;15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.UNAIDS. 2013 UNAIDS Report on the Global AIDS Epidemic. 2013. [Google Scholar]
- 17.Ma Z, Lu FX, Torten M, Miller CJ. The number and distribution of immune cells in the cervicovaginal mucosa remain constant throughout the menstrual cycle of rhesus macaques. Clin Immunol. 2001;100:240–249. doi: 10.1006/clim.2001.5058. [DOI] [PubMed] [Google Scholar]
- 18.Sodora DL, Gettie A, Miller CJ, Marx PA. Vaginal transmission of SIV: assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res Hum Retroviruses. 1998;14(Suppl 1):S119–123. [PubMed] [Google Scholar]
- 19.Parakkal PF, Gregoire AT. Differentiation of vaginal epithelium in the normal and hormone-treated rhesus monkey. Biol Reprod. 1972;6:117–130. doi: 10.1093/biolreprod/6.1.117. [DOI] [PubMed] [Google Scholar]
- 20.Marx PA, Spira AI, Gettie A, Dailey PJ, Veazey RS, Lackner AA, Mahoney CJ, Miller CJ, Claypool LE, Ho DD, Alexander NJ. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2:1084–1089. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 21.Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, Ketas T, Marx PA, Klasse PJ, Burton DR, Moore JP. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 22.Veazey RS, Klasse PJ, Ketas TJ, Reeves JD, Piatak M, Jr, Kunstman K, Kuhmann SE, Marx PA, Lifson JD, Dufour J, Mefford M, Pandrea I, Wolinsky SM, Doms RW, DeMartino JA, Siciliano SJ, Lyons K, Springer MS, Moore JP. Use of a small molecule CCR5 inhibitor in macaques to treat simian immunodeficiency virus infection or prevent simian-human immunodeficiency virus infection. J Exp Med. 2003;198:1551–1562. doi: 10.1084/jem.20031266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veazey RS, Springer MS, Marx PA, Dufour J, Klasse PJ, Moore JP. Protection of macaques from vaginal SHIV challenge by an orally delivered CCR5 inhibitor. Nat Med. 2005;11:1293–1294. doi: 10.1038/nm1321. [DOI] [PubMed] [Google Scholar]
- 24.Burton DR, Hessell AJ, Keele BF, Klasse PJ, Ketas TA, Moldt B, Dunlop DC, Poignard P, Doyle LA, Cavacini L, Veazey RS, Moore JP. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc Natl Acad Sci U S A. 2011;108:11181–11186. doi: 10.1073/pnas.1103012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barouch DH, Klasse PJ, Dufour J, Veazey RS, Moore JP. Macaque studies of vaccine and microbicide combinations for preventing HIV-1 sexual transmission. Proc Natl Acad Sci U S A. 2012;109:8694–8698. doi: 10.1073/pnas.1203183109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein K, Veazey RS, Warrier R, Hraber P, Doyle-Meyers LA, Buffa V, Liao HX, Haynes BF, Shaw GM, Shattock RJ. Neutralizing IgG at the portal of infection mediates protection against vaginal simian/human immunodeficiency virus challenge. J Virol. 2013;87:11604–11616. doi: 10.1128/JVI.01361-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lederman MM, Veazey RS, Offord R, Mosier DE, Dufour J, Mefford M, Piatak M, Jr, Lifson JD, Salkowitz JR, Rodriguez B, Blauvelt A, Hartley O. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science. 2004;306:485–487. doi: 10.1126/science.1099288. [DOI] [PubMed] [Google Scholar]
- 28.Veazey RS, Klasse PJ, Schader SM, Hu Q, Ketas TJ, Lu M, Marx PA, Dufour J, Colonno RJ, Shattock RJ, Springer MS, Moore JP. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005;438:99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- 29.Veazey RS, Ketas TA, Klasse PJ, Davison DK, Singletary M, Green LC, Greenberg ML, Moore JP. Tropism-independent protection of macaques against vaginal transmission of three SHIVs by the HIV-1 fusion inhibitor T-1249. Proc Natl Acad Sci U S A. 2008;105:10531–10536. doi: 10.1073/pnas.0802666105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veazey RS, Ling B, Green LC, Ribka EP, Lifson JD, Piatak M, Jr, Lederman MM, Mosier D, Offord R, Hartley O. Topically applied recombinant chemokine analogues fully protect macaques from vaginal simian-human immunodeficiency virus challenge. J Infect Dis. 2009;199:1525–1527. doi: 10.1086/598685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veazey RS, Ketas TJ, Dufour J, Moroney-Rasmussen T, Green LC, Klasse PJ, Moore JP. Protection of rhesus macaques from vaginal infection by vaginally delivered maraviroc, an inhibitor of HIV-1 entry via the CCR5 co-receptor. J Infect Dis. 2010;202:739–744. doi: 10.1086/655661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otten RA, Adams DR, Kim CN, Jackson E, Pullium JK, Lee K, Grohskopf LA, Monsour M, Butera S, Folks TM. Multiple vaginal exposures to low doses of R5 simian-human immunodeficiency virus: strategy to study HIV preclinical interventions in nonhuman primates. J Infect Dis. 2005;191:164–173. doi: 10.1086/426452. [DOI] [PubMed] [Google Scholar]
- 33.Kim CN, Adams DR, Bashirian S, Butera S, Folks TM, Otten RA. Repetitive exposures with simian/human immunodeficiency viruses: strategy to study HIV pre-clinical interventions in non-human primates. J Med Primatol. 2006;35:210–216. doi: 10.1111/j.1600-0684.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 34.McNicholl JM, Henning TC, Vishwanathan SA, Kersh EN. Non-Human Primate Models of Hormonal Contraception and HIV. Am J Reprod Immunol. 2014 doi: 10.1111/aji.12246. [DOI] [PubMed] [Google Scholar]
- 35.Radzio J, Spreen W, Yueh Y, Mitchell J, Jenkins L, Garcia-Lerma JG, Heneine W. Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2014. [Google Scholar]
- 36.Dumond JB, Yeh RF, Patterson KB, Corbett AH, Jung BH, Rezk NL, Bridges AS, Stewart PW, Cohen MS, Kashuba AD. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. AIDS. 2007;21:1899–1907. doi: 10.1097/QAD.0b013e328270385a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicol MR, Kashuba AD. Pharmacologic opportunities for HIV prevention. Clin Pharmacol Ther. 2010;88:598–609. doi: 10.1038/clpt.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown KC, Patterson KB, Malone SA, Shaheen NJ, Prince HM, Dumond JB, Spacek MB, Heidt PE, Cohen MS, Kashuba AD. Single and multiple dose pharmacokinetics of maraviroc in saliva, semen, and rectal tissue of healthy HIV-negative men. J Infect Dis. 2011;203:1484–1490. doi: 10.1093/infdis/jir059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dumond JB, Patterson KB, Pecha AL, Werner RE, Andrews E, Damle B, Tressler R, Worsley J, Kashuba AD. Maraviroc concentrates in the cervicovaginal fluid and vaginal tissue of HIV-negative women. J Acquir Immune Defic Syndr. 2009;51:546–553. doi: 10.1097/QAI.0b013e3181ae69c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsibris AM, Pal U, Schure AL, Veazey RS, Kunstman KJ, Henrich TJ, Klasse PJ, Wolinsky SM, Kuritzkes DR, Moore JP. SHIV-162P3 infection of rhesus macaques given maraviroc gel vaginally does not involve resistant viruses. PloS one. 2011;6:e28047. doi: 10.1371/journal.pone.0028047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Keele BF, Li H, Keating S, Norris PJ, Carville A, Mansfield KG, Tomaras GD, Haynes BF, Kolodkin-Gal D, Letvin NL, Hahn BH, Shaw GM, Barouch DH. Low-dose mucosal simian immunodeficiency virus infection restricts early replication kinetics and transmitted virus variants in rhesus monkeys. J Virol. 2010;84:10406–10412. doi: 10.1128/JVI.01155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reese M, Ford S, Bowers G, Humphreys J, Webster L, Gould E, Polli J, Generaux G, Johnson M, Clark P, Watson C, Lou Y, Piscitelli S. International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy; Washington, DC. 2014. [Google Scholar]
- 44.Jeong H, Choi S, Song JW, Chen H, Fischer JH. Regulation of UDP-glucuronosyltransferase (UGT) 1A1 by progesterone and its impact on labetalol elimination. Xenobiotica. 2008;38:62–75. doi: 10.1080/00498250701744633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radzio J, Hanley K, Mitchell J, Ellis S, Deyounks F, Jenkins LT, Hanson D, Heneine W, Garcia-Lerma JG. Physiologic doses of depot-medroxyprogesterone acetate do not increase acute plasma simian HIV viremia or mucosal virus shedding in pigtail macaques. AIDS. 2014;28:1431–1439. doi: 10.1097/QAD.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 46.Spreen W, Ford SL, Chen S, Wilfret D, Margolis D, Gould E, Piscitelli S. GSK1265744 Pharmacokinetics in Plasma and Tissue Following Single-Dose Long-Acting (LA) Injectable Administration in Healthy Subjects. J Acquir Immune Defic Syndr. 2014 doi: 10.1097/QAI.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 47.Malcolm RK, Veazey RS, Geer L, Lowry D, Fetherston SM, Murphy DJ, Boyd P, Major I, Shattock RJ, Klasse PJ, Doyle LA, Rasmussen KK, Goldman L, Ketas TJ, Moore JP. Sustained release of the CCR5 inhibitors CMPD167 and maraviroc from vaginal rings in rhesus macaques. Antimicrob Agents Chemother. 2012;56:2251–2258. doi: 10.1128/AAC.05810-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, Kiwanuka N, Kigozi G, Kiddugavu M, Lutalo T, Nalugoda F, Wabwire-Mangen F, Meehan MP, Quinn TC. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 49.Pilcher CD, Tien HC, Eron JJ, Jr, Vernazza PL, Leu SY, Stewart PW, Goh LE, Cohen MS, Quest S, Duke C UN CE AH IV. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis. 2004;189:1785–1792. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]
- 50.Hel Z, Stringer E, Mestecky J. Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocr Rev. 2010;31:79–97. doi: 10.1210/er.2009-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quinn TC, Overbaugh J. HIV/AIDS in women: an expanding epidemic. Science. 2005;308:1582–1583. doi: 10.1126/science.1112489. [DOI] [PubMed] [Google Scholar]
- 52.Vishwanathan SA, Guenthner PC, Lin CY, Dobard C, Sharma S, Adams DR, Otten RA, Heneine W, Hendry RM, McNicholl JM, Kersh EN. High susceptibility to repeated, low-dose vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J Acquir Immune Defic Syndr. 2011;57:261–264. doi: 10.1097/QAI.0b013e318220ebd3. [DOI] [PubMed] [Google Scholar]
- 53.Stone M, Keele BF, Ma ZM, Bailes E, Dutra J, Hahn BH, Shaw GM, Miller CJ. A limited number of simian immunodeficiency virus (SIV) env variants are transmitted to rhesus macaques vaginally inoculated with SIVmac251. J Virol. 2010;84:7083–7095. doi: 10.1128/JVI.00481-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winner B, Peipert JF, Zhao Q, Buckel C, Madden T, Allsworth JE, Secura GM. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366:1998–2007. doi: 10.1056/NEJMoa1110855. [DOI] [PubMed] [Google Scholar]
- 55.Rossi G, Frediani S, Rossi R, Rossi A. Long-acting antipsychotic drugs for the treatment of schizophrenia: use in daily practice from naturalistic observations. BMC psychiatry. 2012;12:122. doi: 10.1186/1471-244X-12-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giagulli VA, Triggiani V, Corona G, Carbone D, Licchelli B, Tafaro E, Resta F, Sabba C, Maggi M, Guastamacchia E. Evidence-based medicine update on testosterone replacement therapy (TRT) in male hypogonadism: focus on new formulations. Curr Pharm Des. 2011;17:1500–1511. doi: 10.2174/138161211796197160. [DOI] [PubMed] [Google Scholar]
- 57.Harouse JM, Gettie A, Eshetu T, Tan RC, Bohm R, Blanchard J, Baskin G, Cheng-Mayer C. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIV(SF162P3) J Virol. 2001;75:1990–1995. doi: 10.1128/JVI.75.4.1990-1995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harouse JM, Gettie A, Tan RC, Blanchard J, Cheng-Mayer C. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science. 1999;284:816–819. doi: 10.1126/science.284.5415.816. [DOI] [PubMed] [Google Scholar]
- 59.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Plasma PK of individual GSK744 LA-treated rhesus macaques throughout the PK study.
Fig. S2. Correlation of rectal tissue GSK744 concentrations by processing method prior to freezing.
Fig. S3. Correlation of cervical tissue GSK744 concentrations from individual samples.
Fig. S4. GSK744 plasma concentrations from Depo-Provera-treated rhesus macaques compared with humans.
Fig. S5. Consensus sequence analysis of SHIV integrase-coding regions from plasma of infected GSK744 LA-treated macaques.
Fig. S6. Minimum number of T/F variants was estimated from plasma collected within one week of detection of viremia.
Table S1. Summary of time of detection for plasma viral RNA (vRNA), proviral DNA, and anti-SHIV antibodies (Ab).
Table S2. Susceptibility of single-cycle recombinant viruses with the integrase-coding regions of SHIV162P3 viral stock, FM26, FG95 and mutants to GSK744
Table S3. Env single-genome analysis summary.