Abstract
Autoimmune diseases are a major cause of morbidity, and their incidence and prevalence continue to rise. Treatments for these diseases are non-specific and result in significant adverse effects. Targeted therapies may help in improving the risk : benefit ratio associated with treatment. Immunological memory is an important feature of the vertebrate immune system that results in the production of cells that are long-lived and able to respond to antigens in a more robust manner. In the setting of autoimmunity this characteristic becomes detrimental due to the ongoing response to a self-antigen(s). These memory cells have been shown to play key roles in various autoimmune diseases such as type 1 diabetes, multiple sclerosis and psoriasis. Memory T cells and B cells can be identified based on various molecules expressed on their surface. Memory T cells can be divided into three main categories – central memory, effector memory and resident memory cells. These subsets have different proliferative potential and cytokine-producing abilities. Utilizing differentially expressed surface molecules or downstream signalling pathway proteins in these cells it is now possible to target memory cells while sparing naive cells. We will discuss the various available options for such a strategy and several potential strategies that may yield successful therapies in the future.
Keywords: autoimmune disease, Immunological memory, memory T cell, memory B cell, targeted therapy
Introduction
The prevalence and incidence of autoimmune disease continues to rise across the globe 1–3. Several of these diseases affect individuals in young or middle age, and thus have major societal costs in addition to the high level of individual morbidity 4. Treatments for autoimmune disorders have largely been non-specific and involved immunosuppression targeting the adaptive immune system. While effective, these broad-spectrum approaches led to significant adverse effects, including increased risk of infections and toxicity to non-immune cells. More specific therapies targeting individual cell populations could lead potentially to reductions in these adverse effects. Refining the targets to include cells that are pathogenic, while sparing other components of the adaptive immune system, could lead to a more acceptable risk : benefit ratio.
Immunological memory is an important feature of the adaptive immune system that allows it to mount an intensified immune response to a previously recognized antigen, and plays an important role in the defence against infectious pathogens 5. This particular attribute may, however, be an effective target in autoimmune disorders, in which the ability of the immune system to recognize an autoantigen more readily would be detrimental. We will explore briefly the generation and characteristics of memory cells and the importance of memory cells in autoimmune disease and will then address the current and possible future methods of targeting these cells.
T cell memory
Generation of memory T cells
There are two leading theories regarding the generation of memory cells: linear and divergent 6. In the linear model, the encounter of an antigen-specific naive CD4+ or CD8+ T cell (Tnaive) can lead to activation followed by proliferation and differentiation into cytokine-producing effector T cells (Teff) 7. Following the acute response, the majority of these cells undergo apoptosis in a contraction phase with a small proportion persisting and differentiating into memory T cells (Tmem). In the divergent model, activated Tnaive cells can differentiate directly into the memory phenotype bypassing the Teff phase 8. While the production of both effector and memory cells from the asymmetrical division of T cells has been demonstrated unequivocally, the extent to which this process leads to the production of the memory cell population is uncertain (Fig. 1) 9. Recent studies provide evidence in favour of a model in which Tnaive cells can heterogeneously produce different Tmem cell subsets 10,11. This may depend upon different factors, such as the strength of T cell receptor (TCR) stimulation, dendritic cell (DC) interactions and cytokine signalling 12–14. These memory cell subsets serve as precursors for Teff cells. The precise mechanisms governing differentiation into a particular memory cell subset are unknown.
Fig 1.
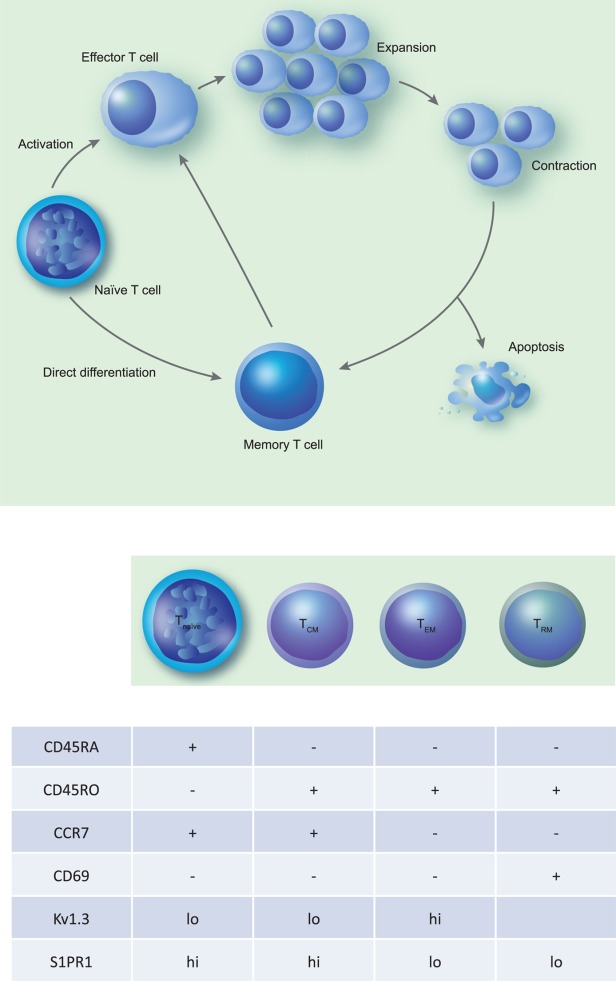
Generation of memory T cells and common surface markers of various subtypes of memory T cells. (a) The process of generation of memory T cells. Naive T cells undergo activation following engagement of the T cell receptor (TCR) and appropriate co-stimulatory signals, subsequently undergoing proliferation and differentiation into effector T cells. Later in the immune response effector T cells undergo a contraction phase resulting in either apoptosis or differentiation into long-lived memory T cells. Additional pathways for generation of memory cells include direct differentiation of memory T cells from naïve cells and production of effector and memory T cells from naive cells by asymmetric cell division. (b) The common surface markers in tabular form used to identify the naive and memory T cells such as CD45RA, CD45RO, CCR7 and CD69. In addition, it lists some other markers that are expressed differentially on these cell populations and could potentially be used to target specific memory T cell populations.
Subsets of memory T cells
Memory T cells are identified by the expression of the marker CD45RO and absence of CD45RA (found on Tnaive cells) 15,16. In addition, compared to Tnaive cells they also have increased expression of CD2, CD11a and CD44 15. They are long-lived and proliferate in a more rapid fashion when exposed to antigen, differentiating to give rise to cytokine-producing Teff cells. Three major subtypes of Tmem cells are now recognized: central memory cells (TCM), effector memory cells (TEM) and resident memory cells (TRM) 17,18.
TCM cells are CCR7+ and CD62Lhi and retain their ability to circulate to secondary lymphoid tissues 18. These cells have a greater proliferative capacity and are longer-lived. They are thought to be important for production of Teff cells following antigen recall 19,20. By virtue of being CCR7− and CD62Llo, TEM cells circulate to peripheral non-lymphoid tissues and provide immediate effector functions. They are thought to be shorter-lived than TCM cells, have lower proliferate capacity but greater cytokine production 19,20. A more recently described class of memory cells is the TRM subset. Similar to TEM, these cells are CCR7−, but express additional markers CD69 with or without CD103 21. TRM subsets of CD4+ and CD8+ T cells were described initially in mice and were identified subsequently in humans 17,22–25. These cells are localized within tissues and do not circulate to the bloodstream. Their functions are highly dependent upon the tissue in which they are found 12. The role that these cells might play in organ-specific autoimmune disorders is being gradually clarified.
Cytokines important for memory cell production and survival
Studies of the generation of CD4+ memory cells have revealed that interleukin (IL)-7 plays an important role in the formation of memory CD4+ cells 14. Resting T cells express IL-7R and down-regulate this when they are activated. Teff cells that are destined to become memory cells will up-regulate IL-7R. The role of IL-7 was clarified by the lack of a memory response in IL7–/– hosts and in mice with mutations of the IL-7R alpha gene 26,27. IL-15 has been shown to be important for the production of Tmem cells 28–30. Both IL-7 and IL-15 play an important role in the survival of CD4+ and CD8+ memory cells, with IL-7 being more important for CD4+ cells 31,32. In addition to these cytokines, transforming growth factor (TGF)-β is another cytokine that has been found to play an important role in the development of CD8+ TRM in various tissues 23,33. TGF-β increases the expression of CD103, a marker that is characteristic for TRM cells, and blockade of TGF signalling can lead to reduced production of TRM cells. These various cytokines may serve as therapeutic targets for autoimmune memory cells.
Metabolism and T cell phenotype
In recent years, differences in the metabolism of T cells based on their function have been noted. Teff cells utilize glycolysis as a means of production of adenosine triphosphate (ATP) even in the presence of sufficient oxygen, and one potential role of this metabolic-switch in facilitating cytokine production was elucidated recently 34. Conversely, CD8+ and CD4+ Tmem cells have been demonstrated to prefer fatty acid oxidation (FAO) as their means of ATP production while suppressing glycolysis 35,36. These distinct metabolic profiles of Teff and Tmem cells may enable targeting of specific metabolic pathways that are important for the survival and function of memory cells.
Memory cells are important in autoimmune disease
The role of memory cells in various autoimmune diseases has been explored extensively. We will briefly discuss the role as studied in multiple sclerosis (MS), an inflammatory demyelinating disease involving the central nervous system 37.
Studies have shown that memory cells are important in the pathogenesis of MS. A study in paediatric MS showed that patients had a higher percentage of memory T cells similar to that in 20–30-year-older healthy controls 38. Studies in adult MS subjects have demonstrated increased frequencies of Tmem cells, especially TEM cells 39–42. Evidence for the importance of these cells also comes from studies demonstrating that memory cells are enriched in the cerebrospinal fluid (CSF) of patients with MS, and the majority of T cells found in parenchymal MS lesions are TEM cells 43–45.
Similarly, other diseases have been shown to demonstrate changes in the proportion of memory cells in the peripheral circulation as well as the target tissues 46,47.
Markers for pathogenic memory T cells
Because autoimmune disorders are often marked by an ongoing exposure of autoimmune memory cells to the offending antigen, Niesner et al. studied the transcriptomes of repeatedly activated T helper type 1 (Th1) cells and found that the expression of the Twist1 gene was up-regulated and increased incrementally with each restimulation 48. Twist1 has been shown to be a regulator of Th1 cell cytokine production and inhibition of Twist1 function leads to exaggeration of Th1-mediated inflammation. T cells isolated from tissues with rheumatoid arthritis and IBD patients showed extremely high levels of Twist1 expression, providing further support for this as a biomarker for pathogenic memory T cells 49. The identification of other markers defining pathogenic memory cells will help in developing more targeted therapeutic approaches.
Strategies to target memory T cells
Targeting memory T cell surface markers. (a) Alefacept: alefacept is a dimeric fusion protein, consisting of the CD2 binding portion of the leucocyte function-associated antigen-3 (LFA-3) and the Fc portion of human immunoglobulin G, which was initially approved by the Food and Drug Administration (FDA) for treatment of psoriasis 50. CD58 or LFA3 is expressed on antigen-presenting cells (APCs) and is the endogenous ligand for CD2 leading to T cell stimulation 51. As CD2 is expressed highly on Tmem cells, with levels being highest on TEM cells and slightly lower on TCM cells, it was felt that Alefacept would target these cells selectively. Alefacept disrupts the CD2–CD58 interaction and results in depletion of TEM cells and, to a lesser extent, TCM cells in trials in psoriasis and type 1 DM 52,53. In patients with psoriasis alefacept reduced TEM cells numbers in diseased skin as well as in peripheral blood 54. Studies in non-human primates have shown that alefacept can target co-stimulatory blockade resistant TEM cells, which are an important player in autoimmunity 55.
(b) Cytokine signalling blockade: as IL-15 and IL-7 are important in the production and survival of TEM and TCM cells, blockade of signalling though their cytokine receptors could serve as an effective strategy to target memory cells. Janus kinases (Jak) are cytoplasmic tyrosine kinases that play a role in intracellular signalling downstream of type I cytokines such as IL-2, IL-7 and IL-15 56.
Tofacitinib is a potent inhibitor of Jak 1and Jak 3 that has shown efficacy in the treatment of rheumatoid arthritis 57. Tofacitinib has shown efficacy in preventing rejection of transplants and its effect may be related to blocking IL-15 signalling 58. Further studies are required to determine the effect on Tmem cell subsets in subjects treated with tofacitinib.
Ruxolitinib is an inhibitor of Jak 1 and 2 and has been proposed as a possible means of disrupting IL-15 signalling in Tmem cells 59. In a study by Xing et al., the importance of cytotoxic CD8+ TEM phenotype cells in the pathogenesis of alopecia areata was demonstrated 60. The authors then demonstrated the efficacy of Jak 1 and 2 inhibition in an animal model of alopecia areata, and finally the improvement of alopecia areata in patients who were treated with ruxolitinib. Further studies would be required to clearly define changes in the memory cell population in subjects treated with this medication.
(c) S1P receptor modulators: S1P signalling plays a major role in the egress of T cells from lymphoid tissue in concert with other cellular surface molecules such as CCR7, CD69 and CD62L 61. Initial studies demonstrated marked depletion of central memory cells as well as naive cells from the peripheral circulation in patients treated with fingolimod (S1P receptor modulator) 62. TEM cells, which have low receptor S1P1 expression, were not reduced significantly. However, the drug resulted in a marked reduction in Th17 effector cells, and this led to the assumption that TCM cells were the precursors for the majority of Th17 effectors 63. Nevertheless, actions of fingolimod extend beyond lymphocyte trapping in the lymph nodes and can have direct effects on signal transducer and activator of transcription-1 (STAT-3) signalling, which is instrumental for the development of Th17 responses 64.
Also of interest are the effects of S1P signalling in the production and retention of TRM cells. TRM cells express CD69 and have KLF2 decreased activation and low S1P1R expression 65. Forced expression of KLF2 or S1P1R on the TRM cells leads to reduced establishment of a TRM pool. This suggests that modulation of S1P signalling may also work by altering TRM pools; however, further studies are required to confirm this possible mechanism of action.
(d) Kv1.3 inhibition: Kv1.3 is an outward rectifying potassium channel that is up-regulated specifically on TEM cells and appears to be essential for the effector function of these cells' channels by allowing the countercurrent influx of calcium 66. Tnaive cells and TCM cells have a higher expression of KCa3.1 channels and are relatively less dependent on Kv1.3 67. In MS, previous studies have demonstrated an increased presence of CD45RA–CCR7− TEM cells in perivenular infiltrates and MS lesions 44. These cells have an abundance of Kv1.3 channels. Blockade of Kv1.3 channels has been shown to ameliorate experimental autoimmune encephalitis (EAE), a mouse model of MS, and suppress effector memory cell function of human myelin reactive T cells 41,68,69.
Psoriatic skin lesions also show evidence of accumulation of T cells with up-regulation of Kv1.3 channels 70. A study of a Kv1.3 channel blocker ameliorated disease in skin grafts of psoriatic skin in severe compromised immunodeficient (SCID) mice 70. Thus blockade of Kv1.3 channels may serve as an effective treatment targeting autoimmune TEM cells.
(e) Tumour necrosis factor (TNF) family receptor FAS/CD95 targeting: the TNF family receptor FAS plays an important role in maintaining immunological self-tolerance 71. Interestingly, it has been shown that TEM cells are highly susceptible to FAS-mediated apoptosis, while TCM and Tnaive cells are relatively resistant. Administration of FAS ligand led to apoptosis of TEM cells selectively while sparing Tnaive and TCM cells 72. This is thought to be due to more efficient assembly and activation of the FAS-associated death-inducing signalling complex (DISC) in TEM cells. Thus, utilizing FAS ligands may help to eliminate TEM cells in autoimmune diseases.
Targeting memory cell metabolism
Because Tmem cells are long-lived and utilize FAO rather than glycolysis, targeting FAO in these cells may result in reduction in the numbers and function of Tmem cells 36. A recent study demonstrated that, unlike Teff cells, Tmem cells did not utilize extrinsic fatty acids but rather produce fatty acids required for FAO within the cell 35. Lipolysis due to lysosomal acid lipase (LAL) in lysosomes was imperative for the survival of CD8+ Tmem cells. LAL knock-out led to a lack of production of Tmem cells when Teff cells were deprived of antigen and hence went through a contraction phase, underlining the importance of metabolism in the development of Tmem cells. In another study the suppression of FAO in CD4+ Tmem cells led to a reduction in the functional capacity and survival of these cells 36. These studies suggest that disruption of specific metabolic pathways in Tmem cells may be an approach for the specific targeting of this cell population.
Humoral memory
Immunological memory in the humoral arm of the adaptive immune system is mediated through long-lived memory B cells and sustained antibody titres produced from long-lived plasma cells 73. These cells are produced primarily in the germinal centres of secondary lymphoid organs; however, the precise mechanisms leading to differentiation from naive B cells to memory B cells or long-lived plasma cells are unclear (Fig. 2) 74.
Fig 2.
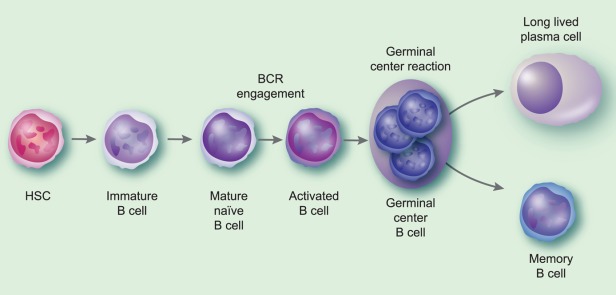
B cell differentiation and generation of memory B cells and long-lived plasma cells. This figure depicts the process of production of memory B cells and long-lived plasma cells. While the majority of memory B cells and long-lived plasma cells are derived from the germinal centre reaction in secondary lymphoid tissues, subsets of each of these types of cells that have extra-follicular origins have been described.
Memory B cells provide enhanced antibody production when restimulated and are characterized by isotype switching and affinity maturation 75. Memory B cells in humans were identified originally as class-switched immunoglobulin D negative cells and have been identified more recently by the expression of CD27. Subsets of B cells that are CD27– but display the characteristics of memory B cells have also been described.
As well as the production of antibodies, memory B cells also play important roles in autoimmunity through cytokine production and antigen presentation to T cells 76,77. Additionally, memory B cells may not require certain survival signals, such as B cell activating factor (BAFF)/BAFF-R interaction, making them resistant to certain drugs targeting these pathways (Belimumab) 78.
Long-lived plasma cells also provide humoral immunological memory and could serve as a source of pathogenic antibodies in autoimmune disease. Long-lived plasma cells can persist for several years and have high expression of CXCR4, which helps them to home to specialized niches high in CXCL12 such as the bone marrow 79. They require several different growth factors for survival, such as IL-6, ligands for CD44 and CD28–B7 interactions 80,81.
Strategies for targeting humoral memory
B cell depletion
In MS, treatment with B cell-depleting agents leads to significant reductions in disease activity 82,83. The basis for this effect appears to be independent of B cell antibody production and may be related to a reduced production of proinflammatory cytokines such as IL-6 or reduced antigen presentation by B cells, which by their sheer numbers are an important APC pool 84. Studies with normal human B cells demonstrated that memory B cells are the major producers of proinflammatory cytokines such as lymphotoxin and TNF-α 77. In patients with MS, B cells produce lower amounts of the anti-inflammatory cytokine IL-10 77. Following B cell depletion, a repopulation with naive B cells that produce larger amounts of IL-10 and lower amounts of proinflammatory cytokines was seen, and could explain the long-term efficacy of this strategy 77,85.
In subjects with immune thrombocytopenic purpura, it was noted that B cell depletion with rituximab led to an increase in the number of splenic long-lived plasma cells. This was thought to be secondary to a change in the splenic micro-environment and could potentially explain the lack of efficacy of B cell depletion in certain autoimmune diseases. In such disorders, a combined strategy that targets both B cells as well as long-lived plasma cells may be required.
Targeted B cell therapies
(a) Atacicept: the importance of memory B cells in the pathogenesis of autoimmune disorders was also demonstrated by the lack of efficacy of atacicept 86,87. Atacicept is a fusion protein consisting of the extracellular domain of the transmembrane activator and CAML interactor (TACI) receptor, bound to the Fc portion of human immunoglobulin 88. This receptor binds B lymphocyte stimulator (BLyS) and a proliferation-inducing ligand (APRIL), which are cytokines involved in B cell proliferation and survival 89. Atacicept was shown to target mature B cells and short-lived plasma cells while sparing memory cells, as well as B cell progenitors 88. In a trial of atacicept in patients with rheumatoid arthritis a transient increase in memory B cells was noted 90. This lack of removal of memory B cells may explain the inefficacy of this approach, given the previous evidence of the role of memory B cells in autoimmunity 76,77.
(b) Tocilizumab: tocilizumab is a humanized monoclonal antibody to the IL-6 receptor that binds both soluble and membrane-bound forms of the receptor. It is approved for use in patients with RA who have had an inadequate response to other therapies 91. IL-6 is a pleiotropic cytokine secreted by several different cell types including T cells, macrophages, fibroblasts, osteoblasts and endothelial cells. It plays an important role in B cell activation and the development of antibody-producing plasma cells. In patients with rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), tocilizumab treatment resulted in a reduction in memory B cells 92,93. Tocilizumab also reduced immunoglobulin levels in patients with SLE and RA, suggesting a reduction in plasma cell numbers 92,94.
The efficacy of tocilizumab suggests that targeting memory B cells may be an effective strategy to treat autoimmune disease.
Future directions
Cautions
Experience with previous ‘targeted' approaches that were found subsequently to have other off-target effects, leading to serious adverse effects, must make us cautious when testing new putative therapies targeting immune memory. Anti-inflammatory medications such as anti-TNF-α agents, that were effective in certain autoimmune disorders, led to the development of inflammatory demyelinating lesions of the CNS 95.
Additionally, although memory cells may play an important role in autoimmunity, they continue to be important for other functions, such as protection against infections and anti-tumour effects. A balanced approach targeting the subsets of cells likely to be the most pathogenic would yield the best balance of risk and benefit.
Better targets for pathogenic memory cells
Targets to define more clearly the factors that cause the generation of different subsets of memory cells would help to develop novel therapies. GWAS studies have now identified several different loci that are associated with autoimmune disease 96,97. Studies are now beginning to link these genes with gene expression data from different cell subtypes to define which memory cell-associated genes are implicated in autoimmune disease 98. Targeting of these gene products may lead to more efficient pathogenic memory cell targeting while preserving helpful immunological memory.
With advances in the understanding of the generation and survival of different memory subsets, insight into the identification of pathogenic memory cells and new approaches to identify cell-specific gene variants in autoimmune disease that may reveal new therapeutic targets, it is likely that memory cell-specific therapies may become a reality in the near future. These could be effective and safe for treating a variety of autoimmune diseases.
Acknowledgments
This work was supported in part by a Sylvia Lawry physician fellowship award from the National Multiple Sclerosis Society (FP-1787-A-1) to P. B.
Disclosure
The authors declare no financial or commercial conflicts of interest.
REFERENCES
- Koch-Henriksen N, Sørensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010;9:520–32. doi: 10.1016/S1474-4422(10)70064-8. [DOI] [PubMed] [Google Scholar]
- Vehik K, Dabelea D. The changing epidemiology of type 1 diabetes: why is it going through the roof? Diabetes Metab Res Rev. 2011;27:3–13. doi: 10.1002/dmrr.1141. [DOI] [PubMed] [Google Scholar]
- Logan I, Bowlus CL. The geoepidemiology of autoimmune intestinal diseases. Autoimmun Rev. 2010;9:A372–8. doi: 10.1016/j.autrev.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223–43. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- Amsen D, Backer RA, Helbig C. Decisions on the road to memory. Adv Exp Med Biol. 2013;785:107–20. doi: 10.1007/978-1-4614-6217-0_12. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen EMM, Sprent J, Surh CD. Generation and maintenance of memory CD4(+) T cells. Curr Opin Immunol. 2009;21:167–72. doi: 10.1016/j.coi.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach C, van Heijst JWJ, Schumacher TNM. The descent of memory T cells. Ann NY Acad Sci. 2011;1217:139–53. doi: 10.1111/j.1749-6632.2010.05830.x. [DOI] [PubMed] [Google Scholar]
- Ahmed R, Bevan MJ, Reiner SL, Fearon DT. The precursors of memory: models and controversies. Nat Rev Immunol. 2009;9:662–8. doi: 10.1038/nri2619. [DOI] [PubMed] [Google Scholar]
- Chang JT, Palanivel VR, Kinjyo I, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–91. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- Buchholz VR, Flossdorf M, Hensel I, et al. Disparate individual fates compose robust CD8+ T cell immunity. Science. 2013;340:630–5. doi: 10.1126/science.1235454. [DOI] [PubMed] [Google Scholar]
- Gerlach C, Rohr JC, Perié L, et al. Heterogeneous differentiation patterns of individual CD8+ T cells. Science. 2013;340:635–9. doi: 10.1126/science.1235487. [DOI] [PubMed] [Google Scholar]
- Hu W, Pasare C. Location, location, location: tissue-specific regulation of immune responses. J Leukoc Biol. 2013;94:409–21. doi: 10.1189/jlb.0413207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair DA, Lefrançois L. Increased competition for antigen during priming negatively impacts the generation of memory CD4 T cells. Proc Natl Acad Sci USA. 2007;104:15045–50. doi: 10.1073/pnas.0703767104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooms H, Wolslegel K, Lin P, Abbas AK. Interleukin-2 enhances CD4+ T cell memory by promoting the generation of IL-7R alpha-expressing cells. J Exp Med. 2007;204:547–57. doi: 10.1084/jem.20062381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders ME, Makgoba MW, Sharrow SO, et al. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988;140:1401–7. [PubMed] [Google Scholar]
- Smith SH, Brown MH, Rowe D, et al. Functional subsets of human helper-inducer cells defined by a new monoclonal antibody, UCHL1. Immunology. 1986;58:63–70. [PMC free article] [PubMed] [Google Scholar]
- Teijaro JR, Turner D, Pham Q, et al. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187:5510–4. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Förster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Champagne P, Ogg GS, King AS, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–11. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- Ellefsen K, Harari A, Champagne P, et al. Distribution and functional analysis of memory antiviral CD8 T cell responses in HIV-1 and cytomegalovirus infections. Eur J Immunol. 2002;32:3756–64. doi: 10.1002/1521-4141(200212)32:12<3756::AID-IMMU3756>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. 2014;14:24–35. doi: 10.1038/nri3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DL, Farber DL. Mucosal resident memory CD4 T cells in protection and immunopathology. Front Immunol. 2014;5:331. doi: 10.3389/fimmu.2014.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Rahimpour A, Ma JZ, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol. 2013;14:1294–301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- Clark RA, Watanabe R, Teague JE, et al. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci Transl Med. 2012;4:117ra7. doi: 10.1126/scitranslmed.3003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JS, Toapanta FR, Salerno-Goncalves R, et al. Characterization and functional properties of gastric tissue-resident memory T cells from children, adults, and the elderly. Front Immunol. 2014;5:294. doi: 10.3389/fimmu.2014.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondrack RM, Harbertson J, Tan JT, et al. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne LC, Dhanji S, Snow JW, et al. Impaired CD8 T cell memory and CD4 T cell primary responses in IL-7R alpha mutant mice. J Exp Med. 2007;204:619–31. doi: 10.1084/jem.20061871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itsumi M, Yoshikai Y, Yamada H. IL-15 is critical for the maintenance and innate functions of self-specific CD8(+) T cells. Eur J Immunol. 2009;39:1784–93. doi: 10.1002/eji.200839106. [DOI] [PubMed] [Google Scholar]
- Schluns KS, Williams K, Ma A, et al. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168:4827–31. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- Waldmann TA. The biology of IL-15: implications for cancer therapy and the treatment of autoimmune disorders. J Invest Dermatol Symp Proc. 2013;16:S28–30. doi: 10.1038/jidsymp.2013.8. [DOI] [PubMed] [Google Scholar]
- Klonowski KD, Williams KJ, Marzo AL, Lefrançois L. Cutting edge: IL-7-independent regulation of IL-7 receptor alpha expression and memory CD8 T cell development. J Immunol. 2006;177:4247–51. doi: 10.4049/jimmunol.177.7.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarajan P, Chen Z. Autoimmune effector memory T cells: the bad and the good. Immunol Res. 2013;57:12–22. doi: 10.1007/s12026-013-8448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bevan MJ. Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity. 2013;39:687–96. doi: 10.1016/j.immuni.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-H, Curtis JD, Maggi LB, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–51. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan D, van der Windt GJW, Huang SC-C, et al. Memory CD8(+) T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity. 2014;41:75–88. doi: 10.1016/j.immuni.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub DD, Hesdorffer CS, Ferrucci L, et al. Distinct energy requirements for human memory CD4 T-cell homeostatic functions. FASEB J. 2013;27:342–9. doi: 10.1096/fj.12-217620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–17. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Balint B, Haas J, Schwarz A, et al. T-cell homeostasis in pediatric multiple sclerosis: old cells in young patients. Neurology. 2013;81:784–92. doi: 10.1212/WNL.0b013e3182a2ce0e. [DOI] [PubMed] [Google Scholar]
- Haegele KF, Stueckle CA, Malin J-P, Sindern E. Increase of CD8+ T-effector memory cells in peripheral blood of patients with relapsing-remitting multiple sclerosis compared to healthy controls. J Neuroimmunol. 2007;183:168–74. doi: 10.1016/j.jneuroim.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Lovett-Racke AE, Trotter JL, Lauber J, et al. Decreased dependence of myelin basic protein-reactive T cells on CD28-mediated costimulation in multiple sclerosis patients. A marker of activated/memory T cells. J Clin Invest. 1998;101:725–30. doi: 10.1172/JCI1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff H, Calabresi PA, Allie R, et al. The voltage-gated Kv1.3 K(+) channel in effector memory T cells as new target for MS. J Clin Invest. 2003;111:1703–13. doi: 10.1172/JCI16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegretta M, Nicklas JA, Sriram S, Albertini RJ. T cells responsive to myelin basic protein in patients with multiple sclerosis. Science. 1990;247:718–21. doi: 10.1126/science.1689076. [DOI] [PubMed] [Google Scholar]
- Kivisäkk P, Mahad DJ, Callahan MK, et al. Expression of CCR7 in multiple sclerosis: implications for CNS immunity. Ann Neurol. 2004;55:627–38. doi: 10.1002/ana.20049. [DOI] [PubMed] [Google Scholar]
- Rus H, Pardo CA, Hu L, et al. The voltage-gated potassium channel Kv1.3 is highly expressed on inflammatory infiltrates in multiple sclerosis brain. Proc Natl Acad Sci USA. 2005;102:11094–9. doi: 10.1073/pnas.0501770102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen KM, Gocke AR, Allie R, et al. Expression of CCR7 and CD45RA in CD4+ and CD8+ subsets in cerebrospinal fluid of 134 patients with inflammatory and non-inflammatory neurological diseases. J Neuroimmunol. 2012;249:86–92. doi: 10.1016/j.jneuroim.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Furuhashi T, Nishida E, et al. Increased population of central memory T cells in circulating peripheral blood of psoriasis patients. J Dermatol Sci. 2013;70:61–4. doi: 10.1016/j.jdermsci.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Matteucci E, Ghimenti M, Di Beo S, Giampietro O. Altered proportions of naïve, central memory and terminally differentiated central memory subsets among CD4+ and CD8 + T cells expressing CD26 in patients with type 1 diabetes. J Clin Immunol. 2011;31:977–84. doi: 10.1007/s10875-011-9573-z. [DOI] [PubMed] [Google Scholar]
- Niesner U, Albrecht I, Janke M, et al. Autoregulation of Th1-mediated inflammation by twist1. J Exp Med. 2008;205:1889–901. doi: 10.1084/jem.20072468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H-D, Radbruch A. Targeting pathogenic T helper cell memory. Ann Rheum Dis. 2011;70(Suppl. 1):i85–7. doi: 10.1136/ard.2010.140954. [DOI] [PubMed] [Google Scholar]
- Krueger GG, Papp KA, Stough DB, et al. A randomized, double-blind, placebo-controlled phase III study evaluating efficacy and tolerability of 2 courses of alefacept in patients with chronic plaque psoriasis. J Am Acad Dermatol. 2002;47:821–33. doi: 10.1067/mjd.2002.127247. [DOI] [PubMed] [Google Scholar]
- Luo L, Sun Z, Cheng H, Luo G. Memory T-cell-specific therapeutics attenuate allograft rejection via mediation of alloreactivity in memory cells. Immunol Lett. 2012;148:53–8. doi: 10.1016/j.imlet.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Rigby MR, DiMeglio LA, Rendell MS, et al. Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Diabetes Endocrinol. 2013;1:284–94. doi: 10.1016/S2213-8587(13)70111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamian F, Lin S-L, Lee E, et al. Alefacept (anti-CD2) causes a selective reduction in circulating effector memory T cells (Tem) and relative preservation of central memory T cells (Tcm) in psoriasis. J Transl Med. 2007;5:27. doi: 10.1186/1479-5876-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers WHPM, van Duijnhoven M, van Erp PEJ, et al. The effect of alefacept on T-cell subsets and cells expressing NK receptors in lesional psoriatic skin: the effects of monotherapy and combination treatment with calcipotriol. J Dermatol Treat. 2008;19:344–50. doi: 10.1080/09546630802050472. [DOI] [PubMed] [Google Scholar]
- Weaver TA, Charafeddine AH, Agarwal A, et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nat Med. 2009;15:746–9. doi: 10.1038/nm.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–90. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann R, Kremer J, Cush J, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367:495–507. doi: 10.1056/NEJMoa1109071. [DOI] [PubMed] [Google Scholar]
- Wojciechowski D, Vincenti F. Tofacitinib in kidney transplantation. Expert Opin Invest Drugs. 2013;22:1193–9. doi: 10.1517/13543784.2013.811231. [DOI] [PubMed] [Google Scholar]
- Mesa RA, Yasothan U, Kirkpatrick P. Ruxolitinib. Nat Rev Drug Discov. 2012;11:103–4. doi: 10.1038/nrd3652. [DOI] [PubMed] [Google Scholar]
- Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043–9. doi: 10.1038/nm.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris CS, Blaho VA, Hla T, Han MH. Sphingosine-1-phosphate receptor 1 signalling in T cells: trafficking and beyond. Immunology. 2014;142:347–53. doi: 10.1111/imm.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehling M, Brinkmann V, Antel J, et al. FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology. 2008;71:1261–7. doi: 10.1212/01.wnl.0000327609.57688.ea. [DOI] [PubMed] [Google Scholar]
- Mehling M, Lindberg R, Raulf F, et al. Th17 central memory T cells are reduced by FTY720 in patients with multiple sclerosis. Neurology. 2010;75:403–10. doi: 10.1212/WNL.0b013e3181ebdd64. [DOI] [PubMed] [Google Scholar]
- Liao J-J, Huang M-C, Goetzl EJ. Cutting edge: alternative signaling of Th17 cell development by sphingosine 1-phosphate. J Immunol. 2007;178:5425–8. doi: 10.4049/jimmunol.178.9.5425. [DOI] [PubMed] [Google Scholar]
- Skon CN, Lee J-Y, Anderson KG, et al. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol. 2013;14:1285–93. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan MD, Chandy KG. Ion channels in the immune system as targets for immunosuppression. Curr Opin Biotechnol. 1997;8:749–56. doi: 10.1016/s0958-1669(97)80130-9. [DOI] [PubMed] [Google Scholar]
- Rangaraju S, Chi V, Pennington MW, Chandy KG. Kv1.3 potassium channels as a therapeutic target in multiple sclerosis. Expert Opin Ther Targets. 2009;13:909–24. doi: 10.1517/14728220903018957. [DOI] [PubMed] [Google Scholar]
- Beeton C, Wulff H, Barbaria J, et al. Selective blockade of T lymphocyte K(+) channels ameliorates experimental autoimmune encephalomyelitis, a model for multiple sclerosis. Proc Natl Acad Sci USA. 2001;98:13942–7. doi: 10.1073/pnas.241497298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Pennington M, Jiang Q, et al. Characterization of the functional properties of the voltage-gated potassium channel Kv1.3 in human CD4+ T lymphocytes. J Immunol. 2007;179:4563–70. doi: 10.4049/jimmunol.179.7.4563. [DOI] [PubMed] [Google Scholar]
- Gilhar A, Bergman R, Assay B, et al. The beneficial effect of blocking Kv1.3 in the psoriasiform SCID mouse model. J Invest Dermatol. 2011;131:118–24. doi: 10.1038/jid.2010.245. [DOI] [PubMed] [Google Scholar]
- Straus SE, Sneller M, Lenardo MJ, et al. An inherited disorder of lymphocyte apoptosis: the autoimmune lymphoproliferative syndrome. Ann Intern Med. 1999;130:591–601. doi: 10.7326/0003-4819-130-7-199904060-00020. [DOI] [PubMed] [Google Scholar]
- Ramaswamy M, Cruz AC, Cleland SY, et al. Specific elimination of effector memory CD4+ T cells due to enhanced Fas signaling complex formation and association with lipid raft microdomains. Cell Death Differ. 2011;18:712–20. doi: 10.1038/cdd.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik MJ, Weisel F. Germinal center selection and the development of memory B and plasma cells. Immunol Rev. 2012;247:52–63. doi: 10.1111/j.1600-065X.2012.01124.x. [DOI] [PubMed] [Google Scholar]
- Pieper K, Grimbacher B, Eibel H. B-cell biology and development. J Allergy Clin Immunol. 2013;131:959–71. doi: 10.1016/j.jaci.2013.01.046. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Mei H, Dörner T, et al. Memory B and memory plasma cells. Immunol Rev. 2010;237:117–39. doi: 10.1111/j.1600-065X.2010.00938.x. [DOI] [PubMed] [Google Scholar]
- Harp CT, Ireland S, Davis LS, et al. Memory B cells from a subset of treatment-naïve relapsing-remitting multiple sclerosis patients elicit CD4(+) T-cell proliferation and IFN-γ production in response to myelin basic protein and myelin oligodendrocyte glycoprotein. Eur J Immunol. 2010;40:2942–56. doi: 10.1002/eji.201040516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duddy M, Niino M, Adatia F, et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol. 2007;178:6092–9. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- Stohl W, Hiepe F, Latinis KM, et al. Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus. Arthritis Rheum. 2012;64:2328–37. doi: 10.1002/art.34400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Y, Waite J, Brewer F, et al. The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity. J Exp Med. 2004;200:1145–56. doi: 10.1084/jem.20041185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassese G, Arce S, Hauser AE, et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol. 2003;171:1684–90. doi: 10.4049/jimmunol.171.4.1684. [DOI] [PubMed] [Google Scholar]
- Njau MN, Kim JH, Chappell CP, et al. CD28-B7 interaction modulates short- and long-lived plasma cell function. J Immunol. 2012;189:2758–67. doi: 10.4049/jimmunol.1102728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–88. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- Kappos L, Li D, Calabresi PA, et al. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet. 2011;378:1779–87. doi: 10.1016/S0140-6736(11)61649-8. [DOI] [PubMed] [Google Scholar]
- Barr TA, Shen P, Brown S, et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med. 2012;209:1001–10. doi: 10.1084/jem.20111675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or A, Calabresi PA, Arnold D, et al. Rituximab in relapsing–remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol. 2008;63:395–400. doi: 10.1002/ana.21363. [DOI] [PubMed] [Google Scholar]
- Richez C, Truchetet M-E, Schaeverbeke T, Bannwarth B. Atacicept as an investigated therapy for rheumatoid arthritis. Expert Opin Invest Drugs. 2014;23:1285–94. doi: 10.1517/13543784.2014.943835. [DOI] [PubMed] [Google Scholar]
- Kappos L, Hartung H-P, Freedman MS, et al. Atacicept in multiple sclerosis (ATAMS): a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Neurol. 2014;13:353–63. doi: 10.1016/S1474-4422(14)70028-6. [DOI] [PubMed] [Google Scholar]
- Hartung H-P, Kieseier BC. Atacicept: targeting B cells in multiple sclerosis. Ther Adv Neurol Disord. 2010;3:205–16. doi: 10.1177/1756285610371146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon SR, Gross JA, Ansell SM, Novak AJ. An APRIL to remember: novel TNF ligands as therapeutic targets. Nat Rev Drug Discov. 2006;5:235–46. doi: 10.1038/nrd1982. [DOI] [PubMed] [Google Scholar]
- Van Vollenhoven RF, Kinnman N, Vincent E, et al. Atacicept in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase II, randomized, placebo-controlled trial. Arthritis Rheum. 2011;63:1782–92. doi: 10.1002/art.30372. [DOI] [PubMed] [Google Scholar]
- Kaly L, Rosner I. Tocilizumab-a novel therapy for non-organ-specific autoimmune diseases. Best Pract Res Clin Rheumatol. 2012;26:157–65. doi: 10.1016/j.berh.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Roll P, Muhammad K, Schumann M, et al. In vivo effects of the anti-interleukin-6 receptor inhibitor tocilizumab on the B cell compartment. Arthritis Rheum. 2011;63:1255–64. doi: 10.1002/art.30242. [DOI] [PubMed] [Google Scholar]
- Shirota Y, Yarboro C, Fischer R, et al. Impact of anti-interleukin-6 receptor blockade on circulating T and B cell subsets in patients with systemic lupus erythematosus. Ann Rheum Dis. 2013;72:118–28. doi: 10.1136/annrheumdis-2012-201310. [DOI] [PubMed] [Google Scholar]
- Illei GG, Shirota Y, Yarboro CH, et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. 2010;62:542–52. doi: 10.1002/art.27221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon AJ, Spain RI, Kruer MC, Bourdette D. Inflammatory neurological disease in patients treated with tumor necrosis factor alpha inhibitors. Mult Scler. 2011;17:1472–87. doi: 10.1177/1352458511412996. [DOI] [PubMed] [Google Scholar]
- The International Multiple Sclerosis Genetics Consortium (IMSGC). Evidence for polygenic susceptibility to multiple sclerosis-the shape of things to come. Am J Hum Genet. 2010;86:621–5. doi: 10.1016/j.ajhg.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecham AH, Patsopoulos NA, Xifara DK, et al. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet. 2013;45:1353–60. doi: 10.1038/ng.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranzini SE, Khankhanian P, Patsopoulos NA. Network-based multiple sclerosis pathway analysis with GWAS data from 15,000 cases and 30,000 controls. Am J Hum Genet. 2013;92:854–65. doi: 10.1016/j.ajhg.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]


