Abstract
Strongly decreased leucocyte counts and a reduced CD4/CD8 T cell ratio in the cerebrospinal fluid (CSF) of natalizumab (NZB)-treated multiple sclerosis (MS) patients may have implications on central nervous (CNS) immune surveillance. With regard to NZB-associated progressive multi-focal leucoencephalopathy, we aimed at delineating a relationship between free NZB, cell-bound NZB, adhesion molecule (AM) expression and the treatment-associated shift in the CSF T cell ratio. Peripheral blood (PB) and CSF T cells from 15 NZB-treated MS patients, and CSF T cells from 10 patients with non-inflammatory neurological diseases and five newly diagnosed MS patients were studied. Intercellular adhesion molecule-1 (ICAM-1), leucocyte function antigen-1 (LFA-1), very late activation antigen-4 (VLA-4), NZB saturation levels, and T cell ratios were analysed by flow cytometry. NZB concentrations were measured by enzyme-linked immunosorbent assay (ELISA). Lower NZB saturation levels (P < 0·02) and a higher surface expression of ICAM-1 and LFA-1 (P < 0·001) were observed on CSF CD8 T cells. CSF T cell ratios (0·3–2·1) and NZB concentrations (0·01–0·42 µg/ml) showed a pronounced interindividual variance. A correlation between free NZB, cell-bound NZB or AM expression levels and the CSF T cell ratio was not found. Extremely low NZB concentrations and a normalized CSF T cell ratio were observed in one case. The differential NZB saturation and AM expression of CSF CD8 T cells may contribute to their relative enrichment in the CSF. The reduced CSF T cell ratio appeared sensitive to steady-state NZB levels, as normalization occurred quickly. The latter may be important concerning a fast reconstitution of CNS immune surveillance.
Keywords: adhesion molecules, CD4/CD8, cerebrospinal fluid, multiple sclerosis, natalizumab, T cell ratio
Introduction
The monoclonal antibody natalizumab (NZB) interferes with immune cell extravasation from the peripheral blood (PB) into tissues by blocking the alpha-4 (CD49d) subunit of very late activation antigen-4 (VLA-4; alpha-4/beta-1 integrin; CD49dCD29). The pathogenic relevance of VLA-4 for multiple sclerosis (MS) was described in the early 1990s by Yednock and co-workers1, who showed that antibodies against the alpha-4 or beta-1 chains interfered with the extravasation of leucocytes into the central nervous system (CNS) in experimental autoimmune encephalomyelitis (EAE). This finding was translated rapidly into the clinical arena and NZB was approved in Europe and reapproved in the United States in 2006 for the treatment of relapsing forms of MS. Via the blocking of leucocyte extravasation, NZB detains immune cells in the peripheral blood. The result is a markedly diminished immune cell transmigration into the CNS, a reduced leucocyte count in the CSF2 and a depletion of dendritic cells within cerebral perivascular spaces3. Disappearance of CSF-specific oligoclonal bands, reduced intrathecal immunoglobulin (Ig)G synthesis and decreased levels of the chemokine CXCL13 are most probably secondary effects of NZB treatment4–7.
Another striking finding is the NZB-induced shift in the ratio of CSF CD4/CD8 T cells (T cell ratio) in favour of CD8 to levels similar to human immunodeficiency virus (HIV)-infected patients8. This is relevant with regard to progressive multifocal leucoencephalopathy (PML), a demyelinating infection of the CNS caused by reactivation of latent JC virus (JCV). Until 2006 PML was seen almost exclusively in severely immune compromised individuals with HIV or cancer. NZB-associated PML is a rare but severe adverse event and up to now it is not completely understood how treatment with NZB facilitates development of PML. Possible scenarios include impaired immune surveillance of the CNS and permissiveness for active JCV replication9.
A CSF T cell ratio in favour of CD4 is considered normal and it is most probably prerequisite for a fully functional CNS immune surveillance. We searched for mechanisms involved in the NZB-associated shift in the CSF T cell ratio in favour of CD8 and analysed CSF and PB immune cells from NZB-treated MS patients undergoing lumbar puncture for exclusion of PML. The intention was to delineate a potential relationship between free and cell-bound NZB, adhesion molecule (AM) expression and the shift in the CSF T cell ratio. The relative accumulation of CD8 T cells or, from another viewpoint, the relative decrease of CD4 T cells in the CSF of NZB-treated patients, may thus be understood more clearly.
Materials and methods
Patients
Blood and CSF were obtained from 15 NZB-treated MS patients (MS-NZB) referred to our hospital for diagnostic lumbar puncture to rule out PML (Table1). The mean age of NZB-treated MS patients was 38 [standard deviation (s.d.) ± 10] years. The mean duration of NZB treatment was 34 (s.d. ± 10) months and the mean time since the last NZB infusion was 3·9 (s.d. ± 1·5) weeks. High-sensitivity polymerase chain reaction (PCR) for the detection of JCV-DNA in the CSF was performed at the Institute for Virology at the Medical University of Vienna. CSF samples from 10 patients diagnosed with non-inflammatory neurological disease (NIND), including depression, mild cognitive impairment and paraesthesia, and from five newly diagnosed MS patients (MSneo) served as controls. The mean age of patients with NIND was 53 (s.d ± 19) years and 31 (s.d. ± 9) in newly diagnosed MS patients. Routine CSF analysis showed a CSF cell count of 1·5 (s.d. ± 1·4) leucocytes/µl in NZB-treated MS patients, 1·4 (s.d. ± 0·7) leucocytes/µl in patients with NIND, and 6·0 (s.d. ± 3·2) leucocytes/µl in newly diagnosed MS patients. The study was approved by the local ethics committee and written consent was obtained from all subjects.
Table 1.
Overview of clinical and laboratory data of the 15 natalizumab (NZB)-treated MS patients.
| NZB* | last NZB† | Routine CSF parameters | NZB (µg/ml) | CD4/CD8 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Age (years) | EDSS | (months) | (weeks) | Cells/µl | QAlb‡ | IgG synthesis§ | OCB¶ | serum | CSF | PB | CSF | |
| 1 | F | 37 | 4·0 | 23 | 3·6 | 1 | 7·5(+) | Negative | Positive | 52 | 0·16 | 2·0 | 0·6 |
| 2 | F | 32 | 5·0 | 39 | n·a· | 1 | 4·1 | Negative | Negative | 65 | 0·11 | 1·5 | 0·4 |
| 3 | M | 52 | 4·0 | 35 | 4·1 | 1 | 8·1(+) | Negative | Negative | 25 | 0·09 | 1·0 | 0·3 |
| 4 | F | 45 | 6·5 | 41 | 5·9 | 1 | 5·0 | Negative | Positive | 4·3 | 0·01 | 2·0 | 2·1 |
| 5 | F | 28 | 6·0 | 60 | 3·9 | 1 | 7·0(+) | Negative | Positive | 20 | 0·05 | 1·7 | 1·4 |
| 6 | F | 20 | 3·5 | 29 | 4·1 | 0 | 3·0 | Negative | Positive | 37 | 0·03 | 1·9 | 1·0 |
| 7 | M | 31 | 6·5 | 51 | 3·3 | 2 | 13·0(+) | Negative | Negative | 52 | 0·23 | 1·7 | 0·9 |
| 8 | F | 53 | 3·0 | 35 | 7·4 | 1 | 3·5 | Positive | Positive | 49·8 | 0·1 | 5·6 | 0·7 |
| 9 | F | 38 | 4·0 | 24 | 4·7 | 1 | 3·3 | Negative | Positive | 15 | 0·03 | 2·1 | 0·4 |
| 10 | M | 40 | 1·5 | 14 | 1·0 | 1 | 10·7(+) | Negative | Positive | 109 | 0·42 | 2·7 | 0·9 |
| 11 | F | 53 | 4·5 | 12 | 2·0 | 1 | 11·2(+) | Negative | Positive | 61 | 0·24 | 1·0 | 0·5 |
| 12 | M | 34 | 4·0 | 64 | 3·6 | 2 | 9·0(+) | Negative | Positive | 59·2 | 0·17 | 2·2 | 0·4 |
| 13 | M | 23 | 2·0 | 4 | 4·0 | 6 | 7·4(+) | Positive | Positive | 39·5 | 0·13 | 0·7 | 0·3 |
| 14 | M | 43 | 4·5 | 67 | 3·1 | 3 | 5·3 | Positive | Positive | 22·3 | 0·07 | 1·6 | 0·7 |
| 15 | F | 37 | 2·0 | 17 | 3·9 | 1 | 3·1 | Negative | Positive | 39·1 | 0·05 | 1·2 | 0·5 |
Duration of NZB treatment.
time since the last NZB infusion.
QAlb (albumin quotient: CSF albumin/serum albumin × 1000) as measure for the blood–CSF-barrier integrity; (+) refers to an increased blood–CSF-barrier permeability.
intrathecal immunoglobulin (Ig)G synthesis evaluated according to Reiber [(1996) Evaluation of blood–CSF barrier function and quantification of the humoral immune response within the CNS. In: Thompson EJ, Trojano M and Livrea P (eds). CSF analysis in multiple sclerosis. Springer-Verlag: Milan, 51–72].
detection of OCB by isoelectric focusing. CSF = cerebrospinal fluid; EDSS = expanded disability status scale; F = female; M = male; OCB = oligoclonal bands; PB = peripheral blood; CD4/CD8 = CD4/CD8 T cell ratio.
Specimens
Enrichment of peripheral blood mononuclear cells (PBMC) from venous blood collected into cell preparation tubes (CPT; Becton Dickinson AG, Basel, Switzerland) was performed as described previously10. Serum was collected from whole blood after centrifugation at 2000 g for 10 min. CSF (7–8 ml) was centrifuged immediately at 350 g for 10 min after collection. Sera and CSF were aliquoted and stored at –80°C until further processing. CSF cells were resuspended in buffer (phosphate buffered saline supplemented with 2.5% bovine serum albumin) and subjected to flow cytometry.
NZB concentrations in the CSF and serum
NZB concentrations of paired CSF and serum samples were determined as described previously by a highly sensitive cross-linking assay11,12 at Sanquin Diagnostic Services (Amsterdam, the Netherlands), with the following modifications: CSF samples were tested in serial dilutions starting at 1:10; background was evaluated using pooled physiological CSF (n = 10).
Monoclonal detection antibodies (mAbs)
Anti-CD3-phycoerythrin Texas Red conjugate (ECD) (clone UCTH1), anti-CD8-phycoerythrin cyanin 5·1 conjugate (PC5) (clone B9·11), anti-CD4-phycoerythrin cyanin 7 conjugate (PC7) (clone SFCI12t4D11), anti-CD49d-fluorescein isothiocyanate (FITC) (alpha-4 subunit of VLA-4; clone HP2/1), anti-CD29-FITC (beta-1 subunit of VLA-4; 4B4), anti-CD11a-FITC [alpha-L subunit of leucocyte function antigen-1 (LFA-1); IM0860U], anti-human IgG4-FITC (clone HP6025) and the corresponding isotype control IgG1-FITC (679·1Mc7) were purchased from Beckman Coulter (Vienna, Austria). Intercellular adhesion molecule-1 (ICAM)-1-FITC (clone RR1/1) and IgG1-FITC (clone P3·6·2·8·1) were obtained from eBioscience (Vienna, Austria).
Flow cytometry
Due to low cell counts in the CSF, six was the maximum number of stainings. Determination of AM expression levels and/or the cellular NZB saturation was omitted if the CSF amount was insufficient: the CSF T cell ratio was therefore determined in 15, AM expression levels in 12 and the NZB saturation of CSF T cells in 10 patients. Immunostainings of PBMC were performed as described previously13. In brief, 100 µl PBMC suspensions (1 × 106 cells/ml) were stained for 30 min on ice in saturating amounts of mAbs. CSF samples were stained in parallel with PBMC samples and in identical concentrations of mAbs. The median fluorescence intensities (MFI) of CSF and PB T cell subsets stained with antibodies specific to ICAM-1, LFA-1 and to the two subunits of VLA-4 were used as measure for AM surface expression.
NZB saturation levels of CSF and PB T cells were determined after in-vitro incubation (30 min on ice) of one cell sample with saturating amounts of NZB (10 µg/ml, representing 100% NZB saturation) and a second aliquot with buffer only (representing in-vivo-bound NZB levels). Unbound NZB was removed by a washing step and bound NZB was detected by flow cytometry using FITC-labelled anti-human IgG4 (anti-huIgG4-FITC). NZB saturation (in %) was calculated from the MFI according to the following formula: MFI anti-hulgG4-FITC of buffer-treated cells/MFI anti-hulgG4 of in-vitro NZB-saturated cells × 100.
Cells were acquired on a Cytometrics FC500 and analysed using CXP software (both from Beckman Coulter, Brea, CA, USA). Cells were gated according to forward- and side-scatter light properties and selected positively for CD3/CD8 and CD3/CD4 expression. AM expression and NZB saturation levels of CD8 and CD4 T cells were analysed. The gating strategy is illustrated in the Supporting information, Figs S1 and S2).
Statistical methods
Data were screened for outliers. For t-tests and correlations, the Kolmogorov–Smirnov test was used to test data for normality. Generalized estimation equation model (GEE) with one fixed factor (NIND, MS-NZB and MS-neo) and CD8 versus CD4 as repeated factor, together with Fisher's least squares difference (LSD) test as post-hoc tests were used for comparisons of means. In this model, the robust estimator for the covariance matrix was used. In addition, a repeated-measures analysis with two repeated factors (PB versus CSF and CD8 versus CD4) together with Fisher's LSD test as post-hoc tests was used. Linear regression analyses with corresponding Pearson's correlation coefficients were performed to compare concentrations of free NZB in the serum and CSF, and to compare AM expression with the CSF and PB CD4/CD8 T cell ratios; 95% confidence intervals (CI) were computed for difference of selected means and for the regression line. All tests were performed two-sided and a P-value less than 5% indicates a statistically significant difference or relation. All computations and illustrations were performed using spss statistics version 21·0 (IBM Germany GmbH, Ehningen, Germany), Microsoft Excel (Microsoft Office 2007, Redmond, WA, USA) and statistica version 10 (Hill and Lewicki 2007).
Results
Free and cell-bound NZB in the PB and CSF from NZB-treated MS patients
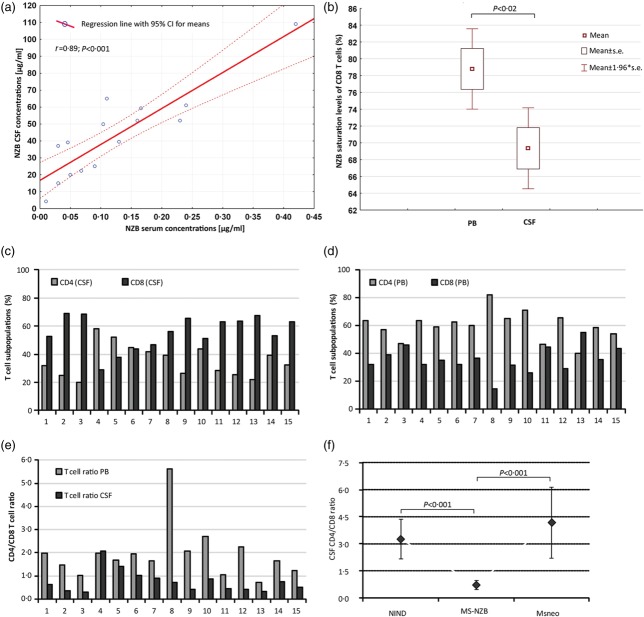
The median NZB concentration was 50·9 µg/ml (range 4·3–109·0) in the serum and 0·11 µg/ml (range 0·01–0·42) in the CSF. The median NZB serum/CSF quotient (QNATS/CSF) was 357 (range 226–1233). For comparison, the median concentration of IgG was 871 mg/dl (range 527–1220) in the serum and 3·3 mg/dl (range 1·9–6·5) in the CSF, resulting in a median IgG serum/CSF quotient (QIgGS/CSF) of 226 (range 119–539). NZB concentrations in the serum and CSF both correlated negatively with the time since the last NZB infusion (serum r = −0·646, P = 0·009; CSF r = −0·565, P = 0·028). A positive correlation was observed between NZB serum and NZB CSF concentrations (r = 0·890, P < 0·001; Fig. 1a).
Fig 1.
Free natalizumab (NZB), cell-bound NZB and the CD4/CD8 T cell ratio. (a) Correlation analysis of NZB concentration levels in paired serum/cerebrospinal fluid (CSF) samples from NZB-treated multiple sclerosis (MS) patients (n = 15) assayed by enzyme-linked immunosorbent assay (ELISA). (b) Mean NZB saturation levels of peripheral blood (PB) and CSF CD8 T cells from NZB-treated MS patients (n = 10) investigated by flow cytometry. Distribution of %CD4 and %CD8 T cell subpopulations in the CSF (c) and PB (d) of NZB-treated MS patients and illustration of the CSF and corresponding PB CD4/CD8 T cell ratios. (f) Comparison of the mean CSF CD4/CD8 T cell ratios of NZB-treated MS patients (n = 15), patients with non-inflammatory neurological disease (NIND) (n = 10) and newly diagnosed MS patients (MSneo, n = 5). Bars (Fig. 1f) represent 95% confidence interval (CI).
Mean NZB saturation levels of CD4 T cells were 82% (s.d. ± 4) in the PB and 78% (s.d. ± 10) in the CSF. Mean NZB saturation levels of CD8 T cells were lower in the CSF (69%, s.d. ± 8) than in the PB (79%, s.d. ± 8; P = 0·016; Fig. 1b), and they were also lower compared to CSF CD4 T cells (82%, s.d. ± 4; P = 0·014). No significant correlation between NZB CSF concentrations and NZB saturation levels of CSF CD8 or CD4 T cells or the CSF T cell ratio was observed.
The CSF CD4/CD8 T cell ratio
We found a T cell ratio in favour of CD8 in the CSF (mean 0·7, s.d. ± 0·5) but not in the PB (mean 1.9, s.d. ± 1·1) of most NZB-treated patients (Fig. 1c–e). The mean CSF T cell ratio of patients with NIND and newly diagnosed MS patients was 3·1 (s.d. ± 1·4) and 4·2 (s.d. ± 1·9), respectively (Fig. 1f).
Of note, the interindividual variability in the CSF T cell ratio in the NZB-treated cohort was pronounced, ranging from 0·3 to 2·1 and in two patients was even above 1·0, thus in favour of CD4.
In patient 4 the CSF ratio was 2·1 and the interval since the last NZB infusion was 6 weeks. The same patient had the lowest NZB concentration in the serum (4·3 µg/ml) and CSF (0·01 µg/ml). In patient 5 the CSF ratio was 1·4 in spite of a 4-weekly treatment interval. NZB concentrations were 20 µg/ml and 0·05 µg/ml in the serum and CSF, respectively. Conversely, in patient 8 the CSF ratio was 0·7, despite an interval of 7–8 weeks since the last NZB infusion. NZB concentrations were 52·2 µg/ml and 0·23 µg/ml in the serum and CSF, respectively.
We tested if the T cell composition of the PB or PB T cell ratio had any influence on the CSF T cell ratio, but found no correlation (r = 0·158; P = 0·57).
AM expression of CSF and PB T cells from NZB-treated MS patients
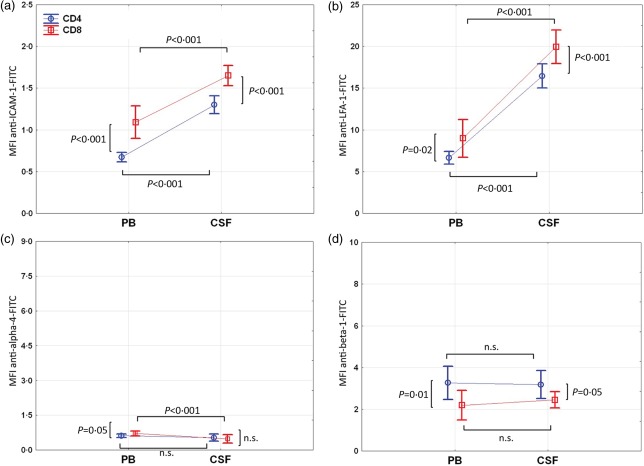
Expression levels of ICAM-1 and LFA-1 were significantly higher on CSF compared to PB CD8 and CD4 T cells (P < 0·001; the following results are the mean differences in ICAM-1 and LFA-1 MFIs between PB and CSF T cells and the corresponding 95% CI of the difference: ICAM-1, CD8: 0·6 (0·5–0·8); ICAM-1, CD4: 0·6 (0·4–0·7); LFA-1, CD8: 10·9 (9·1–12·8); LFA-1, CD4: 9·8 (7·9–11·6); Fig. 3a,b, Table2). Both AM were expressed higher on CSF and PB CD8 compared to CD4 T cells (P < 0·02 for all; the following results are the mean differences in ICAM-1 and LFA-1 MFIs between CD8 and CD4 T cells and the corresponding 95% CI of the difference: ICAM-1, CSF: 0·4 (0·2–0·5); ICAM-1, PB: 0·4 (0·3–0·5); LFA-1, CSF: 3·5 (1·6–5·3); LFA-1, PB: 2·3 (0·5–4·2), Fig. 2a,b; Table2).
Table 2.
Overview of AM expression levels on PB and/or CSF T cells from the three patient groups.
| 1. CSF versus PB T cells in NZB-treated MS patients (n = 12) | ||||||||
|---|---|---|---|---|---|---|---|---|
| CD8 T cells | CD4 T cells | |||||||
| CSF | PB | Difference in MFI | P-value | CSF | PB | Difference in MFI | P-value | |
| MFI (SD) | MFI (s.d.) | mean (95% CI) | MFI (s.d.) | MFI (s.d.) | mean (95% CI) | |||
| ICAM-1 | 1·7 (0·2) | 1·1 (0·3) | 0·6 (0·4–0·7) | <0·001 | 1·3 (0·2) | 0·7 (0·1) | 0·6 (0·5–0·8) | <0·001 |
| LFA-1 | 19·9 (3·2) | 8·9 (3·6) | 10·9 (9·1–12·8) | <0·001 | 16·5 (2·3) | 6·7 (1·2) | 9·8 (7·9–11·6) | <0·001 |
| alpha-4 | 0·5 (0·3) | 0·7 (0·2) | 0·2 (0·1–0·3) | <0·001 | 0·5 (0·2) | 0·6 (0·1) | 0·1 (0·0–0·2) | 0·14 |
| beta-1 | 2·5 (0·6) | 2·1 (1·1) | 0·3 (−0·5–1·0) | 0·47 | 3·2 (1·1) | 3·3 (1·2) | 0·1 (0·6–0·8) | 0·81 |
| 2. CSF CD8 T cells versus CSF CD4 T cells in NZB-treated patients, patients with NIND and newly diagnosed MS patients | ||||||||
|---|---|---|---|---|---|---|---|---|
| NZB-treated MS patients (n = 12) | Patients with NIND (n = 10) | |||||||
| CD8 | CD4 | Difference in MFI | P-value | CD8 | CD4 | Difference in MFI | P-value | |
| MFI (s.d.) | MFI (s.d.) | mean (95% CI) | MFI (s.d.) | MFI (s.d.) | mean (95% CI) | |||
| ICAM-1 | 1·7 (0·2) | 1·3 (0·2) | 0·4 (0·2–0·5) | <0·001 | 1·4 (0·3) | 1·1 (0·2) | 0·2 (0·1–0·3) | <0·001 |
| LFA-1 | 19·9 (3·2) | 16·5 (2·3) | 3·5 (1·6–5·3) | <0·001 | 21·4 (5·4) | 13·8 (2·9) | 7·6 (5·5–9·7) | <0·001 |
| alpha-4 | 0·5 (0·3) | 0·5 (0·2) | 0·1 (0·0–0·2) | 0·26 | 4·1 (1·3) | 5·1 (2·2) | 1·0 (0·1–1·8) | 0·028 |
| beta-1 | 2·5 (0·6) | 3·2 (1·1) | 0·7 (0·0–1·5) | 0·05 | 5·9 (1·1) | 8·7 (1·4) | 2·8 (2·3–3·3) | <0·001 |
| Newly diagnosed MS patients (n = 5) | ||||||||
|---|---|---|---|---|---|---|---|---|
| CD8 | CD4 | Difference in MFI | P-value | |||||
| MFI (s.d.) | MFI (s.d.) | mean (95% CI) | ||||||
| ICAM-1 | 1·4 (0·1) | 1·0 (0·1) | 0·4 (0·3–0·4) | <0·001 | ||||
| LFA-1 | 21·9 (1·6) | 13·8 (0·5) | 8·2 (7·1–9·2) | <0·001 | ||||
| alpha-4 | 4·4 (0·9) | 4·8 (0·8) | 0·4 (0·2–0·6) | 0·001 | ||||
| beta-1 | 5·9 (0·5) | 9·3 (1·0) | 3·5 (3·1–3·8) | <0·001 | ||||
| 3. CSF T cells of NZB-treated patients versus CSF T cells from patients with NIND or newly diagnosed MS patients | ||||||||
|---|---|---|---|---|---|---|---|---|
| CD8 T cells | CD4 T cells | |||||||
| MS-NZB | NIND | Difference in MFI | P value | MS-NZB | NIND | Difference in MFI | P-value | |
| MFI (s.d.) | MFI (s.d.) | mean (95% CI) | MFI (s.d.) | MFI (s.d.) | mean (95% CI) | |||
| ICAM-1 | 1·7 (0·2) | 1·4 (0·3) | 0·3 (0·1–0·5) | 0·05 | 1·3 (0·2) | 1·1 (0·2) | 0·2 (0·0–0·3) | 0·077 |
| LFA-1 | 19·9 (3·2) | 21·4 (5·4) | 1·4 (−2·2–5·0) | 0·442 | 16·5 (2·3) | 13·8 (2·9) | 2·7 (0·6–4·8) | 0·011 |
| alpha-4 | 0·5 (0·3) | 4·1 (1·3) | 3·7 (2·9–4·4) | <0·001 | 0·5 (0·2) | 5·1 (2·2) | 4·6 (3·2–5·9) | <0·001 |
| beta-1 | 2·5 (0·6) | 5·9 (1·1) | 3·5 (2·8–4·3) | <0·001 | 3·2 (1·1) | 8·7 (1·4) | 5·6 (4·6–6·5) | <0·001 |
| CD8 T cells | CD4 T cells | |||||||
| MS-NZB | MSneo | Difference in MFI | P value | MS-NZB | MSneo | Difference in MFI | P-value | |
| MFI (s.d.) | MFI (s.d.) | mean (95% CI) | MFI (s.d.) | MFI (s.d.) | mean (95% CI) | |||
| ICAM-1 | 1·7 (0·2) | 1·4 (0·1) | 0·2 (0·1–0·4) | 0·001 | 1·3 (0·2) | 1·0 (0·1) | 0·3 (0·2–0·4) | <0·001 |
| LFA-1 | 19·9 (3·2) | 21·9 (1·6) | 2·0 (0·2–4·1) | 0·207 | 16·5 (2·3) | 13·8 (0·5) | 2·7 (1·4–4·0) | <0·001 |
| alpha-4 | 0·5 (0·3) | 4·4 (0·9) | 3·9 (3·1–4·7) | <0·001 | 0·5 (0·2) | 4·8 (0·8) | 4·2 (3·6–4·9) | <0·001 |
| beta-1 | 2·5 (0·6) | 5·9 (0·5) | 3·4 (2·9–3·9) | <0·001 | 3·2 (1·1) | 9·3 (1·0) | 6·1 (5·2–7·1) | <0·001 |
AM = adhesion molecule; CI = confidence interval; CSF = cerebrospinal fluid; MFI = median fluorescence intensity; MSneo = newly diagnosed MS patients; MS-NZB = NZB-treated MS patients; NIND = non-inflammatory neurological disease; NZB = natalizumab; PB = peripheral blood; s.d. = standard deviation.
Fig 2.
Adhesion molecule (AM) expression levels in the peripheral blood (PB) and cerebrospinal fluid (CSF) of natalizumab (NZB)-treated patients. (a) Intercellular adhesion molecule-1 (ICAM-1), (b) leucocyte function antigen-1 (LFA-1), (c) alpha-4 and (d) beta-1 median fluorescence intensity (MFI) levels of CD4 (open circles) and CD8 (open squares) T cells in the PB and CSF from NZB-treated patients (n = 12) are shown. Bars represent 95% confidence interval (CI); n.s. = not significant.
Surface expression levels of the alpha-4 subunit of VLA-4 on CSF and PB T cells were decreased pronouncedly and approximated background MFI levels of the isotype (negative) control. The pronounced decrease was proof that NZB treatment was effective, because NZB and the anti-alpha-4 detection antibody clone HP2/1 share the same epitope. Cell-bound NZB, therefore, interferes with binding of the detection antibody. For this reason, we did not go into detail concerning differences in residual anti-alpha-4-FITC MFIs between PB and CSF T cells (Fig. 2c; Table2).
Expression levels of the beta-1 subunit of VLA-4 were similar on CD8 and CD4 T cells in the CSF and PB. Unlike ICAM-1 and LFA-1, beta-1 was higher expressed on CD4 compared to CD8 T cells in the CSF (P = 0·048) and PB (P = 0·01).
AM expression of CSF T cells from NZB-treated MS patients versus NIND and MS at first diagnosis
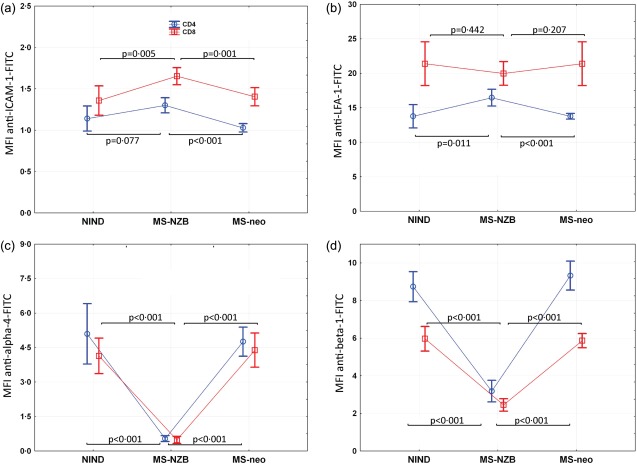
We compared AM expression levels of CSF T cells from the NZB-treated MS patients with those from patients with NIND (n = 10) and newly diagnosed MS patients (n = 5), primarily to confirm observations attributable to NZB treatment. An exact overview of results is given in Table2.
Both CD8 and CD4 T cells in the CSF from patients with NIND and newly diagnosed MS patients showed clearly higher expression levels of both VLA-4 subunits than those from NZB-treated patients (P < 0·001 for all; the following results are the mean differences in alpha-4 and beta-1 MFIs between NZB-treated patients and patients with NIND/newly diagnosed MS patients and the corresponding 95% CI: alpha-4, CD8: 3·7 (2·9–4·4)/3·9 (3·1–4·7); alpha-4, CD4: 4·6 (3·2–5·9)/4·2 (3·6–4·9); beta-1, CD8: 3·5 (2·8–4·3)/3·4 (2·9–3·9); beta-1, CD4: 5·6 (4·6–6·5)/6·1 (5·2–7·1); Fig. 3c,d). As in NZB-treated patients, beta-1 expression levels of CSF CD4 T cells from both patients with NIND and newly diagnosed MS patients, were higher than those of CD8 T cells (P < 0·001; mean difference in beta-1 MFI on CSF T cells from patients with NIND/newly diagnosed MS patients and the corresponding 95% CI: 2·8 (2·3–3·3)/3·5 (3·1–3·8). Of note, alpha-4 expression levels of CSF CD4 T cells from the two control groups were also higher than those of CD8 T cells (NIND, P = 0·028; newly diagnosed MS patients, P = 0·001; mean difference in alpha-4 MFI on CSF T cells from patients with NIND/newly diagnosed MS patients and the corresponding 95% CI: 1·0 (0·1–1·8)/0·4 (0·2–0·6).
Fig 3.
Adhesion molecule (AM) expression levels in the cerebrospinal fluid (CSF) of natalizumab (NZB)-treated patients and controls. (a) Intercellular adhesion molecule-1 (ICAM-1), (b) leucocyte function antigen-1 (LFA-1), (c) alpha-4 and (d) beta-1 median fluorescence intensity (MFI) levels of CD4 (small squares) and CD8 (large squares) T cells in the CSF of NZB-treated MS patients (n = 12), patients with non-inflammatory neurological disease (NIND) (n = 10) and newly diagnosed multiple sclerosis (MS) patients (MSneo, n = 5) were compared. Bars represent 95% confidence interval (CI); n.s. = not significant.]
We further observed higher expression levels of ICAM-1 on CSF CD8 T cells from NZB-treated patients compared to those from patients with NIND [P = 0·005; mean difference (95% CI) in ICAM-1 MFI: 0·3 (0·05–0·51)] and newly diagnosed MS patients [P = 0·001; mean difference (95% CI) in ICAM-1 MFI: 0·2 (0·01–0·4); Fig. 3a]. The same trend was observed upon comparing ICAM-1 expression levels of CSF CD4 T cells between patient groups (NIND, P = 0·077; mean difference (95% CI) in ICAM-1 MFI: 0·2 (0·0–0·3); newly diagnosed MS patients (P < 0·001; mean difference (95% CI) in ICAM-1 MFI: 0·3 (0·2–0·4)). Expression levels of LFA-1, too, were higher on CSF CD4 T cells from NZB-treated patients (NIND, P = 0·011; mean difference (95% CI) in LFA-1 MFI: 2·7 (0·6–4·8); newly diagnosed MS patients, P < 0·001; mean difference (95% CI) in LFA-1 MFI: 2·7 (1·4–4·0); Fig. 3b). No differences were observed concerning LFA-1 expression levels on CSF CD8 T cells.
Discussion
We investigated a possible relationship between the NZB-associated shifts in the CD4/CD8 T cell ratio in the CSF and free and cell-bound NZB and/or expression levels of ICAM-1, LFA-1 and VLA-4 on T cells in the CSF and PB. We observed a pronounced interindividual variability in T cell ratios, but a relationship to one of the investigated parameters could not be delineated. Our data, however, provide evidence concerning three possible factors affecting the CSF T cell ratio: steady-state levels of free NZB, lower NZB-saturation levels of CSF CD8 T cells and higher surface expression of ICAM-1 and LFA-1 on CSF CD8 compared to CSF CD4 T cells.
A reduced CSF T cell ratio in NZB-treated MS patients but not in patients with NIND or newly diagnosed MS patients was in agreement with previous studies8,14,15. In addition, we found a pronounced variance of CSF T cell ratios which could not be correlated with corresponding PB T cell ratios or CD8 and CD4 T cell frequencies. In two patients the CSF T cell ratios were even in favour of CD4, and unaltered compared to their PB T cell ratios. In one patient this could be explained by a 6 weeks' treatment-free interval, extremely low NZB serum and CSF concentrations, and a slight increase in the alpha-4 detection of CSF and PB T cells. In contrast, a third patient still had a decreased CSF T cell ratio despite a treatment-free interval of 7–8 weeks. NZB serum and CSF concentrations in this patient were quite high, however, and surface levels of unbound alpha-4 accordingly low. Of note, normalization of the CSF T cell ratio does not necessarily involve a recovery of immune cell transmigration. Stueve et al.8,16 reported persistently low CSF leucocyte counts despite a normalized CSF T cell ratio 6 months after cessation of NZB. Our data show that the T cell ratio appears sensitive to the individual NZB turnover rate, and that normalization can occur quickly. Steady-state NZB levels are apparently one important factor for the NZB-induced decrease in the CSF T cell ratio.
Free NZB was detected in the CSF of all NZB-treated patients and CSF levels correlated with those in serum. CSF NZB concentrations, however, were low and approximately a factor of 350 less than in the serum. For comparison, the factor between CSF and serum IgG from the same patients was approximately 225. The difference might have originated from a lower passage of NZB across the blood–CSF barrier. Indeed, previous findings showed IgG1 and IgG3 as main CSF IgG subtypes and the lowest IgG index for IgG417. NZB has an IgG4 framework, and IgG4 antibodies reportedly have unique biological properties, such as Fab-arm exchange and poor interactions with FcγRs and complement18,19.
Regardless of the lower levels of free NZB in the CSF, they apparently suffice to keep down respectively interfere with VLA-4 expression on CSF T cells. NZB saturation levels of CSF CD4 T cells were 80% and approximated those of PB T cells. NZB saturation levels of CSF CD8 T cells were significantly lower, and a second possible link to the relative enrichment of CD8 T cells in the CSF.
NZB treatment is known to impact upon the surface expression of VLA-4 negatively and, to a lesser extent, of LFA-1 on PB immune cells20–24. Here we provide evidence that NZB affects diminished alpha-4 detection and a clear decrease in the associated beta-1 subunit on CSF T cells, as expression levels in the PB and CSF were similarly low.
Conversely, both LFA-1 and ICAM-1 were expressed more highly on CSF compared to PB T cells. This was consistent with their supposed memory cell phenotype as CSF T cells25–27, and may allow some compensation for the lack of functional alpha-4, especially as we observed higher ICAM-1 levels on CSF CD8 T cells from NZB-treated patients compared to patients with NIND and newly diagnosed MS patients. In this context, they could have contributed to the relative enrichment of CSF CD8 T cells during NZB treatment, which is in line with evidence that T cells are capable of using alternative adhesion molecules for restricted lymphocyte trafficking, as reported for T helper type 1728,29.
With regard to limitations, we acknowledge a relatively small sample size, an MS collective with symptoms of disease activity despite NZB treatment and low abundant CSF cell populations. A lumbar puncture, however, requires a clear indication. CSF from NZB-treated MS patients, therefore, is rare, and we strengthened our data by including CSF data from patients with NIND and newly diagnosed MS patients.
In conclusion, we showed that CSF concentrations of free NZB, although low, apparently suffice to interfere with VLA-4 expression on CSF T cells and that the CSF T cell ratio normalizes quickly in the absence of steady-state NZB levels. The latter might be of importance concerning a fast reconstitution of CNS immune surveillance function. Functional correlation did not provide evidence of a prominent role of one single AM, but higher expression levels of ICAM-1 and LFA-1 on CSF CD8 compared to CD4 T cells in concert with other AM still waiting to be identified may provide the basis for the relative accumulation of CSF CD8 T cells in NZB-treated patients.
Disclosures
A. H. received travel support for scientific meetings from Merck Serono, Genzyme, Novartis and Teva; G. P. received travel support for scientific meetings from Biogen-Idec, Bayer and Merck Serono; P. W. received travel support for scientific meetings from Merck Serono, Biogen-Idec, Bayer and Novartis; K. O. received travel support for scientific meetings from Bayer, Biogen-Idec, Genzyme, Merck Serono and Sanofi-Aventis; J. S. received personal compensations for consulting services and/or speakers honoraria and/or support for congress presentations from Biogen-Idec, Terumo, Merck-Serono and Genzyme; W. H. has nothing to disclose; E. H. B. received financial support for research activities from Abbott Diagnostics, Roche Molecular Diagnostics and Roche Diagnostics Austria; S. A. has nothing to disclose; T. R. reports receiving payment for lectures from AbbVie and Pfizer; D. K., The Biologicals Laboratory of Sanquin Diagnostic Services, with Desiree van der Kleij as Head of this Laboratory, performs assays for biological levels and anti-drug antibodies for many pharmaceutical industries and hospitals; E. T. received financial support for research activities and/or personal compensation for consulting services from Eisai, Ever-Neuropharma, Medtronics, Pfizer, Sunovion, New Bridge, Gerot-Lanacher, Biogen-Idec, Sanovi, Bial, Cyberonics, Novartis, Actavis and UCB Pharma; J. K. received financial support for research activities and/or personal compensation for consulting services from Almirall, Bayer, Biogen-Idec, Genzyme, Medtronic, Merck-Serono, Novartis and Sanofi-Aventis.
Supporting Information
Additional Supporting information may be found in the online version of this article at the publisher's web-site:
Fig. S1. Flow cytometric analysis of adhesion molecule (AM) expression levels of cerebrospinal fluid (CSF) T cells. Shown are the gating strategy and representative dot-plots of one natalizumab (NZB)-treated multiple sclerosis (MS) patient (left panel) and one patient with non-inflammatory neurological disease (NIND) (right panel). CD4 T cells (pink) and CD8 T cells (dark blue) were positively selected from the CD3 T cell population. Of note, we used quadrants and percentages in the figure for clearly illustrating different AM expression levels between the NZB-treated MS patient and the NIND control, whereas in the text and for analysis median fluorescence intensities (MFI) were used. ECD = phycoerythrin Texas Red conjugate; FITC = fluorescein isothiocyanate; ICAM-1 = intercellular adhesion molecule-1; LFA-1 = leucocyte function antigen-1; PC5 = phycoerytrhin cyanin5.1 conjugate; PC7 = phycoerythrin cyanin7 conjugate; SS = side-scatter.
Fig. S2. Flow cytometric analysis of natalizumab (NZB) saturation of cerebrospinal fluid (CSF) T cells. Shown are the gating strategy and representative dot-plots of two NZB-treated multiple sclerosis (MS) patients. Cell-bound NZB (in-vivo levels and after in-vitro saturation treatment) was detected on CD T cells (pink) and CD8 T cells (dark blue) with a fluorescein isothiocyanate (FITC)-labelled anti-human immunoglobulin (Ig)G4 antibody. Of note, we used quadrants and percentages in the figure for clearly illustrating different levels of cell-bound NZB, whereas in the text and for analysis median fluorescence intensities (MFI) were used. EC = phycoerythrin Texas Red conjugate; ICAM-1 = intercellular adhesion molecule-1; LFA-1 = leucocyte function antigen-1; PC5 = phycoerytrhin cyanin5.1 conjugate; PC7 = phycoerythrin cyanin7 conjugate.
References
- Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–6. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- Stuve O, Cepok S, Elias B, et al. Clinical stabilization and effective B-lymphocyte depletion in the cerebrospinal fluid and peripheral blood of a patient with fulminant relapsing–remitting multiple sclerosis. Arch Neurol. 2005;62:1620–3. doi: 10.1001/archneur.62.10.1620. [DOI] [PubMed] [Google Scholar]
- del Pilar MM, Cravens PD, Winger R, et al. Decrease in the numbers of dendritic cells and CD4+ T cells in cerebral perivascular spaces due to natalizumab. Arch Neurol. 2008;65:1596–603. doi: 10.1001/archneur.65.12.noc80051. [DOI] [PubMed] [Google Scholar]
- Sellebjerg F, Bornsen L, Khademi M, et al. Increased cerebrospinal fluid concentrations of the chemokine CXCL13 in active MS. Neurology. 2009;73:2003–10. doi: 10.1212/WNL.0b013e3181c5b457. [DOI] [PubMed] [Google Scholar]
- Villar LM, Garcia-Sanchez MI, Costa-Frossard L, et al. Immunological markers of optimal response to natalizumab in multiple sclerosis. Arch Neurol. 2012;69:191–7. doi: 10.1001/archneurol.2011.971. [DOI] [PubMed] [Google Scholar]
- von Glehn F, Farias AS, de Oliveira AC, et al. Disappearance of cerebrospinal fluid oligoclonal bands after natalizumab treatment of multiple sclerosis patients. Mult Scler. 2012;18:1038–41. doi: 10.1177/1352458511428465. [DOI] [PubMed] [Google Scholar]
- Harrer A, Tumani H, Niendorf S, et al. Cerebrospinal fluid parameters of B cell-related activity in patients with active disease during natalizumab therapy. Mult Scler. 2013;19:1209–12. doi: 10.1177/1352458512463483. [DOI] [PubMed] [Google Scholar]
- Stuve O, Marra CM, Bar-Or A, et al. Altered CD4+/CD8+ T-cell ratios in cerebrospinal fluid of natalizumab-treated patients with multiple sclerosis. Arch Neurol. 2006;63:1383–7. doi: 10.1001/archneur.63.10.1383. [DOI] [PubMed] [Google Scholar]
- Schwab N, Schneider-Hohendorf T, Posevitz V, et al. L-Selectin is a possible biomarker for individual PML risk in natalizumab-treated MS patients. Neurology. 2013;81:865–71. doi: 10.1212/WNL.0b013e3182a351fb. [DOI] [PubMed] [Google Scholar]
- Sellner J, Koczi W, Harrer A, et al. Glatiramer acetate attenuates the pro-migratory profile of adhesion molecules on various immune cell subsets in multiple sclerosis. Clin Exp Immunol. 2013;173:381–9. doi: 10.1111/cei.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rispens T, Leeuwen A, Vennegoor A, et al. Measurement of serum levels of natalizumab, an immunoglobulin G4 therapeutic monoclonal antibody. Anal Biochem. 2011;411:271–6. doi: 10.1016/j.ab.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Rispens T, Vennegoor A, Wolbink GJ, Polman CH, Killestein J. Natalizumab remains detectable in patients with multiple sclerosis long after treatment is stopped. Mult Scler. 2012;18:899–901. doi: 10.1177/1352458511431073. [DOI] [PubMed] [Google Scholar]
- Harrer A, Pilz G, Einhaeupl M, et al. Lymphocyte subsets show different response patterns to in vivo bound natalizumab – a flow cytometric study on patients with multiple sclerosis. PLOS ONE. 2012;7:e31784. doi: 10.1371/journal.pone.0031784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowarik MC, Pellkofer HL, Cepok S, et al. Differential effects of fingolimod (FTY720) on immune cells in the CSF and blood of patients with MS. Neurology. 2011;76:1214–21. doi: 10.1212/WNL.0b013e3182143564. [DOI] [PubMed] [Google Scholar]
- Kowarik MC, Grummel V, Wemlinger S, et al. Immune cell subtyping in the cerebrospinal fluid of patients with neurological diseases. J Neurol. 2014;261:130–43. doi: 10.1007/s00415-013-7145-2. [DOI] [PubMed] [Google Scholar]
- Stuve O, Marra CM, Jerome KR, et al. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol. 2006;59:743–7. doi: 10.1002/ana.20858. [DOI] [PubMed] [Google Scholar]
- Di PF, Gredler V, Kuenz B, et al. Features of intrathecal immunoglobulins in patients with multiple sclerosis. J Neurol Sci. 2010;288:147–50. doi: 10.1016/j.jns.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol. 2010;10:301–16. doi: 10.1038/nri2761. [DOI] [PubMed] [Google Scholar]
- Davies AM, Rispens T, Ooijevaar-de HP, et al. Structural determinants of unique properties of human IgG4-Fc. J Mol Biol. 2014;426:630–44. doi: 10.1016/j.jmb.2013.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrer A, Wipfler P, Einhaeupl M, et al. Natalizumab therapy decreases surface expression of both VLA-heterodimer subunits on peripheral blood mononuclear cells. J Neuroimmunol. 2011;234:148–54. doi: 10.1016/j.jneuroim.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Jilek S, Mathias A, Canales M, et al. Natalizumab treatment alters the expression of T-cell trafficking marker LFA-1 alpha-chain (CD11a) in MS patients. Mult Scler, In press. 2013 doi: 10.1177/1352458513513208. ; published online 20 November 2013. DOI: 10.1177/1352458513513208. [DOI] [PubMed] [Google Scholar]
- Niino M, Bodner C, Simard ML, et al. Natalizumab effects on immune cell responses in multiple sclerosis. Ann Neurol. 2006;59:748–54. doi: 10.1002/ana.20859. [DOI] [PubMed] [Google Scholar]
- Wipfler P, Oppermann K, Pilz G, et al. Adhesion molecules are promising candidates to establish surrogate markers for natalizumab treatment. Mult Scler. 2011;17:16–23. doi: 10.1177/1352458510383075. [DOI] [PubMed] [Google Scholar]
- Defer G, Mariotte D, Derache N, et al. CD49d expression as a promising biomarker to monitor natalizumab efficacy. J Neurol Sci. 2012;314:138–42. doi: 10.1016/j.jns.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Giunti D, Borsellino G, Benelli R, et al. Phenotypic and functional analysis of T cells homing into the CSF of subjects with inflammatory diseases of the CNS. J Leukoc Biol. 2003;73:584–90. doi: 10.1189/jlb.1202598. [DOI] [PubMed] [Google Scholar]
- Kivisakk P, Mahad DJ, Callahan MK, et al. Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci USA. 2003;100:8389–94. doi: 10.1073/pnas.1433000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen KM, Gocke AR, Allie R, et al. Expression of CCR7 and CD45RA in CD4+ and CD8+ subsets in cerebrospinal fluid of 134 patients with inflammatory and non-inflammatory neurological diseases. J Neuroimmunol. 2012;249:86–92. doi: 10.1016/j.jneuroim.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V, Heink S, Petermann F, et al. Th17 lymphocytes traffic to the central nervous system independently of alpha4 integrin expression during EAE. J Exp Med. 2011;208:2465–76. doi: 10.1084/jem.20110434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Hohendorf T, Rossaint J, Mohan H, et al. VLA-4 blockade promotes differential routes into human CNS involving PSGL-1 rolling of T cells and MCAM-adhesion of TH17 cells. J Exp Med. 2014;211:1833–46. doi: 10.1084/jem.20140540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Flow cytometric analysis of adhesion molecule (AM) expression levels of cerebrospinal fluid (CSF) T cells. Shown are the gating strategy and representative dot-plots of one natalizumab (NZB)-treated multiple sclerosis (MS) patient (left panel) and one patient with non-inflammatory neurological disease (NIND) (right panel). CD4 T cells (pink) and CD8 T cells (dark blue) were positively selected from the CD3 T cell population. Of note, we used quadrants and percentages in the figure for clearly illustrating different AM expression levels between the NZB-treated MS patient and the NIND control, whereas in the text and for analysis median fluorescence intensities (MFI) were used. ECD = phycoerythrin Texas Red conjugate; FITC = fluorescein isothiocyanate; ICAM-1 = intercellular adhesion molecule-1; LFA-1 = leucocyte function antigen-1; PC5 = phycoerytrhin cyanin5.1 conjugate; PC7 = phycoerythrin cyanin7 conjugate; SS = side-scatter.
Fig. S2. Flow cytometric analysis of natalizumab (NZB) saturation of cerebrospinal fluid (CSF) T cells. Shown are the gating strategy and representative dot-plots of two NZB-treated multiple sclerosis (MS) patients. Cell-bound NZB (in-vivo levels and after in-vitro saturation treatment) was detected on CD T cells (pink) and CD8 T cells (dark blue) with a fluorescein isothiocyanate (FITC)-labelled anti-human immunoglobulin (Ig)G4 antibody. Of note, we used quadrants and percentages in the figure for clearly illustrating different levels of cell-bound NZB, whereas in the text and for analysis median fluorescence intensities (MFI) were used. EC = phycoerythrin Texas Red conjugate; ICAM-1 = intercellular adhesion molecule-1; LFA-1 = leucocyte function antigen-1; PC5 = phycoerytrhin cyanin5.1 conjugate; PC7 = phycoerythrin cyanin7 conjugate.